Current chronic diseases are a reflection of the Westernized diet that features a decreased consumption of indigestible dietary fiber metabolized by gut bacteria to butyrate, which has a critical role in colonic homeostasis. The authors report on initiating a semivegetarian diet (SVD) for patients with inflammatory bowel disease. There was no untoward effect of the SVD. The remission rate with combined infliximab and SVD for patients with newly diagnosed Crohn Disease was 100%. Maintenance of remission on SVD without scheduled maintenance therapy with biologic drugs was 92% at 2 years. The authors recommend a high fiber intake to treat Crohn Disease.
Abstract
Current chronic diseases are a reflection of the westernized diet that features a decreased consumption of dietary fiber. Indigestible dietary fiber is metabolized by gut bacteria, including Faecalibacterium prausnitzii, to butyrate, which has a critical role in colonic homeostasis owing to a variety of functions. Dietary fiber intake has been significantly inversely associated with the risk of chronic diseases. Crohn disease (CD) is not an exception. However, even authors who reported the inverse association between dietary fiber and a risk of CD made no recommendation of dietary fiber intake to CD patients. Some correspondence was against advocating high fiber intake in CD.
We initiated a semivegetarian diet (SVD), namely a lacto-ovo-vegetarian diet, for patients with inflammatory bowel disease. Our SVD contains 32.4 g of dietary fiber in 2000 kcal. There was no untoward effect of the SVD. The remission rate with combined infliximab and SVD for newly diagnosed CD patients was 100%. Maintenance of remission on SVD without scheduled maintenance therapy with biologic drugs was 92% at 2 years. These excellent short- and long-term results can be explained partly by SVD. The fecal bacterial count of F prausnitzii in patients with CD is significantly lower than in healthy controls. Diet reviews recommend plant-based diets to treat and to prevent a variety of chronic diseases. SVD belongs to plant-based diets that inevitably contain considerable amounts of dietary fiber. Our clinical experience and available data provide a rationale to recommend a high fiber intake to treat CD.
INTRODUCTION
There are interesting articles published on Crohn disease (CD) and dietary fiber.1–3 The cohort study by Ananthakrishnan et al1 showed that a long-term intake of dietary fiber was associated with a lower risk of CD. The authors of the study and its editorial2 advocated to the public the consumption of a recommended amount of dietary fiber (25 g/day for women and 38 g/day for men) (Table 1). However, there was no statement regarding CD patients.1,2 Stein and Cohen3 were against advocating high fiber intake in CD without any clear reason. This misleads physicians and patients into continuance of the conventional low-residue diet for CD. Our clinical experience and available data favor the recommendation of a high fiber diet for CD.
Table 1.
Summarized amounts of dietary fiber from references
| Reference | Subject | Diet | Amount of dietary fiber | Efficacy for CD |
|---|---|---|---|---|
| Ananthakrishnan et al, 20131 | Nurses | The highest quintile of dietary fiber intake (median) | 24.0 g/day | Decreased risk of CD |
| The lowest quintile of dietary fiber intake (median) | 11.6 g/day | Not applicable | ||
| Heaton et al, 197915 | Patients with CD | An unrefined-carbohydrate, fiber-rich diet | 33.4 ± 1.8 g/day | Decrease in admissions |
| Ritchie et al, 198716 | Patients with CD | An unrefined-carbohydrate, fiber-rich diet (median at 2 years) | 27.9 g/day | No effect |
| Control: a refined-carbohydrate diet (median at 2 years) | 18.1 g/day | Not applicable | ||
| Chiba et al, 201018 | Patients with CD | Semivegetarian diet | 32.4 ± 2.1 g/2000 kcal/day | Relapse prevention |
| (Soluble dietary fiber) | (6.8 ± 0.7 g/2000 kcal/day) | |||
| (Insoluble dietary fiber) | (23.3 ± 1.6 g/2000 kcal/day) | |||
| Kaplan, 20132 | Recommendation for women | Not applicable | 25 g/day | Not applicable |
| Recommendation for men | 38 g/day | |||
| Chiba et al, 201018 | Recommendation for women | Not applicable | 17 g/day | Not applicable |
| Recommendation for men | 20 g/day |
CD = Crohn disease.
DIETARY FIBER IN HEALTH AND DISEASE
Effect of Dietary Fiber
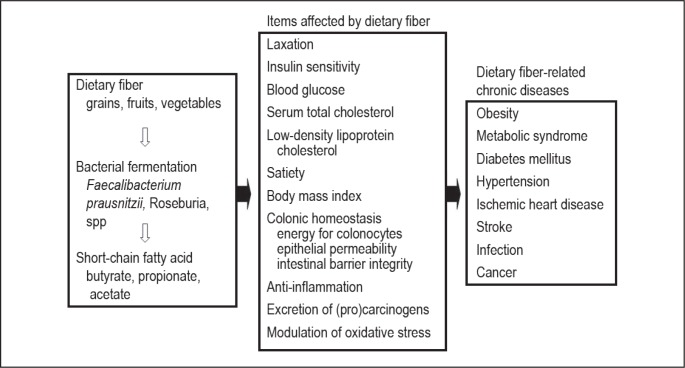
Dietary fiber is known to 1) improve laxation by increasing bulk and reducing transit time of feces through the bowel; 2) increase excretion of bile acid, estrogen, and fecal procarcinogens and carcinogens by binding to them; 3) lower serum cholesterol; 4) slow glucose absorption and improve insulin sensitivity; 5) lower blood pressure; 6) promote weight loss; 7) inhibit lipid peroxidation; and 8) provide anti-inflammatory properties (Figure 1).4 After a large prospective cohort study, Park et al4 found that dietary fiber intake was significantly inversely associated with risk of total death and death from cardiovascular disease, infectious diseases, and respiratory diseases in both men and women. Dietary fiber intake was also related to a lower risk of death from cancer in men (Figure 1). Among specific sources of dietary fiber, fiber from grains showed the most consistent inverse association with risk of total and cause-specific death. Namely, current chronic diseases are related to decreased consumption of dietary fiber—which is a part of dietary Westernization.5 In evaluating the effects of dietary Westernization we are apt to stress adverse effects of increased consumption of animal protein or animal fat, but it is equally important to stress the drawbacks of decreased consumption of dietary fiber.
Figure 1.
Dietary fiber, its effect on and relationship to chronic diseases.
Mechanism of the Effect of Dietary Fiber through the Gut Microflora
Our understanding of the mechanisms of the effect of dietary fiber has advanced since Burkitt et al,6 on the basis of epidemiologic data, postulated that the high incidence of colon cancer, diverticulosis, irritable bowel syndrome, and hemorrhoids as well as atherosclerosis, coronary artery disease, diabetes, obesity, and hyperlipidemia is secondary to prolonged fiber deprivation.7 Indigestible dietary fiber is metabolized to short-chain fatty acids, primarily acetate, propionate, and butyrate, by gut bacteria.8 Short-chain fatty acids serve as a major energy source for colonocytes. Among short-chain fatty acids, butyrate has a critical role in colonic homeostasis owing to a variety of functions: inhibiting inflammation and carcinogenesis, reinforcing various components of the colonic defense barrier, decreasing oxidative stress, and providing a satiety sensation.8 These beneficial effects overlap naturally with those of dietary fiber (Figure 1). Therefore, it is reasonable to postulate a sequence of shortage of dietary fiber, decreased butyrate, and loss of homeostasis that leads to chronic diseases (Figure 1). Recent observations that gut microflora is formed by diet9 and an introduction of the concept that gut microflora is an environmental factor in obesity studies underline the critical role of diet.10 A supporting example of the intestinal metabolism of dietary phosphatidylcholine and cardiovascular risk was recently published.11
Faecalibacterium prausnitzii is one of the most abundant commensal bacteria in the human intestinal microflora of healthy adults, representing more than 5% of the total bacterial population. F prausnitzii is known to produce butyrate, and its production is associated with dietary fiber.12 A meta-analysis shows that the fecal bacterial count of F prausnitzii in patients with inflammatory bowel disease (IBD) is significantly lower than in healthy controls, particularly in CD (Figure 1).13 Whether this finding is observed even before treatment for IBD or whether it is a secondary effect of the current low-residue diet in IBD is to be elucidated.
DIETARY FIBER AND CROHN DISEASE
Case Control Study
Three studies described by Hou et al14 on pre-illness dietary fiber consumption and CD showed that high fiber intake decreased CD risk. One study showed statistical significance in those consuming more than 22.1 g/day compared with less than 13.8 g/day (odds ratio, 0.12; 95% confidence interval, 0.04–0.37).
Cohort Study
The cohort study by Ananthakrishnan et al,1 in which 170,776 women in the Nurses’ Health Study were followed up for 26 years, found that the highest quintile for consuming dietary fiber (median, 24.0 g/day) was associated with a 40% reduction in risk of CD compared with the lowest quintile (11.6 g/day) (Table 1). This was the first prospective cohort study on a large scale determining the relationship between dietary fiber and the risk of CD. It was concluded that long-term intake of dietary fiber is associated with a lower risk of CD.
Intervention Study
There have been two dietary intervention studies in CD focusing on dietary fiber. Heaton et al15 reported significant efficacy of an unrefined-carbohydrate, fiber-rich diet in CD compared with a control diet in the number of hospital admissions, the duration of hospitalizations, and total number of days in the hospital. In this study, dietary fiber was 33.4 ± 1.8 g/day (Table 1). However, a controlled multicenter trial by Ritchie et al16 in 1987 was not able to reproduce the effect (Table 1). To our knowledge, there has been no subsequent intervention study focusing on dietary fiber. The above conflicting results seem to be because of the difference in subjects that happened while evaluating the maintenance effect of a concomitant elemental diet during infliximab therapy.17 The early use of infliximab is more effective than late use in CD, which seemed to result in the conflicting effects of an elemental diet. Most patients (69%) were newly diagnosed (within 3 months) in the study by Heaton et al,15 whereas most patients (54%) were long-standing with intestinal resection in the study by Ritchie et al.16 Recently diagnosed cases might be more responsive to dietary manipulation than long-standing cases.
METHODS AND RESULTS
Our Experience
Expanding our knowledge in gut microflora led to the concept that the greatest environmental factor in IBD is diet-associated gut microflora.10 The microflora is disrupted in IBD mainly by a Westernized diet: increased consumption of animal fat, animal protein, and sugar as well as decreased consumption of dietary fiber. The conventional recommended diet for IBD is a low-residue diet that stems from a fear of irritating the bowel with dietary fiber. However, there is no evidence that such a diet is ideal for IBD.18 A low-residue diet that lacks nondigestible carbohydrates might accelerate the dysbiosis in IBD.19,20 Hoping to increase beneficial bacteria in the gut, we initiated a semivegetarian diet (SVD) replacing the low-residue diet.18 Our SVD is a lacto-ovo-vegetarian diet with an additional serving of fish once a week and meat once every 2 weeks. We provided SVD during hospitalization for 22 consecutive adult CD cases: 14 men and 8 women, age 19 to 77 years (median, 26.5 years), with enterocolitis (11), enteritis (1), or colitis (10). Seventeen patients had active CD whereas 5 patients had undergone resective surgery immediately before the intervention. With regard to the active CD patients, 12 were experiencing the initial onset and 5 were experiencing relapse, the median disease duration was 8.0 months (range, 1 to 74 months), and the main medication was standard induction therapy with infliximab in 16 patients and sulfasalazine in 1. SVD was initiated on the same day as infusion of infliximab. With regard to the postoperative CD patients, 1 was experiencing initial onset with 2 years of disease duration and 4 were experiencing relapse with disease duration more than 8 years; the intervention began on postoperative day 12 to 25; and the main medication was metronidazole 750 mg/day. Initially approximately 800 or 1100 kcal/day was given, and calories were gradually increased to a maximum of approximately 30 kcal/kg standard body weight. The median length of SVD was 49 days for those with active CD and approximately 3 weeks for those with postoperative CD. Remission was defined as the disappearance of active symptoms of CD. All active CD patients obtained remission: the CD active index significantly decreased from 255 ± 169 (mean ± SD) on admission to 46 ± 24 at week 6 (p < 0.0001).
Those patients who achieved clinical remission, either medically or surgically, were provided dietary guidance for SVD before discharge and were advised to maintain SVD after discharge. None of the patients took infliximab or immunosuppressants in the follow-up study. Our SVD contains 32.4 ± 2.1 g of dietary fiber (soluble dietary fiber, 6.8 ± 0.7 g; insoluble dietary fiber, 23.3 ± 1.6 g) in 2000 kcal. The amount is far in excess of the recommended amount for the Japanese population, 17 g/day for women and 20 g/day for men (Table 1).
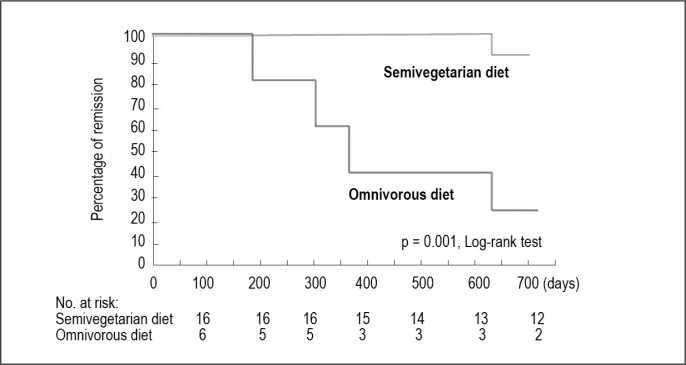
Each patient’s dietary pattern was assessed by means of a food-frequency questionnaire. When the following 2 conditions were fulfilled, it was regarded as SVD. One is that a patient follows the principle of SVD: daily intake of rice, vegetables, and fruits, and occasional intake of fish, meat, and other animal-based foods. The other is that a patient refrains from foods reported as risk factors for IBD in or outside Japan. A diet that did not fulfill these 2 conditions was regarded as an omnivorous diet. Compliance to SVD was 100% among inpatients and 73% among outpatients. There was no untoward effect of SVD in our study. The remission rate with combined infliximab and SVD for newly diagnosed CD was 100% (unpublished observation). Maintenance of remission on SVD was 92% at 2 years (Figure 2).18,21 This was obtained without scheduled maintenance therapy with biologic drugs. Because these excellent short- and long-term results are not obtained by the current low-residue diet they can partly be explained by SVD.
Figure 2.
Life table estimate of maintaining remission with semivegetarian diet or omnivorous diet.
Reprinted with kind permission from the Japanese Society of Digestion and Absorption. Chiba M. Efficacy of semi-vegetarian diet in Crohn’s disease [abstract in English]. Digestion & Absorption 2010:33(3):336–45.
CONCLUSION
Although the precise mechanism is to be determined, epidemiology provides convincing evidence that a plant-based diet is a healthy diet providing therapeutic and/or preventive effects against current major chronic diseases.22–24 Available data suggest the rationale to use dietary fiber in the treatment of IBD.25 We believe a plant-based diet not only is effective for gut inflammation but also promotes the general health of IBD patients.18,26 A plant-based diet inevitably contains considerable amounts of dietary fiber. A high amount of dietary fiber is not harmful and seems to be favorable for CD.
Acknowledgments
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
Cure
Diet cures more than the lancet.
— Spanish proverb
References
- 1.Ananthakrishnan AN, Khalili H, Konijeti GG, et al. A prospective study of long-term intake of dietary fiber and risk of Crohn’s disease and ulcerative colitis. Gastroenterology. 2013 Nov;145(5):970–7. doi: 10.1053/j.gastro.2013.07.050. DOI: http://dx.doi.org/10.1053/j.gastro.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaplan GG. Does consuming the recommend daily level of fiber prevent Crohn’s disease? Gastroenterology. 2013 Nov;145(5):925–7. doi: 10.1053/j.gastro.2013.09.033. DOI: http://dx.doi.org/10.1053/j.gastro.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Stein AC, Cohen RD. Dietary fiber intake and Crohn’s disease. Gastroenterology. 2014 Apr;146(4):1133. doi: 10.1053/j.gastro.2013.12.044. DOI: http://dx.doi.org/10.1053/j.gastro.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 4.Park Y, Subar AF, Hollenbeck A, Schatzkin A. Dietary fiber intake and mortality in the NIHAARP diet and health study. Arch Intern Med. 2011 Jun 27;171(12):1061–8. doi: 10.1001/archinternmed.2011.18. DOI: http://dx.doi.org/10.1001/archinternmed.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller SN, Hackler LR. Changes in the crude fiber content of the American diet. Am J Clin Nutr. 1978 Sep;31(9):1510–4. doi: 10.1093/ajcn/31.9.1510. [DOI] [PubMed] [Google Scholar]
- 6.Burkitt DP, Walker AR, Painter NS. Effect of dietary fibre on stools and the transit-times, and its role in the causation of disease. Lancet. 1972 Dec 30;2(7792):1408–12. doi: 10.1016/s0140-6736(72)92974-1. DOI: http://dx.doi.org/10.1016/S0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 7.Pitchumoni CS, Hertan HI, Yerra N. Current status of dietary fiber. Trop Gastroenterol. 1988 Jul-Aug;9(3):113–22. [PubMed] [Google Scholar]
- 8.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008 Jan 15;27(2):104–19. doi: 10.1111/j.1365-2036.2007.03562.x. DOI: http://dx.doi.org/10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- 9.De Filippo C, Cavalieri D, Di Paola M, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14691–6. doi: 10.1073/pnas.1005963107. DOI: http://dx.doi.org/10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiba M, Tsuda H, Abe T, Sugawara T, Morikawa Y. Missing environmental factor in inflammatory bowel disease: diet-associated gut microflora. Inflamm Bowel Dis. 2011 Aug;17(8):E82–3. doi: 10.1002/ibd.21745. DOI: http://dx.doi.org/10.1002/ibd.21745. [DOI] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013 Apr 25;368(17):1575–84. doi: 10.1056/NEJMoa1109400. DOI: http://dx.doi.org/10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benus RF, van der Werf TS, Welling GW, et al. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr. 2010 Sep;104(5):693–700. doi: 10.1017/S0007114510001030. DOI: http://dx.doi.org/10.1017/S0007114510001030. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: a meta-analysis and systemic review of the literature. Gastroenterol Res Pract. 2014;2014:872725. doi: 10.1155/2014/872725. DOI: http://dx.doi.org/10.1155/2014/872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011 Apr;106(4):563–73. doi: 10.1038/ajg.2011.44. DOI: http://dx.doi.org/10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- 15.Heaton KW, Thornton JR, Emmett PM. Treatment of Crohn’s disease with an unrefined-carbohydrate, fibre-rich diet. Br Med J. 1979 Sep 29;2(6193):764–6. doi: 10.1136/bmj.2.6193.764. DOI: http://dx.doi.org/10.1136/bmj.2.6193.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritchie JK, Wadsworth J, Lennard-Jones JE, Rogers E. Controlled multicentre therapeutic trial of an unrefined carbohydrate, fibre rich diet in Crohn’s disease. Br Med J (Clin Res Ed) 1987 Aug 29;295(6597):517–20. doi: 10.1136/bmj.295.6597.517. DOI: http://dx.doi.org/10.1136/bmj.295.6597.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiba M, Tsuji T, Komatsu M. Conflicting results on the efficacy of enteral nutrition during infliximab maintenance therapy for Crohn’s disease are correct. Dig Dis Sci. 2014 Jan;59(1):227–8. doi: 10.1007/s10620-013-2954-9. DOI: http://dx.doi.org/10.1007/s10620-013-2954-9. [DOI] [PubMed] [Google Scholar]
- 18.Chiba M, Abe T, Tsuda H, et al. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol. 2010 May 28;16(20):2484–95. doi: 10.3748/wjg.v16.i20.2484. DOI: http://dx.doi.org/10.3748/wjg.v16.i20.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker AW, Ince J, Duncan SH, et al. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. ISME J. 2011 Feb;5(2):220–30. doi: 10.1038/ismej.2010.118. DOI: http://dx.doi.org/10.1038/ismej.2010.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012 Aug;4(8):1059–119. doi: 10.3390/nu4081095. DOI: http://dx.doi.org/10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chiba M. Efficacy of semi-vegetarian diet in Crohn’s disease [abstract in English] Digestion & Absorption. 2010;33(3):336–45. [Google Scholar]
- 22.McEvoy CT, Temple N, Woodside JV. Vegetarian diets, low-meat diets and health: a review. Public Health Nutr. 2012 Dec;15(12):2287–94. doi: 10.1017/S1368980012000936. DOI: http://dx.doi.org/10.1017/S1368980012000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Cancer Research Fund; American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: American Institute for Cancer Research; 2007. Nov, [Google Scholar]
- 24.Tuso PJ, Ismail MH, Ha BP, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. DOI: http://dx.doi.org/10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galvez J, Rodríguez-Cabezas ME, Zarzuelo A. Effects of dietary fiber on inflammatory bowel disease. Mol Nutr Food Res. 2005 Jun;49(6):601–8. doi: 10.1002/mnfr.200500013. DOI: http://dx.doi.org/10.1002/mnfr.200500013. [DOI] [PubMed] [Google Scholar]
- 26.Chiba M, Ohno H, Ishii H, Komatsu M. Plant-based diets in Crohn’s disease [Letter] Perm J. 2014 Fall;18(4):94. doi: 10.7812/TPP/14-117. DOI: http://dx.doi.org/10.7812/TPP/14-117. [DOI] [PMC free article] [PubMed] [Google Scholar]