Atherosclerosis associated with high dietary intake of meats, fat, and carbohydrates remains the leading cause of mortality in the US. Polyphenols derived from dietary plant intake have protective effects on vascular endothelial cells, possibly as antioxidants that prevent oxidation of low-density lipoprotein. This review provides a mechanistic perspective of the evidence for protection by a plant-based diet against atherosclerotic coronary artery disease.
Abstract
A plant-based diet is increasingly becoming recognized as a healthier alternative to a diet laden with meat. Atherosclerosis associated with high dietary intake of meat, fat, and carbohydrates remains the leading cause of mortality in the US. This condition results from progressive damage to the endothelial cells lining the vascular system, including the heart, leading to endothelial dysfunction. In addition to genetic factors associated with endothelial dysfunction, many dietary and other lifestyle factors, such as tobacco use, high meat and fat intake, and oxidative stress, are implicated in atherogenesis. Polyphenols derived from dietary plant intake have protective effects on vascular endothelial cells, possibly as antioxidants that prevent the oxidation of low-density lipoprotein. Recently, metabolites of L-carnitine, such as trimethylamine-N-oxide, that result from ingestion of red meat have been identified as a potential predictive marker of coronary artery disease (CAD). Metabolism of L-carnitine by the intestinal microbiome is associated with atherosclerosis in omnivores but not in vegetarians, supporting CAD benefits of a plant-based diet. Trimethylamine-N-oxide may cause atherosclerosis via macrophage activation. We suggest that a shift toward a plant-based diet may confer protective effects against atherosclerotic CAD by increasing endothelial protective factors in the circulation while reducing factors that are injurious to endothelial cells. The relative ratio of protective factors to injurious endothelial exposure may be a novel approach to assessing an objective dietary benefit from a plant-based diet. This review provides a mechanistic perspective of the evidence for protection by a plant-based diet against atherosclerotic CAD.
INTRODUCTION
Despite the remarkable work done by physicians to treat coronary artery disease (CAD), heart disease is still the leading cause of death in the US.1 A Western diet containing large amounts of sugar, salt, cholesterol, and fat can lead to diabetes mellitus, high blood pressure, hyperlipidemia, obesity, and CAD. Major CAD risk factors, such as tobacco use, hypercholesterolemia, hypertension, and diabetes, have all been found to cause vascular endothelial cell (VEC) injury and dysfunction.2 VEC injury and dysfunction lead to atherogenesis, atherosclerosis, and atherothrombotic CAD. Recent literature suggests that lifestyle management that includes a diet of mostly plants may help prevent and reverse CAD.3 Plant-based nutrition is the predominant consumption of plant-based, whole foods to obtain macronutrients (carbohydrates, protein, and fats), micronutrients (vitamins and minerals), and bioactive components (eg, flavonoids, plant sterols, polyphenols) that optimize body function. It is a conscious and mindful decision to maximize the health benefits per calorie while minimizing potential harmful exposures. A plant-based diet is by definition low in fat, cholesterol, salt, animal products, and sugar. As a result, a plant-based diet is associated with a lower incidence of CAD and thus lower costs associated with the treatment of CAD. Therefore, changing from a Western diet to a plant-based diet may be a simple, low-cost intervention that prevents atherothrombotic CAD.
The primary aim of the plant-based diet is to maximize the consumption of nutrient-dense plant foods while minimizing processed foods, added sugars, oils, and animal-based foods.3 A plant-based diet encourages lots of vegetables and fruits and is low in fat.4 Broadly defined, a plant-based diet has significant health benefits, and studies have shown that a plant-based diet can be an effective treatment for obesity,5–9 diabetes,10–14 hypertension,15 hyperlipidemia,16 and heart disease.17,18
The Lifestyle Heart Trial found that 82% of patients diagnosed with heart disease who followed this plant-based diet program had some level of regression of atherosclerosis and 91% had a reduction in the frequency of angina episodes, whereas 53% of the control group, fed the American Heart Association diet, had progression of atherosclerosis.17 In addition, the study showed a reduction in low-density lipoprotein (LDL) (37.2%) that is similar to results achieved with lipid-lowering medications. Similarly, other researchers showed that compared with a control group, the plant-based diet group had a 73% decrease in coronary events and a 70% decrease in all-cause mortality.15 In 1998, a collaborative analysis using original data from 5 prospective studies was reviewed and showed that, compared with nonvegetarians, vegetarians had a 24% reduction in ischemic heart disease death rates.19
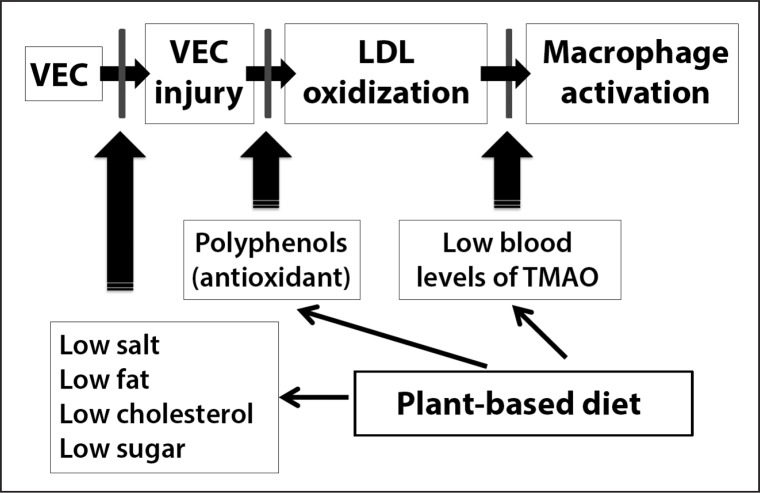
Atherothrombotic CAD is a largely preventable condition that is characterized by the formation of atherosclerotic plaques. Atherosclerotic lesions are the result of an excessive, inflammatory-fibro-proliferative response to various forms of insult to VEC lining the coronary arteries.2 VECs are active and dynamic cells involved in maintaining homeostasis in both disease and health.20 The main function of VECs is to modulate nitric oxide production, leukocyte and platelet adhesion, and leukocyte (macrophage) transmigration. The three main stages of atherogenesis that lead to atherosclerosis are outlined in Table 1 and shown in Figure 1 and include VEC injury, LDL oxidation, and macrophage activation. VECs become dysfunctional after they are injured, resulting in an inflammatory response. Dysfunctional VECs lose the ability to produce nitric oxide and to prevent adhesion of platelets, LDL, and monocytes to the VEC. As a result, LDL and monocytes adhere and migrate into the subendothelial space. In the subendothelial space, LDL becomes oxidized and promotes monocyte transformation to macrophages. In addition, more macrophages are recruited to the area of abnormal endothelium by the expression of cell adhesion molecules and cytokines and are activated by oxidized LDL (OxLDL).21 Macrophages absorb the OxLDL, leading to further activation and enlargement and creating foam cells and fibrous plaques.22–24 Activated macrophages secrete effector molecules that kill cells, degrade the extracellular matrix, and increase vascular smooth muscle cell apoptosis, promoting plaque formation and destabilization. Over time and with repeated VEC injury, arteries narrow and become diseased with the penultimate rupture of unstable plaques in coronary arteries, leading to complete occlusion of the artery, ischemia, and death.
Table 1.
Steps of artherogenesis1
| VEC injury | LDL oxidation | Macrophage activation |
|---|---|---|
| VEC dysfunction | Chemokine released by VEC | Macrophages release cytokines that recruit more monocytes to subendothelial space |
| Decrease nitric oxide ➔ vasoconstriction | Monocytes adhere to VEC | Marcophages take up oxidized LDL and become foam cells |
| Platelet and monocyte adhesion | Monocytes migrate to subendothelial cell space | Foam cells release cytokines that cause smooth muscle formation resulting in formation of fibrous cap |
| LDL uptake and LDL migration to subendothelial space | Monocytes differentiate into macrophages | Fibrous cap becomes unstable and ruptures, resulting in thrombosis and blood vessel occlusion |
Libby P, Ridker RM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011 May 19;473(7347):317–25. DOI: http://dx.doi.org/10.1038/nature/0146.
LDL = low-density lipoprotein; VEC = vascular endothelial cells.
Figure 1.
Potential plant-based diet targets to prevent coronary artery disease.
A new look at the steps of atherogenesis paradigm takes into account new information on plant-based diets. A plant-based diet could be considered a medicine that prevents and treats atherogenesis by multiple pathways. A diet low in fat, low in cholesterol, low in salt, and low in red meat may decrease vascular endothelial cell (VEC) injury. Polyphenols may decrease oxidation of low-density lipoprotein (LDL) and prevent oxidized LDL (OxLDL)-induced monocyte adhesion to VEC, monocyte transformation into macrophages, and foam cell formation. Reducing red meat intake may decrease trimethylamine-N-oxide (TMAO) formation. Decreasing TMAO formation inhibits atherogenesis by down-regulating macrophage uptake of OxLDL. Because there is no direct way to measure atherogenesis, it may be necessary to continue to monitor LDL cholesterol and OxLDL levels, hypertension, and blood sugar levels. In the future, it may be possible to monitor substances such as polyphenols and TMAO that may help to identify patients at risk for atherogenesis before they actually progress to atherosclerosis, plaque rupture, and myocardial infarction.
CORONARY ARTERY DISEASE PREVENTION
Vascular Endothelial Cell Injury
VECs play a key role in the regulation of vascular homeostasis, and increasing evidence suggests that alterations in endothelial function contribute to the pathogenesis and clinical expression of CAD.25 Causes of initial VEC injury include elevated LDL levels,2 elevated blood sugar levels,26 diabetes,27 and high blood pressure.28 Recent studies show that lifestyle management with diet may prevent diabetes,29 lower blood pressure levels,30 lower LDL levels,31 and prevent CAD events and death.32 Therefore, changing to a plant-based diet may decrease CAD mortality by interrupting or reversing the process of atherogenesis.17,18 A plant-based diet decreases the risks associated with atherothrombotic CAD and may down-regulate the atherogenesis inflammatory initiation process by decreasing the intake of substances found in processed foods, added sugars, oils, and meats that promote atherogenesis with a reciprocal increased intake of bioactive substances found in plants that protect the endothelium and inhibit atherogenesis.
Low-Density Lipoprotein Oxidation
Epidemiologic data have shown that people with a high consumption of fruit and vegetables are at a lower risk of death compared with those with a low consumption.33 Data from the Nurses’ Health Study demonstrated an inverse relationship between fruit and vegetable intake and CAD, and each additional serving of leafy green vegetables per day resulted in an 11% decreased risk for CAD.34 Other epidemiologic studies suggest that higher polyphenol intake from a plant-based diet is associated with decreased risk for CAD.35–37 Studies have also shown that decreased intake of flavonoids may be associated with an increased risk of myocardial infarction.38 A systemic review by Mente et al39 in 2009 suggested there is strong evidence that a plant-based diet may protect against CAD whereas a Western diet may promote CAD. One mechanism by which a plant-based diet may promote health is via the positive effects of polyphenols.
A review by Vita25 suggests that the reduced risk of CAD events may be related to the beneficial effect polyphenols have on endothelial cell function. On the basis of cell culture studies, polyphenols may positively affect critical steps in atherogenesis, including LDL oxidation, nitric oxide release, inflammation, oxidative stress, cell adhesion, foam cell formation, smooth muscle cell proliferation, and platelet aggregation.40 Evidence suggests that individuals with the highest polyphenols intake have modestly reduced risks for CAD.36,41–46 There is increasing evidence that flavonoids may have beneficial effects on VEC control of thrombosis, inflammation, and vascular tone.25 Polyphenols may also have beneficial effects limiting platelet adhesion and aggregation that can precipitate acute coronary syndromes after plaque rupture. Diet may also influence the effects of risk factors on VEC function. Prospective studies have shown that endothelial dysfunction is associated with an increased risk of CAD events.47–49 Many interventions known to reduce CAD risk have the ability to reverse endothelial dysfunction.50 These findings suggest that endothelial function may have utility as a surrogate marker of CAD risk. Furthermore, endothelial function has evolved into a clinically useful end point for studies of potential interventions for the prevention or treatment of CAD.
Nitric oxide released from VEC via the endothelial nitric oxide synthase is a vasoprotective molecule.51 In addition to promoting vasodilatation, VEC nitric oxide has anti-atherosclerotic properties that include inhibition of platelet aggregation, of leukocyte adhesion, and of smooth muscle cell proliferation.51 Therefore, VEC nitric oxide production via endothelial nitric oxide synthase is a significant target to prevent and to treat CAD. Leikert et al52 showed that an alcohol-free red wine polyphenol extract strongly increases nitric oxide release, endothelial nitric oxide synthase, and endothelial nitric oxide synthase expression after long-term incubation of human endothelial cells.
To explore this possibility, researchers investigated the effects of tea consumption on endothelial function. Tea contains an assortment of water-soluble antioxidant flavonoids that have been shown to have a beneficial effect on endothelial function and possibly nitric oxide production.53 Other flavonoid-containing beverages, such as grape products, have been shown to improve endothelial function and LDL oxidation in adults with angiographically proven CAD.54
Zamora-Ros and colleagues55 evaluated the relationship between total urinary polyphenols and all-cause mortality during a 12-year period among older adult participants. The study population included 807 men and women aged 65 years and older from Tuscany, Italy. During the 12-year follow-up, 274 participants died. At enrollment, total urinary polyphenols excretion tended to be greater in participants who survived than in those who died. In the multivariable Cox model, participants in the highest tertile of total urinary polyphenols at enrollment had a mortality rate lower than those in the lowest tertile. The authors concluded that total urinary polyphenols is an independent risk factor for mortality among community-dwelling older adults and that a high dietary intake of polyphenols may be associated with longevity. Studies have shown that polyphenols may have an effect on LDL oxidation. In one study, dietary polyphenols reduced plasma LDL oxidation by 20%.56 In another study, resveratrol and alcohol-free wine polyphenols protected LDL from oxidation.57 These data suggest that polyphenols found in fresh fruits and vegetables may help prevent atherogenic lesions by down-regulating oxidation of LDL.
Macrophage Activation
Consuming large amounts of red meat is a risk factor for heart disease. Researchers prospectively followed 84,136 women aged 30 to 55 years in the Nurses’ Health Study during a 26-year period.58 The authors reported that higher intakes of red meat were significantly associated with elevated risk of heart disease. A subsequent study reached a similar conclusion in a cohort of male physicians. Men in the highest quintile for red meat consumption had a 24% increase in risk of heart failure compared with men in the lowest quintile of consumption.59
This strong association between heart disease and consumption of red meat is further supported by recent studies. Studies in animals and humans showed a mechanistic link between intestinal microbial metabolism of nutrients in red meat (choline and L-carnitine) and CAD through the production of a pro-atherosclerotic metabolite called trimethylamine-N-oxide (TMAO).60 Another article published in Nature Medicine62 reported that omnivorous human subjects were found to produce more TMAO than did individuals who consumed primarily a plant-based diet (vegans or vegetarians). A recent study in the New England Journal of Medicine63 reported that TMAO levels are predictors of CAD. The researchers followed 4007 patients, primarily men, undergoing elective angiography for evaluation of possible coronary disease; none had experienced acute coronary syndromes for 3 years. Elevated TMAO levels were associated with an increased risk of major cardiovascular events (hazard ratio for highest vs lowest TMAO quartile, 2.54; 95% confidence interval, 1.96 to 3.28; p < 0.001). Wang et al61 reported that TMAO produced from red meat nutrients may play a role in promoting atherosclerosis by possibly activating macrophages and foam cells.
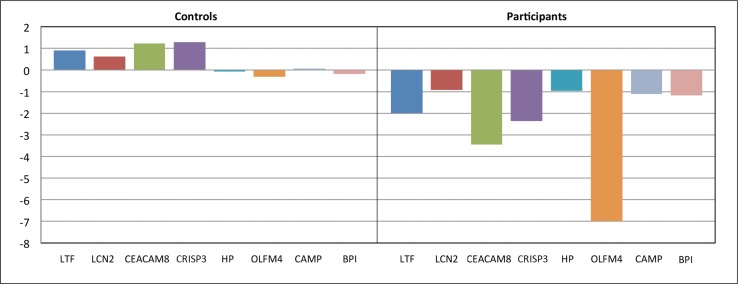
To further study the effect that diet may have on risk factors for atherosclerosis and macrophage activation, Ellsworth et al31 looked at CAD risk factor reduction and peripheral blood gene expression in patients who participated in a year-long lifestyle management program compared with a control group that did not participate. The one-year lifestyle management program included a plant-based diet, 180 minutes per week of moderate aerobic exercise, and a stress-management program. Among participants in the lifestyle-change program, at one year, the prevalence of hypertension decreased from 41% to 17%, obesity rates decreased from 60% to 37%, and the rate of dyslipidemia decreased from 54% to 37%. The researchers also found that lifestyle modification effectively reduced expression of pro-inflammatory genes associated with neutrophil (macrophage) activation and the pathogenesis of atherosclerosis (Figure 2). Gene expression was not modified by medications in the matched control group.
Figure 2:
Proinflammatory gene expression and lifestyle modification.1
- Gene Symbol = Gene Name (Gene Ontology Biologic Process)
- LTF = lactotransferrin (immune response)
- LCN2 = lipocalin (transporter activity)
- CEACAM8 = carcinoembryonic antigen-related (immune response)
- CRISP3 = cysteine-rich secretory protein 3 (immune response; defense response)
- HP = haptoglobin protein (defense response)
- OLFM4 = olfactomedin4 (cell adhesion)
- CAMP = cathelicidin antimicrobial peptide (defense response)
- BPI = bactericidal/permeability-increasing protein (immune response; lipid binding)
1. Ellsworth DL, Croft DT, Weyandt J, et al. Intensive cardiovascular risk reduction induces sustainable changes in expression of genes and pathways important to vascular function. Circ Cardiovasc Genet 2014 Apr 1;7(2):151–60. DOI: http://dx.doi.org/10.1161/CIRCGENETICS.113.000121.
DISCUSSION
Plant-based nutrition can prevent diabetes, high blood pressure, and CAD events.3 The benefits of a predominantly plant-based nutritional regimen deserve further consideration on the basis of the information presented in this article. Compared with people who frequently consume red meat, people who eat less red meat and consume more vegetables have lower body mass index, lower systolic blood pressure, lower serum levels of LDL, and thinner blood vessel intimal medial wall thickness.63 Finally, consumption of a heavily plant-based diet has been shown to result in less oxidative stress and less micro-inflammation compared with omnivores’ meat-centric diet.64 Reducing risk factors for atherosclerosis with a plant-based diet may help us to understand the potential a plant-based diet has on down-regulating the process of atherogenesis and atherothrombotic CAD. Therefore, food may be medicine and the power of lifestyle management in disease prevention should not be ignored. Compared with drug therapy, angiography/stent placement, and bypass surgery, a plant-based diet may be a low-cost intervention that can prevent and reverse atherosclerotic CAD.18
Ninety-three percent of Americans are omnivores65 and are unlikely to give up meat. Historically, humans have consumed meat in varying amounts based upon environmental accessibility to plant-based foods and general food scarcity. Industrialization has allowed meat to become a central part of our food culture and our plates, and it is highly regarded as the best source of protein. The data presented in this article suggest that once we develop a plant-based diet micro-biota, our bacteria will not convert phosphatidylcholine or L-carnitine to TMAO with the occasional consumption of meat. In addition, the data presented in this article suggest that polyphenols from fresh fruits and vegetables increased survival and that elevated TMAO levels from red meat were associated with decreased survival. In light of this new information, we may consider measuring the ratio of polyphenols to TMAO as an indicator of atherogenesis risk. Reducing the risk factors for atherogenesis will significantly reduce the downstream risk of atherosclerosis, atherothrombotic CAD, and myocardial infarction.
Finally, as more data become available in the literature, we may want to consider how healthy eating can systemically prevent atherosclerosis. In this article, we present a proposed mechanism for how a plant-based diet may prevent atherosclerosis and CAD events (Figure 1). This includes 1) prevention of VEC injury by eating foods low in sugar, salt, and fat; 2) prevention of LDL oxidation by increased intake of fresh fruits and vegetables containing antioxidants, such as polyphenols; and 3) prevention of macrophage activation by decreasing intake of red meat, by exercise, and by stress reduction.
CONCLUSION
VEC injury may occur in individuals who do not have clinically active CAD but who have risk factors for CAD, such as smoking, hypercholesterolemia, diabetes, and hypertension.41 Because abnormal endothelial function is an early marker of CAD, the endothelium appears to be an ideal target for preventive therapy. Because we do not have specific markers for endothelial cell dysfunction, evidence presented in this article suggests that levels of urinary polyphenols (as a marker of fruit and vegetable consumption), blood TMAO levels (as a marker for red meat consumption), and proinflammatory gene expression of genes involved in atherogenesis may indicate the VEC milieu that promotes VEC health versus VEC injury. Elevating levels of blood polyphenols and lowering blood TMAO levels by eating a plant-based diet may promote health of VECs and prevent atherosclerotic CADs by at least three different proposed mechanisms. More research in this area is needed so in the future we may have objective measures of healthy eating that will promote VEC wellness years before the development of atherosclerosis and CAD.
Acknowledgments
Mary Corrado, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Go AS, Mozaffarian D, Roger VL, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014 Jan 21;129(3):e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. DOI: http://dx.doi.org/10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999 Jan 14;340(2):115–26. doi: 10.1056/NEJM199901143400207. DOI: http://dx.doi.org/10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 3.Tuso PJ, Ismail MH, Ha BJ, Bartolotto C. Nutritional update for physicians: plant-based diets. Perm J. 2013 Spring;17(2):61–6. doi: 10.7812/TPP/12-085. DOI: http://dx.doi.org/10.7812/TPP/12-085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaney D, Diehl H. The optimal diet: the official CHIP cookbook. Hagerstown, MD: Autumn House Publishing; 2009. Jan 1, [Google Scholar]
- 5.Berkow SE, Barnard N. Vegetarian diets and weight status. Nutr Rev. 2006 Apr;64(4):175–88. doi: 10.1111/j.1753-4887.2006.tb00200.x. DOI: http://dx.doi.org/10.1111/j.1753-4887.2006.tb00200.x. [DOI] [PubMed] [Google Scholar]
- 6.Farmer B, Larson BT, Fulgoni VL, 3rd, Rainville AJ, Liepa GU. A vegetarian dietary pattern as a nutrient-dense approach to weight management: an analysis of the national health and nutrition examination survey 1999–2004. J Am Diet Assoc. 2011 Jun;111(6):819–27. doi: 10.1016/j.jada.2011.03.012. DOI: http://dx.doi.org/10.1016/j.jada.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Beydoun MA. Meat consumption is associated with obesity and central obesity among US adults. Int J Obes (Lond) 2009 Jun;33(6):621–8. doi: 10.1038/ijo.2009.45. DOI: http://dx.doi.org/10.1038/ijo.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tonstad S, Butler T, Yan R, Fraser GE. Type of vegetarian diet, body weight, and prevalence of type 2 diabetes. Diabetes Care. 2009 May;32(5):791–6. doi: 10.2337/dc08-1886. DOI: http://dx.doi.org/10.2337/dc08-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabaté J, Wien M. Vegetarian diets and childhood obesity prevention. Am J Clin Nutr. 2010 May;91(5):1525S–1529S. doi: 10.3945/ajcn.2010.28701F. DOI: http://dx.doi.org/10.3945/ajcn.2010.28701F. [DOI] [PubMed] [Google Scholar]
- 10.Rosell M, Appleby P, Spencer E, Key T. Weight gain over 5 years in 21,966 meat-eating, fish-eating, vegetarian, and vegan men and women in EPIC-Oxford. Int J Obes (Lond) 2006 Sep;30(9):1389–96. doi: 10.1038/sj.ijo.0803305. DOI: http://dx.doi.org/10.1038/sj.ijo.0803305. [DOI] [PubMed] [Google Scholar]
- 11.Campbell TC, Campbell TM., II . The China Study: the most comprehensive study of nutrition ever conducted and the startling implications for diet, weight loss, and long-term health. Dallas, TX: BenBella Books; 2006. May 11, [Google Scholar]
- 12.Snowdon DA, Phillips RL. Does a vegetarian diet reduce the occurrence of diabetes? Am J Public Health. 1985 May;75(5):507–12. doi: 10.2105/ajph.75.5.507. DOI: http://dx.doi.org/10.2105/AJPH.75.5.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vang A, Singh PN, Lee JW, Haddad EH, Brinegar CH. Meats, processed meats, obesity, weight gain and occurrence of diabetes among adults: findings from Adventist Health Studies. Ann Nutr Metab. 2008;52(2):96–104. doi: 10.1159/000121365. DOI: http://dx.doi.org/10.1159/000121365. [DOI] [PubMed] [Google Scholar]
- 14.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care. 2006 Aug;29(8):1777–83. doi: 10.2337/dc06-0606. DOI: http://dx.doi.org/10.2337/dc06-0606. [DOI] [PubMed] [Google Scholar]
- 15.de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999 Feb;99(6):779–85. doi: 10.1161/01.cir.99.6.779. DOI: http://dx.doi.org/10.1161/01.CIR.99.6.779. [DOI] [PubMed] [Google Scholar]
- 16.Appleby PN, Thorogood M, McPherson K, Mann JI. Associations between plasma lipid concentrations and dietary, lifestyle and physical factors in the Oxford Vegetarian Study. J Hum Nutr Diet. 1995 Oct;8(5):305–14. DOI: http://dx.doi.org/10.1111/j.1365-277X.1995.tb00324.x. [Google Scholar]
- 17.Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998 Dec 16;280(23):2001–7. doi: 10.1001/jama.280.23.2001. DOI: http://dx.doi.org/10.1001/jama.280.23.2001. Erratum in: JAMA 1999 Apr 21;281(15):1380. DOI: http://dx.doi.org/10.1001/jama.281.15.1380. [DOI] [PubMed] [Google Scholar]
- 18.Esselstyn CB., Jr Resolving the coronary artery disease epidemic through plant-based nutrition. Prev Cardiol. 2001 Autumn;4(4):171–7. doi: 10.1111/j.1520-037x.2001.00538.x. DOI: http://dx.doi.org/10.1111/j.1520-037X.2001.00538.x. [DOI] [PubMed] [Google Scholar]
- 19.Key TJ, Fraser GE, Thorogood M, et al. Mortality in vegetarians and non-vegetarians: a collaborative analysis of 8300 deaths among 76,000 men and women in five prospective studies. Public Health Nutr. 1998 Mar;1(1):33–41. doi: 10.1079/phn19980006. DOI: http://dx.doi.org/10.1079/PHN19980006. [DOI] [PubMed] [Google Scholar]
- 20.Vane JR, Born GVR, Welzel D, editors. The endothelial cell in health and disease. Stuttgart, Germany: Schattauer GmbH; 1995. Dec, [Google Scholar]
- 21.Boyle JJ. Macrophage activation in atherosclerosis: pathogenesis and pharmacology of plaque rupture. Curr Vasc Pharmacol. 2005 Jan;3(1):63–8. doi: 10.2174/1570161052773861. DOI: http://dx.doi.org/10.2174/1570161052773861. [DOI] [PubMed] [Google Scholar]
- 22.Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: a multifactorial process. Exp Clin Cardiol. 2002 Spring;7(1):40–53. [PMC free article] [PubMed] [Google Scholar]
- 23.Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Lüscher TF. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 1998 Jun 30;97(25):2494–8. doi: 10.1161/01.cir.97.25.2494. DOI: http://dx.doi.org/10.1161/01.CIR.97.25.2494. [DOI] [PubMed] [Google Scholar]
- 24.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–5. doi: 10.1073/pnas.88.11.4651. DOI: http://dx.doi.org/10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vita JA. Polyphenols and cardiovascular disease: effects on endothelial and platelet function. Am J Clin Nutr. 2005 Jan;81(1 Suppl):292S–297S. doi: 10.1093/ajcn/81.1.292S. [DOI] [PubMed] [Google Scholar]
- 26.Williams SB, Goldfine AB, Timimi FK, et al. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998 May 5;97(17):1695–701. doi: 10.1161/01.cir.97.17.1695. DOI: http://dx.doi.org/10.1161/01.CIR.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 27.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Lee BK, Creager MA. Impaired endothelium-dependent vasodilation in patients with insulin-dependent diabetes mellitus. Circulation. 1993 Dec;88(6):2510–6. doi: 10.1161/01.cir.88.6.2510. DOI: http://dx.doi.org/10.1161/01.CIR.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 28.Tsao PS, Niebauer J, Buitrago R, et al. Interaction of diabetes and hypertension on determinants of endothelial adhesiveness. Arterioscler Thromb Vasc Biol. 1998 Jun;18(6):947–53. doi: 10.1161/01.atv.18.6.947. DOI: http://dx.doi.org/10.1161/01.ATV.18.6.947. [DOI] [PubMed] [Google Scholar]
- 29.Diabetes Prevention Program Research Group. Knowler WC, Fowler SE, Hamman RF, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009 Nov 14;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. DOI: http://dx.doi.org/10.1016/S0140-6736(09)61457-4. Erratum in: Lancet 2009 Dec 19;374(9707):2054. DOI: http://dx.doi.org/10.1016/S0140-6736(09)62154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokoyama Y, Nishimura K, Barnard ND, et al. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med. 2014 Apr;174(4):577–87. doi: 10.1001/jamainternmed.2013.14547. DOI: http://dx.doi.org/10.1001/jamainternmed.2013.14547. [DOI] [PubMed] [Google Scholar]
- 31.Ellsworth DL, Croft DT, Jr, Weyandt J, et al. Intensive cardiovascular risk reduction induces sustainable changes in expression of genes and pathways important to vascular function. Circ Cardiovasc Genet. 2014 Apr 1;7(2):151–60. doi: 10.1161/CIRCGENETICS.113.000121. DOI: http://dx.doi.org/CIRCGENETICS.113.000121. [DOI] [PubMed] [Google Scholar]
- 32.Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013 Jul 8;173(13):1230–8. doi: 10.1001/jamainternmed.2013.6473. DOI: http://dx.doi.org/10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agudo A, Cabrera L, Amiano P, et al. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain) Am J Clin Nutr. 2007 Jun;85(6):1634–42. doi: 10.1093/ajcn/85.6.1634. Erratum in: Am J Clin Nutr 2008 Oct;88(4):1181. [DOI] [PubMed] [Google Scholar]
- 34.Hung HC, Joshipura KJ, Jiang R, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004 Nov 3;96(21):1577–84. doi: 10.1093/jnci/djh296. DOI: http://dx.doi.org/10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 35.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007 Mar;85(3):895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 36.Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997 May;65(5):1489–94. doi: 10.1093/ajcn/65.5.1489. [DOI] [PubMed] [Google Scholar]
- 37.Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996 Feb 24;312(7029):478–81. doi: 10.1136/bmj.312.7029.478. DOI: http://dx.doi.org/10.1136/bmj.312.7029.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC. Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr. 2002 May;75(5):880–6. doi: 10.1093/ajcn/75.5.880. [DOI] [PubMed] [Google Scholar]
- 39.Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009 Apr 13;169(7):659–69. doi: 10.1001/archinternmed.2009.38. DOI: http://dx.doi.org/10.1001/archinternmed.2009.38. [DOI] [PubMed] [Google Scholar]
- 40.Ginter E, Simko V. Plant polyphenols in prevention of heart disease. Bratisl Lek Listy. 2012;113(8):476–80. doi: 10.4149/bll_2012_105. DOI: http://dx.doi.org/10.4149/BLL_2012_105. [DOI] [PubMed] [Google Scholar]
- 41.Brown CA, Bolton-Smith C, Woodward M, Tunstall-Pedoe H. Coffee and tea consumption and the prevalence of coronary heart disease in men and women: results from the Scottish Heart Health Study. J Epidemiol Community Health. 1993 Jun;47(3):171–5. doi: 10.1136/jech.47.3.171. DOI: http://dx.doi.org/10.1136/jech.47.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996 Mar 25;156(6):637–42. DOI: http://dx.doi.org/10.1001/archinte.156.6.637. [PubMed] [Google Scholar]
- 43.Rimm EB, Katan MB, Ascherio A, Stampfer MJ, Willett WC. Relation between intake of flavonoids and risk for coronary heart disease in male health professionals. Ann Intern Med. 1996 Sep 1;125(5):384–9. doi: 10.7326/0003-4819-125-5-199609010-00005. DOI: http://dx.doi.org/10.7326/0003-4819-125-5-199609010-00005. [DOI] [PubMed] [Google Scholar]
- 44.Yochum L, Kushi LH, Meyer K, Folsom AR. Dietary flavonoid intake and risk of cardiovascular disease in postmenopausal women. Am J Epidemiol. 1999 May 15;149(10):943–9. doi: 10.1093/oxfordjournals.aje.a009738. DOI: http://dx.doi.org/10.1093/oxfordjournals.aje.a009738. [DOI] [PubMed] [Google Scholar]
- 45.Sesso HD, Gaziano JM, Buring JE, Hennekens CH. Coffee and tea intake and the risk of myocardial infarction. Am J Epidemiol. 1999 Jan 15;149(2):162–7. doi: 10.1093/oxfordjournals.aje.a009782. DOI: http://dx.doi.org/10.1093/oxfordjournals.aje.a009782. [DOI] [PubMed] [Google Scholar]
- 46.Mukamal KJ, Maclure M, Muller JE, Sherwood JB, Mittleman MA. Tea consumption and mortality after acute myocardial infarction. Circulation. 2002 May 28;105(21):2476–81. doi: 10.1161/01.cir.0000017201.88994.f7. DOI: http://dx.doi.org/10.1161/01.CIR.0000017201.88994.F7. [DOI] [PubMed] [Google Scholar]
- 47.Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000 Apr 25;101(16):1899–906. doi: 10.1161/01.cir.101.16.1899. DOI: http://dx.doi.org/10.1161/01.CIR.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 48.Halcox JP, Schenke WH, Zalos G, et al. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002 Aug 6;106(6):653–8. doi: 10.1161/01.cir.0000025404.78001.d8. DOI: http://dx.doi.org/10.1161/01.CIR.0000025404.78001.D8. [DOI] [PubMed] [Google Scholar]
- 49.Perticone F, Ceravolo R, Pujia A, et al. Prognostic significance of endothelial dysfunction in hypertensive patients. Circulation. 2001 Jul 10;104(2):191–6. doi: 10.1161/01.cir.104.2.191. DOI: http://dx.doi.org/10.1161/01.CIR.104.2.191. [DOI] [PubMed] [Google Scholar]
- 50.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003 Oct 1;42(7):1149–60. doi: 10.1016/s0735-1097(03)00994-x. DOI: http://dx.doi.org/10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- 51.Albini A, Tosetti F, Li VW, Noonan DM, Li WW. Cancer prevention by targeting angiogenesis. Nat Rev Clin Oncol. 2012 Sep;9(9):498–509. doi: 10.1038/nrclinonc.2012.120. DOI: http://dx.doi.org/10.1038/nrclinonc.2012.120. [DOI] [PubMed] [Google Scholar]
- 52.Leikert JF, Räthel TR, Wohlfart P, Cheynier V, Vollmar AM, Dirsch VM. Red wine polyphenols enhance endothelial nitric oxide synthase expression and subsequent nitric oxide release from endothelial cells. Circulation. 2002 Sep 24;106(13):1614–7. doi: 10.1161/01.cir.0000034445.31543.43. DOI: http://dx.doi.org/10.1161/01.CIR.0000034445.31543.43. [DOI] [PubMed] [Google Scholar]
- 53.Hodgson JM, Puddey IB, Burke V, Watts GF, Beilin LJ. Regular ingestion of black tea improves brachial artery vasodilator function. Clin Sci (Lond) 2002 Feb;102(2):195–201. DOI: http://dx.doi.org/10.1042/CS20010120. [PubMed] [Google Scholar]
- 54.Stein JH, Keevil JG, Wiebe DA, Aeschlimann S, Folts JD. Purple grape juice improves endothelial function and reduces the susceptibility of LDL cholesterol to oxidation in patients with coronary artery disease. Circulation. 1999 Sep 7;100(10):1050–5. doi: 10.1161/01.cir.100.10.1050. DOI: http://dx.doi.org/10.1161/01.CIR.100.10.1050. [DOI] [PubMed] [Google Scholar]
- 55.Zamora-Ros R, Rabassa M, Cherubini A, et al. High concentrations of a urinary biomarker of polyphenol intake are associated with decreased mortality in older adults. J Nutr. 2013 Sep;143(9):1445–50. doi: 10.3945/jn.113.177121. DOI: http://dx.doi.org/10.3945/jn.113.177121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carmeli E, Fogelman Y. Antioxidant effect of polyphenolic glabridin on LDL oxidation. Toxicol Ind Health. 2009 May-Jun;25(4–5):321–4. doi: 10.1177/0748233709103034. DOI: http://dx.doi.org/10.1177/0748233709103034. [DOI] [PubMed] [Google Scholar]
- 57.Frémont L, Belguendouz L, Delpal S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polyunsaturated fatty acids. Life Sci. 1999;64(26):2511–21. doi: 10.1016/s0024-3205(99)00209-x. DOI: http://dx.doi.org/10.1016/S0024-3205(99)00209-X. [DOI] [PubMed] [Google Scholar]
- 58.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010 Aug 31;122(9):876–83. doi: 10.1161/CIRCULATIONAHA.109.915165. DOI: http://dx.doi.org/10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ashaye A, Gaziano J, Djoussé L. Red meat consumption and risk of heart failure in male physicians. Nutr Metab Cardiovasc Dis. 2011 Dec;21(12):941–6. doi: 10.1016/j.numecd.2010.03.009. DOI: http://dx.doi.org/10.1016/j.numecd.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013 May;19(5):576–85. doi: 10.1038/nm.3145. DOI: http://dx.doi.org/10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011 Apr 7;472(7341):57–63. doi: 10.1038/nature09922. DOI: http://dx.doi.org/10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013 Apr 25;368(17):1575–84. doi: 10.1056/NEJMoa1109400. DOI: http://dx.doi.org/10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang SY, Zhang HJ, Sun SY, et al. Relationship of carotid intima-media thickness and duration of vegetarian diet in Chinese male vegetarians. Nutr Metab (Lond) 2011 Sep 19;8(1):63. doi: 10.1186/1743-7075-8-63. DOI: http://dx.doi.org/10.1186/1743-7075-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sebeková K, Boor P, Valachovicová M, et al. Association of metabolic syndrome risk factors with selected markers of oxidative status and microinflammation in healthy omnivores and vegetarians. Mol Nutr Food Res. 2006 Sep;50(9):858–68. doi: 10.1002/mnfr.200500170. DOI: http://dx.doi.org/10.1002/mnfr.200500170. [DOI] [PubMed] [Google Scholar]
- 65.Palmer A, Ovsepian N. 2011 vegetarian and vegan stats [Internet] Norfolk, VA: People for the Ethical Treatment of Animals; 2011. [cited 2014 Sep 4]. Available from: www.peta.org/living/food/2011-vegetarian-vegan-stats/. [Google Scholar]