Abstract
Ground cover vegetation is often added or allowed to generate to promote conservation biological control, especially in perennial crops. Nevertheless, there is inconsistent evidence of its effectiveness, with studies reporting positive, nil or negative effects on pest control. This might arise from differences between studies at the local scale (e.g. orchard management and land use history), the landscape context (e.g. presence of patches of natural or semi-natural vegetation near the focal orchard), or regional factors, particularly climate in the year of the study. Here we present the findings from a long-term regional monitoring program conducted on four pest species (Bactrocera oleae, Prays oleae, Euphyllura olivina, Saissetia oleae) in 2,528 olive groves in Andalusia (Spain) from 2006 to 2012. Generalized linear mixed effect models were used to analyze the effect of ground cover on different response variables related to pest abundance, while accounting for variability at the local, landscape and regional scales. There were small and inconsistent effects of ground cover on the abundance of pests whilst local, landscape and regional variability explained a large proportion of the variability in pest response variables. This highlights the importance of local and landscape-related variables in biological control and the potential effects that might emerge from their interaction with practices, such as groundcover vegetation, implemented to promote natural enemy activity. The study points to perennial vegetation close to the focal crop as a promising alternative strategy for conservation biological control that should receive more attention.
Introduction
Habitat management is a conservation biological pest control strategy focused on manipulating the environment to enhance natural enemy populations [1], [2]. A common conservation biological control practice in perennial crops is the establishment and management of a ground cover [3], which often consists of an inter-tree herbaceous vegetation strip, although it can extend as a continuous covering across the crop [4]. Ground cover can be formed by single species or a polyculture, with species typically belonging to Poaceae and Fabaceae [5], [6] and other herbs [7], [8] and is often comprised of self-regenerating vegetation [9], [10]. Ground cover can improve the diversity of vegetation, especially if replacing bare ground, and creates new habitat structure [1]. As a result, the abundance [9] and diversity of natural enemies increase [8], [11], which might promote higher rates of predation and parasitism of insect pests by natural enemies [12]. The identity of ground cover species can however benefit some pests, so ‘selective plants’ have been recommended such that only natural enemies derive benefit [13].
Despite any increase in the abundance of predatory and parasitoid insects led by the use of ground covers, it is still uncertain whether this will translate into reduced pest densities. Much of the existing literature focuses on the effects of ground covers on natural enemies, implicitly assuming that an increase in their abundance or diversity will necessarily lead to a decrease in pest abundance. However, some studies have demonstrated that this does not consistently occur, as intraguild predation and other biotic processes can diminish the impact of natural enemy species assemblages on herbivore populations [14–16]. Further, those studies that explicitly investigate the effects of ground cover on herbivore abundance provide inconsistent evidence ([17] and references herein). For example, according to different studies ground cover in peach orchards could either decrease [18], [19] or increase the abundance of pests [5], [20]. Their use in other orchards (apple, pear, citrus, olive) has yielded likewise either positive or nil results in terms of biological control (see S1 Appendix for a comprehensive review of the literature).
Inconsistent findings might arise from differences in local conditions (e.g. crop management, land use history), the landscape context (e.g. surrounding natural or semi-natural vegetation), and variables operating at a regional scale (e.g. climatic conditions of the region at the time when the study was conducted) [1], [21]-[23]. The influence of factors operating at these different scales is often not apparent in studies conducted at one single locality and that have no representation of heterogeneity at other scales or temporal replication. In this study we present evidence from a long-term (2006–2012) regional assessment conducted in 2,528 olive orchards in the Andalusia region, Spain, in order to assess the effect of ground cover on the abundance of pest herbivores. Through this research, we aim to provide a robust conclusion on the utility of ground cover in olive orchards as well as to tease-apart the relative importance of other unmeasured effects over a range of scales (local, landscape, regional). Our study is unique in terms of the large spatial (2,528 orchards) and temporal (seven years) replication of samples using the same methodology, which allows detection and quantification of the effect of ground cover on different pest species, while accounting for non-specific random variance at the local, landscape and regional scale.
Materials and Methods
Data collection
The Warning and Information Plant Protection Network (WIPPN) of Andalusia (Spain) monitors the populations of potentially dangerous arthropods and provides guidelines to farmers on how to treat pests. It has several monitoring stations (MS) spread throughout the region, each covering an area of ca. five hectares. These are part of private olive groves that have an agreement with the administration to monitor pests. All these farms are handled under integrated protection management criteria (IPM) so insecticide treatments are made whenever a pest population exceeds relevant threshold levels. Since 2006, technicians of the WIPPN have collected data on olive pest numbers, together with information about the orchards, including presence of ground cover vegetation following a prescribed protocol [24] (Fig. 1). Ground cover vegetation in these sites is comprised of naturally generating plants, chiefly grasses interspersed with broadleaved plants. No detailed data exist for botanical composition of the groundcovers so, for the purposes of this analysis, groundcovers were not categorised. In this work we use data collected by the WIPPN from 2006 to 2012 to investigate the effects of ground cover on four of the most common pests found in this crop: Prays oleae (Bern.) (Lepidoptera: Yponomeutidae) (the olive moth), Bactrocera oleae (Rossi) (Diptera Tephritidae) (the olive fly), Euphyllura olivina Costa (Hemiptera: Psyllidae) (the olive psyllid), and Saissetia oleae (Olivier) (Hemiptera: Coccidae (the olive scale).
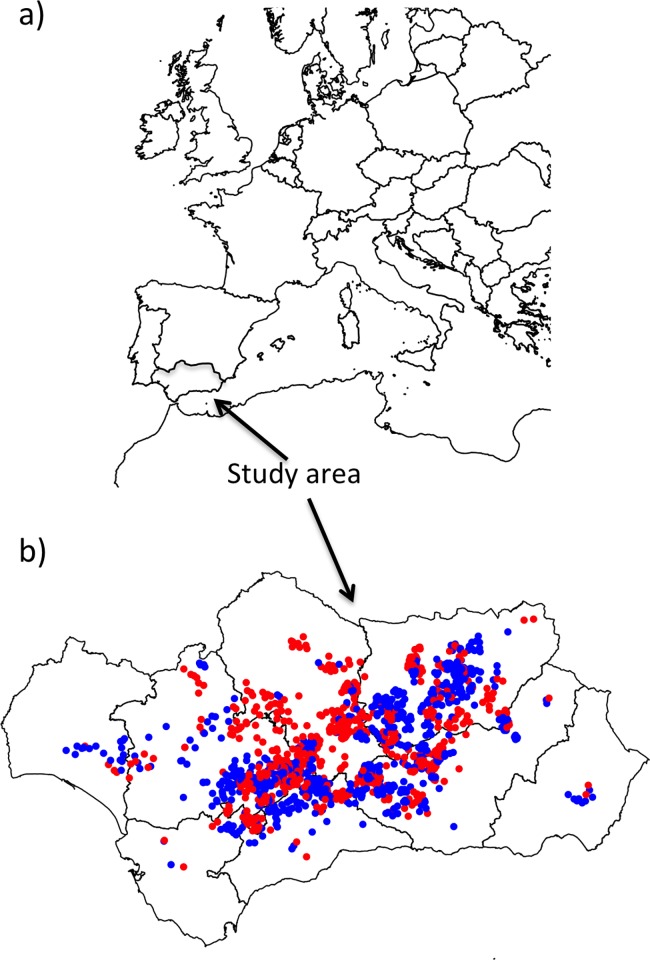
Figure 1. Map of the Andalusian region, Spain, and the monitoring stations used in this study.
Red points represent ground cover MS while blue points represent bare soil MS before pairing.
Prays oleae. The olive moth is one of the principal insect pests in olive groves. The damage caused by this insect can reduce olive production at a level of 50–60%, which equates to 8–11 kg per tree in modern cultivars [25]. It has three generations: phylophagous (leaf generation), anthophagous (flower generation), and carpophagous (fruit generation) [26]. The two latter are the most damaging. The leaf generation overwinters inside the leaves and is not very abundant. The flower generation is the most abundant of the three, and attacks the floral buttons, drastically reducing the potential fruit set. The larvae of the fruit generation, which develop inside the olive stone, cause its premature fall, decreasing crop productivity.
To measure abundance of adults, two funnel traps, baited with the sex pheromone Z-7 tetradecenal, were located in every MS and were checked every week from the beginning of March to the end of November (Table 1). A major issue is that these traps catch simultaneously adults from the three generations. The leaf and flower generations partly overlap through time, but can be set apart from the fruit generation across time. In addition, a total of 400 inflorescences were collected from mid March to the end of May at each MS, and percentage of inflorescences with larvae was used as a proxy to population size of the flower generation. Similarly, a total of 400 olive fruits were taken from the beginning of August to the end of September at each MS, and the percentage of fruits with larvae of the fruit generation was calculated (Table 1). Percentages were transformed to number of successes (infested) and failures (not infested) for the corresponding response variables, over a total of 400 inflorescences and fruits respectively.
Table 1. Response variables used for each of the four species analysed in this study, family error used in the generalized linear mixed models, and number of observations (i.e. number of monitoring stations) available for each response variable for all the years.
| Species | Response variable | Type of response variable | GLM error distribution | Number of MSs used |
|---|---|---|---|---|
| P. oleae | ||||
| Funnel traps adults generation 1&2 | Count | Negative binomial | 2418 | |
| Larvae / inflorescence | Proportion | Binomial | 1984 | |
| Funnel traps adults generation 3 | Count | Negative binomial | 1114 | |
| Larvae / fruit | Proportion | Binomial | 2160 | |
| B. oleae | ||||
| Funnel traps adults | Count | Negative binomial | 2118 | |
| Sticky traps adults | Count | Negative binomial | 2528 | |
| Damaged fruits | Proportion | Gaussian | 754 | |
| E. olivina | Nymphs / inflorescence | Proportion | Binomial | 276 |
| S. oleae | Living forms / shoot | Count | Negative binomial | 354 |
Bactrocera oleae. The olive fruit fly oviposits in the olive fruit mesocarp, where larvae develop through three instar stages [26] depending on environmental conditions, from July to late autumn, the most harmful being that occurring in autumn. Tunnelling and falling of fruits caused by the larvae of the olive fruit can be very high, causing in some years the almost complete loss of olive production, where no pest management measures are undertaken. Populations of this insect were estimated weekly at each MS from the beginning of June to the end of December, by means of three Mc-Phail traps and three sticky traps baited with the pheromones diammonium phosphate and dioxaspiro [5.5] undecane, respectively. The damage caused by the olive fly was also estimated by counting the proportion of fruits damaged by this insect during the same period as for sticky and Mc-Phail traps (Table 1).
Euphyllura olivine. The olive psyllid is a major pest in North Africa [27]. In European Mediterranean countries it is usually considered a secondary pest but could become a more serious threat to olive crops as a result of climate change which would cause a shift of the species range northwards in Europe [28]. The nymphs of this insect attack the young shoots and inflorescences causing damages to fruits (30%) and inflorescences (70%) [27]. It has three or four generations per year, the first being the most damaging. From mid March to the end of May, the number of inflorescences, out of 400 per MS, which had nymphs of olive psyllid was used to estimate the damage caused by this insect (Table 1).
Saissetia oleae. The olive scale is widely distributed and attacks several fruit trees. This pest infests leaves and twigs, sucks the tree sap and produces large amounts of honeydew, which serves as a substrate for sooty mould fungi; the latter interferes with photosynthetic and respiratory processes of the plant and affects the quality of the fruit. This results in yield decreases and rejection of fruit for export [29]. To quantify the presence of this pest, the total number of individuals caught in 200 shoots was counted weekly at each MS during June (Table 1).
Data processing and analyses
For each pest species we selected response variables that represent—directly or indirectly—population size-related measures and for which there were enough observations (> 250) in the database to conduct reliable statistical analyses (Table 1).
We used the maximum value attained throughout the year for response variables that were measured weekly. We chose peak of abundance instead of cumulative abundance, as there was a high variability in the number of observations per orchard. The dates of first and last assessment and duration of data collection (i.e. number of samples per growing season) differed between sites, years and response variable. This precluded analysis of total or cumulative pest values because site and year data were not comparable. Accordingly, we analysed peak values for each variable to check whether using peak rather than cumulative numbers distorted measures of pest metrics. We correlated peak abundance with cumulative abundance for data sets where there was a consistent number of observations. Correlations between peak of abundance and estimated cumulative abundance for all response variables were always high (minimum r = 0.773) (S2 Appendix).
To account for variability at the landscape scale, we compared paired samples of MSs with ground cover and bare soil. Pairing was performed by selecting for each monitoring station with ground cover vegetation the nearest monitoring station with bare soil (up to a maximum distance of 10 km). We repeated this procedure for all MSs until there were no more possible pairs and disregarded data from remaining unpaired sites. Pairing was also constrained within cultivars in order to maintain as much homogeneity as possible. We repeated the pairing every year because not all MSs had data available every year. Consequently, the number of observations used for analysis was not constant for each response variable (Table 1). Because of that, we avoided to use a time series framework to include the variable year. Doing this would force us to withdraw data from many monitoring stations, weakening the statistical power of our models. Therefore, we considered year as a geographic regional influence because: (1) all data taken within a given year share similar macroclimatic conditions; (2) if macroclimatic conditions have an influence on pest abundance, we are effectively modelling such effects on the response variable (i.e. pest abundance) by incorporating year as a random effect.
We used generalized linear mixed models (GLMMs) to analyse the effect of ground cover on the different response variables by accounting for random effects at different spatial scales: plot, pair, and year. Depending on the nature of the response variable and post-hoc evaluation of model residuals we used different error distributions. A Gaussian error distribution was used for raw proportions (e.g. 75% of fruits damaged by B. oleae). A negative binomial error distribution was selected for count data, whereas a binomial error distribution was chosen for proportions where we had information on the number of successes (infestation) and failures (no infestation) of a particular event (e.g. 300 fruits with and 100 fruits without larvae of P. oleae) (Table 1). Random factors were used to model potential structures of autocorrelation in the dataset and were attributed to unmeasured features that can be found at different scales, specifically: (1) 'plot' represents local scale variability for MS that have been sampled at different years, and might thus have a similar response throughout time due to crop management related issues such as intensity of pesticide application, land use history, topography or micro-climatic conditions or even different types of ground cover that might have an important effect on pest abundance [13], [30]-[32]; (2) 'pair' refers to the geographical pairing of MS. Paired MS might share a common response to pests, regardless they have ground cover or bare soil, due to landscape-scale factors such as crop diversity, landscape diversity, landscape complexity or presence of patches of natural or semi-natural vegetation and their related measures such as mean patch size, edge density, and so on [21], [33], [34]; and (3) 'year' represents particular macro-climatic conditions such as temperature and humidity. This climatic features might equally affect all plots at the regional level since it is well known that some years are climatically more suitable for pest outbreaks than others [22], [35].
We built all possible combinations of random and fixed factors. 'Pair' was nested within 'year', but plot was not nested within 'pair', because the assignment of each 'plot' to a 'pair' might change from one year to the next. Overall we fitted 15 models that were compared using the Akaike Information Criterion (AIC). Models with a difference in AIC > 2 indicate that the worse model has virtually no support and could be omitted. Following Nakagawa and Schielzeth [36], we calculated the R2 to have an account of the variability supported by models with Gaussian or binomial error distribution. Though this approach cannot be applied to models with a negative binomial error distribution (S. Nagakawa, personal communication), it allows two components of R2 to be calculated: (1) a marginal R2 that only takes into account the variability explained by fixed effects; and (2) a conditional R2 that accounts for the variability supported by both the fixed and random effects. When ground cover was included in at least one of the best models, we quantified its effect in relation to the different sources of random variability (local, landscape and regional) for each response variable. To achieve this, we calculated the difference between ground cover and bare soil from the fixed effects estimated model parameters, and added a random error from the corresponding variance term estimated by the model at each scale of variability. We repeated this procedure 1000 times and plot the 95% confidence intervals of such predictions.
All the analyses were performed in R [37], and its packages’lme4' [38] and “glmmADMB” [39].
Results
Model selection indicated that there were either one or two best models for each pest response variable. In all cases, except for E. olivina and S. oleae, at least one of the best models included the fixed and all the random effects (Table 2). Alternative best models for some response variables (P. oleae funnel traps in generation 3; B. oleae funnel traps and fruit damage; S. oleae, just with regional as random factor selected) excluded the fixed effect of ground cover. For E. olivina, the best model excluded the regional-scale random effect (Table 2). S. oleae displayed four best models, all including regional factor as random effect but also the combination of this with local and landscape factors separately. For all best models for which it was possible to calculate the R2, we found a much higher conditional than marginal R2 (Table 2).
Table 2. Comparison of alternative models (using AICc) for the response variables tested in the study.
| Model | Species and response variable | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P. oleae | B. oleae | E. olivina | S. oleae | ||||||||
| Funnel traps generation 1&2 | Funnel traps generation 3 | Larvae/inflorescence | Larvae/fruit | Funnel traps | Sticky traps | Damaged fruits | Presence/inflorescence | Presence/shoot | |||
| Fixed effect | Random effects | ||||||||||
| Ground cover | No | 41931.20 | 14938.70 | 59937.78 | 212352.6 | 22579.60 | 32829.80 | 5151.03 | 8841.59 | 3168.52 | |
| Local | 41090.40 | 14724.66 | 18800.90 | 86147.74 | 22082.00 | 32562.00 | 5131.30 | 2491.27 | 3170.52 | ||
| Landscape | 40940.20 | 14607.88 | 16803.58 | 69063.21 | 21970.20 | 32542.40 | 5064.25 | 2015.06 | 3168.68 | ||
| Regional | 41510.80 | 14637.70 | 45024.71 | 187061.4 | 22313.20 | 32204.60 | 5049.47 | 7223.38 | 3165.70 | ||
| Local+Landscape | 40668.60 | 14594.02 | 7935.64 | 23439.52 | 21795.40 | 32396.40 | 5045.40 | 1294.32 | 3170.68 | ||
| Local+Regional | 40656.00 | 14493.26 | 16280.28 | 77530.91 | 21628.60 | 32203.20 | 5023.11 | 1673.95 | 3167.70 | ||
| Landscape+Regional | 40732.60 | 14430.82 | 16661.74 | 68987.29 | 21753.00 | 32371.00 | 4995.03 | 2009.70 | 3167.40 | ||
| Local+Landscape+Regional | 40398.20 | 14411.26 | 7803.70 | 23397.89 | 21507.80 | 32128.60 | 4978.63 | 1297.49 | 3169.40 | ||
| No ground cover | Local | 41094.20 | 14723.68 | 18799.81 | 86150.07 | 22080.20 | 32563.60 | 5130.32 | 2502.77 | 3171.88 | |
| Landscape | 40943.80 | 14607.50 | 16841.12 | 69461.36 | 21968.40 | 32545.20 | 5063.33 | 2026.55 | 3171.80 | ||
| Regional | 41508.80 | 14635.70 | 45059.58 | 187405.7 | 22316.80 | 32580.20 | 5048.25 | 7233.54 | 3167.28 | ||
| Local+Landscape | 40712.12 | 14592.90 | 7940.64 | 23466.91 | 21794.40 | 32401.00 | 5044.49 | 1300.44 | 3172.80 | ||
| Local+Regional | 40658.00 | 14491.78 | 16298.67 | 77539.27 | 21627.00 | 32205.40 | 5021.93 | 1683.19 | 3169.28 | ||
| Landscape+Regional | 40735.20 | 14430.04 | 16699.28 | 69385.44 | 21751.00 | 32374.40 | 4994.11 | 2021.19 | 3169.20 | ||
| Local+Landscape+Regional | 40401.00 | 14409.88 | 7810.15 | 23424.78 | 21506.60 | 32132.60 | 4977.29 | 1303.72 | 3172.80 | ||
| N.A. | N.A. | 0.00165 | 0.00562 | N.A. | N.A. | 0.00046 | 0.01245 | N.A. | |||
| N.A. | N.A. | 0.41615 | 0.56952 | N.A. | N.A. | 0.62922 | 0.73679 | N.A. | |||
The best model (lowest AICc) is indicated in boldface type. The marginal (m) and conditional (c) R2, when its calculation was possible, refer to the best model.
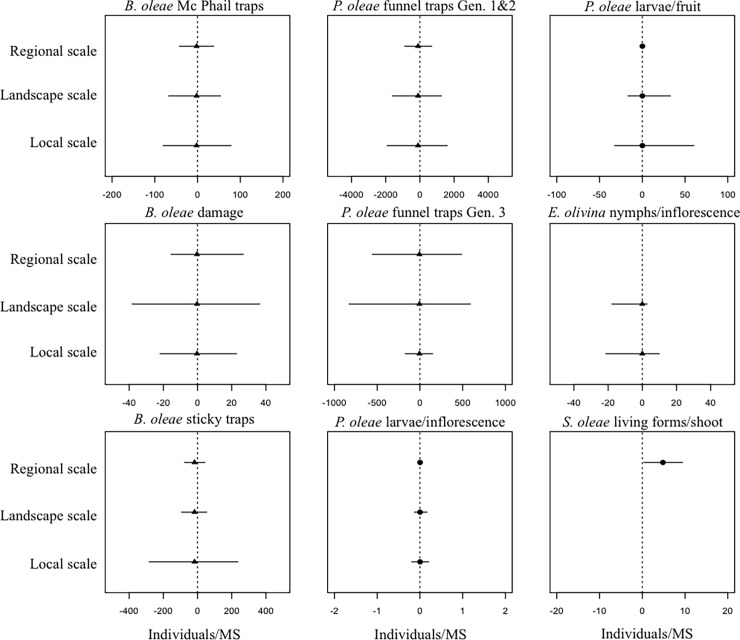
Model estimations showed that, even if ground cover was included in the best model, it had a minor effect in absolute terms compared to random variability. This was particularly the case for local (i.e. plot) and landscape (i.e. pair) scales, although regional variability was also important for most of the response variables. However, for S. oleae random variability associated with factors at the regional scale reported an effect comparable to ground cover (Fig. 2).
Figure 2. Best model estimations for the differences between ground cover and bare soil for all the response variables.
Positive values represent an increase in pest abundance in the presence of ground cover (filled circle) whereas negative values represent a decrease in pest abundance in the presence of ground cover (filled triangle). Bars represent a 95% of the confidence interval of the random effects predictions (± σ2) at a local, landscape, and regional scale. If a random factor is not included in the selected best model (Table 2), no 95% confidence interval bars were drawn.
Discussion
The results of this study indicate that ground cover, as a single entity and compared to bare ground, is not an effective measure to reduce pest abundance in olive groves. This is evident in the absence of this term in some of the best fit models, the low amount of variability explained by this factor in those models where it was present, and its small absolute effect—as quantified by estimated model parameters—in relation to the random factors. In contrast, random effects, explained a greater proportion of the observed variability in all response variables. Such factors represent the effect of variables operating at different spatial and temporal scales, whose variability has not been specifically measured, and which might have an influence in the response variable. Although the processes operating behind random factors are unknown, here we provide an account of some of the potential variables that might be explaining the high variability observed in pest abundance or related response variables (e.g. pest damage to crop) at different spatial scales.
At the local scale, crop management related issues such as intensity of pesticide application, land use history, topography or micro-climatic features, might have an important effect on pest abundance. The botanical composition of ground cover [40], its density [41], the width of the strip [42], or the height of grasses might also have an influence on pest abundance [43]. The lack of data on any variability in groundcover types in the available data set, however, precludes testing for any optimal forms of vegetation or management. The abundance of B. oleae has been demonstrated to be influenced by site elevation [30]. Another factor that could influence pest populations is the history of pesticide use [31] and the nature of the active constituent in insecticides [31]. Intensive use of pesticides, particularly broad spectrum types could depress the local community of natural enemies thereby favouring herbivores independently of direct effects on pest populations. Further, any lack of nearby non-crop vegetation would increase the detrimental effects of pesticide, as natural enemies will have no refuge from which to recolonize sprayed crops [17], [33], [44].
Landscape-scale factors relate largely to the composition and connectivity of vegetation including other crops, natural or semi-natural vegetation, at ranges up to 10 km from the crop that are known to affect the presence of natural enemies in crops [21], [33], [34]. In Andalusia, as well as in other Mediterranean regions, olive groves can adopt a variety of forms, from highly intensified crops where landscape heterogeneity is very low, to less intensively managed crops in areas with high landscape heterogeneity, particularly in mountainous areas [45]. In olive groves, areas of herbaceous vegetation and areas of woody vegetation near olive crops, and smaller patches of woody vegetation within olive groves, decreased the abundance of P. oleae and E. olivina [47]. Landscape-related features such as mean patch size, edge density, and landscape diversity, might likewise help reduce B. oleae abundance [48], [49]. This does not necessarily mean that the more the natural vegetation the more effective the biological control. Indeed, some studies seem to point out to complex interactions between different landscape and local features [50], [51], sometimes reinforcing their mutual role in biological control, and some other times counteracting their effects [52]. For example, when both habitats were present in an olive crop, the abundance of some groups of natural enemies (spider and parasitoids) was positively influenced by adjacent vegetation, whereas this effect was lower or even reversed in bare soil plots [50]. Habitats provided by surrounding natural vegetation can also produce the opposite effect by removing, rather than providing, natural enemies to the crop [53]. Overall, these landscape scale variables are well known to have an influence on pest abundance in crops [21], [33], [46] so it was not our aim to specifically test for such effects. Rather, this study assessed the relative impact of groundcover vegetation in relation to other variables that would be operating at the scale of landscape as well as more local and regional scales.
At a regional scale, inter-annual climatic variability might have a major influence on pest abundance. Changes in temperature and humidity could alter the phenology of pests and natural enemies and, therefore, influence insect population growth rate [22], [35]. Humidity can affect herbivores such as S. oleae, as larger populations of this insect have been found near rivers or creeks [54]. This would explain why the amount of variability explained by this factor was higher for S. oleae than for other species. In a recent study in olive groves [47], we detected a high variability in the response of E. olivina and P. oleae to ground cover and different surrounding natural vegetation features between two consecutive years, and attributed these changes to climatic variability between years.
From a farmer perspective, local conditions are too varied to be accounted for when outlining pest control strategies. Similarly, nothing can be done to account for inter-annual climatic variability, even thought the response of pests to any treatment, including ground cover, might be dependent on climatic conditions [35]. Landscape-scale factors, on the other hand, can be taken into account by farmers and are thus subjected to be included in the design of strategies against pests. Farmers could potentially manage vegetation at a whole-farm scale to provide features that promote biological control such as refuge habitat for natural enemies. Farmers could also site production areas on parts of their property to best benefit from any vegetation beyond their ownership boundary that might provide similar benefits. Thus, future research should pay more attention to the landscape context, particularly to perennial non-crop vegetation surrounding or nearby the crop, and to the interactions between these structures and ground cover. Although our study has demonstrated that ground cover by itself is not particularly efficient in terms of biological control, investigating different kinds of vegetation and management practices could identify particular options that do strongly promote biological control. Crucially, however, this analysis of a large data set indicated clearly that groundcovers of the type that is common in Spanish olive groves does not strongly promote biological control. Finally, we acknowledge that other ecosystem services, such as soil fertility and prevention of soil erosion [55], [56], or pollination enhancement [57], can be important so need to be considered in future design of functional groundcovers.
Supporting Information
Based on and expanded from Simon et al. (2010). The effect of ground cover on pest control is considered to be positive, null or negative when either the density of the pest arthropod of the fruit tree and fruit damage is lower, equal or higher, respectively, compared with control.
(DOCX)
(DOCX)
Data Availability
Data are from the Red de Alerta e Información Fitosanitaria (RAIF) de Andalucía (Spain), provided by the Andalusia Government. Requests to access data may be submitted through: http://www.juntadeandalucia.es/agriculturaypesca/portal/contacto.html.
Funding Statement
The authors thank Mr. Ricardo Alarcón Roldán, Head of the Crop Protection Service of the Junta de Andalusia (Spain), for providing the data to develop this work. This study was grant-aided by Junta de Andalucía (PO7-AGR-2747), project REMEDINAL-2 (Comunidad de Madrid, S2009/AMB-1783), and the Ministry of Education of Spain (D. P. FPU grant AP-2007-03970). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Landis DA, Wratten SD, Gurr GM (2000) Habitat management to conserve natural enemies of arthropod pests in agriculture. Annu Rev Entomol 45: 175–201. [DOI] [PubMed] [Google Scholar]
- 2. Paredes D, Campos M, Cayuela L (2013. a) El control biológico de plagas de arthrópodos por conservación: técnicas y estado del arte. Ecosistemas 22(1): 56–61. [Google Scholar]
- 3. Boller EF, Häni F, Poehling HM (2004) Ecological infrastructures: ideabook on functional biodiversity at the farm level. Lindau: IOBC. [Google Scholar]
- 4. Smith MW, Arnold DC, Eikenbary RD, Rice NR, Shiferaw A, et al. (1996) Influence of ground cover on beneficial arthropods in pecan. Biol Control 6: 164–176. 8885080 [Google Scholar]
- 5. McClure MS, Andreadis TG, Lacy HG (1982) Manipulating orchard ground cover to reduce the invasion by leafhopper vectors X-disease. J Econ Entomol 75: 64–68. [Google Scholar]
- 6. Stephens MJ, France CM, Wratten SD, Frampton C (1998) Enhancing biological control of leafrollers (Lepidoptera: Tortricidae) by sowing buckwheat (Fagopyrum esculentum) in an orchard. Biocontrol Sci Techn 8: 547–558. [Google Scholar]
- 7. Song BZ, Wu HY, Kong Y, Zhang J, Du YL, et al. (2010) Effects of intercropping with aromatic plants on the diversity and structure of an arthropod community in a pear orchard. Biocontrol 55: 741–751. [Google Scholar]
- 8. Beizhou S, Zhang J, Jinghui H, Hongying W, Yun K, et al. (2011) Temporal dynamics of the arthropod community in pear orchard intercropped with aromatic plants. Pest Manag Sci 67: 1107–1114. 10.1002/ps.2156 [DOI] [PubMed] [Google Scholar]
- 9. Silva EB, Franco JC, Vasconcelos T, Branco M (2010) Effect of ground cover vegetation on the abundance and diversity of beneficial arthropods in citrus orchards. Bull Entomol Res 100: 489–499. 10.1017/S0007485309990526 [DOI] [PubMed] [Google Scholar]
- 10. Aguilar-Fenollosa E, Pascual-Ruiz S, Hurtado MA, Jacas JA (2011) Efficacy and economics of ground cover management as a conservation biological control strategy against Tetranychus urticae in clementine mandarin orchards. Crop Prot 30:1328–1333. [Google Scholar]
- 11. Ditner N, Oliver B, Beck J, Brick T, Nagel P, et al. (2013) Effects of experimentally planting non-crop flowers into cabbage fields on the abundance and diversity of predators. Biodivers Conserv 22: 1049–1061. [Google Scholar]
- 12. Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, et al. (1980) Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu Rev Ecol Sys 11: 41–65. [Google Scholar]
- 13. Baggen LR, Gurr GM (1998) The influence of food on Copidosoma koehleri (Hymenoptera: Encyrtidae), and the use of flowering plants as a habitat management tool to enhance biological control of potato moth, Phthorimaea operculella (Lepidoptera: Gelechiidae). Biol Control 11: 9–17. 9445092 [Google Scholar]
- 14. Rosenheim JA, Kaya HK, Ehler LE, Marois JJ, Jaffe BA (1995) Intraguild predation among biological control agents: theory and evidence. Biol Control 5: 303–335. [Google Scholar]
- 15. Hodge MA (1999) The implications of intra-guild predation for the role of spiders in biological control. J Arachnol 27: 351–362. 10433675 [Google Scholar]
- 16. Finke DL, Denno RF (2004) Predator diversity dampens trophic cascades. Nature 429: 407–410. [DOI] [PubMed] [Google Scholar]
- 17. Simon S, Bouvier JC, Debras JF, Sauphanor B (2010) Biodiversity and pest management in orchard systems. A review. Agron Sustain Dev 30: 139–152. [Google Scholar]
- 18. Dong J, Wu X, Zhang Q, Jin X, Ding J, et al. (2005) Evaluation of lucerne cover crop for improving biological control of Lyonetia clerkella (Lepidoptera: Lyonetiidae) by means of augmenting its predators in peach orchards. Great Lakes Entomology 38: 186–200. [Google Scholar]
- 19. Wan NF, Ji XY, Jiang JX, Dan JG (2011) Effects of ground cover on the niches of main insect pests and their natural enemies in peach orchard. Chinese Journal of Ecology 30: 30–39. [Google Scholar]
- 20. Meagher RL, Meyer JR (1990) Influence of ground cover and herbicide treatments on Tetranychus urticae populations in peachorchards. Exp Appl Entomol 9: 149–158. [Google Scholar]
- 21. Thies C, Tscharntke T (1999) Landscape structure and biological control in agroecosystems. Science 285: 893–895. [DOI] [PubMed] [Google Scholar]
- 22. Logan JD, Wolesensky W, Joern A (2006) Temperature-dependent phenology and predation in arthropods systems. Ecol Modell 196: 471–482. [Google Scholar]
- 23. Perovic DJ, Gurr GM, Raman A (2010) Effect of landscape composition and arrangement on biological control agents in a simplified agricultural system: A cost-distance approach. Biol Control 52: 263–270. 10.1002/dev.20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Junta de Andalucia (2012) Protocolo de campo para seguimiento del cultivo. http://www.juntadeandalucia.es/agriculturaypesca/portal/export/sites/default/comun/galerias/galeriaDescargas/minisites/raif/manuales_de_campo/ProtocolosCampos_Olivar.pdf. Accessed 2013 Sep 15.
- 25. Ramos P, Campos M, Ramos JM (1998) Long-term study on the evaluation of yield and economic losses caused by Prays oleae Bern. in a olive crop of Granada (Southern Spain). Crop Prot 17: 645–647. [Google Scholar]
- 26. Arambourg Y (1986) Traite d'entomologie Oleicole. Madrid: Consejo Oleícola Internacional. [Google Scholar]
- 27. Jarraya A (2004) Principaux nuisibles des plantes Cultivées et des denrées stockées en Afrique du Nord. Leur biologie. leurs ennemis naturels. leurs dégâts. leur contrôle. Tunis: Edition Climat Publication; [Google Scholar]
- 28. Malumphy C (2011) Olive psyllid Euphyllura olivina (Hemiptera: Psyllidae), a Mediterranean pest of olive breeding outdoors in Britain. British Journal of Entomology and Natural History 24: 17–21. [Google Scholar]
- 29. Argov Y, Rössel Y (1993) Biological control of the Mediterranean black scale (Hom.: Coccidae) in Israel. Entomophaga 38: 89–100. [Google Scholar]
- 30. Castrignano A, Boccaccio L, Cohen Y, Nestel D, Kounatidis I, et al. (2012) Spatio-temporal population dynamics and area-wide delineation of Bactrocera oleae monitoring zones using multi-variate geostatistics. Precision Agriculture 13: 421–441. [Google Scholar]
- 31. Croft BA, Brown WA (1975) Responses of arthropods natural enemies to insecticide. Annu Rev Entomol 20: 285–335. [DOI] [PubMed] [Google Scholar]
- 32. Valentine BJ, Gurr GM, Thwaite WG (1996) Efficacy of the insect growth regulators tebufenozide and fenoxycarb for lepidopteran pest control in apples, and their compatibility with biological control for integrated pest management. Aust J Exp Agric 36: 501–506. [Google Scholar]
- 33. Bianchi F, Booij CJH, Tscharntke T (2006) Sustainable pest regulation in agricultural landscapes: a review on landscape composition, biodiversity and natural pest control. Proc R Soc Lond B Biol Sci 273: 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bowie MH, Gurr GM, Hossain Z, Baggen LR, Frampton CM (1999) Effects of distance from field edge on aphidophagous insects in a wheat crop and observations on trap design and placement. Int J Pest Manage 45: 69–73. [Google Scholar]
- 35. Martinat PJ (1987) The role of climatic variation and weather in forest insect outbreaks. In: Barbosa P, Schultz JC, editors. Insect Outbreaks. New York: Academic Press Inc; pp. 241–268. [Google Scholar]
- 36. Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed effect models. Methods Ecol Evol 4: 283–294. [Google Scholar]
- 37.R Development Core Team (2012) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. Available: http://www.R-project.org.
- 38.Bates D, Maechler M, Bolker B, Walker S (2013) lme4: Linear mixed-effects models using Eigen and S4. R package version 1.0–5
- 39.Skaug H, Fournier D, Nielsen A, Magnusson A, Bolker B (2013) Generalized Linear Mixed Models using AD Model Builder. R package version 0.7.5.
- 40. Begum M, Gurr GM, Wratten SD, Hedberg PR, Nicol HI (2006) Using selective food plants to maximize biological control of vineyards pests. J Appl Ecol 43: 547–554 [Google Scholar]
- 41. Colloff MJ, Lindsay EA, Cook DC (2013) Natural pest control in citrus as an ecosystem service: Integrating ecology, economics and management at the farm scale. Biol Control 67: 170–177. [Google Scholar]
- 42. Hardman JM, Franklin JL, Bostanian NJ, Thistlewood HMA (2011) Effect of the width of the herbicide strip on mite dynamics in apple orchards. Exp Appl Acarol 53: 215–234. 10.1007/s10493-010-9397-1 [DOI] [PubMed] [Google Scholar]
- 43. Tsitsilas A, Hoffman AA, Weeks AR, Umina PA (2011) Impact of groundcover manipulations within windbreaks on mite pests and their natural enemies. Aust J Entomol 50: 37–47. [Google Scholar]
- 44. Gurr GM, Kvedaras OL (2010) Synergizing biological control: Scope for sterile insect technique, induced plant defences and cultural techniques to enhance natural enemy impact. Biol Control 52: 198–207. [Google Scholar]
- 45. Duarte J, Campos M, Guzmán JR, Beaufoy G, Farfan MA, et al. (2009) Olivar y biodiversidad. In: Sostenibilidad de la producción de olivar en Andalucía. Sevilla: Junta de Andalucía. [Google Scholar]
- 46. Rusch A, Bommarco R, Jonsson M, Smith HG, Ekbom B (2013) Flow and stability of natural pest control services depend on complexity and crop rotation at the landscape scale. J App Ecol 50: 345–354. [Google Scholar]
- 47. Paredes D, Cayuela L, Gurr GM, Campos M (2013. b) Effect of non-crop vegetation types on conservation biological control of pests in olive groves. PeerJ 1: e116 10.7717/peerj.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Boccaccio L, Petacchi R (2009) Landscape effects on the complex of Bactrocera oleae parasitoids and implications for conservation biological control. BioControl 54: 607–616. [Google Scholar]
- 49. Ortega M, Pascual S, (2013) Spatio-temporal analysis of the relationship between landscape structure and the olive fruit fly Bactrocera oleae (Diptera: Tephritidae). Agr For Ent 10.1111/afe.12030. [DOI] [Google Scholar]
- 50. Paredes D, Cayuela L, Campos M (2013. c) Synergistic effects of ground cover and adjacent vegetation on natural enemies of olive insect pests. Agric Ecosys Environ 173: 72–80. [Google Scholar]
- 51. Woltz JM, Isaacs R, Landis DA (2012) Landscape structure and habitat management differentially influence insect natural enemies in an agricultural landscape. Agric Ecosyst Environ 152, 40–49. [Google Scholar]
- 52. Martin EA, Reinekingb B, Seoc B, Steffan-Dewenterr I (2013) Natural enemy interactions constrain pest control in complex agricultural landscapes. Proc Natl Acad Sci U S A 110: 5534–5539. 10.1073/pnas.1215725110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Blitzer EJ, Dormann CF, Holzschuh A, Klein AM, Rand TA, et al. (2012) Spillover of functionally important organism between managed and natural habitats. Agric Ecosys Environ 146: 34–43. [Google Scholar]
- 54.De Andrés F (2001) Enfermedades y plagas del olivo. Jaén: Riquelme y Vargas Ediciones.
- 55. Cullen R, Warner KD, Jonsson M, Wratten SD (2008) Economics and adoption of conservation biological control. Biol Control 45: 272–280. 10.1597/06-195 [DOI] [PubMed] [Google Scholar]
- 56. Hartwig NL, Ammon HU (2002) 50th Anniversary—Invited article—Cover crops and living mulches. Weed Sci 50:688–699. [Google Scholar]
- 57. Tscheulin T, Neokosmidis L, Petanidou T, Settele J (2011) Influence of landscape context on the abundance and diversity of bees in Mediterranean olive groves. Bull Entomol Res 101:557–564 10.1017/S0007485311000149 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Based on and expanded from Simon et al. (2010). The effect of ground cover on pest control is considered to be positive, null or negative when either the density of the pest arthropod of the fruit tree and fruit damage is lower, equal or higher, respectively, compared with control.
(DOCX)
(DOCX)
Data Availability Statement
Data are from the Red de Alerta e Información Fitosanitaria (RAIF) de Andalucía (Spain), provided by the Andalusia Government. Requests to access data may be submitted through: http://www.juntadeandalucia.es/agriculturaypesca/portal/contacto.html.