Abstract
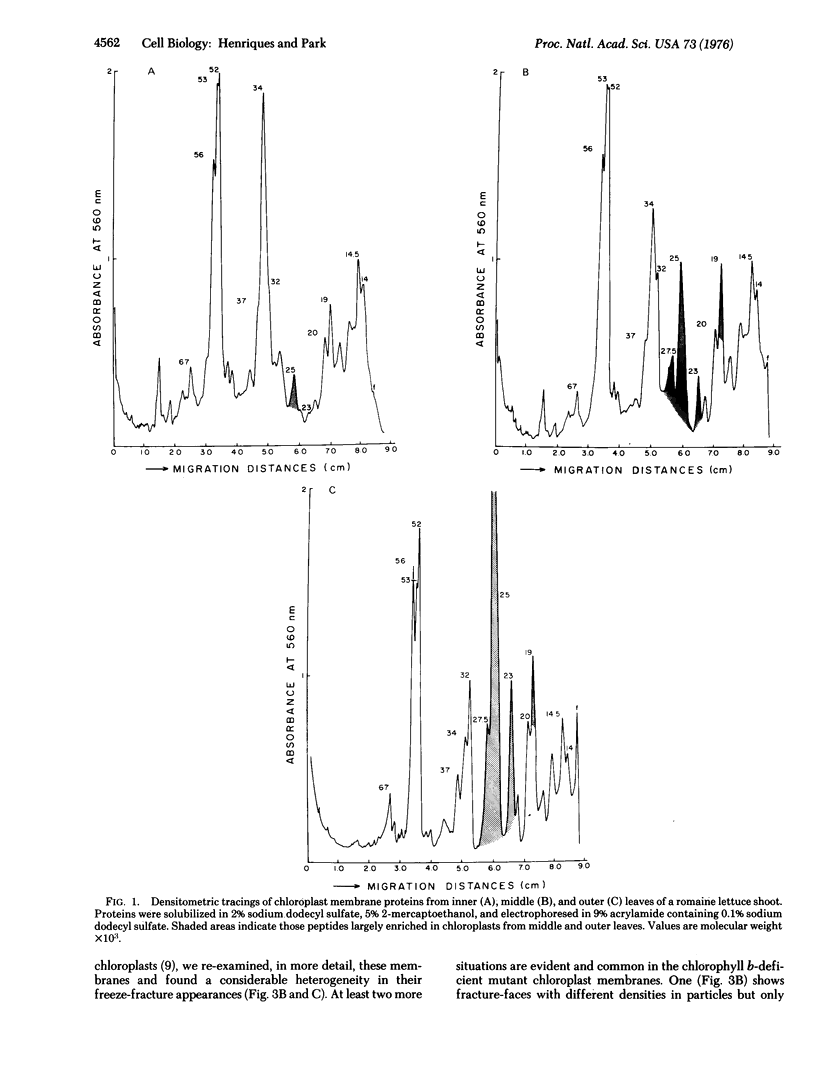
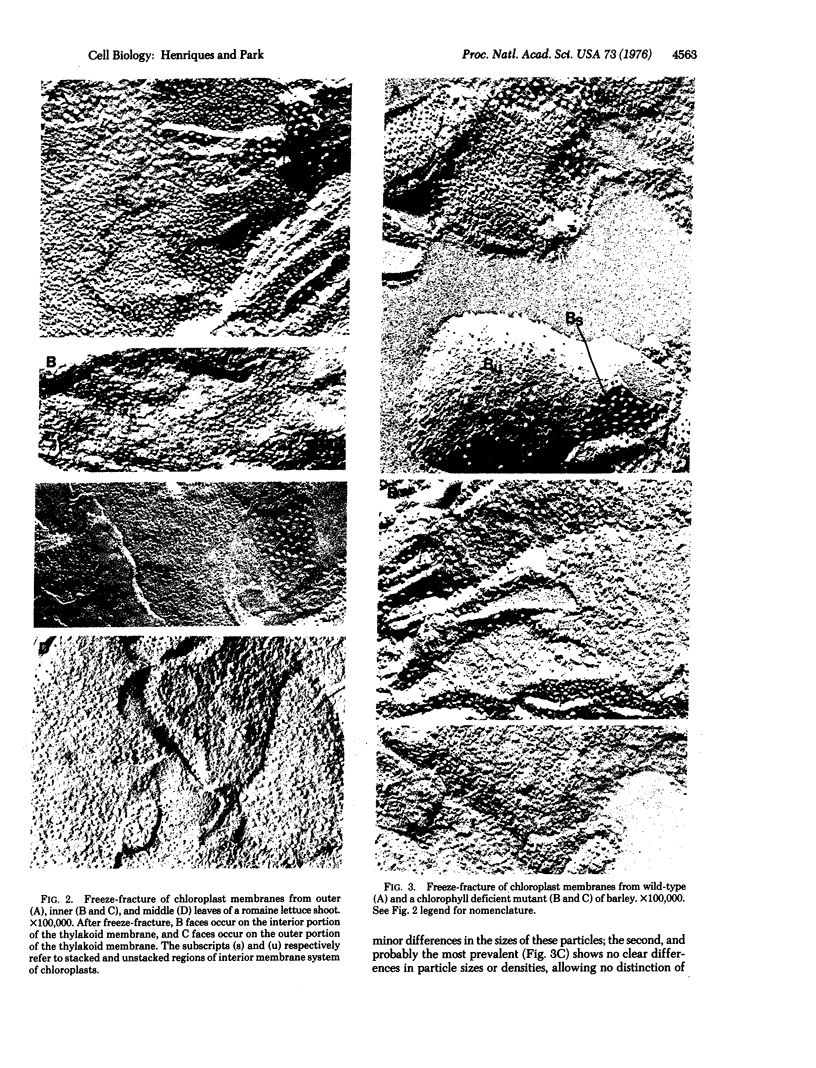
A comparative study of polypeptide composition and freeze-fracture morphology of chloroplast membranes isolated from different, but photosynthetically active, regions of a romaine lettuce shoot is presented. Chloroplasts prepared from outer dark-green leaves possess a fully developed light-harvesting chlorophyll-protein complex, have low chlorophyll a:b ratios and display fracture faces similar to those found in other higher plant chloroplast membranes; chloroplasts from leaves more to the interior of the shoot, have a much lower content of chlorophyll, show high chlorophyll a:b ratios, are depleted in components of the light-harvesting chlorophyll-protein complex, and exhibit extensive modifications of their fracture faces. We have also re-examined the freeze-fracture morphology of chloroplast membranes from a barley b-deficient mutant that lacks the light-harvesting chlorophyll-protein complex. A tentative interpretation of our findings suggests correlating the assembly of the light-harvesting chlorophyll-protein complex into the chloroplast membranes with the appearance of large freeze-fracture B face particles in the stacked interior-membrane region, and differentiation of typical fracture faces.
Keywords: membrane peptide, freeze-fracture, chloroplast
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The molecular organization of chloroplast thylakoids. Biochim Biophys Acta. 1975 Aug 15;416(2):191–235. doi: 10.1016/0304-4173(75)90007-5. [DOI] [PubMed] [Google Scholar]
- Butler W. L., Kitajima M. Energy transfer between photosystem II and photosystem I in chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):72–85. doi: 10.1016/0005-2728(75)90190-5. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Compositional characteristics of a chloroform/methanol soluble protein fraction from spinach chloroplast membranes. Biochim Biophys Acta. 1976 May 14;430(2):312–320. doi: 10.1016/0005-2728(76)90087-6. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Further Chemical and Morphological Characterization of Chloroplast Membranes from a Chlorophyll b-less Mutant of Hordeum vulgare. Plant Physiol. 1975 Apr;55(4):763–767. doi: 10.1104/pp.55.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highkin H. R., Boardman N. K., Goodchild D. J. Photosynthetic Studies on a Pea-mutant Deficient in Chlorophyll. Plant Physiol. 1969 Sep;44(9):1310–1320. doi: 10.1104/pp.44.9.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nobel P. S., Zaragoza L. J., Smith W. K. Relation between Mesophyll Surface Area, Photosynthetic Rate, and Illumination Level during Development for Leaves of Plectranthus parviflorus Henckel. Plant Physiol. 1975 Jun;55(6):1067–1070. doi: 10.1104/pp.55.6.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R. B. Substructure of chloroplast lamellae. J Cell Biol. 1965 Oct;27(1):151–161. doi: 10.1083/jcb.27.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rntzen C. J., Dilley R. A., Crane F. L. A comparison of chloroplast membrane surfaces visualized by freeze-etch and negative staining techniques; and ultrastructural characterization of membrane fractions obtained from digitonin-treated spinach chloroplasts. J Cell Biol. 1969 Oct;43(1):16–31. doi: 10.1083/jcb.43.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Staehelin L. A. Chloroplast membrane structure. Intramembranous particles of different sizes make contact in stacked membrane regions. Biochim Biophys Acta. 1975 Oct 10;408(1):1–11. doi: 10.1016/0005-2728(75)90153-x. [DOI] [PubMed] [Google Scholar]
- Strotmann H., Hesse H., Edelmann K. Quantitative determination of coupling factor CF1 of chloroplasts. Biochim Biophys Acta. 1973 Aug 31;314(2):202–210. doi: 10.1016/0005-2728(73)90135-7. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Highkin H. R. Composition of the photosynthetic apparatus of normal barley leaves and a mutant lacking chlorophyll b. Eur J Biochem. 1974 Jan 3;41(1):109–116. doi: 10.1111/j.1432-1033.1974.tb03250.x. [DOI] [PubMed] [Google Scholar]
- Thornber J. P., Stewart J. C., Hatton M. W., Bailey J. L. Studies on the nature of chloroplast lamellae. II. Chemical composition and further physical properties of two chlorophyll-protein complexes. Biochemistry. 1967 Jul;6(7):2006–2014. doi: 10.1021/bi00859a019. [DOI] [PubMed] [Google Scholar]