Abstract
Cotton (Gossypium spp.) fibers are single-cell trichomes that arise from the outer epidermal layer of seed coat. Here, we isolated a R3-MYB gene GhCPC, identified by cDNA microarray analysis. The only conserved R3 motif and different expression between TM-1 and fuzzless-lintless mutants suggested that it might be a negative regulator in fiber development. Transgenic evidence showed that GhCPC overexpression not only delayed fiber initiation but also led to significant decreases in fiber length. Interestingly, Yeast two-hybrid analysis revealed an interaction complex, in which GhCPC and GhTTG1/4 separately interacted with GhMYC1. In transgenic plants, Q-PCR analysis showed that GhHOX3 (GL2) and GhRDL1 were significantly down regulated in −1–5 DPA ovules and fibers. In addition, Yeast one-hybrid analysis demonstrated that GhMYC1 could bind to the E-box cis-elements and the promoter of GhHOX3. These results suggested that GhHOX3 (GL2) might be downstream gene of the regulatory complex. Also, overexpression of GhCPC in tobacco led to differential loss of pigmentation. Taken together, the results suggested that GhCPC might negatively regulate cotton fiber initiation and early elongation by a potential CPC-MYC1-TTG1/4 complex. Although the fibers were shorter in transgenic cotton lines than in the wild type, no significant difference was detected in stem or leaf trichomes, even in cotton mutants (five naked seed or fuzzless), suggesting that fiber and trichome development might be regulated by two sets of genes sharing a similar model.
Introduction
Trichomes are epidermal hairs found in most land plants. Only some cells are committed to form trichomes. The specificity of development and differentiation of the trichome cell makes it a perfect model to study the regulation of cell development and cell fate determination. Trichome development and root epidermal patterning have been studied in depth in the model plant Arabidopsis thaliana. In Arabidopsis, the R2R3-MYB protein GLABRA1 (GL1) [1], the basic helix–loop–helix proteins GLABRA3 (GL3) or ENHANCER OF GLABRA3 (EGL3) [2, 3] and the WD40 protein TRANSPARENT TESTA GLABRA1 (TTG1) [4] form a combinatorial regulatory complex MYB-bHLH-TTG1 to positively regulate trichome patterning. Furthermore, this complex regulates the downstream gene GL2, encoding a homeodomain (HD-Zip) protein required for normal trichome development [5]. In Arabidopsis, four homologous single MYB proteins, CAPRICE (CPC) [6], TRIPTYCHON (TRY) [7] and ENHANCER OF TRY and CPC1 and 2 (ETC1 and ETC2) [8, 9] have been identified as negative regulators of trichome initiation and patterning. These inhibitory proteins contain a DNA binding domain but no transcriptional activation domain. Protein interaction analysis in yeast has suggested that TRY or CPC can interrupt the functionality of the “activating” GL1-bHLH-TTG1 complex through competitive interaction with bHLH [3, 7, 10]. Therefore, these factors may work as negative transcriptional regulators [11]. Fortunately, the development of cotton fibers and Arabidopsis trichomes share a similar regulatory mechanism involving closely related transcription factors as well as a similar lateral inhibition signaling pathway [12], making these trichomes an excellent system for cotton fiber research.
Cotton (Gossypium spp.) fiber cells are seed trichomes derived from the epidermal layer of the cotton seed coat. The molecular components responsible for regulating fiber cell differentiation have not been fully characterized [13]. In cotton, transcription factors also play an important role in regulating fiber elongation. Overexpression of GaMYB2, which is homologous to AtGL1, rescues trichome formation in the gl1 mutant and induces the production of seed trichomes in Arabidopsis [14]. Moreover, constitutive overexpression of GhMYB25 in transgenic tobacco results in an increase in branched long-stalked leaf trichomes [15]. Correspondingly, GhMYB25-silenced cotton exhibits alterations in the timing of rapid fiber elongation, resulting in short fibers, dramatic reductions in trichomes on other parts of the plant and reductions in seed production [16], while suppression of GhMYB25-like results in cotton plants with fiberless seeds [17]. An HD-Zip IV family transcription factor, G. barbadense Meristem Layer 1 (GbML1), interacts with GhMYB25 and specifically binds to the L1 box of GbRDL1 [18]. GbPDF1 is involved in cotton fiber initiation through interacting with three proteins (PPIP1–PPIP3) [13]. Therefore, it appears that these transcription factors may require companion proteins to work with.
Here, we isolated GhCPC from Gossypium TM-1 0DPA ovules, which is homologous to AtCPC and encodes a negative regulator of cotton fiber elongation. Our work aimed to identity whether GhCPC could interact with other proteins to form a MYB-bHLH-WD40 complex that regulates fiber elongation. Our results showed that GhCPC could interact with a bHLH gene, GhMYC1, while GhMYC1 also interacts with GhTTG1 and GhTTG4. In 35S::CPC transgenic lines, the levels of transcripts of the downstream genes GhHOX3 and GhRDL1 were significantly reduced. Using the yeast one-hybrid system, we found that GhMYC1 could bind the promoter of GhHOX3. These results indicate that cotton fiber elongation may be regulated by a similar regulatory complex to that of Arabidopsis trichome development.
Materials and Methods
Plant Materials
The cotton lines used in this study included TM-1, the fiberless or fuzzless-lintless mutants XZ142fls, MD17 and SL1-7-1, the naked seed or fuzzless-linted mutants N1N1 and n2n2, Gossypium raimondii, G. herbaceum var. africanum, (MD17×TM-1)F2, two RILs [(MD17×TM-1)F5 and (SL1-7-1×TM-1)F5 populations] and transgenic lines. All of these lines were cultivated in Nanjing, China. Transgenic tobacco lines were grown in a greenhouse at Nanjing Agricultural University. Ovules and fibers were collected for analysis at different developmental stages and immediately frozen in liquid nitrogen. The day of anthesis was defined as 0 DPA.
Gene Clone and Sequence Analysis
Genomic DNA and total RNA were extracted as described [19, 20]. Specific primers (CPC-full-F/R, S1 Table) were designed by electronic cloning to amplify the full-length GhCPC genomic sequence and cDNA sequence from TM-1. The promoters of GhCPC and GrCPC were amplified by TAIL-PCR [21]. The PCR products were ligated to T-vector pMD19 (Takara, Dalian, China) and then sequenced using GenScript (Nanjing, China). Amino acid sequences from different plants species were chosen using Blast software from NCBI. Alignments of homologous peptide sequences were carried out with ClustalX software (version 1.81) [22] and a neighbor joining phylogenetic tree was constructed using MEGA 4.1 [23].
Q-PCR analysis
Ovules and fibers were collected from different plants from the same lines at the same time and place with three biological replicates. Total RNA samples (2 μg per reaction) of different tissues were reverse transcribed into cDNAs using AMV reverse transcriptase. The expression of genes involved in this study was analyzed by quantitative real time-PCR (Q-PCR). Q-PCR assays were performed in a 7500 Real-Time PCR System (Applied Biosystems) using First Start Universal SYBR Green Master (Roche). The cotton Histone3 gene (AF024716) was used as an internal control and the relative expression levels of the genes were calculated using the comparative threshold cycle method. The amplification efficiency of each gene was calculated. The Q-PCR cycles were as follows: (1) 95°C, 10 min; (2) 40 cycles of 95°C for 15 s, 60°C(temperature varied for different primers, S1 Table) for 30 s and 72°C for 30 s; (3) a melting curve analysis from 65 to 95°C (1 s hold per 0.2°C increase) to check the specificity of the amplified product. Relative expression levels were determined by the 2-ΔCt method.
Yeast two-hybrid assay
The yeast two-hybrid assay was performed using the Matchmaker GAL4 Two-Hybrid System following the manufacturer’s protocol (Clontech). Full-length cDNA of GhCPC was fused to the GAL4-DNA-binding domain of the bait vector pGBKT7 and transformed into yeast strain Y187. A cDNA library from cotton ovules and fibers (0–20 DPA) was constructed by transforming yeast strain AH109 with ds cDNA and the pGADT7-Rec vector according to the manufacturer’s instructions. The library host strain was mated with bait strain Y187, and the mating mixture was then spread onto SD/–His/–Leu/–Trp medium and incubated at 30°C for 3–4 days. The transformants were selected and the positive clones were isolated and retransformed to bait strains to test their interaction using pGBKT7-53/pGADT7 as the positive control and pGBKT7-Lam/pGADT7 as the negative control.
Yeast one-hybrid assay
The yeast one-hybrid assay was performed using the MATCHMAKER onehybrid system (Clontech). The HOX3 promoter region (−1,798–1, 229 bp) was amplified from the TM-1 genome and fragments of 4×E-box-WT and 4×E-box-Mutant were synthesized by GeneScript Bio-Technology Co., Ltd. These three fragments were ligated into the HindIII- XhoI sites of pAbAi. The bait constructs were linearized with BstBI and integrated into the yeast genome (strain Y1H). Various concentrations of Aureobasidin A (AbA; Clontech, Cat.NO.630446) on SD/-Ura medium were used to identify the basal expression of AUR1-C. The ORF of GhMYC1 was ligated to the GAL4 activation domain in pGAD424. Yeast transformants were tested on SD/-Ura medium containing 50ng/ml AbA.
Plasmid construction and plant transformation
Cotton transformation was carried out as described [24]. To construct the overexpression and antisense vectors, two pairs of primers containing XbaI and SmaI sites were used to amplify GhCPC and the PCR products were ligated into pBI121 vectors, respectively.
The constructs were introduced into Agrobacterium strain LBA4404 for transformation. Cotyledon and hypocotyl explants from G. hirsutum cv. W0 were transformed using Agrobacterium-mediated transformation and selections were performed on kanamycin sulfate-containing medium. After approximately 10 months of in vitro culture and selection, the putative transgenic plants were transferred to a greenhouse for further screening and analysis. Homozygosis of transgenic plants was determined by segregation analysis based on the presence or absence of the kanamycin selection marker NPTII using specific primers for PCR detection as described above [25]. Another primer set was used to determine the presence of the promoters and the GhCPC transgene.
The homozygous transgenic lines were developed by pedigree selection and further used to test fiber length of T3 generation plants in Nanjing in 2012. Fiber samples of transgenic lines and the wild type were tested at the Supervision, Inspection and Test Center of Cotton Quality, Ministry of Agriculture in China.
Scanning electron microscopy of initial fibers at 0 DPA
To detect the differences in initial fibers between CPC-overexpression transgenic lines and the wild type, ovules were simultaneously collected from the same position and fixed in 3% glutaraldehyde. After washing, dehydration, incubation and drying, the cotton ovules were examined under an S-3000N SEM (Japan).
Accession numbers
Sequence data of genes in this article are deposited in GenBank as follows: GhCPC (FJ402930), GhHOX3 (AY626159), GaRDL1 (AY641990), GhTRY (JN997398), GhTRY (JN997398), GhTTG1 (JQ005876), GhTTG2 (AF530910), GhTTG3 (AF530911), GhTTG4 (AF530912), GhDEL61 (JN997401), GhDEL65 (JN997400), AtCPC (819249), AtTRY (835401).
GhETC1 and GhMYC1 have not been uploaded onto NCBI.
Results
Isolation and Characterization of GhCPC in Cotton
In a previous study, we detected an EST with significantly different expression levels between TM-1 and fuzzless-lintless cotton mutants. Here, we isolated the gene from TM-1 0 DPA ovules and designated it as GhCPC (FJ402930), which is homologous to AtCPC. Sequence analysis showed that this gene encoded the same amino acids as contig16590, but not as contig17149 (S1 Fig.).
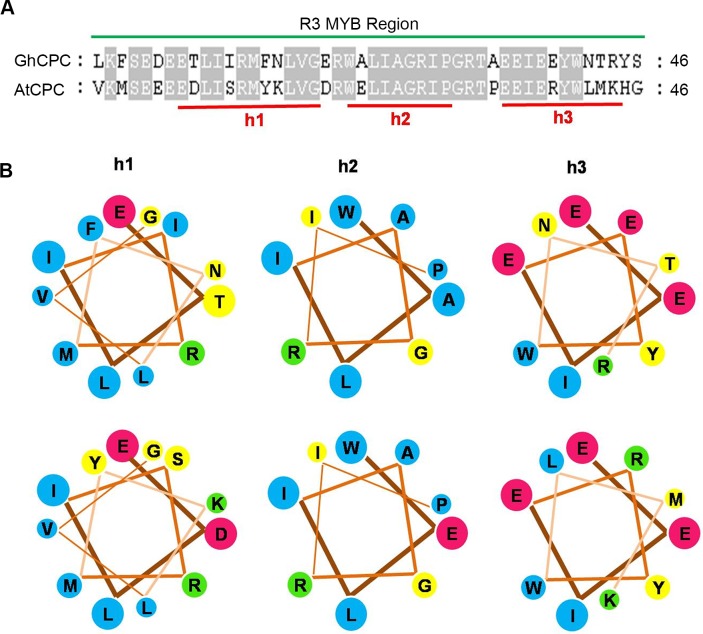
Both GhCPC and AtCPC encode a single R3 MYB repeat protein which shares 65% sequence identity. Like c-MYB, the R3 motif is composed of three helices (h1, h2, h3; Fig. 1A and 1B) [26, 27]. A comparison of the helices of GhCPC and AtCPC revealed that h1, h2 and h3 were similar. The high degree of homology between GhCPC and AtCPC suggests that these genes may have similar functions in controlling trichome or cotton fiber development through a potential MYB-bHLH-TTG1 regulatory complex.
Figure 1. Protein comparison of GhCPC (R3 MYB) and AtCPC (R3 MYB).
A. Sequence alignment of GhCPC and AtCPC R3 proteins. Shaded letters indicate identical residues. Green lines shows the positions of the three helices (h1, h2, h3) forming R3 MYB. B. Helical diagrams of h1, h2 and h3 in GhCPC R3 and AtCPC R3 with non polar residues in blue, polar residues in yellow, acidic residues in red and basic residues in green.
We also identified CPC proteins in other species (G. raimondii, Arabidopsis thaliana, Theobroma cacao, Vitis vinifera, Populus trichocarpa, Oryza sativa, Zea mays, Sorghum bicolor, Setaria italica, Panicum virgatum and Brachypodium distachyon), but CPC proteins were only found in the eudicots, with none detected in monocots, according to genome annotation (http://phytozome.net/). These results suggest that CPC proteins did not differentiate along with the differentiation of eudicots and monocots. Sequence alignment revealed a domain conserved among different species that was critical for their function (S2 Fig.).
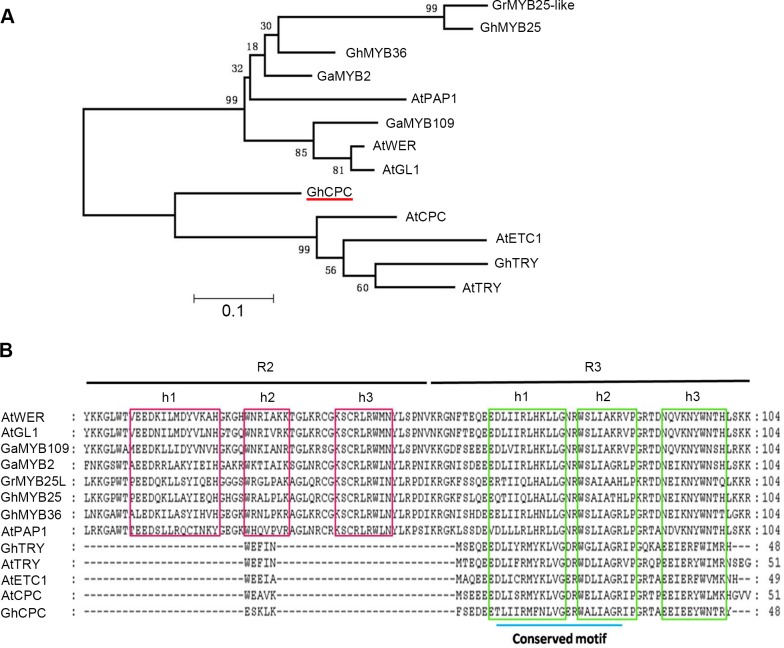
The MYB gene family is one of the most important transcription factor gene families in the plant kingdom. Comparative analysis of a gene family may reveal important adaptive changes at the protein level and thereby provide insights that relate structure to function. To provide a framework for identifying the evolution of R2R3 MYB and R3 MYB, a neighbor joining phylogenetic tree was constructed based on the R2R3 and R3 amino acid sequences (Fig. 2A). This tree has three distinct branches consisting of the genes GhMYB25 (GaMYB2, GhMYB25, GhMYB25-like, AtPAP1), GaMYB109 (GaMYB109, AtWER, AtGL1) and GhCPC (GhCPC, GhTRY, AtETC1, AtCPC, AtTRY; Fig. 2A). The full-length R2R3 MYB genes encode two DNA-binding domains, the R2 and R3 domain (Fig. 2B). Each domain has three conserved helices, and the structure of the third helix is essential for sequence-specific DNA binding. The first and second helixes of the R3 domain contain a conserved motif involved in MYB-bHLH interactions (Fig. 2B) [27–29]. As AtCPC, GhCPC also has only an R3 domain and exhibits a loss of DNA transcriptional activation of the R2 MYB domain. Previous studies showed AtCPC negatively regulated the trichome development by a MYB-bHLH-TTG1 complex [30, 31]. So we presumed that GhCPC might perform its function also as a negative regulatory gene in fiber development.
Figure 2. Phylogenetic tree and amino acid sequence alignment among the R2R3 MYB and R3 MYB regions.
A. Neighbor joining phylogenetic tree of the amino acid sequence of the R2R3 MYB regions (AtWER, AtGL1, AtPAP1, GhMYB2, GhMYB25, GhMYB25-like, GhMYB36, GhMYB109) and R3 MYB regions (AtETC1, AtTRY, AtCPC, GhTRY, GhCPC). B. Sequence alignment of R2R3 MYB and R3 MYB members using ClustalX software. R2 and R3 domains are marked with black bars under the corresponding residues. Three helices of both the R2 and R3 domains are indicated with red and green boxes, respectively. The conserved MYB-bHLH interaction motif on the first and second helices of the R3 domain is underlined with a blue bar.
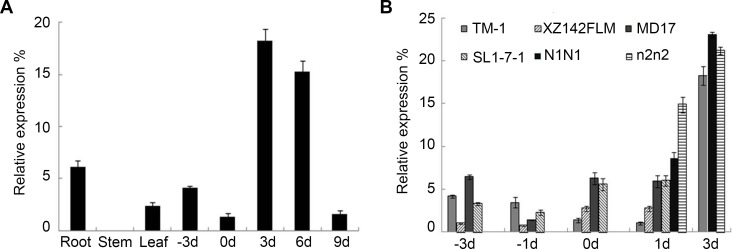
To explore whether GhCPC functions as a negative transcriptional regulator of fiber initiation and elongation, we performed expression analysis of GhCPC in TM-1 and three fiberless or fuzzless-lintless mutants using quantitative real time-PCR (Q-PCR; Fig. 3). The results showed that the peak of GhCPC expression occurred in 3 DPA ovules during fiber elongation. The expression of GhCPC was at a lower level in leaves and roots, while no transcripts were detected in stems (Fig. 3A). Moreover, fuzzless-lintless (XZ142 fls, MD17, SL1-7-1) and fuzzless-linted or naked-seed (N1N1, n2n2) mutants had a significantly higher expression level than TM-1 in 0, 1 and 3 DPA ovules, while the opposite result was obtained in −1 DPA ovules (Fig. 3B). These results suggest that CPC may contribute to early fiber initiation and elongation and act as an inhibitor in the development of fuzzy seeds with lint.
Figure 3. Q-PCR analysis of GhCPC expression in different cotton species.
A. Spatial and temporal expression patterns in roots, stems, leaves, −3–0 DPA ovules and 3–9 DPA fibers in TM-1. B. Differential expression pattern in ovules of wild type (TM-1), three fiberless mutants (XZ142 FLM, MD17 and SL1-7-1) and two naked-seed mutants (N1N1 and n2n2).
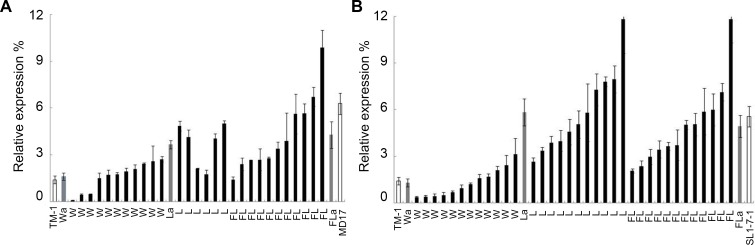
To further examine the transcript levels of GhCPC in fuzzy-linted, fuzzless-linted and fuzzless-lintless cotton, we examined the expression of GhCPC among the three phenotypes in two RIL populations, (MD17 ×TM-1) and (SL1-7-1×TM-1) RILs(F5). First, we harvested 0–1 DPA ovules from 11 linted-fuzzy (wild type), six linted-fuzzless (La) and 11 lintless-fuzzless (FLa) lines derived from (MD17×TM-1) RILs (F5). The highest relative expression level of GhCPC was found in the fuzzless-lintless lines and the lowest was found in fuzzy-linted lines (Fig. 4A). Moreover, the average expression level was significantly lower in the wild type than in the La and FLa types, while it was lower in La than in FLa. However, the expression of GhCPC was lower in La and FLa than in MD17. In the other RIL population, GhCPC had a similar expression pattern in different lines. Specifically, we selected 12 wild type, 11 La and 12 FLa lines from (SL1-7-1×TM-1) RILs (F5). Similar to (MD17×TM-1) RILs (F5), GhCPC had a higher transcript levels in mutant type than wild type, while the highest transcript levels appeared in both the La and FLa lines (Fig. 4B). GhCPC expression was lower in FLa than in SL1-7-1, while the expression of this gene was also lower in the wild type than in TM-1. Though the wild type or lintless-fuzzless type lines had a similar phenotype to the corresponding parents, no identical expression level was detected. We presumed that the inhibition of GhCPC might be resulted from allele polymerization or genetic models and these also need further research to clarify.
Figure 4. Q-PCR analysis of GhCPC expression in two RIL populations.
A, B showed different expression pattern in 0–1 DPA ovules of pure lines of (MD17×TM-1) and (SL1-7-1×TM-1) RIL F5 population, respectively. W, L and FL on abscissa represent linted-fuzzy, linted-fuzzless and lintless-fuzzless lines, respectively. Wa, La and FLa on abscissa represent the average expression levels in the linted-fuzzy, linted-fuzzless and lintless-fuzzless lines, respectively.
Overexpression of GhCPC Leads to Significant Decreases in fiber Length
To investigate whether overexpression of GhCPC would inhibit cotton fiber elongation, we generated CPC overexpression constructs driven by the 35S promoter and introduced these constructs into cotton (W0) via Agrobacterium tumefaciens-mediated transformation. Finally, we obtained 19 independent sense transgenic lines (T0), but only two antisense transgenic lines were obtained due to poor regeneration. We performed detailed phenotype and molecular analyses in the T3 generation plants. Compared to the wild type plants, which were separated from the T0 generation, overexpression of GhCPC resulted in shorter fibers in the GhCPC overexpression transgenic lines. However, no distinct difference was observed in the antisense transgenic lines, although the expression of GhCPC was lower in these lines than in the wild type (S3 Fig.), possibly because other genes, such as GhTRY or GhETC1, compensated for the loss of expression of GhCPC in the antisense lines.
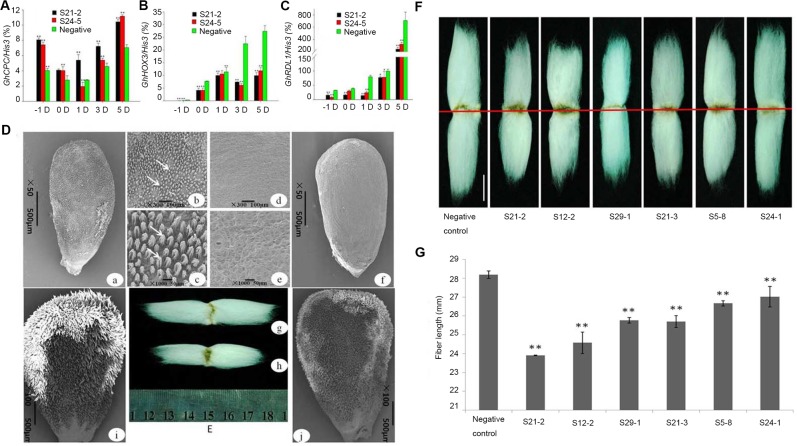
In the GhCPC overexpression transgenic lines, we examined the expression level of GhCPC in −1, 0, 1, 3, 5-DPA ovules and fibers by Q-PCR. The result showed a similar expression trend that observed in TM-1. The accumulation of GhCPC transcripts was significantly higher in the ovules and fibers of the overexpression lines than in the wild type (Fig. 5A). Scanning electron microscopy (SEM) of the ovules of wild type plants revealed normal differentiation and rapid emergence of fiber cells from the surface of the ovule at 0 DPA. By contrast, the surfaces of the ovules from the transgenic plants S21-2 were smooth, with no appearance of fiber initiation (Fig. 5D). However, at 1 DPA, the fibers of CPC-overexpression plants began to develop, although their lengths were much shorter than those of the wild type. These results suggest that overexpression of GhCPC is first effective in fiber initiation. Integrating the regulatory model of Arabidopsis trichome development and the role of GhHOX3 and GhRDL1 in fiber elongation [14], we presumed that GhHOX3 and GhRDL1 are downstream genes of GhCPC. In the sense transgenic lines S21-2 and S29-1 (T3), Q-PCR showed that the transcript levels of GhHOX3 were significantly reduced compared to the wild type, which was also true for GhRDL1 (Fig. 5B–C). Mature fibers on S21-2 T3 homozygous transgenic plants were significantly shorter (23.90 ± 0.01 mm) than those of the wild type (28.14 ± 0.12 mm, t-test, p-value ≤ 0.01; Fig. 5E), while the other transgenic lines also exhibited shorter fibers to various degrees (Fig. 5F-G). These results suggest that CPC plays an important role in the early stages of fiber cell differentiation, and overexpression of CPC not only delayed initial fiber development, but it also repressed early fiber elongation. However, there were no significant changes in the trichome phenotype of leaves between CPC overexpression transgenic lines and the wild type,besides the number of trichomes between S21-2 (23.0 ± 1.7) and the wild type (23.4 ± 1.8) in the same field of view (20× stereomicroscope; S6 Fig.).
Figure 5. Compared to the wild type, overexpression of GhCPC leads to delayed fiber differention and decreased fiber length in the T3 generation Wild type was separated from the T0 generation.
A, B and C respectively show significantly different expression of GhCPC, GhHOX3 and GhRDL1 in CPC sense transgenic lines S21-2 and wild type. D, Different initial development of fiber cells of 0 DPA between CPC-overexpression T3 S21-2 and wild type . a, b, c Wild type ovules exhibited normal differentiation and rapid emergence of fiber cells from the surface (a-c). However, CPC-overexpression ovules exhibited the opposite morphology in which the surfaces of ovules from the transgenic plants were smooth with no appearance of fiber initiation. The fiber cells were observed at 50×, 300× and 1,000× magnification. E, Mature fibers in the wild type (g) and S21-2 (h), respectively, corresponding to D-a and D-f. F, Mature fiber comparison among wild type and CPC overexpression lines. The white line represents 1 mm. G, Measurement of fiber length showed that the fiber length in transgenetic lines was shorter than that in the wild type. Bars represent SD of three measurements and ** represent p-value ≤ 0.01 (t-test).
Interaction of proteins of the CPC-MYC1-TTG1/4 regulatory complex by yeast two-hybrid assays
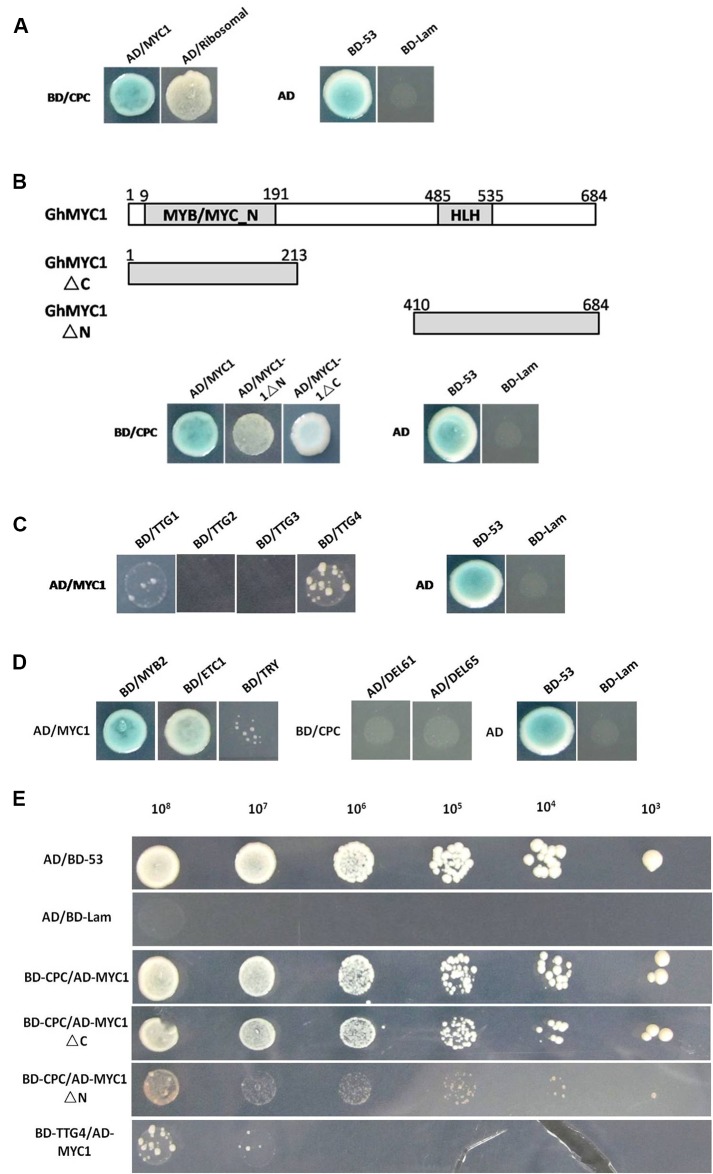
Previous studies have shown that AtCPC can interact with AtGL3, while AtTTG1 also interacts with AtGL3 [7, 32]. Examining similar interactions between GhCPC and other factors would improve our understanding of the role of GhCPC during cotton fiber differentiation. To identify whether GhCPC could interact with other proteins, the full-length GhCPC fused to the GAL4-DNA binding domain was used to screen a fiber yeast two-hybrid library using cDNAs from ovules and fibers from −3 DPA to 25 DPA. Two candidate interacting proteins were identified. One of the candidate proteins, GhMYC1, contained a MYC_N domain and a bHLH domain (S5 Fig.) and the other was a 60S ribosomal protein, named GhRibosomal, by homologous.
Yeast co-transformed with BD-GhCPC and AD-GhMYC1 or AD-GhRibosomal grew on SD/–Ade/–His/–Leu/–Trp selective medium and activated the reporter gene to produce a blue end product with X-α-Gal (Fig. 6A). Since GhMYC1 contains two conserved motifs involved in MYC_N (aa 9–191) and bHLH (aa 485–535), the GhMYC1 protein was divided into two fragments, the N terminus and the C terminus. Furthermore, using MYC1-truncated derivatives of the N terminus (deletion of C, ΔC) or C terminus (deletion of N, ΔN) fragments, we found that both MYC1ΔC and MYC1ΔN were sufficient to interact with CPC separately, while the interaction with MYC1ΔC was stronger than that of MYC1ΔN, but not as strong as that of the whole protein (Fig. 6B, E). These results suggested that the MYC1 amino terminal truncations differed in their ability to independently activate reporter gene transcription when fused to the AD, and both parts were together responsible for the interaction.
Figure 6. Interactions of different proteins.
A. Yeast two-hybrid assays examining the interactions between CPC, MYC1 and ribosomal proteins. The vectors pGADT7/pGBKT-53 and pGADT7/pGBKT-Lam were separately used as positive and negitave controls. B. Mapping of trucated domains of MYC1 to bind to CPC. As shown, both the MYB/MYC domain and the bHLH domain are required for the interaction with CPC. C. Of the four WD40 proteins, only TTG1 and TTG4 had weak interactions with CPC. D. Interactions between different function genes. E. Different concentrations (cell/ml) were plated onto SD/-Ade-Leu-Trp-His medium to examine the intensity of the interaction in the positve control by the yeast two-hybrid assay.
Like GhMYC1, both GhDEL61 and GhDEL65, which are homologous to AtGL3, belong to the bHLH super family. Therefore, these two bHLH genes fused to the activation domain were separately co-transformed with GhCPC. Interestingly, no interactions were observed among the proteins (Fig. 6D), although protein sequence analysis showed that the conserved domains of the three bHLH proteins shared high identity (S6 Fig.).
The fact that GhCPC strongly interacted with GhMYC1 suggests that there might be a regulatory complex consisting of CPC-MYC1-TTG in fiber development. Therefore, we examined whether GhMYC1 could interact with the WD40 genes (GhTTG1-4 homologous to AtTTG1) in cotton. Further study showed that GhMYC1 could interact with GhTTG1 and GhTTG4, but the interaction between them was not strong, while no interactions were observed with GhTTG2 or GhTTG3 (Fig. 6C); perhaps these genes may take part in different complexes in different pathways.
Together, these data demonstrate that the CPC-MYC1-TTG1/4 regulatory complex may play an important role in fiber elongation. Similar to Arabidopsis, the model for the role of this complex in cotton fiber development involves inhibition with a feedback mechanism. According to this model, GhCPC regulates fiber initiation and directs the production of the fibers by forming a non-activating complex, which does not promote the transcription of the downstream gene GhHOX3.
MYC1 directly binds to ProGhHOX3
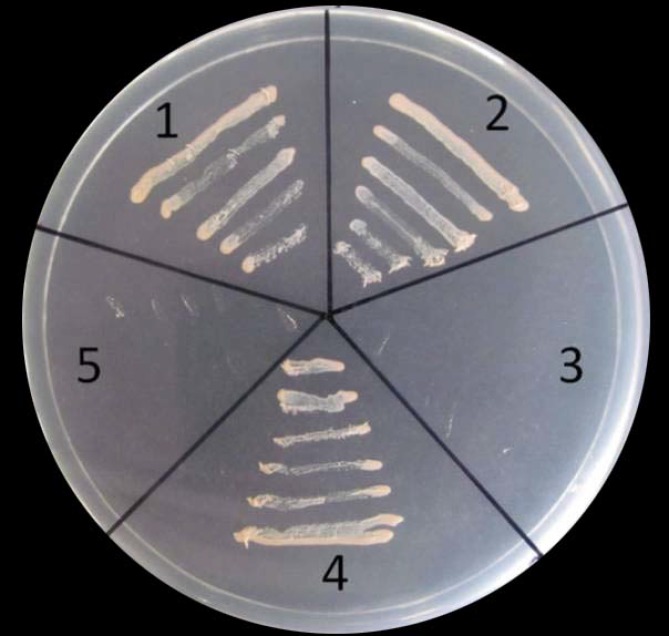
In the CPC sense transgenic lines, significantly decreased expression of GhHOX3 was observed compared to the wild type. Previous in vitro and in vivo binding assays showed that the bHLH domain can bind to the G-box cis-element (CACGTG) and G-box-like motifs (AACGTG or CATGTG) [33–35]. In a broader sense, E-box elements (CANNTG) served as binding sites of bHLH transcription factors [36]. Sequence analysis by PLACE (http://www.dna.affrc.go.jp/htdocs/PLACE/) revealed that the GhHOX3 promoter (~2 kb) contained seven E-box cis-elements, one of which is a G-box cis-element. To determine whether MYC1 directly binds to the promoter of GhHOX3, we performed DNA-protein interaction analysis using the yeast one-hybrid system. Three DNA fragments were respectively inserted into the pAbAi vector. One contained part of the ProGhHOX3 and the other two were 4×E-box-WT (four tandem repeats of CACGTG) and 4×E-box-Mutant (four tandem repeats of CATAGA).
Yeast one-hybrid analysis showed that GhMYC1 could bind to the ProGhHOX3 and 4×E-box-WT. However, when CACGTG was mutated to CATAGA, no binding activity to 4×E-box-Mutant was found (Fig. 7). These results indicate that the bHLH protein MYC1 is able to recognize and bind to the E-box motif and function as a transcription activator in yeast. The results also suggest that GhMYC1 plays an important role in regulating the expression of GhHOX3. Also, like a bridge, when CPC binds to GhMYC1, they form a negative regulatory complex to inhibit downstream gene expression. If some positive regulators bind to GhMYC1, it may act as a promoter of fiber elongation.
Figure 7. Transcriptional activation of ProHOX3, 4×E-box-WT and 4×E-box-Mutant by GhMYC1.
(1) Yeast Y1H integrating ProHOX3, (2) 4×E-box-WT, (3) 4×E-box-Mutant or (4) p53-pAbAi were respectively transformed with GhMYC1.
Discussion
The potential role of GhCPC in cotton fiber elongation
Both cotton fibers and Arabidopsis trichomes are epidermal hairs, and they may share similar molecular mechanisms in the regulation of cell development. In Arabidopsis, AtCPC overexpression caused fewer trichomes than normal. Thus, the CPC gene determines the fate of epidermal cell differentiation in Arabidopsis [6]. In this study, GhCPC only had a conserved R3-domain with three helices and exhibits a loss of DNA transcriptional activation of the R2 MYB domain. AtCPC is a negative regulator of trichome initiation and patterning in Arabidopsis [6]. Since the R3 motif of GhCPC is homologous to AtCPC in terms of amino acid sequence, this protein may also have a similar function in fiber development. Expression analysis of GhCPC in TM-1, fiberless mutants and two RIL populations showed that GhCPC might serve as a negative regulator of fiber elongation. Previous studies had suggested that CPC played a critical role in determining the fate of epidermal cell differentiation in Arabidopsis [6]. We obtained a similar result in transgenic cotton plants. GhCPC overexpression led to significant decreases in fiber length, especially in 0 DPA ovules with no fiber protuberances, which is analogous to the dramatically reduced number of trichomes observed in 35S::CPC and 35S::ETC1 transgenic Arabidopsis [6, 8]. This study showed that GhCPC delayed fiber elongation but did not completely inhibit cotton fiber early development, like GhMYB25-like silenced line [17]. These results reveal that GhCPC is a regulator of fiber elongation and that cotton fibers may share a similar model of cell differentiation with Arabidopsis trichomes.
GhCPC functions through the potential formation of the CPC-MYC1-TTG1/4 regulatory complex
A network of three classes of proteins consisting of bHLH, MYB transcription factors and a WD40 repeat protein (TRANSPARENT TESTA GLABRA1, TTG1) act in concert to activate trichome initiation and patterning [11]. Evidence for the existence of the TTG1-bHLH-MYB complex is based entirely on protein interaction studies performed using the yeast two-hybrid system [2, 3, 28]. Protein interaction analysis has shown that AtCPC binds to the GL1-bHLH-TTG1 complex through competitive interactions with bHLH to inhibit trichome initiation when AtCPC is over expressed [6, 37, 38]. Our yeast two-hybrid data also show that GhCPC strongly interacted with a bHLH protein, GhMYC1, which is involved in gene regulation. GhMYC1 could interact with GhTTG1 and GhTTG4 to produce the CPC-MYC1-TTG1/4 complex, which regulates fiber elongation in cotton.
In the regulatory complex, each partner may have similar or opposite genes on functions. In cotton, two bHLH proteins, GhDEL61 and GhDEL65, are homologous to AtGL1 and share 29% and 33% identity with GhMYC1, respectively. Amino sequence analysis among these three bHLH factors indicated that MYC_N motif of the N-terminal region and the HLH motif domain were highly conserved (S5 Fig.). Interestingly, there were no interactions detected between GhCPC and GhDEL61 or GhDEL65 in our yeast two-hybrid assays. Similarly, GhMYC1 also had strong interactions with GaMYB2 and GhETC1 but weak interactions with GhTRY. Perhaps some different amino of conserved domains or non-conserved domains may block the interactions. The yeast two-hybrid assay results indicated that the proteins had distinct functions and evolutionary patterns or might have different favorite partner proteins. The results also suggested that different proteins might take part in different pathways to regulate different biological processes. Simultaneously, evidence showed that GaMYB2 and GhHOX3 had specific ways in which they cooperate to activate the GhRDL1 promoter and for each pair of transcription factors, the effects seemed to be synergistic rather than additive [14]. Since GhCPC overexpression could decrease GhRDL1 expression, we presumed that GhCPC and GaMYB2 might have opposite effects on fiber elongation by competing to bind to GhMYC1.
GLABRA2 (GL2) is a direct target of the GL1-GL3-TTG2 complex and is directly regulated by GL1 [39, 40]. In the current study, the levels of GhHOX3 (homologous to AtGL2) transcripts in the transgenic lines were lower than those of the negative control. Since gel mobility shift experiments and yeast one-hybrid assays have previously shown that AtCPC does not bind to DNA [41], it is also unlikely that GhCPC represses GhHOX3 by directly binding to its cis-elements sites to repress gene transcription. Similarly, in cotton, GhCPC may prevent the complex from binding to the promoter of GL2. In Arabidopsis, AtMYC2 can directly target the E-box cis-element of the promoters of TPS11 and TPS21 [42], suggesting that the regulatory complex is indeed able to bind to the DNA fragments of the downstream genes. In the current study, the transcripts of GhRDL1 were also significantly repressed by GhCPC overexpression in 35S::CPC transgenic cotton lines. When GhHOX3 genes driven by the 35S promoter was transferred into Arabidopsis, the RDL-P3 promoter was activated to regulate GUS expression [14], while it could also transduce GA signal to promote fiber cell elongation [44]. Taken together, the results support a classical model in which GhCPC, GhMYC1 and GhTTG1/4 might form the CPC-MYC1-TTG1/4 complex to regulate the expression of GhHOX3, and subsequently, this GL2 protein regulates the downstream target gene GhRDL1 to control fiber elongations (Fig. 8). Analysis of the bHLH domain of GhMYC1 and the promoter of GhHOX3 has shown that this conserved domain could recognize and bind to the E-box cis-element. Our yeast one-hybrid data confirmed this speculation.
Figure 8. The potential CPC-MYC1-TTG1 regulatory complex in cotton.
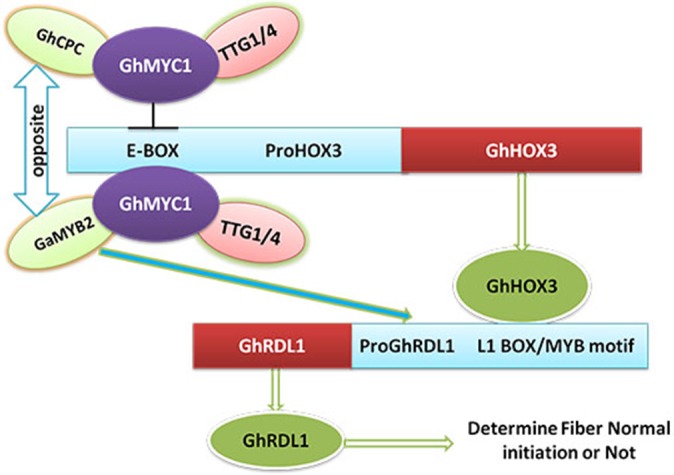
In this network, CPC-MYC1-TTG4 complex can regulate the expression levels of GhHOX3 and GhRDL1 by binding to their promoters. GhCPC and GaMYB2 may have opposite effects on fiber development by completely binding to GhMYC1.
In tobacco, flowers of AtCPC overexpression plants display an unexpected defect in pigmentation [43]. In light of the existence of the CPC-MYC1-TTG4 complex, GhCPC may have a similar function in regulating the production of anthocyanin. To further investigate the role of GhCPC and how conserved this role is across plant species, 35S::CPC was constitutively over expressed in tobacco (Nicotiana tabacum). In the transgenic lines, alterations in petal color also led to a differential loss of pigmentation (S7 Fig.). The results also indicate that GhCPC is involved in regulating anthocyanin biosynthesis, also possibly via the MYB-bHLH-WD40 complex. Further direct evidence of the function of this complex in regulating anthocyanin biosynthesis should be obtained in future studies.
Differences in the trichome/fiber regulatory mechanism between cotton and Arabidopsis
Elucidating the regulatory pathway of GhCPC opens up new avenues for understanding the existence and importance of the CPC-MYC1-TTG1/4 complex in regulating fiber elongation and raises many questions about this regulatory network. Although they are both unicellular epidermal hairs, cotton fibers are distinct from Arabidopsis trichomes, while cotton fibers are produced in the seed and are unbranched and extremely elongated [45]. In this study, we also found that the regulatory system of cotton is not entirely identical to that of Arabidopsis. GhCPC overexpression produces seeds with shorter fibers in cotton, but no significant changes were found in leaf or stem trichomes, besides changes in the fibers of transgenic cotton and fiberless mutants (XZ142 FLM and MD17 FLM). We also found that there was no tissue-specific expression pattern for GhCPC. The silenced GhMYB25-like transcripts cotton produced fiberless seeds, but normal trichomes elsewhere [17], whereas GhHD-1 silenced cottons were almost completely glabrous (hairless) and showed a delay but later normal seed fiber initiation [46]. Therefore, we hypothesize that the development of cotton fibers and leaf (or stem) trichomes is regulated by a similar model, but some genes suited for cotton fibers but not for leaf (or stem) trichomes. Here, we expounded the potential mechanism of GhCPC in regulating fiber development, although the findings of this study are similar to the Arabidopsis model, but the development of cotton fibers is much more complex than that of Arabidopsis trichomes. Compared to the complex and enormous regulatory network, short of enough evidences blocked the confirmation of the complex in fiber development. Elucidating the differences between these systems may further explain the specificity of the molecular regulatory mechanism of plant trichome development.
Supporting Information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
Acknowledgments
We thank Prof. Dr. Wangzhen Guo for his support and comments during the preparation of this manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was financially supported in part by grants from the National Science Foundation of China (31330058) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Oppenheimer DG, Herman PL, Sivakumaran S, Esch J, Marks MD (1991) A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67: 483–493. [DOI] [PubMed] [Google Scholar]
- 2. Payne CT, Zhang F, Lloyd AM (2000) GL3 encodes a bHLH protein that regulates trichome development in Arabidopsis through interaction with GL1 and TTG1. Genetics 156: 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang F, Gonzalez A, Zhao M, Payne CT, Lloyd A (2003) A network of redundant bHLH proteins functions in all TTG1-dependent pathways of Arabidopsis. Development 130: 4859–4869. [DOI] [PubMed] [Google Scholar]
- 4. Walker AR, Davison PA, Bolognesi-Winfield AC, James CM, Srinivasan N, et al. (1999) The TRANSPARENT TESTA GLABRA1 locus, which regulates trichome differentiation and anthocyanin biosynthesis in Arabidopsis, encodes a WD40 repeat protein. The Plant cell 11: 1337–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rerie WG, Feldmann KA, Marks MD (1994) The GLABRA2 gene encodes a homeo domain protein required for normal trichome development in Arabidopsis. Genes & development 8: 1388–1399. [DOI] [PubMed] [Google Scholar]
- 6. Wada T, Tachibana T, Shimura Y, Okada K (1997) Epidermal cell differentiation in Arabidopsis determined by a Myb homolog, CPC. Science 277: 1113–1116. [DOI] [PubMed] [Google Scholar]
- 7. Schellmann S, Schnittger A, Kirik V, Wada T, Okada K, et al. (2002) TRIPTYCHON and CAPRICE mediate lateral inhibition during trichome and root hair patterning in Arabidopsis. The EMBO journal 21: 5036–5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kirik V, Simon M, Huelskamp M, Schiefelbein J (2004) The ENHANCER OF TRY AND CPC1 gene acts redundantly with TRIPTYCHON and CAPRICE in trichome and root hair cell patterning in Arabidopsis. Developmental biology 268: 506–513. [DOI] [PubMed] [Google Scholar]
- 9. Kirik V, Simon M, Wester K, Schiefelbein J, Hulskamp M (2004) ENHANCER of TRY and CPC 2 (ETC2) reveals redundancy in the region-specific control of trichome development of Arabidopsis. Plant molecular biology 55: 389–398. [DOI] [PubMed] [Google Scholar]
- 10. Esch JJ, Chen M, Sanders M, Hillestad M, Ndkium S, et al. (2003) A contradictory GLABRA3 allele helps define gene interactions controlling trichome development in Arabidopsis. Development 130: 5885–5894. [DOI] [PubMed] [Google Scholar]
- 11. Zhao M, Morohashi K, Hatlestad G, Grotewold E, Lloyd A (2008) The TTG1-bHLH-MYB complex controls trichome cell fate and patterning through direct targeting of regulatory loci. Development 135: 1991–1999. 10.1242/dev.016873 [DOI] [PubMed] [Google Scholar]
- 12. Schnittger A, Folkers U, Schwab B, Jurgens G, Hulskamp M (1999) Generation of a spacing pattern: the role of triptychon in trichome patterning in Arabidopsis. The Plant cell 11: 1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng F, Tu L, Tan J, Li Y, Nie Y, et al. (2012) GbPDF1 is involved in cotton fiber initiation via the core cis-element HDZIP2ATATHB2. Plant physiology 158: 890–904. 10.1104/pp.111.186742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang S, Wang JW, Yu N, Li CH, Luo B, et al. (2004) Control of plant trichome development by a cotton fiber MYB gene. The Plant cell 16: 2323–2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Y, Machado AC, White RG, Llewellyn DJ, Dennis ES (2006) Expression profiling identifies genes expressed early during lint fibre initiation in cotton. Plant & cell physiology 47: 107–127. [DOI] [PubMed] [Google Scholar]
- 16. Machado A, Wu Y, Yang Y, Llewellyn DJ, Dennis ES (2009) The MYB transcription factor GhMYB25 regulates early fibre and trichome development. The Plant journal: for cell and molecular biology 59: 52–62. [DOI] [PubMed] [Google Scholar]
- 17. Walford SA, Wu Y, Llewellyn DJ, Dennis ES (2011) GhMYB25-like: a key factor in early cotton fibre development. The Plant journal: for cell and molecular biology 65: 785–797. 10.1111/j.1365-313X.2010.04464.x [DOI] [PubMed] [Google Scholar]
- 18. Zhang F, Zuo K, Zhang J, Liu X, Zhang L, et al. (2010) An L1 box binding protein, GbML1, interacts with GbMYB25 to control cotton fibre development. Journal of experimental botany 61: 3599–3613. 10.1093/jxb/erq173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paterson AH, Brubaker CL, Wendel JF (1993) A rapid method for extraction of cotton (Gossypium spp.) genomic DNA suitable for RFLP or PCR analysis. Plant Molecular Biology Reporter 11: 122–127. [Google Scholar]
- 20. Jiang J, Zhang T (2003) Extraction of total RNA in cotton tissues with CTAB-acidic phenolic method. Cotton Sci 15: 166–167. [Google Scholar]
- 21. Liu YG, Whittier RF (1995) Thermal asymmetric interlaced PCR: automatable amplification and sequencing of insert end fragments from P1 and YAC clones for chromosome walking. Genomics 25: 674–681. [DOI] [PubMed] [Google Scholar]
- 22. Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic acids research 25: 4876–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular biology and evolution 24: 1596–1599. [DOI] [PubMed] [Google Scholar]
- 24. Li FF, Wu SJ, Chen TZ, Zhang J, Wang HH, et al. (2009) Agrobacterium-mediated co-transformation of multiple genes in upland cotton. Plant Cell, Tissue and Organ Culture (PCTOC) 97: 225–235. [Google Scholar]
- 25. Jiang Y, Guo W, Zhu H, Ruan YL, Zhang T (2012) Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant biotechnology journal 10: 301–312. 10.1111/j.1467-7652.2011.00662.x [DOI] [PubMed] [Google Scholar]
- 26. Ogata K, Hojo H, Aimoto S, Nakai T, Nakamura H, et al. (1992) Solution structure of a DNA-binding unit of Myb: a helix-turn-helix-related motif with conserved tryptophans forming a hydrophobic core. Proceedings of the National Academy of Sciences of the United States of America 89: 6428–6432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tominaga R, Iwata M, Okada K, Wada T (2007) Functional analysis of the epidermal-specific MYB genes CAPRICE and WEREWOLF in Arabidopsis. The Plant cell 19: 2264–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zimmermann IM, Heim MA, Weisshaar B, Uhrig JF (2004) Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like BHLH proteins. The Plant journal: for cell and molecular biology 40: 22–34. [DOI] [PubMed] [Google Scholar]
- 29. Uhrig JF (2006) Protein interaction networks in plants. Planta 224: 771–781. [DOI] [PubMed] [Google Scholar]
- 30. Serna L, Martin C (2006) Trichomes: different regulatory networks lead to convergent structures. Trends in plant science 11: 274–280. [DOI] [PubMed] [Google Scholar]
- 31. Wang S, Kwak SH, Zeng Q, Ellis BE, Chen XY, et al. (2007) TRICHOMELESS1 regulates trichome patterning by suppressing GLABRA1 in Arabidopsis. Development 134: 3873–3882. [DOI] [PubMed] [Google Scholar]
- 32. Wada T, Kurata T, Tominaga R, Koshino-Kimura Y, Tachibana T, et al. (2002) Role of a positive regulator of root hair development, CAPRICE, in Arabidopsis root epidermal cell differentiation. Development 129: 5409–5419. [DOI] [PubMed] [Google Scholar]
- 33. Boter M, Ruiz-Rivero O, Abdeen A, Prat S (2004) Conserved MYC transcription factors play a key role in jasmonate signaling both in tomato and Arabidopsis. Genes & development 18: 1577–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dombrecht B, Xue GP, Sprague SJ, Kirkegaard JA, Ross JJ, et al. (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. The Plant cell 19: 2225–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hou X, Lee LY, Xia K, Yan Y, Yu H (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Developmental cell 19: 884–894. 10.1016/j.devcel.2010.10.024 [DOI] [PubMed] [Google Scholar]
- 36. Chaudhary J, Skinner MK (1999) Basic helix-loop-helix proteins can act at the E-box within the serum response element of the c-fos promoter to influence hormone-induced promoter activation in Sertoli cells. Mol Endocrinol 13: 774–786. [DOI] [PubMed] [Google Scholar]
- 37. Szymanski DB, Lloyd AM, Marks MD (2000) Progress in the molecular genetic analysis of trichome initiation and morphogenesis in Arabidopsis. Trends in plant science 5: 214–219. [DOI] [PubMed] [Google Scholar]
- 38. Bernhardt C, Lee MM, Gonzalez A, Zhang F, Lloyd A, et al. (2003) The bHLH genes GLABRA3 (GL3) and ENHANCER OF GLABRA3 (EGL3) specify epidermal cell fate in the Arabidopsis root. Development 130: 6431–6439. [DOI] [PubMed] [Google Scholar]
- 39. Ishida T, Hattori S, Sano R, Inoue K, Shirano Y, et al. (2007) Arabidopsis TRANSPARENT TESTA GLABRA2 is directly regulated by R2R3 MYB transcription factors and is involved in regulation of GLABRA2 transcription in epidermal differentiation. The Plant cell 19: 2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morohashi K, Zhao M, Yang M, Read B, Lloyd A, et al. (2007) Participation of the Arabidopsis bHLH factor GL3 in trichome initiation regulatory events. Plant physiology 145: 736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Koshino-Kimura Y, Wada T, Tachibana T, Tsugeki R, Ishiguro S, et al. (2005) Regulation of CAPRICE transcription by MYB proteins for root epidermis differentiation in Arabidopsis. Plant & cell physiology 46: 817–826. [DOI] [PubMed] [Google Scholar]
- 42. Hong GJ, Xue XY, Mao YB, Wang LJ, Chen XY (2012) Arabidopsis MYC2 interacts with DELLA proteins in regulating sesquiterpene synthase gene expression. The Plant cell 24: 2635–2648. 10.1105/tpc.112.098749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang W, Ning G, Lv H, Liao L, Bao M (2009) Single MYB-type transcription factor AtCAPRICE: a new efficient tool to engineer the production of anthocyanin in tobacco. Biochemical and biophysical research communications 388: 742–747. 10.1016/j.bbrc.2009.08.092 [DOI] [PubMed] [Google Scholar]
- 44. Shan CM, Shangguan XX, Zhao B, Zhang XF, Chao LM, et al. (2014) Control of cotton fibre elongation by a homeodomain transcription factor GhHOX3. Nature communications 5: 5519 10.1038/ncomms6519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kim HJ, Triplett BA (2001) Cotton fiber growth in planta and in vitro. Models for plant cell elongation and cell wall biogenesis. Plant physiology 127: 1361–1366. [PMC free article] [PubMed] [Google Scholar]
- 46. Walford SA, Wu Y, Llewellyn DJ, Dennis ES (2012) Epidermal cell differentiation in cotton mediated by the homeodomain leucine zipper gene, GhHD-1. The Plant journal: for cell and molecular biology 71: 464–478. 10.1111/j.1365-313X.2012.05003.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.