Abstract
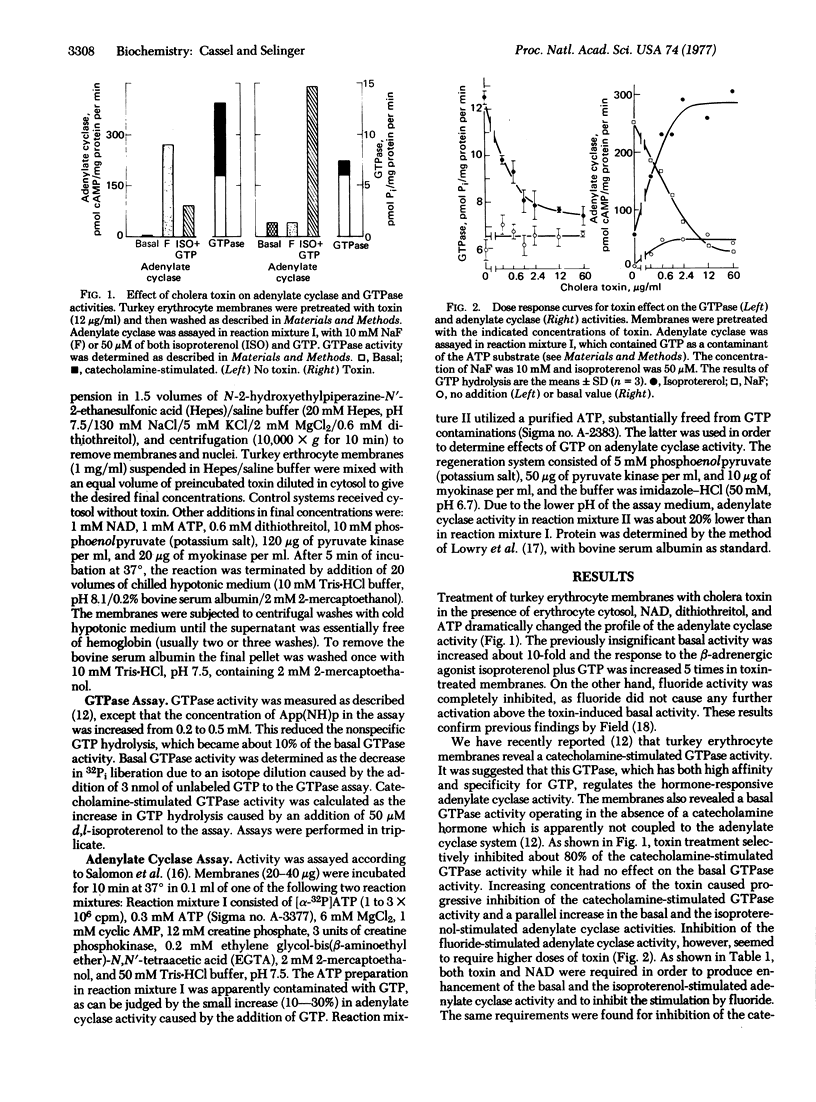
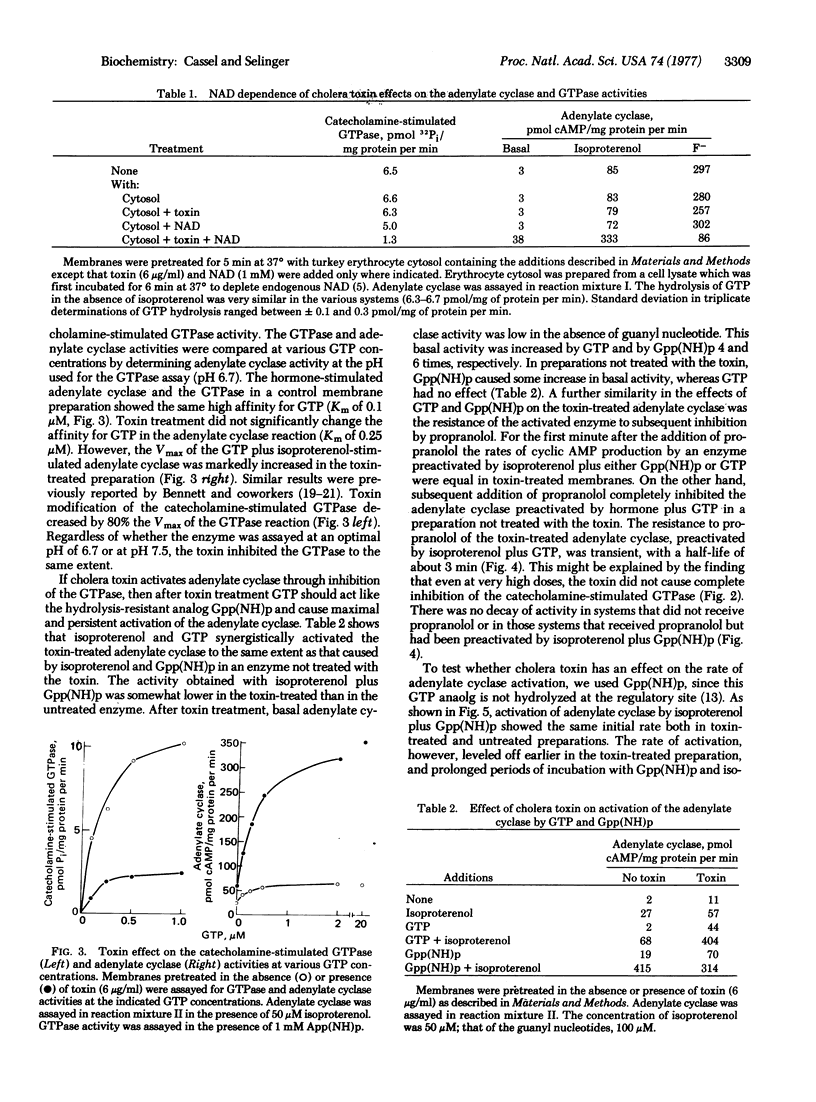
Treatment of turkey erthrocyte membranes with cholera toxin caused an enhancement of the basal and catecholamine-stimulated adenylate cyclase [ATP pyrophosphate-lyase (cyclizing), EC 4.6.1.1] activities. Both of these activities required the presence of GTP. The toxin effect on the adenylate cyclase activity concided with an inhibition of the catecholamine-stimulated guanosinetriphosphatase activity. Inhibition of the guanosinetriphosphatase, as well as enhancement of the adenylate cyclase activity, showed the same dependence on cholera toxin concentrations, and the effect of the toxin on both activities was dependent on the presence of NAD.
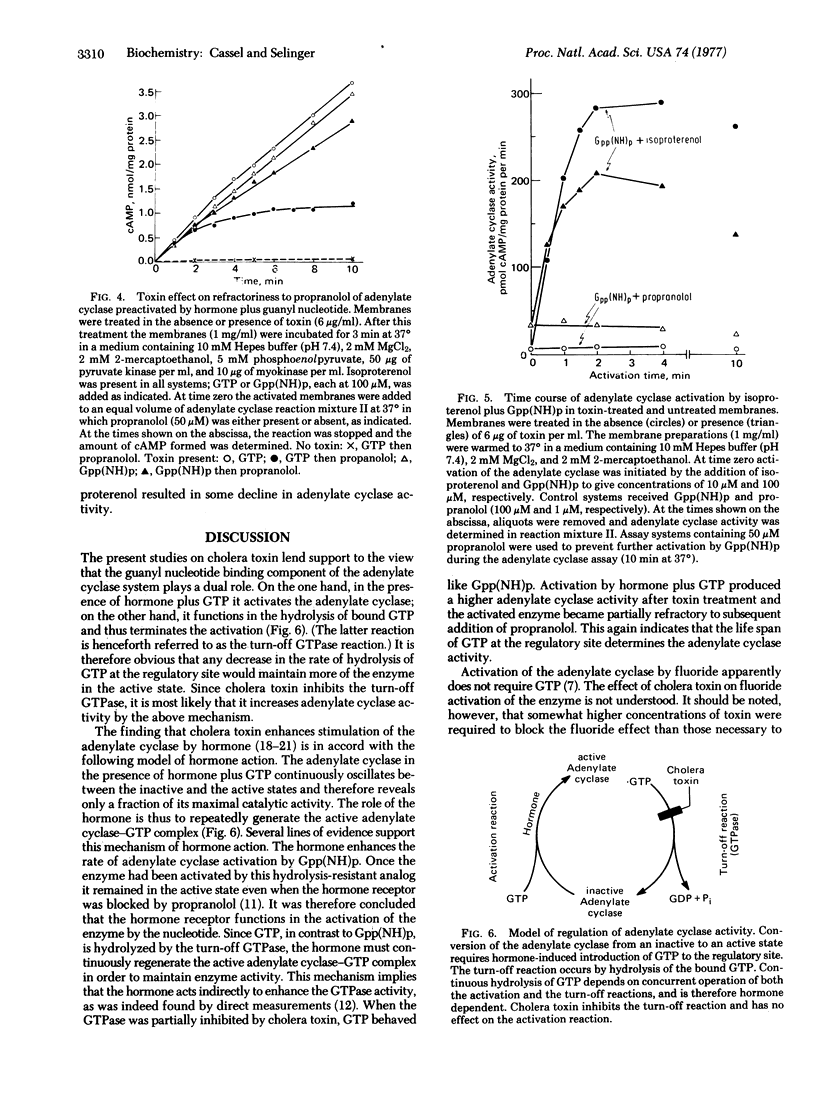
It is proposed that continuous GTP hydrolysis at the regulatory guanyl nucleotide site is an essential turn-off mechanism, terminating activation of the adenylate cyclase. Cholera toxin inhibits the turn-off guanosinetriphosphatase reaction and thereby causes activation of the adenylate cyclase. According to this mechanism GTP should activate the toxin-treated preparation of adenylate cyclase, as does the hydrolysis-resistant analog guanosine 5′-(β,γ-immino)triphosphate [Gpp(NH)p]. Indeed, the toxin-treated adenylate cyclase was maximally activated, in the presence of isoproternol, by either GTP or Gpp(NH)p, while adenylate cyclase not treated with toxin was stimulated by hormone plus GTP to only one-fifth of the activity achieved with hormone plus Gpp(NH)p. Furthermore, the toxin-treated adenylate cyclase activated by isoproterenol plus GTP remained active for and extended period (half-time of 3 min) upon subsequent addition of the β-adrenergic blocker, propranolol. The native enzyme, however, was refractory to propranolol only if activated by Gpp(NH)p but not by GTP.
Keywords: 3′:5′-cyclic AMP, guanyl nucleotide site, catecholamine-stimulated guanosinetriphosphatase, hormone receptor
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett V., Cuatrecasas P. Mechanism of action of Vibrio cholerae enterotoxin. Effects on adenylate cyclase of toad and rat erythrocyte plasma membranes. J Membr Biol. 1975 Jun 3;22(1):1–28. doi: 10.1007/BF01868161. [DOI] [PubMed] [Google Scholar]
- Bennett V., Mong L., Cuatrecasas P. Mechanism of activation of adenylate cyclase by Vibrio cholerae enterotoxin. Relations to the mode of activation by hormones. J Membr Biol. 1975 Nov 7;24(2):107–129. doi: 10.1007/BF01868618. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-induced release of [3H]-Gpp(NH)p from turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Feb;3(1):11–22. [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Mode of action of cholera toxin: stabilization of catecholamine-sensitive adenylate cyclase in turkey erythrocytes. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3299–3303. doi: 10.1073/pnas.71.8.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L., Krans H. M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. V. An obligatory role of guanylnucleotides in glucagon action. J Biol Chem. 1971 Mar 25;246(6):1877–1882. [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y. Evidence for interdependent action of glucagon and nucleotides on the hepatic adenylate cyclase system. J Biol Chem. 1974 Jan 10;249(1):59–65. [PubMed] [Google Scholar]
- Rosen O. M., Rosen S. M. Properties of an adenyl cyclase partially purified from frog erythrocytes. Arch Biochem Biophys. 1969 May;131(2):449–456. doi: 10.1016/0003-9861(69)90417-2. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schramm M., Rodbell M. A persistent active state of the adenylate cyclase system produced by the combined actions of isoproterenol and guanylyl imidodiphosphate in frog erythrocyte membranes. J Biol Chem. 1975 Mar 25;250(6):2232–2237. [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]


