Abstract
Obesity and its related comorbidities can be detrimental for the affected individual and challenge public health systems worldwide. Currently, the only available treatment options leading to clinically significant and maintained body weight loss and reduction in obesity-related morbidity and mortality are based on surgical interventions. This review will focus on two main clinical effects of Roux-en-Y gastric bypass (RYGB), namely body weight loss and change in eating behavior. Animal experiments designed to understand the underlying physiological mechanisms of these post-gastric bypass effects will be discussed. Where appropriate, reference will also be made to vertical sleeve gastrectomy. While caloric malabsorption and mechanical restriction seem not to be major factors in this respect, alterations in gut hormone levels are invariably found after RYGB. However, their causal role in RYGB effects on eating and body weight has recently been challenged. Other potential factors contributing to the RYGB effects include increased bile acid concentrations and an altered composition of gut microbiota. RYGB is further associated with remarkable changes in preference for different dietary components, such as a decrease in the preference for high fat or sugar. It needs to be noted, however, that in many cases, the question about the necessity of these alterations for the success of bariatric surgery procedures remains unanswered.
Keywords: Roux-en-Y gastric bypass, vertical sleeve gastrectomy, gut hormones, restriction, malabsorption, energy expenditure
the obesity epidemic and its related comorbidities constitute a major challenge for both personal health and public health systems worldwide. The enormous increase in knowledge about the physiological mechanisms that control eating and body weight contrasts with the lack of available pharmacological therapies leading to safe, efficient, and long-lasting body weight reductions and amelioration of obesity-related comorbidities. However, recent insights into underlying mechanisms of obesity and bariatric surgery have led to promising perspectives in respect to gut hormone-based strategies against obesity. Nevertheless, best results for maintained weight reduction and improvement of comorbidities are still achieved by surgical means (22, 88, 121, 135, 136). This review aims to summarize key findings in respect to underlying physiological mechanisms of the Roux-en-Y gastric bypass (RYGB) procedure, which is the most commonly performed bariatric operation worldwide and thus, by many is considered as the gold standard in bariatric surgery. Sporadic reference will also be made to vertical sleeve gastrectomy (VSG) when appropriate.
Figure 1 illustrates schematically the preoperative and postoperative anatomy of the gastrointestinal tract after RYGB and VSG operations.
Fig. 1.
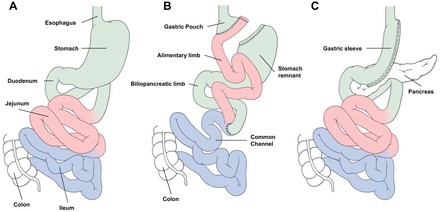
Schematic illustration of the preoperative anatomy (A), postoperative anatomy after Roux-en-Y gastric bypass (RYGB) (B), and vertical sleeve gastrectomy (C).
Both operations reduce eating at least temporarily (19, 140, 143) and lead to changes in food preferences (20, 27, 72). The majority of data collected with the help of preclinical studies seems to be consistent with findings in humans. However, changes in energy expenditure after RYGB seem to be less consistent in humans compared with most animal models, but this may be related to the much larger heterogeneity of study populations in humans compared with laboratory animals (23, 31, 145, 146, 152).
Research with animal models helped to elucidate some of the physiological mechanisms that potentially underlie the treatment success of bariatric surgery. Further, animal experiments allow the assessment of unbiased data that are e.g., independent of nutritional counseling in RYGB patients or other medical advice. Such “independent” measures may be particularly relevant in experiments testing the preference for specific types of food; please see Taste and Food Preferences. Eventually, animal studies may allow the definition of the processes underlying the effects of bariatric surgery and may, therefore, help to develop less invasive treatments than surgery itself.
We realize that the magnitude of some effects induced by RYGB or VSG differs among the different studies. It is surprising, however, that qualitatively, the majority of effects that we describe here seem to be very robust and can be observed irrespective of slight differences in the surgical approach, the laboratory where the studies have been performed, the feeding history of the animals before or after surgery, the duration of presurgical obesity, the animals' weight and adiposity level at the time of surgery, the duration between surgery and metabolic testing, and other factors that may potentially affect the outcome of the study. Hence, because of space limitations, exact details of the experimental designs of the studies cited in this review will be given only when needed.
Current research often focuses on altered concentrations of gut hormones like glucagon-like peptide-1 (GLP-1) or peptide YY (PYY) and metabolites (e.g., bile acids) that are known to affect eating and to modulate nutrient metabolism (6, 16, 17, 29, 41, 66, 67, 70, 73, 114, 133). It needs to be pointed out, however, that association and causality must not be confounded because measurable changes in circulating parameters after bariatric surgery do not necessarily play a causal role for the observed effects of bariatric surgery; hence, it is not yet clear whether these changes alone or in combination are necessary or sufficient for reduced eating or body weight. Figure 2 provides a schematic illustration of potential physiological mechanisms on both the gastrointestinal and systemic levels, as well as behavioral modifications after bariatric surgery.
Fig. 2.
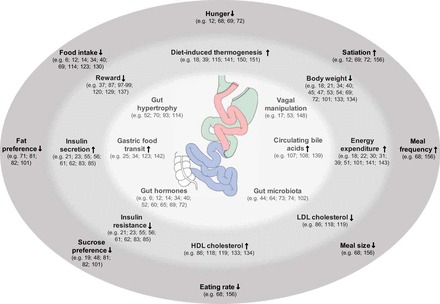
Schematic illustration of potential physiological mechanisms after bariatric surgery. Different mechanisms are arranged on the basis of their level of origin and, therefore, divided into gastrointestinal effects (inner circle, light gray), systemic effects (middle circle, medium gray) and behavioral effects (outer circle, dark gray). Borders between the three levels are transient. Arrows and connections between mechanisms have been intentionally omitted to avoid implication of causal relationships, which in many cases have not yet been established. References are given in parentheses for each mechanism. For further details, please see text.
It is, thus, the aim of this review to present an overview over selected topics with available data on the underlying mechanisms of bariatric surgery (Fig. 2). The main focus will be on the two main clinical effects of Roux-en-Y gastric bypass (RYGB), namely body weight loss and change in eating behavior.
Clinical Effect 1: Body Weight Loss
The obesity epidemic and its related comorbidities constitute a major challenge for both personal health and public health systems worldwide. To date, bariatric surgery and, in particular, the RYGB procedure, which is the most commonly performed bariatric operation worldwide, is the best available therapy for the treatment of obesity and humans and its major comorbidities. Similar to the clinical findings in RYGB patients, RYGB-operated rats or mice are also characterized by a rapid and marked decrease in body weight compared with sham-operated animals (15, 155). Weight loss may result in a decrease of about 20% below the presurgical level within 10–14 days after the intervention. Body weight then plateaus at a relatively constant level, but it has also been reported to start to increase again, perhaps depending on specific characteristics of the surgery (15, 24). However, if weight regain occurs irrespective of the performed bariatric procedure, it appears to be typically slow and does not reach the body weight of respective control animals (24).
Body weight loss after RYGB compared with sham-operated controls can be explained by reduced eating, increased energy expenditure, reduced availability of nutrients for the intermediary metabolism (e.g., caloric malabsorption), and consequently by an altered metabolic efficiency or by a combination of all these factors. The general consensus is that reduced eating and increased energy expenditure may play a much greater role after RYGB surgery than originally thought. This is based on the observation, that in many rat RYGB models, sham-operated rats pair-fed to RYGB rats have a greater body weight than ad libitum-fed RYGB rats; typically, more severe food restriction is required for sham-operated rats to have a body weight trajectory comparable to RYGB rats (body weight-matched rats; BWm). This indicates that energy expenditure may increase after RYGB because caloric malabsorption appears to be only of little importance at least when animals are fed a typical rat diet, which is relatively low in fat content (15, 19, 132, 143).
The decrease in body weight after RYGB is largely due to a decrease in body fat mass; lean body mass is often also reduced but proportionally less (19, 103, 158). Severe food restriction in sham-operated BWm control rats also reduces body adiposity, and general body composition, as well as body fat distribution, seems to be similar in BWm and RYGB rats and approaches that of lean control animals on a low-fat diet (19, 158). Hence, at least in rodents at the whole body level, RYGB-induced changes in body composition seem to reflect to a large degree directly the result of caloric restriction.
Systematic studies are scarce, but several reports indicate that the weight or composition of individual organs may also change after RYGB. Total liver weight may be unchanged, but liver fat content typically decreases after RYGB (56, 57). The reduction in hepatic steatosis after RYGB may be related to changes in key signaling pathways that control hepatic lipogenesis and fatty acid oxidation (106), as well as to changes in the mitochondrial function in hepatocytes (105). Specific changes in the skeletal system indicate a marked decrease in bone mineralization and bone weight with potentially negative consequences on bone stability; the underlying mechanisms will be discussed later in this review (1, 142). It is important to note that not all organs regress but that some organs in fact increase in size or weight after RYGB surgery. The best studied example is the gastrointestinal tract and, in particular, the small intestine where specific segments show a marked hypertrophic reaction after RYGB surgery (19, 53, 71, 95); the latter may potentially be linked to altered metabolic pathways in the intestinal epithelium (116, 148) and also to the increase in total energy expenditure.
Energy Expenditure
The human literature is not entirely consistent in respect to RYGB-induced changes in overall energy expenditure (23, 31, 32, 42, 152, 153). A recent review indicated that total energy expenditure in humans often decreases due to the decrease in fat-free and fat mass but that diet-induced thermogenesis is often increased (please see also below) (145). Nonetheless, some studies in humans report an increase in total energy expenditure, similar to the data in rodents. Discrepant findings may in some cases be explained by the fact that energy expenditure was often measured only over short periods of time and then extrapolated to a 24-h period; hence, true RYGB effects may have been overlooked. Further, necessary control groups were not always included in the studies.
Compared to the clinical studies, data in rats and mice are much more consistent. RYGB reduces spontaneous food intake and eventually lowers body weight. However, in most rat RYGB models, lower caloric intake is only one mechanism leading to body weight loss. Pair feeding of sham-operated rats to the level of the ad libitum intake in RYGB rats reduces body weight much less compared with the RYGB procedure. Hence, to reduce body weight in sham-operated rats to a similar level of RYGB rats requires more severe food restriction. Consequently, altered energy expenditure after RYGB seems to contribute, at least in part, to this phenomenon (19, 70, 133). It needs to be mentioned that pair-feeding typically imposes a very nonphysiological pattern of eating because in most studies, pair-fed rats may receive their daily allocated amount of food at once; this feeding pattern may have independent effects on energy expenditure.
Nonetheless, body weight reduction by dieting usually leads to an adaptive physiological response to reduce energy expenditure; this response helps the body to minimize the potential negative effects of long-term energy restriction in a state of negative energy balance (“starvation response”) (128). RYGB seems to prevent or at least reduce this adaptive response, i.e., body weight loss in RYGB rats is not associated with the same decrease in energy expenditure that parallels weight loss by food restriction (19, 143, 152). The phenomenon is reminiscent of findings showing that the central infusion of leptin (which may mimic a higher fat content in the periphery) prevents the decrease in energy expenditure subsequent to reduced eating (50); however, leptin's causal role for energy expenditure control after RYGB remains unclear. One possible mechanism may involve a lowering of the leptin “set point” at which a certain concentration of plasma leptin may be perceived by the hypothalamus as a state of energy insufficiency (43). Then lower leptin levels would not be perceived as a state of energy insufficiency after RYGB, and, consequently, compensatory mechanisms, including an increased food intake or decreased energy expenditure may not be triggered. However, it is unclear whether leptin signaling is altered, but an increased leptin receptor expression in the rat hypothalamus after RYGB (48) or a higher affinity of these receptors may play a role (43). Nonetheless, the necessity of altered leptin signaling for RYGB to exert its effects is questionable because various studies have shown that RYGB reduces body weight in a number of leptin-deficient rat models (85, 124, 129).
A number of studies were published in recent years addressing the correct analysis of energy expenditure data in animals of markedly differing body weight and body adiposity (98). In the case of RYGB rats, when energy expenditure determined by indirect calorimetry is corrected for body weight, energy expenditure is usually higher in RYGB rats than in sham-operated ad libitum-fed and weight-matched controls (19). However, when calculating total energy expenditure uncorrected for body weight, energy expenditure is not consistently increased after RYGB (2). It is important to note, however, that the comparison to the weight-matched controls is always positive. In some, but not all, studies, the change in energy expenditure was paralleled by a lower respiratory quotient, indicating that fat oxidation is increased over carbohydrate oxidation (19, 40, 132). However, the latter may have been rather related to body weight loss than representing a specific surgical effect as body weight-matched sham-operated controls show a similar response.
RYGB and VSG have often been claimed to produce very similar changes in metabolism. In respect to energy expenditure, the available literature indicates, however, that while part of RYGB's effect on body weight is due to increased energy expenditure or a lack of decreased energy expenditure after weight loss, this may not be an important factor in the body weight loss induced by VSG (117, 140). In most studies, the body weight loss of pair-fed animals was comparable to VSG rats. Further, many studies also report at least some regain of body weight after VSG in rats or mice, which may be explained by the different role of altered energy expenditure after RYGB vs. VSG, respectively.
At present, mechanisms underlying altered energy expenditure after RYGB remain unclear. As RYGB increases postprandial levels of GLP-1, which has been previously implicated in the control of energy expenditure, we recently tested whether acute modulation of the GLP-1 system influences the RYGB-induced changes in energy expenditure. We found, however, that neither acute stimulation nor blockade of GLP-1 receptors with exendin-4 or exendin-9, respectively, alters energy expenditure in any group. In other words, energy expenditure was higher after RGYB than sham operation, but remained unaltered by the manipulation of the GLP-1 system (2). Similar observations were also recently reported using a GLP-1 receptor-deficient model. However, it needs to be mentioned that the baseline effect of RYGB on energy expenditure was minor in this model (155).
From a whole body standpoint, it seems that neither increased spontaneous physical activity nor a general increase in body temperature can explain these findings as core body temperature was rather lower after RYGB (but also in weight-matched controls). However, heat dissipation was not assessed separately (19). While VSG may lead to a slight increase in locomotor activity, RYGB in rodents has been shown to enhance nonactivity based, i.e., resting energy expenditure (117). Similarly, we observed that energy expenditure in RYGB rats was particularly increased during the light phase (19), which approximates resting energy expenditure more than during the dark phase when rats are more active. We also found that RYGB rats showed a slightly higher core temperature during the light phase compared with weight-matched sham-operated controls, which might, in part, have been due to increased food consumption and, hence, differences in diet-induced thermogenesis. In fact, diet-induced thermogenesis in response to a 5-g test meal was higher after RYGB than in body weight-matched control animals (19). According to our own unpublished data, this effect may be more pronounced in rats fed a high-fat diet, which may contribute to the reversal of diet-induced obesity after RYGB. Considering the fact that RYGB rats increase their meal frequency compared with sham-operated or weight-matched rats (19, 132), the overall contribution of diet-induced thermogenesis may actually be higher in RYGB rats. Similar studies in respect to the role of postprandial energy expenditure after RYGB have recently been reported in a number of studies in human RYGB patients; postprandial energy expenditure was elevated compared with weight-matched control groups or compared with patients receiving vertical banded gastroplasty (40, 152, 153).
In a recent study, brown adipose tissue activity (BAT) remained unaltered after RYGB, which is consistent with the lack of increased core body temperature (52). Further, we recently observed that the temperature measured directly in BAT did not differ between RYGB and sham rats, and that β-3 agonist-induced increases in BAT temperature were similar (unpublished results). Other organ-specific factors that may contribute to increased energy expenditure after RYGB-like altered energy efficiency of skeletal muscle have not been tested so far. In a recent pilot study, we found that mitochondrial respiration in the liver of RYGB rats was increased, but the number of mitochondria decreased; hence, the influence on energy metabolism in the liver remains unclear.
We postulate that the increase in total energy expenditure in RYGB rats may be explained, at least in part, by a higher energy requirement of the massively hypertrophied small intestine (see also below; Refs. 19, 53, 95). The hypertrophy should probably be seen as an adaptive measure that helps to maintain an almost unaltered availability of ingested nutrients, despite the fact that nutrients and major digestive juices from the liver and pancreas only mix rather distally in the small intestine, i.e., at the junction of the Roux and biliopancreatic limbs into the common channel (Fig. 1). The entire small bowel increased its total weight by ∼75% after RYGB. Small intestinal oxygen consumption has been estimated to make up for about 20% of total oxygen consumption (44), indicating that gut hypertrophy may at least partly explain the higher energy requirement that, ultimately, may contribute to maintenance of body weight loss. In a seminal paper, Saeidi and colleagues have recently shown that energy metabolism in the gastrointestinal tract is reprogrammed in a way that the gastrointestinal tract becomes a major organ of glucose utilization, which possibly helps to secure its increased metabolic needs (116).
Clinical Effect 2: Change of Eating Behavior
Role of mechanical restriction.
It is a well-known fact that eating decreases after RYGB, although the underlying mechanisms remain unclear. Do patients or animals after RYGB eat less because the amount of food that can be eaten is limited by the size of the gastric pouch leading to mechanical restraint? Or do they eat less because they actively chose to eat less due to reduced hunger, earlier satiation, or prolonged satiety? Further, do RYGB patients or rats change their preference for certain types of food components, such as sucrose or fat, because they experience changes in taste perceptions (e.g., such as a decreased detection thresholds) or because fat and sugar ingestion causes discomfort, such as visceral malaise? Answers to these questions may be complex and various factors may, in fact, combine.
Human studies convincingly suggest that one major factor contributing to reduced overall eating is that RYGB patients are in fact less hungry before they initiate eating and that a meal of a given size leads to more satiation than in respective control patients (13). The similarities in meal patterns between humans and rats after RYGB suggest that physiological mechanisms of meal controls may dominate over psychosocial factors (including food counseling) and ultimately contribute to the reduction in eating.
In other words, the reduction in eating seems to be a controlled process; depending on the test paradigm applied, RYGB rats may, in fact, show increased “wanting,” which is an indicator of food-seeking behavior (131), despite the reduction in caloric intake once eating is initiated. Further, RYGB rats seem to initiate more licking behavior to sweet or fat stimuli in brief access tests (83). Together, these results indicate that reduced eating after RYGB seems to be a voluntary process that is not guided by an inability or unwillingness to approach food or to ingest but that may be related to (enhanced) mechanisms of satiation, i.e., once food ingestion has started. Accordingly, studies in humans have shown that premeal hunger is not higher and postmeal satiation is not lower after RYGB despite an overall lower food intake (73).
RYGB leads to a significant reduction in eating compared with ad libitum-fed sham-operated rats. The main reduction in overall eating in RYGB rats is due to a reduction in dark phase intake and a slight increase in light phase intake (19). Typical changes in meal pattern in rodent models of RYGB and in RYGB patients consist in the ingestion of smaller but more frequent meals that are consumed at slower eating rates, in rats at least during the dark phase (69, 158). An interesting observation is that the (more frequent) dark-phase meals are smaller in RYGB rats but that the (less frequent) light phase meals may actually be larger compared with sham-operated control rats. Consequently, RYGB rats consume meals of similar sizes during the dark and light phase compared with the large dark phase and small light phase meals in sham-operated controls (79).
The exact reasons for altered feeding patterns are unknown, but the idea that RYGB decreases eating due to mechanical restraint can probably be rejected. There exists basically no evidence in the available literature that this factor may play an important role. In contrast, the small gastric pouch does not seem to represent a major mechanical obstacle, as food transit is very rapid postoperatively. Most [but not all (144)] studies have shown that ingested food is rapidly drained into the small intestine, indicating that the gastric pouch has no storage capacity; this seems to be true for humans, rats, and mice (26, 35, 125).
There is a list of several other points that further argue against a major impact of mechanical factors causing an altered eating behavior after RYGB surgery. First, systematic studies do not indicate a correlation between the size of the gastrojejunal anastomosis and weight loss in RYGB rats (18). Second, rats do not increase prandial drinking which might suggest an attempt to overcome mechanical constraint through food dilution with water. Third, ad libitum intake in rats or human RYGB patients can be increased markedly by somatostatin analogs, which block the release of (satiating) gastrointestinal hormones but which do not alter the mechanical situation post-RYGB (41, 73). Fourth, RYGB has repeatedly been shown to cause a shift in eating so that intake of high-fat intake is decreased and intake of low-fat food is increased. Hence, even though RYGB rats still ingest a significant part of their overall caloric intake in the form of high-fat food, the animals choose to ingest less calorically dense food than before surgery (e.g., 20, 49, 72, 131, 158) rather than trying to overcome volume restriction in such way. Fifth, RYGB rats treated with the antagonist of the melanocortin 3/4 receptor SHU9119 were able to ingest twice the amount of food than RYGB control animals (94). Finally, RYGB rats seem to be able to ingest very large amounts of food if metabolically required. Temporary restriction of food access in weight-stable RYGB rats creates a negative energy balance that is immediately compensated by overeating large amounts of food once the restricted access to food is terminated (79). Consistent with our own observations, temporary restriction of food access also lowers body weight in VSG and control rats below their respective “set point”; however, all rats, including the VSG rats, overeat to compensate for the food restriction as soon as the animals are allowed to feed freely (140). Further, pregnant and lactating VSG rats are able to markedly increase the ingested amounts of food similar to control animals if the metabolic needs are present (47). Hence, overall, most findings argue against an important role of mechanical constraint as a major factor of reduced eating and altered meal patterns after RYGB or VSG, and they argue against a fixed “ceiling” effect for food intake after RYGB.
Taste and Food Preferences
RYGB seems to be associated with some interesting changes in eating behavior in respect to food preferences (please see Ref. 84 for a comprehensive review). RYGB patients often seem to decrease their preference for calorically dense food items, such as desserts and fast foods (103, 107), while reporting an increased preference for fruits and vegetables. This may perhaps be related to changes in detection thresholds postoperatively, e.g., for sucrose. It is important to mention that it is not entirely clear whether such changes in eating behavior are a prerequisite for the overall reduction in caloric intake after RYGB. Not least as the effect size of some preference changes reported in RYGB patients seem to be only modest and a transient phenomenon (84).
More robust data have been obtained from well-controlled studies in rat RYGB models. Data with a two-choice test paradigm between high-fat or low-fat diets are very consistent across laboratories. When given the choice, the postoperative intake of high-fat food decreases markedly compared with sham-operated and body weight-matched animals; relative, and in some cases, also absolute intake of low-fat food (usually standard rat chow) increased (20, 27, 49, 72, 117, 158).
Further, testing RYGB rats with liquid solutions containing increasing concentrations of fat (Intralipid) or sugar (sucrose, fructose) vs. water (two-bottle tests) also indicated that the typical strong preference for higher concentrations of fat or sugar that is observed in sham-operated and intact rats seems to be gone after RYGB (20, 49, 72, 158). Moreover, RYGB compensated for the amount of calories ingested via the test solutions, and this was not observed in sham-operated rats; in other words, RYGB rats maintained a constant caloric intake across increasing concentrations of fat solution, while sham-operated rats increased their overall caloric intake along with the higher-fat concentrations offered (72). Overall, these studies indicated that solutions with high-calorie content were preferred less after RYGB surgery. Importantly, such preference shifts between RYGB and sham animals were not seen when rats were tested for the preference of noncaloric stimuli, such as salty, sour, or bitter solutions (20).
Two-bottle intake tests do not allow a distinction between oral, preabsorptive, or postabsorptive mechanisms to explain the decrease in fat and sugar preference in RYGB rats. A frequently used technique that yields information mainly in respect to the oral detection of ingested fluids is the so-called brief access test; this technique allows distinguishing oral from post-oral effects. Animals are offered a battery of bottles that contain different concentrations of the test solutions vs. water; the bottles are only presented for short time intervals during which the animals are allowed to lick from one spout at a time, but the ingested volume and, hence, postoral effects are small. The number of approaches to the bottles can be taken as a parameter of how much the animal wants to ingest the respective stimulus (“appetitive behavior”), the licking rate during presentation of a specific bottle is a parameter of how much the animal likes to ingest that stimulus (“consumatory behavior”) (137, 138).
Various papers have been published using this technique, but the results have not been as uniform as with the preference tests for different types of solid diets (49, 83, 131). Shin and colleagues (131) reported an increase in the relative preference for low-concentrated sucrose or fat solutions and a decrease at higher concentrations compared with high-fat fed obese rats. Similarly, Hajnal et al. (49) also reported an increase in the relative preference for low-concentrated sucrose, but this was only observed in obese, but not in relatively lean, RYGB rats. Hence, RYGB rats liked the low concentrations of sucrose and fat more than sham-operated rats, at least under some conditions. In our own studies, however, we found no decrease in the concentration-dependent licking response to sucrose in chow-fed RYGB rats; to our surprise, RYGB rats actually approached the drinking spouts more often than sham rats, indicating an increased appetitive behavior (83). The reasons for the partly discrepant results across studies is not entirely clear, but may depend on the exact test paradigm, prior experience of the animals with the taste stimulus, the obesity state of the animals, their feeding paradigm and diet, and perhaps differences in the surgical technique, such as size of the gastric pouch.
Nonetheless, it seems clear that during brief access tests, RYGB does not uniformly lead to a shift toward consumption of lower-concentrated sucrose solutions (indicating increased liking of lower and decreased liking of higher concentrations) and, hence, the rats' taste-guided responses to sucrose may not always decrease after RYGB. Nonetheless, RYGB rats seem to be more sensitive to detect sucrose because the lowest sucrose concentration that triggered a significant response and the concentration that triggered the maximal response, respectively, in taste-sensitive neurons of the parabrachial nucleus were lower in RYGB than in control rats (49). The underlying physiology at the cellular level (e.g., taste receptors in taste buds, centrally processing neurons) has not yet been defined. Nonetheless, these findings are consistent with studies in humans that indicate that the detection threshold for sucrose seems to be shifted to lower concentrations when comparing postoperative to preoperative individuals (20) and that the preferred sucrose concentration decreased after RYGB (107). Whether the latter effects are unconditioned or conditioned responses remains to be studied.
Role of Conditioned Taste Aversion in Reduced Fat Preference
Conditioned taste aversion may at least partly contribute to the reduction in fat preference after RYGB, and this aversive response may then lead to a progressive avoidance of the intake of large amounts of fat through a learning process. In humans, these physiological processes may be enhanced or even triggered by nutritional counseling; for this reason, experiments in animal models may produce clearer results.
Hence, we and others recently investigated one possible mechanism for reduced fat preference after RYGB (72). We hypothesized that if RYGB reduced eating and, in particular, high-fat intake because of the induction of visceral malaise, animals might progressively reduce their fat intake by learning that lower fat intake helps them to avoid symptoms of visceral illness. This would be consistent with the observation in a number of studies using RYGB rats that the lower-fat preference after the RYGB intervention progresses over time (72, 131, 158). Indeed, it was found that administration of an amount of fat comparable to that of a single high-fat meal produced a conditioned taste aversion response in RYGB rats (72).
The mechanisms of the fat-induced aversion after RYGB remain unknown. It has been speculated that elevated GLP-1 and PYY levels may contribute to this response because both hormones activate neurons in the brain stem areas that mediate aversive responses (51, 64), and both hormones can induce aversions at least at higher doses (51, 64). However, a causal role of GLP-1 or PYY in the fat aversion after RYGB has not been formally tested.
Changes in Food Reward After RYGB
Aversion and reward are the two sides of a coin in the same category of taste processing. In other words, while aversions discourage an individual from ingesting specific nutrients or tastes, reward promotes this behavior. Of course, increased aversion and decreased reward (or vice versa) may not be mutually exclusive. Dopamine signaling is intimately involved in mediating reward-related behaviors, and some of the major brain regions involved in reward processing are the mesolimbic areas, including the striatum, ventral tegmental area, and the nucleus accumbens (8).
A number of studies that used functional magnetic resonance imaging investigated reward-related behaviors in RYGB patients. In these studies, bypass patients were tested for changes in brain activities when being presented with different pictures of eating or noneating-related cues. Brain activation was reduced after surgery in the brain reward areas, and this was typically more pronounced when food items with a high caloric density were presented (99–101, 122). Further, RYGB patients also differed from patients undergoing gastric banding because activation in brain reward areas was only reduced in the former compared with body weight-matched unoperated controls (122). In the latter study, the observed differences in reward activation appeared to be associated with elevations of GLP-1 and PYY; such an association was, however, questioned by Ochner et al. (100) because they observed major differences in the activation pattern of reward areas in fasted but not in patients postprandially, i.e., when major RYGB-induced elevations in GLP-1 and PYY occur (100). Overall, a decrease in the reward value of food may be partly responsible for the decrease in eating after RYGB, and, in particular, for the decrease in calorically dense high-fat and sweet food items.
Several studies investigated directly whether RYGB reduces eating, in general, and the preference for high-fat and sweet food, in particular, by a reduction in the reward value of these food items. Miras et al. (89) recently tested the willingness of RYGB patients before and after surgery to “work” for sweet and fatty candies in a so-called progressive ratio test (89); individuals had to produce increasing numbers of mouse clicks on a computer to obtain the candy reward, and the results were then compared with normal-weight controls. Interestingly, RYGB patients were much less willing to work for the reward after the operation, and this reduction appeared to be more pronounced in patients with a bigger body mass index loss. The mouse clicking for a nonfat, nonsweet vegetable candy was not affected. In other words, the reward value after RYGB was reduced selectively for the sweet and fat reward (89).
Only a few studies are available that tested potential mechanisms underlying the RYGB-induced decrease in the food-associated reward, and these studies are not conclusive. On the basis of findings that dopamine signaling plays an important role in reward pathways, Steele et al. (139) used the positron emission tomography technology in RYGB patients to determine dopamine binding in brain reward areas. They found that the expression of the dopamine D2 receptor seems to increase after gastric bypass, and they interpreted their findings that the overstimulation of the reward system in obese individuals may have led to a D2 receptor downregulation; this effect may then be normalized after RYGB (139). It is important to note that the interpretation of such data is inherently difficult, but one may postulate that these findings indicate that less food is necessary to produce comparable levels of reward after RYGB, so that the amount of food eaten is decreased. On the other hand, Dunn et al. (38) found a decrease in D2 receptor-mediated binding; their interpretation is that extracellular dopamine signaling after RYGB may have led to a downregulation of the D2 receptors; however, local dopamine release has not yet been studied under such conditions.
Potential Physiological Mechanisms of Roux-en-Y Gastric Bypass
Introduction.
The physiological mechanisms underlying the effects of RYGB surgery are currently the subject of intensive research. Changes induced by RYGB are manifold, and it still needs to be determined which of these changes are causally and functionally relevant for the observed RYGB effects. It is, therefore, of critical importance to go beyond the demonstration of an association alone between observed effects and physiological RYGB-induced alterations.
One important avenue of research is the investigation of alterations in the synthesis and secretion of factors that may have a hormonal action. This is plausible for purely practical reasons, because it is relatively easy to study changes in concentrations of a large number of blood-borne factors after RYGB and compare their levels with appropriate control groups. Further, for at least some of these factors, their action can easily be manipulated either by enhancing their action with agonists or by blocking their action e.g., with specific antagonists or using transgenic animals that lack a receptor for that specific factor.
The idea that blood-borne factors may play an important role for post-RYGB physiology is primarily based on seminal experiments by Atkinson and Brent (7), who showed that the injection of plasma collected postprandially from rats with an intestinal bypass reduced eating in intact, recipient rats compared with intact rats receiving postprandial plasma from sham-operated controls (7). This effect was absent when plasma from fasted bypass or sham-operated animals was injected into recipient rats. Hence, it appears that the circulating blood from bypass animals contains some factors that play an important role for the changes in eating behavior after RYGB surgery (7). Of course, it is theoretically equally possible that a factor that would normally be present is missing in the blood after RYGB, hence producing some sort of disinhibition.
So far, research has mainly concentrated on elevated concentrations of gut hormones and bile acids after RYGB surgery in rats and humans. Indeed, there is some evidence that gastrointestinal hormones may play a causal role for some clinical effects of RYGB. For example, somatostatin analogs which block the release of gastrointestinal hormones have been shown to increase eating in RYGB patients (70, 73) and RYGB-operated rats (41) compared with respective controls (i.e., gastric banding patients and sham-operated rats, respectively). It has to be noted, however, that somatostatin treatment is obviously very unspecific, as it blocks also many other physiological functions, including motor effects. Furthermore, long-term effects (which may result in a blunted decrease in body weight after RYGB) have not been tested yet. A second indirect line of support for a potentially causal role of gastrointestinal hormones comes from studies demonstrating that RYGB patients with greater weight loss had higher GLP-1 and PYY levels than patients who lost a lesser amount of weight [good vs. poor responders; see (35, 73)].
It needs to be mentioned that at least in some studies, and perhaps more consistently in rodent RYGB models than in RYGB patients, gut hormone levels are not only increased postprandially, but at least, in some cases, also in the fasted state. At present, the relevance of increased fasted gut hormone levels are unknown, and it remains unclear whether increased fasted levels may contribute to a reduced feelings of hunger in RYGB patients (13).
Gastrointestinal Hormones
The gut-brain axis is a major component for the control of eating and depends largely on the release of gastrointestinal hormones that have either an orexigenic or anorexigenic action (96). Hence, decreased release of orexigenic and increased release of anorexigenic hormones after RYGB provides a plausible explanation for RYGB's effects on eating and body weight. In fact, one of the most consistent findings in RYGB patients and animal models and the most frequently proposed mechanisms contributing to reduced eating and body weight after RYGB surgery is the increased secretion of gut hormones, in particular, the L-cell products GLP-1 and PYY, but also amylin and CCK. It needs to be stressed, however, that the majority of correlative data contrasts the few data showing causality.
A decrease in the release of the only known peripheral orexigenic hormone ghrelin has been discussed as an underlying cause for reduced eating after RYGB, but even correlative data are not clear (29, 59). Typically, ghrelin levels increase during fasting and decrease after food intake (72). Some but not all studies reported that the rise in ghrelin levels that can usually be observed after diet-induced weight loss is absent after RYGB (29, 59). It is, however, unclear whether the observed changes in ghrelin concentrations are physiologically relevant modulators of eating. Further, it has been shown recently that the VSG procedure, which seems to produce similar hormonal changes as RYGB (108), results in similar weight loss in ghrelin-deficient vs. respective control mice (25). However, comparable data from RYGB models are not available.
Data are more consistent for increased levels of anorexigenic gastrointestinal hormones after RYGB. CCK was the first gastrointestinal hormone shown to contribute to the physiological control of eating, in general, and of meal size, in particular (11, 68). Recent data have shown that postprandial CCK levels are higher after RYGB than in respective controls (35, 62). Similar data have been reported for patients undergoing a jejunoileal bypass operation, which typically shows similar hormonal changes than RYGB (97). Interestingly, however, one study reported that the highest postprandial CCK concentrations were observed in RYGB patients that had a relatively poor response to the surgery (35). The causal contribution of elevated CCK release to reduced eating (and eventually reduced body weight gain) after RYGB is not clear; while the CCK antagonist devazepide increased eating in a female rat model of RYGB, devazepide's effect was similar in sham-operated control animals (5). Hence, the blockade of CCK alone does not seem to reveal a stronger role of CCK in the control of eating in RYGB vs. control animals.
More data are available for the two L-cell derived hormones GLP-1 and PYY, and basically all studies—at least those that we are aware of—reported higher postprandial levels of GLP-1 and PYY after RYGB (e.g., 41, 66, 70, 72, 73, 133); many papers also reported elevated baseline levels of these hormones, at least in rat models of RYGB [reviewed in e.g., (90)]. Circulating GLP-1 and PYY are mainly released by the enteroendocrine L cells, which make direct contact with the gut lumen and which are, therefore, believed to sense the arrival and passage of nutrients along the gastrointestinal tract (30, 34). Nonetheless, the exact mechanisms that are responsible for the increase in postprandial GLP-1 and PYY are still hypothetical; even more so, this is true for elevated baseline levels.
A plausible explanation for the increase in postprandial L-cell secretion is the exposure of the gut lumen to higher concentrations of undigested nutrients. This has usually been thought to be mainly due to an effect in more distal parts of the small intestine, which have a higher density of L-cells embedded in the mucosa (87). It is, however, important to note that the absolute number of L-cells is far higher in the proximal small intestine compared with the distal small intestine (53), indicating that such an effect on L-cells could also be due to the exposure of the Roux limb to nutrients entering this limb immediately after ingestion (35). Of note, experiments testing the causal role of such effects for the exaggerated L-cell response are still pending. Further, L-cell secretions can also be triggered by the parasympathetic nervous system (113). It is however, unknown whether this effect is altered after RYGB compared with control patients or sham-operated animals, respectively. One very popular explanation for increased L-cell secretions is the enhanced stimulation of L-cells by bile acids (3, 58, 147). Please see Bile Acids for details.
A remarkable feature of RYGB-operated rats is a marked hypertrophy and hyperplasia of the gut mucosa in the alimentary (Roux) limb and common channel (19, 71). This general hypertrophy is paralleled by a proportional increase in the absolute number of L-cells. In other words, the absolute L-cell number is increased, while the regional density remains unaltered compared with sham-operated control animals (53, 95, 116). It is plausible that this hypertrophy contributes to an exaggerated GLP-1 response after RYGB, at least at later time points. However, elevated gut hormones can already be observed within a few days after surgery (73), i.e., at a time when this hypertrophic response presumably is still negligible so that it can hardly explain the very rapid increase in L-cell secretions immediately after RYGB surgery.
There is also evidence that the basal and postprandial concentrations of the pancreatic β-cell hormone amylin are elevated after RYGB (133). Amylin is cosynthesized and coreleased with insulin in response to eating. Amylin's satiating action depends on direct activation of brain centers like the area postrema (77, 78, 86) and perhaps the ventral tegmental area (76, 80). Amylin has been shown to enhance the eating inhibitory effect of other satiating hormones like CCK (76, 80). Hence, it is plausible that higher amylin levels may also contribute to reduce eating after RYGB, but its causal role has not been tested yet. Similar to GLP-1 and PYY, it is also unclear how RYGB increases basal and postprandial amylin secretion in rats. It seems unlikely that a direct effect of glucose (or other carbohydrate metabolites) at the pancreatic β-cell plays a role because glucose concentrations are either unchanged or rather decreased (in diabetic individuals) after RYGB (120, 121). One possible option might be that increased amylin levels are caused by a stimulating effect of GLP-1 on β-cell secretion, but this has so far not been tested after RYGB.
Causal Role of Gastrointestinal Hormones in RYGB's Effects
The single or combined action of satiating hormones such as CCK, GLP-1, PYY and amylin provides a plausible explanation for the decrease in meal size observed after RYGB (69, 132, 133). Further, as mentioned above, RYGB patients clustered into a group of “good responders” had a significantly higher postprandial GLP-1 and PYY response than “poor responders” (35, 73). However, similar to the uncertainty of how exactly RYGB leads to elevated postprandial and basal concentrations of these hormones, the causal role of these elevations for reduced eating and eventually body weight after RYGB is far from clear. Of course, because RYGB changes the secretion patterns of a multitude of factors (many of which may still be unknown), it seems evident that the manipulation of one single factor may not be able reveal the complete mechanism(s) underlying the effects of RYGB surgery. In other words, the physiological post-RYGB system may be redundant enough that the blockade of single factors may not be sufficient to abolish RYGB or VSG-induced effects. Some experiments briefly mentioned above are consistent with the general idea of an important role of increased gut hormone secretion after RYGB because the blockade of gut hormone release with somatostatin analogs increased eating in RYGB patients or rats, respectively (41, 73).
So far, most efforts that tried to establish a relevant role for single factors in RYGB-mediated effects failed. Not surprisingly, most efforts concentrated on GLP-1, but blockade of GLP-1 signaling in GLP-1 receptor knockout mice or intraventricular administration of the GLP-1 antagonist exendin-9 in rats was unable to demonstrate a specific single role of GLP-1 for RYGB-mediated effects on eating, energy expenditure, or body weight (2, 155). In a recent study, GLP-1 receptor knockout mice appeared to respond to RYGB surgery because their body weight loss, reduction in fat mass, decrease in food intake, and alteration in fat preference was similar to wild-type control mice (155). Hence, these results correspond to earlier findings in GLP-1 receptor knockout mice undergoing VSG, which also responded similarly to sham-operated controls (154). Further, chronic intraventricularly administered exendin-9 blunted the effect of RYGB on body weight, but sham-operated animals receiving similar treatment also increased body weight compared with vehicle-treated control animals to at least the same extent (155). We were also unable to detect a more prominent role of GLP-1 receptor blockade for the control of eating or energy expenditure in RYGB than in sham-operated rats in our studies (2, 5). Hence, while GLP-1's effects can clearly be observed to be functional in RYGB animals, there is little evidence so far that the enhanced release and action of GLP-1 alone can explain differences in eating and body weight between RYGB and sham-operated control animals. It is important to note, however, that several studies indicate that the enhanced GLP-1 release is, at least in part, responsible for the improved glucose metabolism after RYGB (e.g., 63, 126).
Similar to GLP-1, the selective blockade of CCK receptors with a specific CCK-1 receptor antagonist or of PYY receptors with a PYY-2 receptor antagonist, respectively, did not increase eating more in RYGB than in control rats (5, 155). The latter contrasts with a study in mice undergoing gastrointestinal bypass in which sham-operated, but not PYY knockout, mice showed a reduction in body weight compared with vehicle-treated controls (28). The reason for the apparent discrepancy between the latter two studies is unknown; whether it may be related to the surgical or experimental procedure or the early time point after surgery when the PYY knockout animals were tested, remains to be studied.
Bile Acids
Circulating bile acid (BA) concentrations are reduced in obese compared with lean individuals, and RYGB has been reported to normalize the blunted postprandial bile acid excursions (4). Hormonal effects of circulating BA have been a matter of intense study in recent years because apart from their role as major detergents involved in fat digestion, bile acids act through a variety of receptors, including the FXR and the TGR5, to influence nutrient metabolism and energy expenditure. Next to the gut hormones GLP-1 and PYY, elevated concentrations of BA are the most commonly named (potential) mechanism for RYGB-mediated effects on nutrient and energy metabolism (e.g., Refs. 109–111, 141).
RYGB normalizes the blunted postprandial bile acid excursions in patients (4). Interestingly, while the portal vein concentrations of BA increase massively after a meal in normal rats, systemic BA levels seem to be unaltered and do not increase above fasting levels. After RYGB, however, systemic BA levels also increase massively and may, therefore, be responsible for some RYGB-induced effects (unpublished data).
Increased bile acids may also explain the increased secretion of GLP-1 and PYY after RYGB. However, an effect on L-cell secretion actually requires higher bile acid concentrations in the intestinal lumen because the pertinent bile acid receptor TGR5 that mediates increased L-cell secretions is located in the luminal membrane of L-cells (37). Published data are scarce, but our preliminary data show that the concentration of total bile acids in the common channel of RYGB rats was almost twice as high as that in the terminal ileum of control animals. Nonetheless, the causal contribution of bile acids to increased L-cell secretion after RYGB has not yet been tested; unfortunately, specific antagonists of TGR5 are not available, and to our knowledge, respective experiments with RYGB-operated TGR5 knockout mice have not been performed yet. Further, experiments using sequesters of bile acids to reduce BA-induced GLP1 release may not be helpful to define a causal link between BA and GLP-1 secretions post-RYGB because secondary effects of the sequesters may actually lead to increased rather than decreased GLP-1 release (127). In other words, the direct link between elevated (gut) levels of bile acids and increased L-hormone secretion after RYGB remains unclear. Further, a recent study indicated that it seems unlikely that bile acids are responsible for the early increase of GLP-1 and PYY post-RYGB (141).
Through an FXR-mediated pathway in the intestine, BAs have been found to stimulate the postprandial release of FGF19, a protein that regulates glucose disposal and lipid homeostasis, and acts through FGFR4 to modulate BA synthesis in the liver (119). Further, as mentioned above, BAs may directly stimulate the release of GLP-1 from enteroendocrine cells via the TGR5 receptor. However, the sites of action of BAs likely extend beyond the intestine. BAs increase energy expenditure (EE) in skeletal muscle and in brown adipose tissue (BAT) (151). The same holds true for postprandial thermogenesis, in particular; because BAT functions in the thermic effect of feeding and because postprandial levels of circulating BAs are strongly correlated with postprandial EE in lean individuals (102), it seems plausible that elevated BAs post-RYGB contribute to the increased postprandial EE.
It is important to mention that the direct causal role of elevated BAs in the effects of RYGB on energy expenditure has not yet been tested. Alterations in BA levels in rodent models of bariatric surgery generally parallel the findings in humans, and a recent elegant study in VSG-operated mice indicated that BA signaling via the FXR may be a necessary component in many VSG-induced effects (e.g., effects on body weight, glucose tolerance, etc.) (115). However, similar studies have not yet been performed after RYGB.
Gut Microbiota
Numerous studies in recent years indicated that metabolic processes induced and orchestrated by the gut microbiome play an important role in whole body metabolism in health and disease. Transplantation studies in humans and experimental animals also indicated that changes in the gut microbiome are not only bystanders in metabolic alterations in disease but that they may actually play a causal role. Just to give one example, it has been shown that the transfer of gut microbiota from obese rodents to germ-free animals leads to an increase in eating and body weight compared with germ-free animals receiving microbiota from lean animals (reviewed in Ref. 33).
In respect to bariatric surgery, the changes in the gut microbiota seem to directly contribute to reduced body weight and adiposity after RYGB (75), and in humans, variations of gut microbiota after RYGB were associated with changes in the expression of various genes in white adipose tissue, indicating a direct link between these two processes (65).
Findings that RYGB affects the gut microbiota composition, that it reverses the microbiota from an obese to a lean phenotype and that the changes may be specific to the various limbs post-gastric bypass have been reported repeatedly. We recently showed that the RYGB-induced changes in the microbiota of the alimentary limb and the common channel are similar to changes seen after weight loss by dieting (104). Further, alterations in the gut microbiota after RYGB seem to be similar in various species, including humans, rats, and mice (45, 74, 75), and they seem to be independent of the weight change or caloric restriction per se. It was also reported that the transfer of the gut microbiota from RYGB-treated mice to nonoperated, germ-free mice resulted in weight loss and decreased fat mass in the recipient animals relative to recipients of microbiota from animals undergoing sham surgery (75). Hence, the changes in the gut microbiota seem to directly contribute to reduced body weight and adiposity after RYGB (75).
Overall, changes in gut microbiota may be directly associated with the altered control of energy balance post-bypass, and the postsurgical modulation of gut microbiota may significantly contribute to the beneficial metabolic effects of RYGB surgery. The exact underlying mechanisms are a field of intensive research; open questions are e.g., how altered gut microbiota may influence energy expenditure and whether such changes can actually be assessed by the commonly used technique of indirect calorimetry; which microbiota-derived metabolites may contribute to altered secretions of gut hormones; which bile acid metabolites may derive from microbial metabolism; and how these metabolites may affect host metabolism.
CNS Eating and Body Weight Controls after RYGB
The hindbrain with the area postrema (AP), the nucleus of the solitary tract (NTS), the lateral parabrachial nucleus (PBN), and the hypothalamus play crucial roles in the control of eating (123). Within the hypothalamus, the balance between the activities of NPY-POMC neuronal circuits is critical for the maintenance of body weight (68, 123). It is clear that the peripheral signals that induce the changes in eating behavior and energy expenditure after RYGB need to be transmitted to the brain. This may occur either via vagal or nonvagal afferent nerve signaling or directly via the blood circulation (12).
Remarkably little is known about specific effects of RYGB (or other bariatric surgery procedures) on the brain centers that are involved in eating control. Most of the recent animal studies and some studies in humans have examined the role of the melanocortin system, which seems to be central to many peripheral and brain-derived stimuli that control eating and body weight (55, 92, 93, 157). In humans, the sufficiency of one functional MC4r gene for effective RYGB outcome was indicated in some studies, including RYGB or VSG-operated humans (157). However, the important role of the melanocortin system was supported by findings in humans with a specific variant of the MC4 gene [MC4r(I251L)], which is associated with a better metabolic status; in fact, carriers of this variant had improved surgery outcome (92, 157).
Published data in rodent RYGB or VSG models seem to indicate marked species differences. While homozygous and heterozygous melanocortin-4 receptor (MC4r) knockout rats appeared to be fully responsive to weight-reducing VSG surgery (93), homozygous MC4r knockout mice lost less weight after RYGB than heterozygous knockout or wild-type mice (55).
The specific role of the MC4r in cholinergic preganglionic neurons of the parasympathetic and the sympathetic system after RYGB was recently further investigated in an animal model of diet-induced obesity mice. In these mice, the RYGB-induced body weight loss was mainly due to an increase in energy expenditure. The mice also had improved insulin sensitivity mainly in the liver, but not skeletal muscle or adipose tissue. Most of these effects were absent in MC4r knockout mice, but similar to the study described above with complete MC4r knockouts (55), one functional allele was sufficient to rescue the effect of RYGB; this study clearly shows that functional MC4 receptors are required for the full effects of RYGB on energy expenditure, body weight, and glucose metabolism in mice (55). This study also shed some light on the potential sites of MC4 signaling that are widely distributed throughout the brain. Interestingly, the genetic reintroduction of the MC4r in key autonomic neurons in the brain stem, including the cholinergic preganglionic motor neurons of the dorsal motor nucleus of the vagus, reinstated the effect of RYGB on insulin sensitivity, but not on body weight or obesity; in the latter respect, the mice behaved like (full) MC4r knockouts. In contrast, the reintroduction of the MC4r in cholinergic preganglionic neurons of both the parasympathetic and the sympathetic system reinstated the RYGB effect on eating, body weight, and adiposity; in this case, the improved insulin sensitivity was only secondary to weight loss (157). Hence, different populations of MC4rs seem to be critical for the mediation of specific aspects of RYGB surgery.
A recent study has shown that the eating inhibitory effect and subsequent body weight loss after RYGB seem to depend, at least in part, on vagal transmission because both effects were more pronounced when part of the subdiaphragmatic vagal innervation (specifically the paraesophageal neurovascular bundle) was preserved during RYGB surgery (18); the critical nerves may come directly from the intestines because a lesion of the specific vagal innervation of the portal vein and liver had no effect on the RYGB outcome (130). Further, the decreased excitability of vagal efferent neurons in the dorsal motor nucleus of the vagus that resulted from diet-induced obesity could be reversed by RYGB in rats (14); this was accompanied by an improved response of these neurons to CCK and GLP-1. Yet, other recent studies have indicated that at least for the short term, vagal dissection may actually increase the effects of RYGB on body weight (54, 150); it needs to be stressed, however, that animals with a complete lack of gastrointestinal vagal innervation often are seriously compromised in the early postoperative period so that unspecific effects, also in control animals, early after surgery cannot be excluded. Nonetheless, given the somewhat contradictory findings about the role of the vagus in RYGB, it will be important to study the specific role of other vagal fibers in more detail.
Changes in Gut Morphology after RYGB
The RYGB procedure is associated with typical changes in morphology of the intestinal mucosa (19, 71). This has most consistently been described in RYGB rats, and the phenomenon seems to be less marked in RYGB mice (125). In rats, the total length of the small intestine remains unaltered, but a marked increase in wet weight of the small intestine indicates segmental hypertrophy after RYGB. Specifically, muscular and mucosal layers were significantly thicker in the alimentary (Roux) limb after RYGB compared with anatomically corresponding intestinal segments in sham-operated controls; both mucosal crypt depth and villi height increased (19, 71). Depending on the dimensions of the various limbs after RYGB, similar changes may also be observed in the common channel of some RYGB models, but not in the biliopancreatic limb (53, 95).
The underlying mechanisms leading to hypertrophy of the intestinal mucosa, including muscle layers, remain unknown. Mechanical or chemical factors, or a combination of both, may be involved. It is however, intriguing that a hypertrophic response can only be found in intestinal segments exposed to ingested food (53, 95). One possible explanation is linked to an increased release of GLP-2 from intestinal L-cells [(53, 71); see also below], facilitating intestinal hypertrophy in conjunction with intraluminal factors, such as stimulation by nutrients. Overall, it may be postulated that hypertrophy of certain intestinal segments in RYGB animals represents an adaptive response to optimize nutrient digestion and absorption under conditions in which nutrients and digestive juices from the pancreas and liver mix more distally than under physiological conditions. Similar responses can be seen in experiments in which segments of the small intestine have been surgically removed (36, 60).
Because of the potential importance of gut hormones for the beneficial effects of RYGB and other types of bariatric surgery, several groups also investigated whether RYGB modifies the distribution or density of enteroendocrine cells in the gastrointestinal tract (53, 95). Consistent with the general hypertrophy of the intestinal mucosa, there are clear indications for an adaptive increase in the number of endocrine cells. This translates into an increase in the absolute number of L-cells releasing GLP-1, GLP-2, and PYY, and of CCK-immunoreactive cells. While major effects were seen in the alimentary limb and the common channel, no changes were observed in the biliopancreatic limb. Interestingly, however, the regional density of enteroendocrine cells remained unaltered (53, 95).
When testing the specific expression of preproglucagon (for GLP-1) and PYY in the intestinal segments, the mRNA expression per cell was only increased in the common channel, but not in the alimentary limb (53); hence, it seems that both the (general) stimulus that leads to intestinal hypertrophy (perhaps induced by increased GLP-2 secretions) and the presence of nutrients plus bile acids and gastric or pancreatic juices may be necessary to increase gastrointestinal hormone production at the level of individual cells. Overall, the data clearly indicate that the hormonal secretory capacity of the small intestine increases after RYGB (53).
Digestibility and Nutrient Absorption after RYGB
The reduction in caloric intake and a (relative) increase in energy expenditure play important roles for the weight loss after RYGB. However, another factor that needs to be considered for the animals' whole body energy balance is the efficiency of the animal to use the ingested nutrients for conversion into body tissue or for energy production. In other words, it is important to consider the potential loss of ingested nutrients via the feces due to malabsorption (other sources of energy loss like fermentation gases have not been studied). At least when RYGB rats are fed standard-chow diets, digestion and absorption of nutrients seem to be almost equivalent to sham-operated animals, and fecal energy loss does not seem to be higher in RYGB rats (19). Thus, even though the gastrointestinal anatomy is significantly rearranged after RYGB and even though the stomach, duodenum, and proximal jejunum are excluded from the flow of ingested food (and, hence, the ability to absorb digested nutrients), it seems that the total digestive and absorptive capacity of the intestines still suffices to avoid maldigestion and subsequent caloric malabsorption under many dietary conditions. This may at least partly be due to the massive hypertrophy of the small bowel that is observed predominantly in those gut segments that are still in contact with nutrients [i.e., alimentary (Roux) limb and common channel; see Fig. 1] (19, 53, 71, 95).
Under conditions of high-fat feeding, macronutrient maldigestion and malabsorption may, at least in part, contribute to the negative energy balance of RYGB compared with sham-operated rats (21, 143); in other words, caloric malabsorption may contribute to the RYGB-induced body weight loss if animals are maintained on a high-fat diet (21, 132, 143). In rats operated in our own laboratory, temporary malabsorption was also occasionally observed under conditions of high-fat feeding (e.g., 60% fat by calories), but the contribution to body weight loss seemed to be minor. A recent study by Canales et al. (21) also indicated that fat malabsorption may be a direct consequence of RYGB surgery (21). In that study, it was, however, surprising that the relative fat absorption appeared to be higher when the rats were fed a 40% fat diet compared with a 10% fat diet; given that a limited capacity to absorb dietary fat in RYGB rats may have led to a ceiling effect for absolute fat transport capacity, we would have expected rather the opposite when fat absorption was expressed as percent absorption.
Metabolic effects.
Bariatric surgery has entered a new era some years ago when it became clear that weight loss surgery, and, in particular, RYGB, not only reduces the obesity burden but that it is also leads to a significant reduction in obesity-associated comorbidities; many of these “secondary” effects were observed early after surgery, hence, at a time when the RYGB-induced body weight loss was still negligible.
The term “bariatric surgery” has, therefore, occasionally been substituted by “metabolic surgery” to acknowledge the fact that these surgical procedures do not merely reduce weight, but ameliorate the deleterious metabolic burden of T2DM; in many instances, even prior to significant weight loss (91). Recent studies also have indicated that bariatric surgery offers additional benefits over intensive medical therapy, e.g., in terms of glucose control in diabetics (88, 120, 121). In addition, the improvement of many metabolic disturbances by weight loss surgery is also reflected in the fact that the guidelines for financial compensation for bariatric interventions differ between obese patients with or without metabolic comorbidities. This review will not go into details of the major metabolic effects of RYGB because some excellent articles have been published recently (10, 46, 81, 88, 120, 121, 149). However, a recent study reported that pair-feeding in obese patients produced beneficial effects on glycemic control similar to those seen in patients after RYGB. The authors concluded that on the basis of their data, a very low-calorie diet improves insulin sensitivity and β-cell function just as well as RYGB, at least in the short term (61).
Micronutrient Status After RYGB and RYGB Effects on Bone Metabolism
Micronutrients include the essential minerals, trace elements, and vitamins. Knowledge about the direct consequences of RYGB on the micronutrient status is relatively scarce. A recent review summarized the current recommendations for micronutrient supplementation after RYGB (9); for many of the micronutrients, sound scientific evidence underlying these recommendations is missing and may depend more on anecdotal reports than empirical studies. One matter of concern in RYGB patients is their iron status. Physiologically, most iron is absorbed in the upper part of the small intestine where the intraluminal pH is relatively low; however, this segment of the small intestine is now excluded from contact with ingested food. It has been reported that up to 50% of RYGB patients may develop iron-deficient anemia if iron is not substituted appropriately (82).
Here, on the basis of our own studies, we briefly summarize two examples of essential minerals (calcium) and vitamins (vitamin D) in respect to their bioavailability after RYGB. These two micronutrients have been in the focus of recent RYGB research because of the concern about the potential negative side effects of weight loss surgery on changes in bone metabolism (1, 142). RYGB has repeatedly been reported to lead to a marked decrease in bone mineral density (BMD) (156). Long-term data are still scarce, but the effect of RYGB surgery to reduce BMD may persist beyond the first postsurgical year. Various explanations have been brought forward to explain RYGB-induced bone loss, but only few studies have been performed under well-controlled conditions. Explanations include, e.g., malabsorption of vitamin D leading to vitamin D deficiency, impaired intestinal calcium absorption, and secondary hyperparathyroidism leading to increased bone breakdown. Vitamin D supplementation has often been recommended after RYGB; however, well-controlled studies to justify vitamin D supplementation are missing (9). Importantly, BMD seems to decrease after RYGB surgery, despite vitamin D supplementation, and obesity per se is generally also associated with low vitamin D levels (39). Finally, the status for the biologically active form of vitamin D (1,25-dihydroxyvitamin D [1,25(OH)2D]) is less clear and may actually increase postsurgically, according to some reports (134), questioning the usefulness or need for vitamin D supplementation.
Recently, we and others, therefore, addressed the potential mechanisms underlying RYGB-induced bone loss in more detail and under well-controlled conditions (1, 142). In our study (1), we compared a number of bone metabolism-related parameters between RYGB-operated rats, sham-operated ad libitum-fed rats, and sham-operated rats that were body weight-matched to the RYGB animals. In another study (142), sham-operated rats were compared with RYGB rats and VSG rats whose body weight and lean/fat mass distribution was similar to the RYGB rats. Both studies reported a marked bone loss with a reduction in BMD after RYGB; both studies also suggested that the effects were body weight independent because neither sham-operated body weight-matched (1), nor VSG rats (142), showed a decreased BMD. It seems, therefore, possible that the loss in BMD is mainly due to potentially secondary alterations in metabolism and not primary malabsorption of calcium or vitamin D. Secondary hyperparathyroidism did not seem to be involved in postoperative bone loss, and while 25(OH)D was reduced in both studies after RYGB, reduced serum calcium was not consistently observed (1, 142). Interestingly, we found that active 1,25(OH)2D was actually much higher in RYGB rats, probably due to an upregulation of 1,25(OH)2D synthesis (1). This may explain why calcium malabsorption was only a temporary phenomenon and may contribute to bone loss only initially. However, continued urinary losses of calcium and phosphorus and systemic metabolic acidosis may be the critical factors that contribute to sustained bone loss and insufficient normalization of bone metabolism once calcium malabsorption resolves (1); whether the latter is due to an increased calcium resorptive capacity resulting in the hypertrophic Roux limb is not clear.
Summary
The high prevalence of obesity and its associated comorbidities poses an enormous stress on public health systems. Currently, the most effective treatment options are various bariatric surgery procedures, including its gold standard, the Roux-en-Y gastric bypass. These procedures are invasive, but their effect to reduce body weight and to diminish the occurrence and severity of obesity-related comorbidities largely outweighs any other treatment option. Rat and mouse models of RYGB procedures were instrumental in studying the mechanisms that may underlie the beneficial effects of RYGB. The data clearly indicate that the change in gut anatomy leads to a profound change in the gastrointestinal and whole body physiology. Circulating factors seem to be of primary importance for many RYGB-induced effects, but there may also be a specific role of the nervous system, in particular, the autonomic nervous system. It is always important to realize, however, that the multitude of experiments studying association contrasts with the relative paucity of experiments studying causation. Nonetheless, huge efforts have been undertaken in recent years to understand at least part of the mechanisms mediating RYGB's effects directly or indirectly. It is surprising that only very few studies addressed specific effects of RYGB on the central pathways controlling eating, body weight, and adiposity. Among others, this is a largely unexplored area of research despite all of these efforts.
Outlook
Despite recent progress in understanding post-RYGB physiology, we are still far away from the idea of replacing surgery by nonsurgical means. Obviously, this would be an attractive research goal, as RYGB and most other bariatric surgery procedures are usually considered irreversible and as with any surgical intervention, there is always a risk of intraoperative or postoperative complications. However, RYGB seems to change too many factors in gut and whole body physiology that hamper mimicking the most important of these effects e.g., by pharmacological means soon. It further seems unlikely that the manifold changes after RYGB can be only explained by the manipulation of few targetable mechanisms. Moreover, factors that are altered after RYGB may have beneficial and negative effects at the same time. For example, GLP-1 seems to be a major driver in the overall improvement of glucose metabolism post-RYGB (24), but also plays a role in the high occurrence of hypoglycemic episodes in RYGB patients (118).
Therefore, we need to know much more about the underlying mechanisms of RYGB operations. This is even more important as RYGB operations are increasingly performed in patients with only moderate degrees of obesity, but severe and difficult-to-control metabolic disorders, as well as in adolescents without knowing the long-term consequences in these individuals. Finally, known negative side effects of RYGB [e.g., effects on bone metabolism, increased risk for drug abuse (112)] require intensive research efforts in the future.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: T.A.L. and M.B. conception and design of research; T.A.L. and M.B. analyzed data; T.A.L. and M.B. interpreted results of experiments; T.A.L. and M.B. drafted manuscript; T.A.L. and M.B. edited and revised manuscript; T.A.L. and M.B. approved final version of manuscript; M.B. prepared figures.
ACKNOWLEDGMENTS
The authors thank all their collaborators for the contribution to the work performed in our laboratories; in particular, we thank our colleague C. W. Le Roux (University College Dublin), who was the initial driving force behind our projects. We also sincerely thank our collaborators A. Spector and C. Mathes (Florida State University, Tallahassee), K. Abegg, L. Asarian, T. Bächler, C. Corteville, C. Dörig, P. Doytcheva, E. Osto, M. Osto (all University of Zurich), M. Schiesser (University Hospital Zurich), F. Seyfried (University Hospital of Würzburg), N. Vrang and colleagues (Gubra, Copenhagen), N. Geary, and many others. The financial contributions of the Swiss National Science Foundation, the National Institutes of Health, the Zurich Center for Integrative Human Physiology, the Hartmann Müller Stiftung, and the Schweizerische Herzstiftung are gratefully acknowledged.
REFERENCES
- 1.Abegg K, Gehring N, Wagner CA, Liesegang A, Schiesser M, Bueter M, Lutz TA. Roux-en-Y gastric bypass surgery reduces bone mineral density and induces metabolic acidosis in rats. Am J Physiol Regul Integr Comp Physiol 305: R999–R1009, 2013. [DOI] [PubMed] [Google Scholar]
- 2.Abegg K, Schiesser M, Lutz TA, Bueter M. Acute peripheral GLP-1 receptor agonism or antagonism does not alter energy expenditure in rats after Roux-en-Y gastric bypass. Physiol Behav 121: 70–78, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adrian TE, Gariballa S, Parekh KA, Thomas SA, Saadi H, Al KJ, Nagelkerke N, Gedulin B, Young AA. Rectal taurocholate increases L cell and insulin secretion, and decreases blood glucose and food intake in obese type 2 diabetic volunteers. Diabetologia 55: 2343–2347, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 37: 1553–1559, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asarian L, Abegg K, Geary N, Schiesser M, Lutz TA, Bueter M. Estradiol increases body weight loss and gut-peptide satiation after Roux-en-Y gastric bypass in ovariectomized rats. Gastroenterology 143: 325–327, 2012. [DOI] [PubMed] [Google Scholar]
- 6.Ashrafian H, le Roux CW. Metabolic surgery and gut hormones—a review of bariatric entero-humoral modulation. Physiol Behav 97: 620–631, 2009. [DOI] [PubMed] [Google Scholar]
- 7.Atkinson RL, Brent EL. Appetite suppressant activity in plasma of rats after intestinal bypass surgery. Am J Physiol Regul Integr Comp Physiol 243: R60–R64, 1982. [DOI] [PubMed] [Google Scholar]
- 8.Baik JH. Dopamine signaling in food addiction: role of dopamine D2 receptors. BMB Rep 46: 519–526, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol 8: 544–556, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Barres R, Kirchner H, Rasmussen M, Yan J, Kantor FR, Krook A, Naslund E, Zierath JR. Weight loss after gastric bypass surgery in human obesity remodels promoter methylation. Cell Rep 3: 1020–1027, 2013. [DOI] [PubMed] [Google Scholar]
- 11.Beglinger C, Degen L. Fat in the intestine as a regulator of appetite—role of CCK. Physiol Behav 83: 617–621, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 20 Suppl 1: 64–72, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borg CM, le Roux CW, Ghatei MA, Bloom SR, Patel AG, Aylwin SJ. Progressive rise in gut hormone levels after Roux-en-Y gastric bypass suggests gut adaptation and explains altered satiety. Br J Surg 93: 210–215, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Browning KN, Fortna SR, Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol 591: 2357–2372, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bueter M, Abegg K, Seyfried F, Lutz TA, le Roux CW. Roux-en-Y gastric bypass operation in rats. J Vis Exp e3940, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bueter M, Ashrafian H, le Roux CW. Mechanisms of weight loss after gastric bypass and gastric banding. Obes Facts 2: 325–331, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bueter M, le Roux CW. Sir David Cuthbertson Medal Lecture Bariatric surgery as a model to study appetite control. Proc Nutr Soc 68: 227–233, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T, Lutz T, le Roux CW. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 20: 616–622, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bueter M, Lowenstein C, Olbers T, Wang M, Cluny NL, Bloom SR, Sharkey KA, Lutz TA, le Roux CW. Gastric bypass increases energy expenditure in rats. Gastroenterology 138: 1845–1853, 2010. [DOI] [PubMed] [Google Scholar]
- 20.Bueter M, Miras AD, Chichger H, Fenske W, Ghatei MA, Bloom SR, Unwin RJ, Lutz TA, Spector AC, le Roux CW. Alterations of sucrose preference after Roux-en-Y gastric bypass. Physiol Behav 104: 709–721, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Canales BK, Ellen J, Khan SR, Hatch M. Steatorrhea and hyperoxaluria occur after gastric bypass surgery in obese rats regardless of dietary fat or oxalate. J Urol 190: 1102–1109, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carlsson LM, Peltonen M, Ahlin S, Anveden A, Bouchard C, Carlsson B, Jacobson P, Lonroth H, Maglio C, Naslund I, Pirazzi C, Romeo S, Sjoholm K, Sjostrom E, Wedel H, Svensson PA, Sjostrom L. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med 367: 695–704, 2012. [DOI] [PubMed] [Google Scholar]
- 23.Carrasco F, Papapietro K, Csendes A, Salazar G, Echenique C, Lisboa C, Diaz E, Rojas J. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg 17: 608–616, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Chambers AP, Jessen L, Ryan KK, Sisley S, Wilson-Perez HE, Stefater MA, Gaitonde SG, Sorrell JE, Toure M, Berger J, D'Alessio DA, Woods SC, Seeley RJ, Sandoval DA. Weight-independent changes in blood glucose homeostasis after gastric bypass or vertical sleeve gastrectomy in rats. Gastroenterology 141: 950–958, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD, Thorner MO, Pfluger PT, Gutierrez JA, Tschop MH, Sandoval DA, Seeley RJ. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 144: 50–52, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chambers AP, Smith EP, Begg DP, Grayson BE, Sisley S, Greer T, Sorrell J, Lemmen L, Lasance K, Woods SC, Seeley RJ, D'Alessio DA, Sandoval DA. Regulation of gastric emptying rate and its role in nutrient-induced GLP-1 secretion in rats after vertical sleeve gastrectomy. Am J Physiol Endocrinol Metab 306: E424–E432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chambers AP, Wilson-Perez HE, McGrath S, Grayson BE, Ryan KK, D'Alessio DA, Woods SC, Sandoval DA, Seeley RJ. Effect of vertical sleeve gastrectomy on food selection and satiation in rats. Am J Physiol Endocrinol Metab 303: E1076–E1084, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V, Viollet B, Andreelli F, Withers DJ, Batterham RL. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 60: 810–818, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP, Purnell JQ. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 346: 1623–1630, 2002. [DOI] [PubMed] [Google Scholar]
- 30.D'Alessio D. Intestinal hormones and regulation of satiety: the case for CCK, GLP-1, PYY, and Apo A-IV. J Parenter Enteral Nutr 32: 567–568, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Das SK, Roberts SB, McCrory MA, Hsu LK, Shikora SA, Kehayias JJ, Dallal GE, Saltzman E. Long-term changes in energy expenditure and body composition after massive weight loss induced by gastric bypass surgery. Am J Clin Nutr 78: 22–30, 2003. [DOI] [PubMed] [Google Scholar]
- 32.de Castro Cesar M, de Lima Montebelo MI, Rasera I, Jr., de Oliveira AV, Jr., Gomes Gonelli PR, Aparecida Cardoso G. Effects of Roux-en-Y gastric bypass on resting energy expenditure in women. Obes Surg 18: 1376–1380, 2008. [DOI] [PubMed] [Google Scholar]
- 33.Delzenne NM, Neyrinck AM, Backhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol 7: 639–646, 2011. [DOI] [PubMed] [Google Scholar]
- 34.Dhillo WS, Bloom SR. Gastrointestinal hormones and regulation of food intake. Horm Metab Res 36: 846–851, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Jacobsen SH, Clausen TR, Worm D, Hartmann B, Rehfeld JF, Damgaard M, Madsen JL, Madsbad S, Holst JJ, Hansen DL. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. Int J Obes (Lond) 37: 1452–1459, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Dowling RH. Small bowel adaptation and its regulation. Scand J Gastroenterol Suppl 74: 53–74, 1982. [PubMed] [Google Scholar]
- 37.Duboc H, Tache Y, Hofmann AF. The bile acid TGR5 membrane receptor: From basic research to clinical application. Dig Liver Dis 46: 302–312, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn JP, Cowan RL, Volkow ND, Feurer ID, Li R, Williams DB, Kessler RM, Abumrad NN. Decreased dopamine type 2 receptor availability after bariatric surgery: preliminary findings. Brain Res 1350: 123–130, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earthman CP, Beckman LM, Masodkar K, Sibley SD. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: considerations and implications. Int J Obes (Lond) 36: 387–396, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Faria SL, Faria OP, Cardeal MA, de Gouvea HR, Buffington C. Diet-induced thermogenesis and respiratory quotient after Roux-en-Y gastric bypass. Surg Obes Relat Dis 8: 797–802, 2012. [DOI] [PubMed] [Google Scholar]
- 41.Fenske WK, Bueter M, Miras AD, Ghatei MA, Bloom SR, le Roux CW. Exogenous peptide YY3–36 and Exendin-4 further decrease food intake, whereas octreotide increases food intake in rats after Roux-en-Y gastric bypass. Int J Obes (Lond) 36: 379–384, 2012. [DOI] [PubMed] [Google Scholar]
- 42.Flancbaum L, Choban PS, Bradley LR, Burge JC. Changes in measured resting energy expenditure after Roux-en-Y gastric bypass for clinically severe obesity. Surgery 122: 943–949, 1997. [DOI] [PubMed] [Google Scholar]
- 43.Flier JS. Clinical review 94: What's in a name? In search of leptin's physiologic role. J Clin Endocrinol Metab 83: 1407–1413, 1998. [DOI] [PubMed] [Google Scholar]
- 44.Foster DO, Frydman ML. Tissue distribution of cold-induced thermogenesis in conscious warm- or cold-acclimated rats reevaluated from changes in tissue blood flow: the dominant role of brown adipose tissue in the replacement of shivering by nonshivering thermogenesis. Can J Physiol Pharmacol 57: 257–270, 1979. [DOI] [PubMed] [Google Scholar]
- 45.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, Mariat D, Corthier G, Dore J, Henegar C, Rizkalla S, Clement K. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 59: 3049–3057, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ 347: f5934, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci Transl Med 5: 199ra112, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guijarro A, Suzuki S, Chen C, Kirchner H, Middleton FA, Nadtochiy S, Brookes PS, Niijima A, Inui A, Meguid MM. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am J Physiol Regul Integr Comp Physiol 293: R1474–R1489, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Hajnal A, Kovacs P, Ahmed T, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 299: G967–G979, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA 94: 8878–8883, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Halatchev IG, Cone RD. Peripheral administration of PYY(3–36) produces conditioned taste aversion in mice. Cell Metab 1: 159–168, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Hankir M, Bueter M, Gsell W, Seyfried F, Khalil M, Smith KL, Bloom SR, Bell JD, le Roux CW. Increased energy expenditure in gastric bypass rats is not caused by activated brown adipose tissue. Obes Facts 5: 349–358, 2012. [DOI] [PubMed] [Google Scholar]
- 53.Hansen CF, Bueter M, Theis N, Lutz T, Paulsen S, Dalboge LS, Vrang N, Jelsing J. Hypertrophy dependent doubling of L-cells in Roux-en-Y gastric bypass operated rats. PLoS One 8: e65696, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss after Roux-en-Y gastric bypass surgery in rats. Obes Surg In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL, Ma LL, Blaszczyk K, Keogh JM, Cone RD, Farooqi IS, Kaplan LM. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab 97: E1023–E1031, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He B, Chen L, Yu C, Piao D, Wang Y, Han P. Roux-en-Y gastric bypass increases hepatic and peripheral insulin sensitivity in rats with type 2 diabetes mellitus. Surg Obes Relat Dis 10: 485–493, 2014. [DOI] [PubMed] [Google Scholar]
- 57.He B, Piao D, Yu C, Wang Y, Han P. Amelioration in hepatic insulin sensitivity by reduced hepatic lipid accumulation at short-term after Roux-en-Y gastric bypass surgery in type 2 diabetic rats. Obes Surg 23: 2033–2041, 2013. [DOI] [PubMed] [Google Scholar]
- 58.Hirasawa A, Tsumaya K, Awaji T, Katsuma S, Adachi T, Yamada M, Sugimoto Y, Miyazaki S, Tsujimoto G. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 11: 90–94, 2005. [DOI] [PubMed] [Google Scholar]
- 59.Holdstock C, Engstrom BE, Ohrvall M, Lind L, Sundbom M, Karlsson FA. Ghrelin and adipose tissue regulatory peptides: effect of gastric bypass surgery in obese humans. J Clin Endocrinol Metab 88: 3177–3183, 2003. [DOI] [PubMed] [Google Scholar]
- 60.Holst JJ, Sorensen TI, Andersen AN, Stadil F, Andersen B, Lauritsen KB, Klein HC. Plasma enteroglucagon after jejunoileal bypass with 3:1 or 1:3 jejunoileal ratio. Scand J Gastroenterol 14: 205–207, 1979. [DOI] [PubMed] [Google Scholar]
- 61.Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M, McMahon DJ, Korner J. Very low-calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and β-cell function in type 2 diabetic patients. Diabetes 62: 3027–3032, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U, Worm D, Almdal T, Naver LS, Hvolris LE, Rehfeld JF, Wulff BS, Clausen TR, Hansen DL, Holst JJ, Madsbad S. Changes in gastrointestinal hormone responses, insulin sensitivity, and β-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 22: 1084–1096, 2012. [DOI] [PubMed] [Google Scholar]
- 63.Jorgensen NB, Dirksen C, Bojsen-Moller KN, Jacobsen SH, Worm D, Hansen DL, Kristiansen VB, Naver L, Madsbad S, Holst JJ. Exaggerated glucagon-like peptide 1 response is important for improved beta-cell function and glucose tolerance after Roux-en-Y gastric bypass in patients with type 2 diabetes. Diabetes 62: 3044–3052, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kinzig KP, D'Alessio DA, Seeley RJ. The diverse roles of specific GLP-1 receptors in the control of food intake and the response to visceral illness. J Neurosci 22: 10470–10476, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL, Zucker JD, Dore J, Clement K. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 98: 16–24, 2013. [DOI] [PubMed] [Google Scholar]
- 66.Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL, Wardlaw SL. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 90: 359–365, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 30: 1709–1716, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Langhans W, Geary N. Overview of the physiological control of eating. Forum Nutr 63: 9–53, 2010. [DOI] [PubMed] [Google Scholar]
- 69.Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB, Lonroth H, Fandriks L, Olbers T. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 36: 348–355, 2012. [DOI] [PubMed] [Google Scholar]
- 70.le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V, Shurey S, Ghatei MA, Patel AG, Bloom SR. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 243: 108–114, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.le Roux CW, Borg C, Wallis K, Vincent RP, Bueter M, Goodlad R, Ghatei MA, Patel A, Bloom SR, Aylwin SJ. Gut hypertrophy after gastric bypass is associated with increased glucagon-like peptide 2 and intestinal crypt cell proliferation. Ann Surg 252: 50–56, 2010. [DOI] [PubMed] [Google Scholar]
- 72.le Roux CW, Bueter M, Theis N, Werling M, Ashrafian H, Lowenstein C, Athanasiou T, Bloom SR, Spector AC, Olbers T, Lutz TA. Gastric bypass reduces fat intake and preference. Am J Physiol Regul Integr Comp Physiol 301: R1057–R1066, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A, Lonroth H, Fandriks L, Ghatei MA, Bloom SR, Olbers T. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 246: 780–785, 2007. [DOI] [PubMed] [Google Scholar]
- 74.Li JV, Ashrafian H, Bueter M, Kinross J, Sands C, le Roux CW, Bloom SR, Darzi A, Athanasiou T, Marchesi JR, Nicholson JK, Holmes E. Metabolic surgery profoundly influences gut microbial-host metabolic cross-talk. Gut 60: 1214–1223, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liou AP, Paziuk M, Luevano JM, Jr., Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5: 178ra41, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lutz TA. Amylinergic control of food intake. Physiol Behav 89: 465–471, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Lutz TA. Control of energy homeostasis by amylin. Cell Mol Life Sci 69: 1947–1965, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lutz TA. The interaction of amylin with other hormones in the control of eating. Diabetes Obes Metab 15: 99–111, 2013. [DOI] [PubMed] [Google Scholar]
- 79.Lutz TA, Bueter M. Physiological mechanisms behind Roux-en-Y gastric bypass surgery. Dig Surg 31: 13–24, 2014. [DOI] [PubMed] [Google Scholar]
- 80.Lutz TA, Geary N, Szabady MM, Del PE, Scharrer E. Amylin decreases meal size in rats. Physiol Behav 58: 1197–1202, 1995. [DOI] [PubMed] [Google Scholar]
- 81.Maggard-Gibbons M, Maglione M, Livhits M, Ewing B, Maher AR, Hu J, Li Z, Shekelle PG. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA 309: 2250–2261, 2013. [DOI] [PubMed] [Google Scholar]
- 82.Malone M, Alger-Mayer S, Lindstrom J, Bailie GR. Management of iron deficiency and anemia after Roux-en-Y gastric bypass surgery: an observational study. Surg Obes Relat Dis 9: 969–974, 2013. [DOI] [PubMed] [Google Scholar]
- 83.Mathes CM, Bueter M, Smith KR, Lutz TA, le Roux CW, Spector AC. Roux-en-Y gastric bypass in rats increases sucrose taste-related motivated behavior independent of pharmacological GLP-1-receptor modulation. Am J Physiol Regul Integr Comp Physiol 302: R751–R767, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mathes CM, Spector AC. Food selection and taste changes in humans after Roux-en-Y gastric bypass surgery: a direct-measures approach. Physiol Behav 107: 476–483, 2012. [DOI] [PubMed] [Google Scholar]
- 85.Meirelles K, Ahmed T, Culnan DM, Lynch CJ, Lang CH, Cooney RN. Mechanisms of glucose homeostasis after Roux-en-Y gastric bypass surgery in the obese, insulin-resistant Zucker rat. Ann Surg 249: 277–285, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mietlicki-Baase EG, Hayes MR. Amylin activates distributed CNS nuclei to control energy balance. Physiol Behav In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab 35: 518–523, 2009. [DOI] [PubMed] [Google Scholar]
- 88.Mingrone G, Panunzi S, De GA, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G, Rubino F. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med 366: 1577–1585, 2012. [DOI] [PubMed] [Google Scholar]
- 89.Miras AD, Jackson RN, Jackson SN, Goldstone AP, Olbers T, Hackenberg T, Spector AC, le Roux CW. Gastric bypass surgery for obesity decreases the reward value of a sweet-fat stimulus as assessed in a progressive ratio task. Am J Clin Nutr 96: 467–473, 2012. [DOI] [PubMed] [Google Scholar]
- 90.Miras AD, le Roux CW. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 10: 575–584, 2013. [DOI] [PubMed] [Google Scholar]
- 91.Miras AD, le Roux CW. Metabolic surgery: shifting the focus from glycaemia and weight to end-organ health. Lancet Diabetes Endocrinol 2: 141–151, 2014. [DOI] [PubMed] [Google Scholar]
- 92.Mirshahi UL, Still CD, Masker KK, Gerhard GS, Carey DJ, Mirshahi T. The MC4R(I251L) allele is associated with better metabolic status and more weight loss after gastric bypass surgery. J Clin Endocrinol Metab 96: E2088–E2096, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mul JD, Begg DP, Alsters SI, van HG, Duran KJ, D'Alessio DA, le Roux CW, Woods SC, Sandoval DA, Blakemore AI, Cuppen E, van Haelst MM, Seeley RJ. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab 303: E103–E110, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mumphrey MB, Hao Z, Townsend RL, Patterson LM, Morrison CD, Munzberg H, Stylopoulos N, Ye J, Berthoud HR. Reversible hyperphagia and obesity in rats with gastric bypass by central MC3/4R blockade. Obesity (Silver Spring) 22: 1847–1853, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mumphrey MB, Patterson LM, Zheng H, Berthoud HR. Roux-en-Y gastric bypass surgery increases number but not density of CCK-, GLP-1-, 5-HT-, and neurotensin-expressing enteroendocrine cells in rats. Neurogastroenterol Motil 25: e70–e79, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature 444: 854–859, 2006. [DOI] [PubMed] [Google Scholar]
- 97.Naslund E, Gryback P, Hellstrom PM, Jacobsson H, Holst JJ, Theodorsson E, Backman L. Gastrointestinal hormones and gastric emptying 20 years after jejunoileal bypass for massive obesity. Int J Obes Relat Metab Disord 21: 387–392, 1997. [DOI] [PubMed] [Google Scholar]
- 98.Nestoridi E, Kvas S, Kucharczyk J, Stylopoulos N. Resting energy expenditure and energetic cost of feeding are augmented after Roux-en-Y gastric bypass in obese mice. Endocrinology 153: 2234–2244, 2012. [DOI] [PubMed] [Google Scholar]
- 99.Ochner CN, Kwok Y, Conceicao E, Pantazatos SP, Puma LM, Carnell S, Teixeira J, Hirsch J, Geliebter A. Selective reduction in neural responses to high calorie foods following gastric bypass surgery. Ann Surg 253: 502–507, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ochner CN, Laferrere B, Afifi L, Atalayer D, Geliebter A, Teixeira J. Neural responsivity to food cues in fasted and fed states pre and post gastric bypass surgery. Neurosci Res 74: 138–143, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J, Teixeira J. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 209: 128–135, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ockenga J, Valentini L, Schuetz T, Wohlgemuth F, Glaeser S, Omar A, Kasim E, duPlessis D, Featherstone K, Davis JR, Tietge UJ, Kroencke T, Biebermann H, Kohrle J, Brabant G. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab 97: 535–542, 2012. [DOI] [PubMed] [Google Scholar]
- 103.Olbers T, Bjorkman S, Lindroos A, Maleckas A, Lonn L, Sjostrom L, Lonroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 244: 715–722, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav 119: 92–96, 2013. [DOI] [PubMed] [Google Scholar]
- 105.Peng Y, Murr MM. Roux-en-Y gastric bypass improves hepatic mitochondrial function in obese rats. Surg Obes Relat Dis 9: 429–435, 2013. [DOI] [PubMed] [Google Scholar]
- 106.Peng Y, Rideout DA, Rakita SS, Gower WR, Jr., You M, Murr MM. Does LKB1 mediate activation of hepatic AMP-protein kinase (AMPK) and sirtuin1 (SIRT1) after Roux-en-Y gastric bypass in obese rats? J Gastrointest Surg 14: 221–228, 2010. [DOI] [PubMed] [Google Scholar]
- 107.Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 22: E13–E20, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Peterli R, Steinert RE, Woelnerhanssen B, Peters T, Christoffel-Court Gass M, Kern B, von FM, Beglinger C. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg 22: 740–748, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D, Bekker JH, Ghatei MA, Bloom SR, Walters JR, Welbourn R, le Roux CW. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 153: 3613–3619, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pournaras DJ, le Roux CW. Are bile acids the new gut hormones? Lessons from weight loss surgery models. Endocrinology 154: 2255–2256, 2013. [DOI] [PubMed] [Google Scholar]
- 111.Quercia I, Dutia R, Kotler DP, Belsley S, Laferrere B. Gastrointestinal changes after bariatric surgery. Diabetes Metab 40: 87–94, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Reslan S, Saules KK, Greenwald MK, Schuh LM. Substance misuse following Roux-en-Y gastric bypass surgery. Subst Use Misuse 49: 405–417, 2014. [DOI] [PubMed] [Google Scholar]
- 113.Rocca AS, Brubaker PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 140: 1687–1694, 1999. [DOI] [PubMed] [Google Scholar]
- 114.Rubino F, Gagner M, Gentileschi P, Kini S, Fukuyama S, Feng J, Diamond E. The early effect of the Roux-en-Y gastric bypass on hormones involved in body weight regulation and glucose metabolism. Ann Surg 240: 236–242, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R, Wilson-Perez HE, Sandoval DA, Kohli R, Backhed F, Seeley RJ. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 509: 183–188, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saeidi N, Meoli L, Nestoridi E, Gupta NK, Kvas S, Kucharczyk J, Bonab AA, Fischman AJ, Yarmush ML, Stylopoulos N. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science 341: 406–410, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saeidi N, Nestoridi E, Kucharczyk J, Uygun MK, Yarmush ML, Stylopoulos N. Sleeve gastrectomy and Roux-en-Y gastric bypass exhibit differential effects on food preferences, nutrient absorption and energy expenditure in obese rats. Int J Obes (Lond) 36: 1396–1402, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Salehi M, Gastaldelli A, D'Alessio DA. Blockade of glucagon-like peptide 1 receptor corrects postprandial hypoglycemia after gastric bypass. Gastroenterology 146: 669–680, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schaap FG. Role of fibroblast growth factor 19 in the control of glucose homeostasis. Curr Opin Clin Nutr Metab Care 15: 386–391, 2012. [DOI] [PubMed] [Google Scholar]
- 120.Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Brethauer SA, Navaneethan SD, Aminian A, Pothier CE, Kim ES, Nissen SE, Kashyap SR. Bariatric surgery versus intensive medical therapy for diabetes—3-year outcomes. N Engl J Med 370: 2002–2013, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med 366: 1567–1576, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM, Ismail NA, Durighel G, Ahmed AR, Olbers T, Vincent RP, Alaghband-Zadeh J, Ghatei MA, Waldman AD, Frost GS, Bell JD, le Roux CW, Goldstone AP. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 63: 891–902, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature 404: 661–671, 2000. [DOI] [PubMed] [Google Scholar]
- 124.Seyfried F, Bueter M, Spliethoff K, Miras AD, Abegg K, Lutz TA, le Roux CW. Roux-en Y gastric bypass is superior to duodeno-jejunal bypass in improving glycaemic control in Zucker diabetic fatty rats. Obes Surg 24: 1888–1895, 2014. [DOI] [PubMed] [Google Scholar]
- 125.Seyfried F, Lannoo M, Gsell W, Tremoleda JL, Bueter M, Olbers T, Jurowich C, Germer CT, le Roux CW. Roux-en-Y gastric bypass in mice—surgical technique and characterisation. Obes Surg 22: 1117–1125, 2012. [DOI] [PubMed] [Google Scholar]
- 126.Shah M, Law JH, Micheletto F, Sathananthan M, Dalla MC, Cobelli C, Rizza RA, Camilleri M, Zinsmeister AR, Vella A. Contribution of endogenous glucagon-like peptide 1 to glucose metabolism after Roux-en-Y gastric bypass. Diabetes 63: 483–493, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shang Q, Saumoy M, Holst JJ, Salen G, Xu G. Colesevelam improves insulin resistance in a diet-induced obesity (F-DIO) rat model by increasing the release of GLP-1. Am J Physiol Gastrointest Liver Physiol 298: G419–G424, 2010. [DOI] [PubMed] [Google Scholar]
- 128.Shibata H, Bukowiecki LJ. Regulatory alterations of daily energy expenditure induced by fasting or overfeeding in unrestrained rats. J Appl Physiol 63: 465–470, 1987. [DOI] [PubMed] [Google Scholar]
- 129.Shimizu H, Eldar S, Heneghan HM, Schauer PR, Kirwan JP, Brethauer SA. The effect of selective gut stimulation on glucose metabolism after gastric bypass in the Zucker diabetic fatty rat model. Surg Obes Relat Dis 10: 29–35, 2014. [DOI] [PubMed] [Google Scholar]
- 130.Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg 255: 294–301, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 35: 642–651, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shin AC, Zheng H, Townsend RL, Patterson LM, Holmes GM, Berthoud HR. Longitudinal assessment of food intake, fecal energy loss, and energy expenditure after Roux-en-Y gastric bypass surgery in high-fat-fed obese rats. Obes Surg 23: 531–540, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 151: 1588–1597, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sinha N, Shieh A, Stein EM, Strain G, Schulman A, Pomp A, Gagner M, Dakin G, Christos P, Bockman RS. Increased PTH and 1.25(OH)(2)D levels associated with increased markers of bone turnover following bariatric surgery. Obesity (Silver Spring) 19: 2388–2393, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sjostrom L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjostrom CD, Sullivan M, Wedel H. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 351: 2683–2693, 2004. [DOI] [PubMed] [Google Scholar]
- 136.Sjostrom L, Peltonen M, Jacobson P, Sjostrom CD, Karason K, Wedel H, Ahlin S, Anveden A, Bengtsson C, Bergmark G, Bouchard C, Carlsson B, Dahlgren S, Karlsson J, Lindroos AK, Lonroth H, Narbro K, Naslund I, Olbers T, Svensson PA, Carlsson LM. Bariatric surgery and long-term cardiovascular events. JAMA 307: 56–65, 2012. [DOI] [PubMed] [Google Scholar]
- 137.Smith JC. The history of the “Davis Rig”. Appetite 36: 93–98, 2001. [DOI] [PubMed] [Google Scholar]
- 138.Spector AC. Linking gustatory neurobiology to behavior in vertebrates. Neurosci Biobehav Rev 24: 391–416, 2000. [DOI] [PubMed] [Google Scholar]
- 139.Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. Obes Surg 20: 369–374, 2010. [DOI] [PubMed] [Google Scholar]
- 140.Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J, Toure M, Tschop M, Woods SC, Seeley RJ. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 138: 2426–36, 2436, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Steinert RE, Peterli R, Keller S, Meyer-Gerspach AC, Drewe J, Peters T, Beglinger C. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring) 21: E660–E668, 2013. [DOI] [PubMed] [Google Scholar]
- 142.Stemmer K, Bielohuby M, Grayson BE, Begg DP, Chambers AP, Neff C, Woods SC, Erben RG, Tschop MH, Bidlingmaier M, Clemens TL, Seeley RJ. Roux-en-Y gastric bypass surgery but not vertical sleeve gastrectomy decreases bone mass in male rats. Endocrinology 154: 2015–2024, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Stylopoulos N, Hoppin AG, Kaplan LM. Roux-en-Y gastric bypass enhances energy expenditure and extends lifespan in diet-induced obese rats. Obesity (Silver Spring) 17: 1839–1847, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Suzuki S, Ramos EJ, Goncalves CG, Chen C, Meguid MM. Changes in GI hormones and their effect on gastric emptying and transit times after Roux-en-Y gastric bypass in rat model. Surgery 138: 283–290, 2005. [DOI] [PubMed] [Google Scholar]
- 145.Thivel D, Brakonieki K, Duche P, Morio B, Boirie Y, Laferrere B. Surgical weight loss: impact on energy expenditure. Obes Surg 23: 255–266, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Thivel D, Metz L, Julien A, Morio B, Duche P. Obese but not lean adolescents spontaneously decrease energy intake after intensive exercise. Physiol Behav 123: 41–46, 2014. [DOI] [PubMed] [Google Scholar]
- 147.Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab 10: 167–177, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Troy S, Soty M, Ribeiro L, Laval L, Migrenne S, Fioramonti X, Pillot B, Fauveau V, Aubert R, Viollet B, Foretz M, Leclerc J, Duchampt A, Zitoun C, Thorens B, Magnan C, Mithieux G, Andreelli F. Intestinal gluconeogenesis is a key factor for early metabolic changes after gastric bypass but not after gastric lap-band in mice. Cell Metab 8: 201–211, 2008. [DOI] [PubMed] [Google Scholar]
- 149.Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart 98: 1763–1777, 2012. [DOI] [PubMed] [Google Scholar]
- 150.Wang Y, Liu J. Combination of bypassing stomach and vagus dissection in high-fat diet-induced obese rats-a long-term investigation. Obes Surg 20: 375–379, 2010. [DOI] [PubMed] [Google Scholar]
- 151.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 152.Werling M, Olbers T, Fandriks L, Bueter M, Lonroth H, Stenlof K, le Roux CW. Increased postprandial energy expenditure may explain superior long term weight loss after Roux-en-Y gastric bypass compared to vertical banded gastroplasty. PLoS One 8: e60280, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wilms B, Ernst B, Schmid SM, Thurnheer M, Schultes B. Enhanced thermic effect of food after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab 98: 3776–3784, 2013. [DOI] [PubMed] [Google Scholar]
- 154.Wilson-Perez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Perez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like Peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N, Munzberg H, Morrison CD, Drucker DJ, Berthoud HR. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 306: R352–R362, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res 29: 1507–1518, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM, Still CD, Gerhard GS, Burgess SC, Mirshahi T, Aguirre V. Weight-independent effects of Roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology 144: 580–590, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL, Berthoud HR. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 297: R1273–R1282, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


