Abstract
Intermittent hypoxia (IH) has been the subject of considerable research in recent years, and triggers a bewildering array of both detrimental and beneficial effects in multiple physiological systems. Here, we review the extensive literature concerning IH and its impact on the respiratory, cardiovascular, immune, metabolic, bone, and nervous systems. One major goal is to define relevant IH characteristics leading to safe, protective, and/or therapeutic effects vs. pathogenesis. To understand the impact of IH, it is essential to define critical characteristics of the IH protocol under investigation, including potentially the severity of hypoxia within episodes, the duration of hypoxic episodes, the number of hypoxic episodes per day, the pattern of presentation across time (e.g., within vs. consecutive vs. alternating days), and the cumulative time of exposure. Not surprisingly, severe/chronic IH protocols tend to be pathogenic, whereas any beneficial effects are more likely to arise from modest/acute IH exposures. Features of the IH protocol most highly associated with beneficial vs. pathogenic outcomes include the level of hypoxemia within episodes and the number of episodes per day. Modest hypoxia (9–16% inspired O2) and low cycle numbers (3–15 episodes per day) most often lead to beneficial effects without pathology, whereas severe hypoxia (2–8% inspired O2) and more episodes per day (48–2,400 episodes/day) elicit progressively greater pathology. Accumulating evidence suggests that “low dose” IH (modest hypoxia, few episodes) may be a simple, safe, and effective treatment with considerable therapeutic potential for multiple clinical disorders.
Keywords: intermittent hypoxia, pathogenic, review, therapeutic, dose
intermittent hypoxia (ih) has been a topic of considerable research for decades. However, a full understanding of IH and its biological effects is not yet at hand. Whereas some reports claim that IH elicits pathology, others focus on its beneficial effects. This apparent discrepancy may, at least to some extent, be explained by the wide range of experimental procedures/protocols described as “intermittent hypoxia” among investigators. The essential feature of IH is repeated or recurrent episodes of low oxygen (hypoxia), interspersed with periods of normoxia. However, this definition does not begin to capture the range of protocols reported in the literature. There appears to be no real consensus concerning what should be defined as “intermittent hypoxia,” nor is there any real understanding of key variables defining the biological impact of IH. The fundamental goal of this brief review is to assess relevant characteristics leading to beneficial/compensatory vs. maladaptive/pathological outcomes.
Specific IH protocols/paradigms reported in the literature are most often associated with the specific perspective or field of study of the investigators. Reported IH protocols vary greatly in terms of 1) the severity of hypoxia (e.g., the level of hypoxemia, frequently reported as the inspired oxygen percentage); 2) the duration of hypoxia within episodes; 3) the number of hypoxia/reoxygenation cycles (episodes) per day; 4) the pattern of presentation (e.g., multiple episodes per day with a normoxic period until the next day vs. exposures of limited episodes three days per week, etc.); 5) the cumulative duration of exposure (days/weeks/months); and 6) regulation of other relevant variables, such as the prevailing level of arterial carbon dioxide. Comparing literature descriptions of protocols described as “intermittent hypoxia:” 1) the severity of hypoxia within episodes ranges from 2% to 16% inspired oxygen; 2) the duration of hypoxic episodes ranges from 15–30 s to 12 h; 3) the number of cycles per day ranges from 3 to 2,400; 4) the cumulative IH protocol duration ranges from less than 1 h, to between 2 and 90 days; and 5) most long-lasting protocols involve IH on consecutive days, although some use alternating days (e.g., every other day or 3 times per week). Each of these variables must be carefully considered before we can understand the biological impact of IH since, collectively, they define the effective IH “dose” (39).
Since many laboratories apply unique IH paradigms, discrepancies in terminology make generalizations difficult and obstruct efforts to understand the biological impact of IH. On the other hand, it is counterproductive to completely standardize protocols, since such standardization will obscure the range of IH prevalent in life and may prevent an appreciation of the wide-ranging impact of IH on physiology (155). To maximize progress toward greater understanding and ability to manipulate IH protocols for therapeutic advantage in diverse clinical disorders, a detailed understanding of different animal models and mechanisms underlying each particular disorder will be useful to define optimal IH protocols in each condition.
Although we do include some altitude physiology studies, we do not thoroughly review literature reports concerning the use of IH to preacclimatize individuals as a means of improving their performance at high altitude. Further, we do not systematically discuss cellular mechanisms giving rise to the therapeutic vs. pathogenic IH effects. Rather, the purpose of this review was to examine key features of experimental IH protocols that determine their impact on a wide range of physiological systems. We emphasize in vivo animal models, human studies, and lessons from IH-induced respiratory plasticity (the perspective of the authors). Accumulating evidence reviewed here suggests that modest hypoxia (9–16% inspired O2) and low cycle numbers (3–15 episodes per day) often lead to beneficial effects without detectable pathology, whereas protocols utilizing severe hypoxia (3–8% inspired O2) and more episodes per day (48–2,400 episodes/day) elicit pathology (Fig. 1).
Fig. 1.
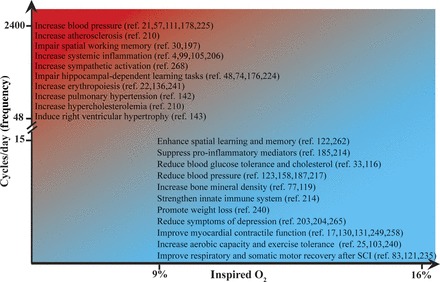
Schematic summarizing factors most influential in determining the balance of beneficial vs. pathogenic intermittent hypoxia (IH) effects. IH protocols consisting of severe hypoxia (2–8% inspired O2) and between 48–2,400 cycles/day are prone to pathology; citations demonstrating pathogenic effects of IH are listed in the upper left quadrant (i.e., high cycle numbers per day with relatively severe hypoxemia within episodes, as indicated by orange/red shading). In contrast, IH protocols consisting of moderate IH (>9% inspired O2; <15 cycles/day) appear to elicit beneficial (potentially “therapeutic”) effects with minimal pathology; citations demonstrating beneficial effects of IH are listed in the lower right quadrant (indicated by blue shading). There is unlikely to be a clear division between protocols giving rise to pathogenic/beneficial effects since there is most likely a gradual transition (39); further, details of this transition may differ in detail among physiological systems. Representative literature concerning the range of IH protocols investigated are summarized in Figs. 2 (pathogenic) and 3 (beneficial).
Respiratory System
Sleep apnea.
Considerable progress has been made in recent decades toward an understanding of pathophysiology and pathogenesis resulting from obstructive and central sleep apnea. Central sleep apnea is often the product of an unstable ventilatory control system, at least in part, due to high chemoreflex responsiveness and variable apneic CO2 thresholds (261). On the other hand, obstructive sleep apnea (OSA) typically occurs in individuals whose narrowed upper airways and reduced upper airway muscle activity during sleep interact to cause airway collapse (222). Although problems with maintenance of upper airway patency result primarily in obstructive sleep apnea, ventilatory control instability can lead to either central or obstructive apnea depending on airway collapsibility.
IH elicits multiple forms of respiratory plasticity expressed as a long-lasting increase in: 1) phrenic nerve activity in anesthetized rats (phrenic long-term facilitation, pLTF) (9, 14); 2) tidal volume in unanesthetized rats (ventilatory long-term facilitation, vLTF) (160, 170); 3) upper airway muscle activity (e.g., hypoglossal long-term facilitation) (34, 200, 218); and 4) the short-term hypoxic (phrenic or) ventilatory response (154, 181). Some have suggested that these forms of respiratory plasticity are compensatory, and reduce the incidence of apneas (1, 128, 139, 199); in contrast, others have suggested that it can have the opposite impact (35, 174, 223). Undoubtedly, each form of plasticity listed above can be beneficial or detrimental depending on prevailing conditions.
The therapeutic potential of IH to treat sleep apnea is uncertain since it has the potential for both stabilizing and destabilizing influences on breathing in anesthetized rats (129) and humans (67). For instance, moderate IH protocols (10, 3-min episodes of 8% O2, 5-min intervals) decreased upper airway resistance in OSA patients (1, 218). On the other hand, this same protocol does not alter upper airway critical closing pressure in OSA patients (198), suggesting that changes in upper airway resistance and caliber can be dissociated from upper airway collapsibility. Chronic IH (5-min episodes of 11–12% O2, 5-min intervals, 12 h/night, 7 days) enhances the hypoxic ventilatory response (114, 192), which elicits beneficial effects on upper airway (UA) patency, yet diminishes breathing stability. An elevated hypoxic ventilatory response (HVR) increases UA dilating muscle activity, thereby decreasing UA resistance (10). On the other hand, an elevated HVR increases respiratory control system “loop gain”, potentially destabilizing breathing and (secondarily) upper airway stability. For example, an exaggerated HVR will cause undershoots in arterial CO2 (35), thereby, reducing ventilatory drive and UA dilator muscle activity. These effects increase the likelihood of subsequent airway collapse. High HVR levels are thought by many to destabilize breathing in OSA. In contrast, IH-induced vLTF might promote breathing stability by ensuring adequate ventilatory drive despite fluctuating CO2 levels near an apneic CO2 threshold (128). In patients with moderate OSA, acute intermittent hypoxia (AIH)-induced increases in the HVR (i.e., progressive augmentation) are greater in the morning, whereas vLTF is greater in the evening (67). If an exaggerated HVR is detrimental and vLTF is beneficial with respect to breathing stability, IH presentations may have greater therapeutic potential in the evening.
Early studies attempting to detect vLTF in humans were performed in poikilocapnic human subjects (i.e., uncontrolled CO2 levels). However, recent evidence shows that IH-induced vLTF is more prominent in individuals with slight, sustained hypercapnia (109, 223). Mateika and Syed (139) propose that moderate acute intermittent hypoxia combined with sustained hypercapnia and continuous positive airway pressure may have therapeutic benefits in sleep apnea patients. The positive airway pressure would maintain upper airway patency. The sustained hypercapnia would promote long-term facilitation in upper airway muscle activity, thereby increasing upper airway patency, as well as vLTF. In principle, the number of apneas would be reduced by sustained hypercapnia since it moves the prevailing arterial CO2 away from the CO2 apneic threshold. Additional work is needed to determine the efficacy and (subsequently) optimal IH protocol to maximize upper airway LTF and vLTF without the destabilizing influence of increased chemoreflex sensitivity in patients with sleep apnea.
Chronic obstructive pulmonary disease.
Chronic obstructive pulmonary disease (COPD) is a category of chronic irreversible lung diseases that includes emphysema, chronic bronchitis and asthma (186). IH training may be useful in patients suffering from COPD. Ukrainian and Russian researchers have used IH training to treat COPD and report beneficial effects, including improved clinical symptoms without unwanted side effects (215).
Randomized, double-blind and controlled clinical trials demonstrate that repetitive mild acute IH (3–5 min of 12–15% O2, 3–5 min normoxic intervals, 5–9 episodes/day, 15 days) elicits beneficial effects in COPD patients, including increased exercise time, baroreflex sensitivity, hypercapnic ventilatory response, total hemoglobin, forced expiratory volume in 1 s, and forced vital capacity (24, 79). However, longer hypoxic intervals in healthy subjects revealed no significant differences between IH-treated vs. control subjects. For example, normobaric hypoxia (3–10 h of continuous hypoxia 12–15% O2 for 7–20 consecutive days) revealed no difference in the hypoxic ventilatory response or ventilatory capacity among groups (97, 232). Similarly, with hypobaric intermittent hypoxia (4,000 or 5,500 m altitude, 3 h/day, 5 days/wk, 4 wk), there were no differences among groups in the HVR or cardiovascular function (61, 73). Accumulating evidence suggests that IH protocols with short hypoxic intervals (3–5 min) are more effective at increasing ventilatory capacity vs. prolonged hypoxic exposures (3–10 h). Despite scarce literature concerning moderate IH in COPD patients, available evidence is promising and raises hope that IH could be used as a complementary therapy with few adverse side effects.
Cardiovascular System
IH training has long been recognized by Russian physician/scientists as a therapeutic approach to prime patients to withstand the stress of diverse disease processes. Their rationale was that adaptations to one stress may increase resistance to another (145). Thereafter, IH training was recognized by the sports medicine community as a useful strategy to enhance aerobic exercise performance (62). For example, IH (2.5 min of 10.5% O2, 1.5-min intervals, 4 h), in combination with low-intensity exercise, improves blood oxygen transport capacity and aerobic endurance and induces altitude acclimatization (29, 98, 101, 194). In this case, “living high” and “training low” promote hematological adaptations that improve aerobic performance without eliciting adverse effects characteristic of more severe IH protocols [e.g., chronic intermittent hypoxia (CIH)].
From the opposite perspective, the association of hypertension and heart disease with OSA has fostered considerable interest in links between IH and cardiovascular disease. Accordingly, more severe and prolonged IH protocols that more closely simulate the IH experienced during OSA were developed. Such CIH protocols significantly increase blood pressure (21, 57, 111, 225), increase right ventricular heart mass (143), and are associated with pulmonary vascular remodeling and hypertension (162). Nonetheless, moderate IH protocols elicit beneficial cardiovascular effects in animal models and humans (17, 163, 213, 258, 260), suggesting an IH dose-response in its physiological impact.
Arterial hypertension.
During hypoxic episodes, chemoreceptor-mediated sympathetic activity increases heart rate, cardiac output, peripheral resistance, and systemic arterial pressure. However, different prolonged IH protocols produce divergent effects on post-IH systemic arterial blood pressure. The hypertensive effects of severe CIH, mimicking OSA, vs. depressor effects of modest IH exemplify this dichotomy. OSA imposes a series of brief, intense hypoxic episodes leading to persistent, maladaptive chemoreflex-mediated activation of the sympathetic nervous system, culminating in hypertension (107, 175, 182). Conversely, accumulating evidence in animal models and humans suggests that moderate IH conditioning may be safe and effective as a means of prevention and/or treatment for systemic hypertension (213, 217).
CIH in humans and rodents elevates blood pressure, and this effect outlasts the period of IH exposure (250). In the rodent studies, the IH dose impacts the magnitude of increased systemic blood pressure. Severe CIH protocols (60–120 episodes/h, 2–5% inspired oxygen, 14–35 consecutive days) increase mean arterial pressure by 9–16 mmHg (21, 57, 111, 225). Moderate CIH protocols (15–20 episodes/h, 6–10% inspired oxygen, 14–70 consecutive days) increase MAP by 12 mmHg (133). Yet milder protocols (10 episodes/h, 10% inspired oxygen, 7 consecutive days) increase MAP less than 2 mmHg in female rats (84). The greatest increases in blood pressure were observed in studies where hypocapnia was prevented via inspired carbon dioxide supplementation. To mimic the episodic asphyxiation imposed by OSA, McGuire and Bradford used combined hypoxia (6–8%) and hypercapnia (12–14%) for 15 s, interspersed with 15-s normoxia/normocapnia (8 h/day, 5 days/wk, 5 wk). This protocol increased diurnal mean systemic arterial pressure by 17 mmHg (142). In young, healthy humans, CIH (13% inspired oxygen, 30 episodes/h, 9 h/day, 14 consecutive days) increases the short-term HVR, blood hemoglobin concentration, and daytime blood pressure (228).
Chronic, severe intermittent hypoxia (57) persistently activates the sympathetic nervous system (55, 112, 182, 219, 220), as well as the renin-angiotensin system (56, 58), increasing blood pressure during apneic events and post-IH wakefulness. Since carotid chemoreceptor denervation prevents CIH-induced hypertension, chemoreceptor or central nervous system (CNS) chemoreflex plasticity underlies this response (57).
The therapeutic potential of IH to treat hypertension has been studied under hypobaric and normobaric conditions. Treatment sessions (30 min, 2–3 h/day, 10–30 days) at simulated altitudes of 1,500–3,500 m (13–17% inspired oxygen) significantly decreased arterial pressure in 60% of hypertensive patients (187). Although hypobaric and normobaric hypoxia elicit similar physiological responses, hypobaric protocols are poorly tolerated by humans (94, 193). Unwanted side effects of hypobaric hypoxia include headache, chest pain associated with insufficient blood flow to the heart, palpitations, and dizziness (53). Mechanisms differentiating normobaric vs. hypobaric hypoxia may include differences in ventilatory patterns, alveolar gas disequilibrium, and acute hypoxic ventilatory responses (193).
Normobaric hypoxia is a more practical way to elicit IH, since it is much simpler to decrease inspired oxygen fraction at atmospheric pressure. Similar to hypobaric IH, normobaric IH normalizes blood pressure in hypertensive patients (123, 213, 217). For example, moderate IH (10, 5 min cycles/day, 10–14% inspired O2, 5-min normoxic intervals) administered to 56 patients with stages I-II hypertension reduced systolic and diastolic blood pressure, heart rate, and peripheral resistance (158). A similar IH protocol (4–10, 3-min cycles/day, 10% inspired O2, 3-min normoxic intervals) decreased blood pressure in hypertensive patients to normotensive levels (123). Furthermore, IH proved safe in elderly patients, reducing clinical symptoms of angina, normalizing lipid metabolism, and microcirculation, and increasing maximal oxygen consumption and exercise tolerance (103).
The antihypertensive effects of moderate IH may arise from increased endothelial NO production (36, 68, 123, 132), which produces vasodilation and opening of reserve capillaries (decreasing peripheral resistance), reduced sympathetic activity (148, 184), minimized calcium overload of vascular smooth muscle (36), improved water and salt metabolism (18), increased antioxidant enzyme activity (7), and increased synthesis of angiogenic growth factors, including VEGF and FGF (248). Finally, moderate IH augments parasympathetic activity similar to altitude acclimatization (90, 190).
Although CIH elicits persistent hypertension similar to OSA (59), moderate IH protocols reduce blood pressure in hypertensive rodent models and humans (213). Again, a major reason for this divergence is that the cardiovascular response depends on the IH “dose”. IH protocols inducing systemic hypertension generally employed brief (6–30 s episodes) and severe (3–9% O2) hypoxemia, as well as prolonged (6–12 h/day) exposures (57, 178, 225). In comparison, moderate IH involves longer hypoxic episodes (45 s to several hours), less severe hypoxia (10–12% O2), and shorter protocol durations per day (1–2 h/day); such protocols do not increase systemic blood pressure in normal rats and actually reduce blood pressure in spontaneously hypertensive rats (213). Whereas “high-dose” CIH elicits sympathetic nervous system activation (31, 112, 182), increased oxidative stress (105, 233), systemic inflammation (5, 23, 202, 212), and persistent hypertension, moderate “low-dose” IH protocols minimally activate and/or dampen these same physiological responses. Such dose-dependent impact of IH on physiological functions likely accounts for many apparent disagreements in the literature, and suggest that “low-dose” IH may be harnessed for therapeutic benefit without invoking comorbidities characteristic of CIH. Indeed, the apparent lack of adverse side effects from low-dose IH that are often encountered with common antihypertensive drugs makes IH an interesting and novel therapeutic strategy to treat systemic hypertension. Before this concept can be advanced, details of the IH dose-response must be understood to optimize benefits while minimizing pathogenesis in each patient. The severity, frequency, and duration of IH episodes are key determinants of its physiological impact (Fig. 1).
Myocardial infarction.
Myocardial infarction remains the major cause of cardiovascular morbidity and mortality, despite advances in drug therapy and interventional procedures (196). The heart adapts to stress, such as brief ischemic episodes that enhance myocardial tolerance to subsequent ischemic incidents (159). Myocardial ischemic tolerance is also induced by IH preconditioning, which exerts cardioprotective effects. For instance, 24-h post-IH pretreatment (40 s of 10% O2, 20 s normoxic intervals, 30 min), rats exhibit reduced myocardial infarct size after global ischemia-reperfusion (17). Similarly, infarct size is decreased in dogs pretreated with IH preconditioning (5–10 min cycles of 9.5–10% inspired O2), as was ventricular tachycardia and fibrillation 24 h after occlusion of left anterior descending coronary artery (130). The same IH protocol in rats reduces cardiac arrhythmias during ischemia and decreases infarct size by 43% (131). Mechanisms suggested to explain these cardioprotective effects may involve β1 adrenergic receptor activation (130) and/or increased nitric oxide production (205).
In humans, moderate IH (5 min, 10–14% O2, 3-min intervals, 15 episodes/day, 3 wk) increases peak oxygen consumption in elderly men (50–70 years old), both with and without coronary artery disease (25). Moreover, during submaximal exercise (cycling at 1 W/kg), heart rate, systolic blood pressure, blood lactate concentration, and perceived exertion are diminished by IH (25). Myocardial protection correlates with the ability of moderate IH (2 min of 10% O2, 2-min intervals, 30 min) to increase myocardial vascularity, coronary blood flow, cardiomyoglobin, and antioxidant enzyme expression (266).
Ischemic tolerance of the heart can also be induced by long-term intermittent hypobaric hypoxia (6, 163, 257). This cardioprotection persists longer than normobaric ischemic preconditioning (20, 26, 164, 263) and is associated with fewer adverse side effects (e.g., right ventricular hypertrophy) vs. chronic sustained hypoxia (6, 92, 173, 256). For example, rats exposed to hypobaric IH (7,000 m altitude, 8 h/day, 35 days) exhibit significantly reduced infarction size and antiarrhythmic protection after 30 min of coronary artery occlusion (164). Cardiac protection by hypobaric IH has been linked to several mechanisms, including greater preservation of Ca2+ homeostasis (32), regulation of calcium/calmodulin-dependent protein kinase II activity (260), reduced myocardial apoptosis (47), and opening of mitochondrial ATP-sensitive potassium channels (102, 165).
Intermittent hypobaric hypoxia is not only protective, but also therapeutic with acute myocardial infarction in animal models, although doubts continue as to whether IH represents a safe technique in postmyocardial infarction patients (32, 45, 78, 165, 249). For instance, intermittent hypobaric hypoxia (404 mmHg, PaO2 ∼84 mmHg, 6 h/day, 14 days) improves postischemic recovery of myocardial contractile function by elevating reactive oxygen species (ROS) production during early reperfusion (249). Seven days after left anterior descending coronary artery ligation, rats exposed to similar protocols showed significantly reduced left ventricular dilation and improved cardiac performance (258). This effect was accompanied by attenuated infarct size, increased coronary blood flow, capillary density, and VEGF expression (258), as well as activation of genes increasing myocardial cell survival (171).
IH increases erythropoietin (EPO) concentrations, stimulating erythropoiesis (22, 136) and increasing hematocrit, blood viscosity, and platelet count (22). Elevated hematocrit increases the risk of ischemic stroke and myocardial infarction (117, 118). On the other hand, other studies found no evidence for alterations in the erythropoietic response with different IH protocols (96, 234). For example, 2-h daily of normobaric IH (13% O2) for 12 days shows no effect on morning plasma EPO concentration (66). Similarly, hypobaric IH at 3,000 m (14% oxygen equivalent) does not enhance erythropoiesis (166), whereas greater simulated altitudes (5,000–6,300 m; ∼7–8% oxygen equivalent) robustly increase several hematological variables (51, 241); thus, a dose-response relationship exists between the severity of hypoxia and erythropoiesis.
Despite abundant literature supporting cardioprotective and therapeutic effects of IH in myocardial infarction, clinical translation remains controversial, possibly due to inadequate ischemic/reperfusion models to simulate human patients (81). Nevertheless, IH appears promising as a therapeutic strategy for coronary heart disease due to its simplicity and long duration of action, with few demonstrated adverse effects (32, 183, 258). However, additional studies are needed to define the most effective IH dose to elicit optimum therapeutic outcomes with minimal patient risk.
Inflammatory/Immune Responses to IH
OSA elicits systemic inflammation (105, 201, 243), and markers of systemic inflammation correlate with cardiovascular disease in both OSA and non-OSA cohorts (105, 150). CIH is hypothesized to activate NF-κB-mediated inflammatory pathways (201), leading to increased expression of the inflammatory mediators TNF-α, IL-6, and c-reactive protein (CRP) (99, 105, 206). CIH-induced inflammation may cause endothelial dysfunction and injury, contributing to atherosclerosis associated with OSA (113). From this perspective, IH is deleterious because of its proinflammatory effects. On the other hand, some reports indicate minimal systemic inflammation in OSA patients (76, 100, 177). Differences in these studies may relate to the specific indicators of systemic inflammation studied, or differences in the effective IH dose in OSA patients.
In contrast to CIH (simulating aspects of OSA), studies using more moderate IH protocols found no evidence for systemic inflammation in rodent models (227). For instance, IH consisting of hypercapnic hypoxia (6 min of 8% O2, 7% CO2; 6 min normoxic/normocapnic intervals, 90 min) does not increase TNF-α or CRP in male piglets (227). Moreover, a single daily isocapnic hypoxia exposure (oxyhemoglobin saturation: 80% ∼ 48 mmHg PaO2, 1 h/day) for 10 consecutive days does not affect markers of systemic inflammation in healthy young men (185).
Inflammation elicited by OSA may relate to multiple factors beyond CIH per se, such as obesity or nocturnal arousal. For example, 12 wk of CIH (30 s 5% O2, 30-s intervals, 12 h/day, 12 wk), increased hepatic TNF-α gene expression only in mice fed a high-cholesterol diet (210). In OSA patients, CRP levels are significantly correlated with the body mass index, esophageal pressures, hip/waist ratio and neck circumference (76). Inflammation may also relate to the frequent arousals experienced by OSA patients (146). Thus, mechanisms of inflammation in OSA patients require further investigation.
Of considerable interest is that some studies suggest that moderate IH protocols actually enhance the innate immune system, while having an overall anti-inflammatory effect. For example, in healthy humans, exposure to 4, 5-min episodes of 10% O2 (5-min room-air intervals, 14 days) augments phagocytic and bactericidal activities of neutrophils, while suppressing proinflammatory mediators such as TNF-α and IL-4 by more than 90% (214). These responses, which persisted at least 7 days post-IH, may augment the body's immune defenses without attendant inflammation.
Together, these studies provide at least some empirical support for the idea that IH can have both proinflammatory and anti-inflammatory actions, depending on the IH dose. Thus, select IH protocols may have clinical applications in patients with ongoing systemic or neural inflammation. However, the potential of IH in immunotherapy has scarcely been explored. Further research is necessary to identify the optimum IH “dose” (e.g., severity of hypoxia, frequency of cycles) to enhance the innate immune system without triggering inflammation (or actually being anti-inflammatory).
Metabolic Responses to IH
According to World Health Organization criteria, metabolic syndrome consists of obesity with associated diabetes mellitus, impaired glucose tolerance, altered fasting glucose levels, and/or insulin resistance (255a). Metabolic syndrome is a chronic, multifactorial disorder resulting from complex interactions between genotype, environment, and physical activity patterns (134). A significant percentage of the obese population suffers from OSA (140), and it has been suggested that prolonged CIH contributes to metabolic syndrome in individuals with OSA. In animal models, typical CIH protocols (40 s 6% O2, 40-s normoxia, 8 h/day, 35 days) alter metabolic hormones; elicit pancreatic β-cell injury (49); and increase sympathetic activation (268), systemic inflammation (4), and levels of the appetite stimulant, neuropeptide Y (230). Collectively, these changes increase food intake, leading to obesity, hypertension, and insulin resistance (189).
In contrast, more moderate IH protocols are reported to have beneficial effects on metabolism, including reduced body weight, cholesterol, and blood sugar levels, as well as increased insulin sensitivity. For example, IH (10–12% O2, 3 times per week, 3–6 wk) with/without physical training (20-min strength-resistance exercises and 30 min high-intensity aerobic exercises) has been proposed as a means of losing weight and increasing aerobic capacity (240) without detrimental effects often associated with prolonged CIH protocols. This method induces physiological adaptations that enhance athletic performance (105, 252).
Mechanisms of moderate IH-induced weight loss may include increased serotonin and/or leptin levels. Acute hypoxia in both humans and rats increases blood serotonin levels, although this finding is strictly correlative (72). Food intake, protein intake, carbohydrate selection, and body weight are all at least partially regulated by serotonin, a molecule known to produce anorexia in rats (72). Moderate IH also reduces body weight by increasing blood leptin concentrations and enhancing liver leptin expression (116). Leptin is a peptide hormone secreted primarily by white adipose tissue, acting on the hypothalamic metabolic control center to reduce energy intake, increase energy consumption, and reduce body fat composition (157). Differentiated human PAZ6 adipocytes cultured for 48 h in 6% oxygen increase leptin mRNA expression, leptin promoter activity, and leptin secretion by two- to three-fold (75). Interestingly, hypoxia (8% O2) for 3 h does not significantly alter in vivo leptin gene expression in rat adipose tissue; however, increased leptin mRNA levels were observed in liver, kidney, and lung tissue (147). Beyond its role in body weight regulation, leptin also plays key roles in inflammation, tissue repair, and angiogenesis; thus, peripheral leptin upregulation during hypoxia may play an important role in tissue repair (52).
Moderate hypoxia (14.6% inspired O2) reduces blood glucose and cholesterol levels (33, 116) and increases insulin sensitivity in subjects with Type 2 diabetes (127). Hypoxia also increases mitochondrial enzymatic activity, glycolysis, and fatty acid oxidation, but reduces cholesterol synthesis (116, 226). Prolonged moderate IH (12 h, 14% O2, 7 days/wk, 4 wk), with or without physical training, improves glucose tolerance and increases glucose transporter (GLUT-4) levels in rats (33). Hypoxia stimulates glucose disposal, independent of contractile activity in rodents (28, 33), isolated human muscle tissue (8), and patients with Type 2 diabetes (127). Further, exercise (60 min at 90% of lactate threshold) enhances the impact of moderate hypoxia on insulin sensitivity (127), suggesting that insulin signaling and insulin-dependent glucose transport are upregulated following hypoxic exercise (33).
Although moderate IH effects in metabolic diseases have not been fully explored, accumulating evidence suggests that moderate IH combined with exercise may help prevent and/or correct metabolic impairment associated with insulin resistance, obesity, and Type 2 diabetes.
Bone
Intermittent hypoxia has positive effects on bone tissue remodeling (77, 119). Rats exposed to IH (10 min, 13% O2, 10-min intervals, 4 h/day, 28 days) increase alkaline phosphatase activity in bone tissue (119), suggesting high osteoblast activity and new bone formation. Moreover, rats exposed to hypobaric IH (430 mmHg ∼ 34 mmHg PaO2; 5 h/day, 5 days/wk, 5 wk) show increased bone mineral density (77), an effect that may result from increased nitric oxide (NO) levels in IH-treated rats since increased bone mineral density was not observed in rats with NO synthase inhibition. High NO levels inhibit bone resorption by inhibiting osteoclast formation and resorptive function of mature osteoclasts (188). Thus, moderate IH protocols may restrain osteoclastic activity and/or stimulate osteoblastic activity, although potential mechanisms leading such effects remain unknown. Further studies are needed to assess IH effects on osteopenia and osteoporosis.
Nervous System
Learning and memory.
OSA causes neurocognitive and behavioral deficits (43). Similarly CIH protocols that simulate IH experienced in OSA cause multiple cognitive deficits in rodents. For instance, 14 days of CIH (90 s of 10% inspired O2, 90-s intervals, 12 h/day, 14 days) during the habitual sleep times of adult male rats reduced REM sleep and impaired hippocampus-dependent learning (74). Both rats and mice display cognitive deficits after CIH, consistent with impaired hippocampal and/or prefrontal cortex function (48, 176, 197, 224).
CIH also triggers hypersomnolence, a typical clinical complaint of OSA patients. CIH during the sleep cycle for 8 consecutive weeks hinders the ability to maintain wakefulness in mice, suggesting that CIH per se contributes to excessive daytime sleepiness in OSA patients (242). In humans, a randomized cross-over design trial demonstrated that CIH (1 min of 6% inspired O2, 1-min intervals, 6 h/day) negatively impacts spatial working memory in healthy young adults (30). High-cycle frequency vs. the cumulative duration of CIH protocol has a greater, detrimental impact on learning and memory.
CIH triggers neuronal apoptosis and cytoarchitectural disorganization in brain regions involved in learning and memory, such as the hippocampal CA1 subfield and the frontoparietal cortex (74). Increased apoptotic activity peaks after 48 h of CIH, and then slowly decreases to levels that remain above normoxic controls (74). CIH reduces N-methyl-d-Aspartate (NMDA) receptor density and the excitability of hippocampal CA1 neurons (179), thereby diminishing the ability of hippocampal neurons to express NMDA-dependent long-term potentiation, a neuronal correlate of memory formation (172). In contrast, moderate IH protocols do not elicit similar CNS pathology. Rats exposed to moderate IH (5 min of 10.5% inspired O2, 5-min normoxic intervals, 10 times/day) either daily for 7 days, or 3 times per week for 4 to 10 wk, show no signs of hippocampal apoptosis or astrogliosis (121, 208, 254).
Deleterious CIH effects may be more pronounced during development. Early-life CIH is associated with anomalous brain development (149, 211) and clinical conditions, such as schizophrenia (42, 191), cerebral palsy, and mental retardation (104). On the other hand, it appears that moderate IH in early life accelerates brain development, leading to greater learning capacity (122, 135, 216, 262). Increased learning capacity has been studied in rodent models, expressed as increased development of conditioned reflexes. Moderate neonatal IH in mice (10.8% O2, 4 h/day from birth to 4 wk of age) enhances performance in Morris water maze and 8-arm radial maze tasks (262). A similar protocol, but with milder hypoxia (16% O2), also enhanced spatial learning and memory in developing mice (122). IH-increased learning capacity is associated with increased brain DNA concentrations, increased neurogenesis (135), and increased expression of proteins involved in synaptic plasticity (122).
The discrepancy among studies seems to be related to differences in the severity of the hypoxic stimuli and the frequency of hypoxic episodes per day. Studies used to mimic aspects of OSA are not expected to have therapeutic benefits due to coincident CNS pathology. On the other hand, moderate IH protocols may enhance learning capacity in developing rodents; the impact of moderate repetitive IH in adults is not yet clear. Moderate repetitive IH protocols (3 times/wk, 10 wk) appear to be safe, offering the possibility that this paradigm may be useful as a therapeutic strategy to elicit functional recovery from motor impairment in multiple clinical conditions (see below).
Brain ischemia and stroke.
IH preconditioning is neuroprotective for subsequent ischemic injury (46, 221). For example, IH preconditioning (8% inspired O2, 4 h/day, 2 wk) reduces the size of infarction, inflammation, and increased blood-brain barrier permeability after 60-min of transient middle cerebral artery occlusion (MCAO) in mice (221). Changes in gene expression differ markedly between harmful ischemia and ischemic preconditioning. Preconditioning seems to attenuate the response to subsequent ischemic incidents, increasing the expression of genes involved in the suppression of metabolic pathways, immune responses, ion-channel activity, and blood coagulation (46).
Some have suggested that IH has therapeutic potential for chronic cerebral ischemia. Brain ischemia is characterized by reduced brain-derived neurotrophic factor (BDNF), diminished synapse formation, and impairments in learning and memory in rodents (50, 110, 238). Seven days post-MCAO in rats, moderate IH (12% O2, 4 h/day, 7 days beginning 7 days post-MCAO) rescues ischemia-induced spatial learning and memory impairment by inducing hippocampal neurogenesis, synaptogenesis, and BDNF expression (237). Moderate IH also reduces infarct size without increasing mortality. In contrast, the same IH protocol administered 1–2 days postischemia increases mortality (236), suggesting that IH may have therapeutic potential for chronic, not acute brain ischemia. Although moderate IH may reduce complications associated with chronic brain ischemia in rats, studies are required to support IH as a potential therapy for cerebral ischemia in humans. If proven to be safe and effective, repeated low-dose IH may confer long-term brain protection in subpopulations of individuals at identified risk for stroke.
Depression.
Since many depressed patients show partial or no response to antidepressants (54), new, more effective treatment options are needed. Preconditioning with mild IH has a preventive/therapeutic effect in rodent models of depression (203, 204). For example, IH preconditioning (10% O2, 2 h/day, 3 days) has an antidepressant effect in learned helplessness, a common model of depression, and returns behavioral and hormonal variables to control levels; this effect was not significantly different from standard antidepressant drugs (203, 204). IH also exerts therapeutic benefits with ongoing depression in rodent models, and in humans. Given the strong link between hippocampal neurogenesis and antidepressant activity (3, 207), and observations that IH enhances neurogenesis in vitro (93) and neuroprogenitor cell proliferation in vivo (264), IH may oppose depression by increasing neurogenesis.
Moderate IH (84 mmHg PaO2, 4 h/day, 14 days) produces antidepressant-like effects in multiple animal models, including the forced swimming test, chronic mild stress, and novelty-suppressed feeding (265). The latter study showed enhanced hippocampal cell proliferation, an effect that requires BDNF-tyrosine kinase (TrkB) signaling. Thus, neurogenic and antidepressant-like IH effects may involve BDNF.
Accumulating evidence supports the concept that moderate IH has protective and therapeutic benefits in human depression, although this evidence is not yet compelling. For example, IH (5 min of 10% inspired O2, 5-min intervals, 120 min/day, 4 wk) reduced depression symptoms in 71% of human patients (15). Further studies are required to understand the therapeutic potential of IH in cognitive/affective disorders.
Intermittent hypoxia induced-respiratory neuroplasticity.
Acute hypoxia increases carotid chemoreceptor activity, thereby increasing breathing in the manner of a classical negative feedback loop. This complex response reflects multiple distinct neural mechanisms operating in different time-domains (175). Factors that differentially elicit these distinct mechanisms include the pattern of hypoxia (intermittent vs. sustained), the duration of exposure (minutes to days), and the severity of the hypoxic stimulus (181). Poikilocapnic hypoxia masks the ventilatory response to hypoxia per se since it reflects stimulation due to hypoxia, offset by ventilatory inhibition from the resulting hypocapnia. Thus, the CO2 status must be considered in any study of ventilation during or after IH (19).
Different IH protocols elicit different cellular and network mechanisms of respiratory plasticity (14, 152, 153, 239). For example, moderate acute IH elicits a long-lasting (hours), serotonin-dependent increase in respiratory motor output known as respiratory long-term facilitation (LTF) (9). LTF appears unique to IH, since it is not evoked by continuous hypoxia of the same cumulative duration (14). Brief IH (3–10 consecutive hypoxic episodes) elicits sustained elevations in phrenic and hypoglossal nerve activity and ventilation in multiple species (9, 27, 60, 82, 137, 144, 154, 239).
Since intermittent carotid sinus nerve stimulation is sufficient to induce LTF (60, 82), it is an expression of central neural vs. peripheral respiratory plasticity. Further, since pLTF elicited by moderate acute IH is blocked by intraspinal administration of serotonin-receptor antagonists (without blocking hypoglossal LTF) (13), pLTF is to be a form of spinal motor plasticity. On the other hand, carotid chemoafferent neuron activation is not essential to observe a form of IH-induced pLTF since (surgical or functional) carotid denervation attenuates, but does not abolish, AIH-induced pLTF (16). However, this form of pLTF may reflect an alternate (serotonin-independent) pathway to long-lasting phrenic motor facilitation revealed once the dominant serotonin-dependent pathway has been eliminated. We have come to realize that multiple, distinct cellular cascades give rise to phenotypically similar, long-lasting phrenic motor facilitation under different conditions (37).
The correlate of pLTF in unanesthetized, spontaneously breathing animals is ventilatory long-term facilitation (vLTF) (160, 170). Although initial studies in humans raised questions about its existence during wakefulness (95, 138, 156), subsequent studies revealed both ventilatory and genioglossus LTF in humans during non-rapid eye movement sleep (35, 180) or in awake humans with modest (background) elevations in arterial carbon dioxide (80, 247).
In longer time domains, or with more severe hypoxic episodes, additional forms of plasticity are elicited by IH (153). For example, CIH (5-min episodes of 10–12% O2, 8–12 h/night, 7 days) elicits serotonin-dependent enhancement of 1) baseline phrenic nerve activity, 2) the short-term hypoxic phrenic response, and 3) phrenic LTF induced by acute intermittent hypoxia (114, 115). CIH-enhanced LTF represents a form of metaplasticity (i.e., the ability of prior experience to alter subsequent plasticity) (2, 153). CIH-induced metaplasticity represents a potential therapeutic advantage in restoring breathing capacity with clinical disorders that cause respiratory insufficiency [e.g., spinal injury, amyotrophic lateral sclerosis ALS)] (151). Unfortunately, CIH also elicits considerable morbidity, including hypertension, hippocampal apoptosis, and cognitive deficits, among others (111, 259, see above). More subtle protocols of repetitive acute intermittent hypoxia (rAIH) have been developed to elicit pLTF metaplasticity without the detrimental effects elicited by CIH. For example, rAIH (e.g., 10, 5-min episodes, 10.5% O2, 5-min intervals, 3 times per week for 10 wk or daily for 7 days) increases the expression of key molecules involved in AIH-induced phrenic LTF without signs of hippocampal apoptosis, astrogliosis, or systemic hypertension (121, 208, 254). Further, rAIH elicits pLTF metaplasticity, enhancing AIH-induced pLTF following pretreatment with daily AIH for 7 days (254), or AIH three times per week for 4 wk (124, 245). Thus, fewer hypoxic episodes per day (even with longer exposure durations) elicit pLTF metaplasticity without pathology. Such protocols have considerable therapeutic potential following, for example, cervical spinal injury (121, 231).
Activation of different cellular cascades within phrenic motor nuclei accounts for many differential effects of varied IH protocols. One hallmark of pLTF is its sensitivity to the pattern of hypoxia; specifically, intermittent, but not sustained, moderate hypoxia elicits pLTF (12). One key difference between intermittent and sustained hypoxia appears to be the level of ROS-dependent inhibition of okadaic acid-sensitive, serine threonine protein phosphatases (125, 253, 255). When ROS are scavenged (125) or their production is blocked (126), pLTF is blocked following moderate AIH. However, by inhibiting spinal serine/threonine protein phosphatases with okadaic acid after reducing ROS formation, pLTF is restored (125). Conversely, with moderate sustained hypoxia, serotonin-dependent phrenic LTF is revealed by spinal okadaic acid administration, suggesting that less ROS-dependent inhibition of the relevant phosphatases occurs with sustained hypoxia (255). In agreement with this hypothesis, spinal okadaic acid has no effect on pLTF following moderate AIH, suggesting that the relevant phosphatases have already been inhibited by some process unique to intermittent, but not sustained, hypoxia (253).
The severity of hypoxia within episodes is a key determinant of the specific cellular mechanisms giving rise to pLTF (167). For example, moderate AIH (3, 5-min hypoxic episodes; PaO2 35–45 mmHg; 5-min intervals) elicits pLTF by a mechanism that requires spinal serotonin type 2 (5-HT2) receptor activation (9, 13, 65), new synthesis of BDNF (11), and activation of its high-affinity receptor TrkB (11, 39), followed by ERK MAPK signaling (88). In contrast, more severe AIH (less than 30 mmHg O2) elicits pLTF by a distinct serotonin-independent mechanism that requires spinal adenosine 2A receptor activation (167). Spinal adenosine 2A (71) and 5-HT7 receptor activation (86) elicit phrenic motor facilitation (pMF, an increase in phrenic motor output elicited by receptor activation) by a mechanism that requires new synthesis of an immature TrkB isoform (not BDNF) and downstream signaling via phosphatidylinositol 3-kinase/protein kinase B (Akt) (not ERK) (71). In longer time domains (days), vascular endothelial growth factor (VEGF) or EPO-induced phrenic motor facilitation might play a role in longer time domains of IH, such as during/after CIH or even chronic sustained hypoxia (39). Spinal VEGF or EPO receptor activation triggers phrenic motor facilitation via mechanisms that require both ERK and Akt signaling (38, 41).
Another contributing factor to the pattern sensitivity of pLTF appears to be balanced cross-talk inhibition between the competing cellular cascades to pLTF described above (44). With moderate AIH, phrenic LTF occurs predominantly via the serotonin/BDNF/ERK-dependent Q pathway to phrenic motor facilitation (37). However, subthreshold activation of the adenosine/TrkB/Akt-dependent S pathway restrains pLTF in this condition via PKA-dependent cross talk inhibition (85, 87). At some level of hypoxemia, extracellular ATP/adenosine builds up enough to convert the system from serotonin-dependent (Q) to adenosine-dependent (S) facilitation (167). With moderate, sustained hypoxia, there appears to be sufficient balance between the serotonin- (Q) and adenosine-dependent (S) pathway activation to cancel each other via balanced cross-talk inhibition (44). The strongest evidence for this is that spinal pretreatment with an adenosine 2A receptor antagonist reveals serotonin-dependent phrenic LTF following moderate sustained hypoxia (44). Thus, a number of factors contribute to the pattern sensitivity of pLTF. The essential point from the perspective of this review is that these distinctions in the pattern and severity of hypoxia protocols are essential if we are to control the specific physiological outcomes for therapeutic benefit.
Collective evidence demonstrates that moderate repetitive AIH can be harnessed as a therapeutic tool to restore lost respiratory (and nonrespiratory) motor output in clinical disorders that cause respiratory insufficiency, such as amyotrophic lateral sclerosis (151, 168), spinal cord injury (121, 151), and sleep apnea (139), or that compromise breathing due to mechanical constraints such as COPD (215).
Breathing in amyotrophic lateral sclerosis.
ALS is a degenerative motor neuron disease, involving death of respiratory motor neurons (267). Patients with ALS invariably develop respiratory muscle weakness, and the most common cause of death is ventilatory failure (108). Transgenic rats overexpressing a mutated form of superoxide dismutase (SOD1G93A) have been studied extensively as an animal model of familial ALS. SOD1G93A mutants exhibit progressive motor neuron death and faithfully mimic important aspects of familial ALS in humans, including compromise of phrenic motor output (120, 161). Interestingly, although more than 60–80% of all phrenic motor neurons die at disease end-stage, phrenic motor output, only decreases between 40 and 50% (168), reflecting the onset of ventilatory failure. Nevertheless, despite major losses of intercostal motor neurons, the ability to increase tidal volume during maximal chemoreceptor stimulation is fully preserved (40). Thus, there is considerable intrinsic capacity to compensate for major losses of key respiratory motor neurons, thereby preserving the capacity to generate tidal volume until late in disease progression. The mechanisms underlying such spontaneous compensation are not yet known, but may reflect forms of plasticity similar to pLTF since surviving phrenic motor neurons at disease end-stage express high levels of BDNF and TrkB protein (209). Nevertheless, respiratory motor neuron death eventually overcomes the capacity for spontaneous compensation, leading to overt ventilatory failure (229).
We recently tested the hypothesis that IH-induced respiratory motor plasticity strengthens synaptic inputs to surviving motor neurons, thereby enhancing respiratory motor output and slowing progression to ventilatory failure in ALS. Indeed, at disease end stage, a single presentation of AIH (3, 5-min episodes, PaO2 35–45 mmHg, 5-min intervals) fully restores the capacity to increase phrenic motor output in anesthetized rats (168). Further studies are needed to understand the potential of repetitive AIH to preserve ventilatory capacity further into disease progression, hopefully improving the quality of life for patients with this devastating disease.
Breathing after spinal cord injury.
Respiratory complications are the leading causes of morbidity and mortality in patients with spinal cord injury (SCI), especially among cervical and higher-thoracic injuries (161a). There are few therapeutic options available after the acute, postinjury period, when mechanisms of spontaneous recovery are exhausted, and additional functional improvement is unlikely (141). Recent work in rodent models has demonstrated that repetitive AIH is a viable therapeutic approach to restore breathing capacity after cervical spinal hemisections (121).
Cervical spinal hemisection at C2 (C2HS) causes persistent deficits in the capacity to increase phrenic motor output (69) and tidal volume in rats (63, 121). In rats with C2HS, even a single presentation of AIH strengthens spinal synaptic pathways to phrenic motor neurons below the hemisection by activating serotonin-dependent neuroplasticity (63, 70). However, the capacity of AIH to induce crossed-spinal synaptic pathways to phrenic motor neurons below the injury is highly dependent on time postinjury. For example, following C2HS, AIH (3 episodes, 5 min at 11% O2, 5-min intervals) induces ipsilateral pLTF (>60 min post-AIH) at 8 wk postinjury, but not at 2 wk postinjury in Sprague-Dawley and Lewis rats (70). This increasing ability to elicit pLTF with time postinjury correlates with spontaneous restoration of serotonergic input to the phrenic motor nucleus below the injury (70). Thus, IH may be more effective at restoring respiratory function in patients with chronic (vs. acute) spinal injury, once descending serotonergic innervation has had sufficient time to recover below the injury to the greatest extent possible. Further, 2 wk post-C2HS, repeated AIH (10, 5-min episodes per day, 10.5% O2, 5-min intervals, 7 consecutive days beginning 7 days post-C2HS) increases the strength of crossed-spinal synaptic inputs to phrenic motor neurons and at least partially restores the capacity to increase tidal volume during maximal chemoreceptor stimulation in rats (121). This functional recovery is accompanied by increased expression of key proteins necessary for AIH-induced phrenic motor plasticity (121), without evidence for hippocampal cell death or reactive gliosis. Detailed mechanisms of rAIH-induced functional recovery are not yet fully explored.
A more aggressive CIH protocol in rats (72, 5-min episodes of 10.5% O2, 5-min intervals, 7 consecutive nights, beginning 7 days postinjury) also induces functional recovery of phrenic motor output and strengthens crossed spinal synaptic inputs to phrenic motor neurons below the injury (64). However, CIH is expected to elicit morbidities, such as systemic hypertension, CNS inflammation, and neuronal death. Since the less severe repetitive AIH elicits similar functional recovery without apparent morbidity (208), such “low-dose” IH protocols have greater clinical potential.
Limb function and walking after spinal cord injury.
Repetitive AIH also elicits sustained functional recovery of forelimb function in rats with partial C2HS (121). rAIH-induced functional improvement is accompanied by increased BDNF and TrkB levels in cervical (C7) motor nuclei innervating the forelimb (121). Although the detailed mechanisms of this functional recovery have not been verified, we suggested that the same serotonin-dependent mechanisms facilitate motor output in respiratory and nonrespiratory motor nuclei (39).
The use of IH to improve limb function in humans with incomplete, chronic SCI has also shown promising results. A single AIH presentation (15, 1-min episodes of 9% O2, 1-min intervals) in incomplete, chronic (>1 year) spinal cord injury patients [American Spinal Cord Injury Association Impairment Scale (AIS) C or D] increases the ability to voluntarily generate plantar flexion for at least 4 h post-AIH (235). Subsequently, in a randomized, double-blind, placebo-controlled, cross-over design study, the impact of repetitive AIH (15 episodes per day, 90 s of 9% O2, 60-s intervals, 5 consecutive days) combined with walking training was studied in 19 chronic, incomplete SCI patients (AIS D) (83). Daily AIH alone increased walking speed 18% 3 days after treatment (10-m walk test), whereas dAIH combined with walking training improved both walking speed and distance (38%) after 5 days and at 1 wk post-dAIH (83). Importantly, no changes in spasticity, heart rate, or cognitive function were noted after dAIH, suggesting that this moderate IH dose is relatively safe in humans.
General Discussion
Intermittent hypoxia has been a subject of considerable investigation from the viewpoint of its adverse and (less widely known) beneficial effects. Recent studies reveal that IH has varied effects on multiple systems and that the magnitude of these effects (and even the direction) depends on the IH dose. Relevant variables include 1) the severity of hypoxemia, 2) the duration of hypoxia, 3) the number of cycles/day, 4) the pattern of IH presentation (e.g., consecutive days vs. alternating days) and 5) the total protocol duration. Of these, the severity of hypoxia and the number of cycles per day appear to be most strikingly correlated with its qualitative effects (Fig. 1). With high cycle numbers and/or severe hypoxic episodes, pathogenesis is more common, although potentially beneficial effects are also observed (Fig. 2). With low cycle numbers per day and/or mild to moderate hypoxic episodes, apparently beneficial effects are more predominant (with considerably less pathology) (Fig. 3). Accumulating evidence suggests that low-dose IH has considerable therapeutic potential to treat multiple clinical conditions (Fig. 1).
Fig. 2.
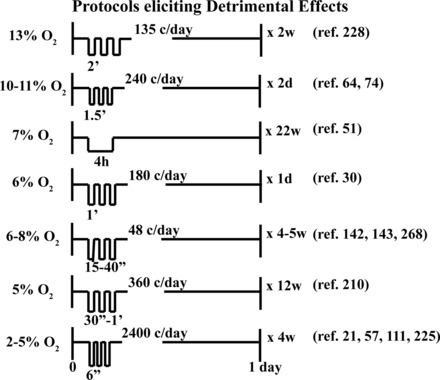
Selected IH protocols reported to elicit pathology. Each protocol depicts the effective IH “dose,” including the severity of hypoxia during each episode (inspired O2), the duration of hypoxic episodes (6 s to 12 h), the number of cycles per day (c/day), and the total time of exposure. Severe protocols (2–8% inspired O2, 48–2,400 c/day) have been shown to elicit detrimental effects in multiple physiological systems (see orange-to-red shading in Fig. 1).
Fig. 3.
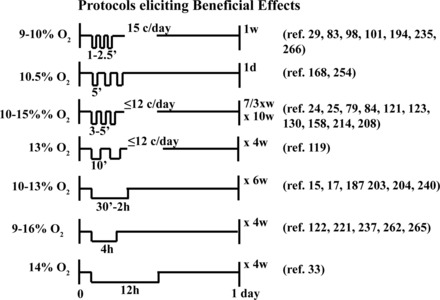
Selected IH protocols reported to elicit beneficial (potentially) therapeutic effects. Each protocol depicts the effective IH “dose,” including the severity of hypoxia during each episode (inspired O2), the duration of hypoxic episodes (6 s to 12 h), the number of cycles per day (c/day), and the total time of exposure. Moderate IH protocols consisting of 9–16% O2 and up to 15 cycles per day elicit beneficial effects in multiple physiological systems (blue shading in Fig. 1).
Detrimental effects of high-dose IH protocols (2–8% O2, 48–2,400 cycles/day) are observed in multiple systems and include systemic hypertension (21, 57, 111, 225), hypercholesterolemia (210), obesity, insulin resistance (189), increased sympathetic activation (268), pulmonary hypertension (142), cognitive deficits (30, 48, 74), and inflammation (4) (Fig. 1). In striking contrast, low-dose IH protocols (9–16% O2, 3–15 cycles/day) reduce arterial hypertension (213), strengthen innate immune responses, reduce inflammation (214), reduce body weight, increase aerobic capacity (240), improve glucose tolerance (33), increase bone mineral density (77), enhance spatial learning and memory (122, 262), rescue ischemia-induced memory impairment (237, 238), reduce symptoms of depression (15), improve postischemic recovery of myocardial contractile function (249), increase respiratory capacity in COPD (79), and increase respiratory and nonrespiratory somatic motor recovery following spinal injuries in rats and humans (83, 121, 235, 244) (Fig. 1). Moreover, repetitive low-dose IH has these benefits without detectable adverse consequences, such as hypertension (254), neuronal cell loss and/or reactive gliosis (121, 208), and systemic inflammation (185, 227).
Detrimental effects induced by high-dose IH often relate to increased oxidative stress and systemic inflammation. Repetitive hypoxia-reoxygenation is in some respects like repeated ischemia-reperfusion, and increases ROS formation (106). Increased ROS production will activate NF-κB and, hence, expression of NF-κB target genes, such as proinflammatory cytokines (e.g., TNF-α, IL-6, and ICAM-1) (106). Such inflammatory molecules have potential to cause cellular damage and endothelial dysfunction, with associated morbidities. In contrast, modest IH protocols do not cause inflammation in humans (214), and may, in fact, strengthen the innate immune system, while suppressing proinflammatory mediator production (214). Thus, there are multiple reasons to suggest that low-dose IH will be simple, safe, and effective in the treatment of diverse clinical disorders that affect multiple body systems. To optimize IH as a therapeutic approach to treat clinical disorders, a balance must be achieved between maximizing benefits (i.e., the highest IH dose possible) without pathology (i.e., not high enough to trigger pathogenesis). It is also important to understand conditions that may undermine the therapeutic efficacy of IH. For example, systemic inflammation (commonly present in SCI patients) undermines hippocampal synaptic plasticity (i.e., learning and memory), as well as spinal respiratory motor plasticity (89, 91, 246). By preventing this undermining effect, we may be able to maximize therapeutic benefits.
Perspectives and Significance
The potential applications of IH in health and pathological states are numerous, but require considerable research to develop protocols that optimize the balance between efficacy and safety in each physiological system. The benefits of this research may be considerable since low-dose IH appears to represent a simple, safe, nonpharmacological method to enhance normal physiological functions, or to restore lost functions (i.e., rehabilitation) in patients with diverse chronic clinical disorders.
GRANTS
This study was supported by National Institutes of Health Grants R01 HL-080209 and R37 HL-69064. A. Navarrete-Opazo was supported by a Fullbright Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.A.N.-O. and G.S.M. prepared figures; A.A.N.-O. drafted manuscript; A.A.N.-O. and G.S.M. edited and revised manuscript; A.A.N.-O. and G.S.M. approved final version of manuscript.
REFERENCES
- 1.Aboubakr SE, Taylor A, Ford R, Siddiqi S, Badr MS. Long-term facilitation in obstructive sleep apnea patients during NREM sleep. J Appl Physiol (1985) 91: 2751–2757, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci 19: 126–130, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 317: 819–823, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep 32: 447–470, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arter JL, Chi DSMG, Fitzgerald SM, Guha B, Krishnaswamy G. Obstructive sleep apnea, inflammation, and cardiopulmonary disease. Front Biosci 9: 2892–2900, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Asemu G, Papousek F, Ostadal B, Kolar F. Adaptation to high altitude hypoxia protects the rat heart against ischemia-induced arrhythmias. Involvement of mitochondrial KATP channel. J Mol Cell Cardiol 31: 1821–1831, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Asha Devi S, Subramanyam MV, Vani R, Jeevaratnam K. Adaptations of the antioxidant system in erythrocytes of trained adult rats: impact of intermittent hypobaric-hypoxia at two altitudes. Comp Biochem Physiol C Toxicol Pharmacol 140: 59–67, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Azevedo JL, Jr, Carey JO, Pories WJ, Morris PG, Dohm GL. Hypoxia stimulates glucose transport in insulin-resistant human skeletal muscle. Diabetes 44: 695–698, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Bach KB, Mitchell GS. Hypoxia-induced long-term facilitation of respiratory activity is serotonin dependent. Respir Physiol 104: 251–260, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Badr MS, Skatrud JB, Dempsey JA. Effect of chemoreceptor stimulation and inhibition on total pulmonary resistance in humans during NREM sleep. J Appl Physiol (1985) 76: 1682–1692, 1994. [DOI] [PubMed] [Google Scholar]
- 11.Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nat Neurosci 7: 48–55, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Baker-Herman TL, Mitchell GS. Determinants of frequency long-term facilitation following acute intermittent hypoxia in vagotomized rats. Respir Physiol Neurobiol 162: 8–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker-Herman TL, Mitchell GS. Phrenic long-term facilitation requires spinal serotonin receptor activation and protein synthesis. J Neurosci 22: 6239–6246, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker TL, Mitchell GS. Episodic but not continuous hypoxia elicits long-term facilitation of phrenic motor output in rats. J Physiol 529: 215–219, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Basovich SN. The role of hypoxia in mental development and in the treatment of mental disorders: a review. Biosci Trends 4: 288–296, 2010. [PubMed] [Google Scholar]
- 16.Bavis RW, Mitchell GS. Intermittent hypoxia induces phrenic long-term facilitation in carotid-denervated rats. J Appl Physiol (1985) 94: 399–409, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Beguin PC, Joyeux-Faure M, Godin-Ribuot D, Levy P, Ribuot C. Acute intermittent hypoxia improves rat myocardium tolerance to ischemia. J Appl Physiol (1985) 99: 1064–1069, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Behm R, Honig A, Griethe M, Schmidt M, Schneider P. Sustained suppression of voluntary sodium intake of spontaneously hypertensive rats (SHR) in hypobaric hypoxia. Biomed Biochim Acta 43: 975–985, 1984. [PubMed] [Google Scholar]
- 19.Bisgard G, Neubauer J. Peripheral and central effects of hypoxia. In: Regulation of Breathing, edited by Dempsey JA, and Pack A. New York: Marcel Dekker, 1995, p. 617–688. [Google Scholar]
- 20.Bolli R. The late phase of preconditioning. Circ Res 87: 972–983, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest 99: 106–109, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brugniaux JV, Pialoux V, Foster GE, Duggan CT, Eliasziw M, Hanly PJ, Poulin MJ. Effects of intermittent hypoxia on erythropoietin, soluble erythropoietin receptor and ventilation in humans. Eur Respir J 37: 880–887, 2011. [DOI] [PubMed] [Google Scholar]
- 23.Budhiraja R, Parthasarathy S, Quan SF. Endothelial dysfunction in obstructive sleep apnea. J Clin Sleep Med 3: 409–415, 2007. [PMC free article] [PubMed] [Google Scholar]
- 24.Burtscher M, Haider T, Domej W, Linser T, Gatterer H, Faulhaber M, Pocecco E, Ehrenburg I, Tkatchuk E, Koch R, Bernardi L. Intermittent hypoxia increases exercise tolerance in patients at risk for or with mild COPD. Respir Physiol Neurobiol 165: 97–103, 2009. [DOI] [PubMed] [Google Scholar]
- 25.Burtscher M, Pachinger O, Ehrenbourg I, Mitterbauer G, Faulhaber M, Puhringer R, Tkatchouk E. Intermittent hypoxia increases exercise tolerance in elderly men with and without coronary artery disease. Int J Cardiol 96: 247–254, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Cai Z, Manalo DJ, Wei G, Rodriguez ER, Fox-Talbot K, Lu H, Zweier JL, Semenza GL. Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108: 79–85, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia in conscious dogs. J Appl Physiol (1985) 73: 2083–2088, 1992. [DOI] [PubMed] [Google Scholar]
- 28.Cartee GD, Douen AG, Ramlal T, Klip A, Holloszy JO. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol (1985) 70: 1593–1600, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Casas M, Casas H, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Rodriguez FA, Viscor G. Intermittent hypobaric hypoxia induces altitude acclimation and improves the lactate threshold. Aviat Space Environ Med 71: 125–130, 2000. [PubMed] [Google Scholar]
- 30.Champod AS, Eskes GA, Foster GE, Hanly PJ, Pialoux V, Beaudin AE, Poulin MJ. Effects of acute intermittent hypoxia on working memory in young healthy adults. Am J Respir Crit Care Med 187: 1148–1150, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Chen L, Einbinder E, Zhang Q, Hasday J, Balke CW, Scharf SM. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med 172: 915–920, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Chiu LL, Chou SW, Cho YM, Ho HY, Ivy JL, Hunt D, Wang PS, Kuo CH. Effect of prolonged intermittent hypoxia and exercise training on glucose tolerance and muscle GLUT4 protein expression in rats. J Biomed Sci 11: 838–846, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhuri S, Pierchala L, Aboubakr SE, Shkoukani M, Badr MS. Long-term facilitation of genioglossus activity is present in normal humans during NREM sleep. Respir Physiol Neurobiol 160: 65–75, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhuri S, Shanidze I, Pierchala L, Belen D, Mateika JH, Badr MS. Effect of episodic hypoxia on the susceptibility to hypocapnic central apnea during NREM sleep. J Appl Physiol (1985) 108: 369–377, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen RA, Weisbrod RM, Gericke M, Yaghoubi M, Bierl C, Bolotina VM. Mechanism of nitric oxide-induced vasodilatation: refilling of intracellular stores by sarcoplasmic reticulum Ca2+ ATPase and inhibition of store-operated Ca2+ influx. Circ Res 84: 210–219, 1999. [DOI] [PubMed] [Google Scholar]
- 37.Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple pathways to long-lasting phrenic motor facilitation. Adv Exp Med Biol 669: 225–230, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dale-Nagle EA, Satriotomo I, Mitchell GS. Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dale EA, Ben Mabrouk F, Mitchell GS. Unexpected benefits of intermittent hypoxia: enhanced motor function. Physiology 29: 39–48, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dale EA, Nashold LJ, Mahamed S, Svendsen CN, Mitchell GS. Sustained ventilatory capacity in a rat model of amyotrophic. FASEB J 20: A1213, 2006. [Google Scholar]
- 41.Dale EA, Satriotomo I, Mitchell GS. Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Population-based case-control study. Br J Psychiatry 179: 403–408, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Decary A, Rouleau I, Montplaisir J. Cognitive deficits associated with sleep apnea syndrome: a proposed neuropsychological test battery. Sleep 23: 369–381, 2000. [PubMed] [Google Scholar]
- 44.Devinney MJ, Huxtable AG, Nichols NL, Mitchell GS. Hypoxia-induced phrenic long-term facilitation: emergent properties. Ann NY Acad Sci 1279: 143–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding HL, Zhu HF, Dong JW, Zhu WZ, Zhou ZN. Intermittent hypoxia protects the rat heart against ischemia/reperfusion injury by activating protein kinase C. Life Sci 75: 2587–2603, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol 8: 398–412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dong JW, Zhu HF, Zhu WZ, Ding HL, Ma TM, Zhou ZN. Intermittent hypoxia attenuates ischemia/reperfusion induced apoptosis in cardiac myocytes via regulating Bcl-2/Bax expression. Cell Res 13: 385–391, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Douglas RM, Miyasaka N, Takahashi K, Latuszek-Barrantes A, Haddad GG, Hetherington HP. Chronic intermittent but not constant hypoxia decreases NAA/Cr ratios in neonatal mouse hippocampus and thalamus. Am J Physiol Regul Integr Comp Physiol 292: R1254–R1259, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Drager LF, Jun JC, Polotsky VY. Metabolic consequences of intermittent hypoxia: relevance to obstructive sleep apnea. Best Pract Res Clin Endocrinol Metab 24: 843–851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enright LE, Zhang S, Murphy TH. Fine mapping of the spatial relationship between acute ischemia and dendritic structure indicates selective vulnerability of layer V neuron dendritic tufts within single neurons in vivo. J Cereb Blood Flow Metab 27: 1185–1200, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Esteva S, Pedret R, Fort N, Torrella JR, Pages T, Viscor G. Oxidative stress status in rats after intermittent exposure to hypobaric hypoxia. Wilderness Environ Med 21: 325–331, 2010. [DOI] [PubMed] [Google Scholar]
- 52.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation, and hematopoiesis. J Leukoc Biol 68: 437–446, 2000. [PubMed] [Google Scholar]
- 53.Farinelli CC, Kayser B, Binzoni T, Cerretelli P, Girardier L. Autonomic nervous control of heart rate at altitude (5050 m). Eur J Appl Physiol Occup Physiol 69: 502–507, 1994. [DOI] [PubMed] [Google Scholar]
- 54.Fava M. Augmentation and combination strategies in treatment-resistant depression. J Clin Psychiatry 62 Suppl 18: 4–11, 2001. [PubMed] [Google Scholar]
- 55.Fletcher EC. Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol (1985) 90: 1600–1605, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Fletcher EC, Bao G, Li R. Renin activity and blood pressure in response to chronic episodic hypoxia. Hypertension 34: 309–314, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Fletcher EC, Lesske J, Culman J, Miller CC, Unger T. Sympathetic denervation blocks blood pressure elevation in episodic hypoxia. Hypertension 20: 612–619, 1992. [DOI] [PubMed] [Google Scholar]
- 58.Foster GE, Hanly PJ, Ahmed SB, Beaudin AE, Pialoux V, Poulin MJ. Intermittent hypoxia increases arterial blood pressure in humans through a renin-angiotensin system-dependent mechanism. Hypertension 56: 369–377, 2010. [DOI] [PubMed] [Google Scholar]
- 59.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol 92: 51–65, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Fregosi RF, Mitchell GS. Long-term facilitation of inspiratory intercostal nerve activity following carotid sinus nerve stimulation in cats. J Physiol 477: 469–479, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fu Q, Townsend NE, Shiller SM, Martini ER, Okazaki K, Shibata S, Truijens MJ, Rodriguez FA, Gore CJ, Stray-Gundersen J, Levine BD. Intermittent hypobaric hypoxia exposure does not cause sustained alterations in autonomic control of blood pressure in young athletes. Am J Physiol Regul Integr Comp Physiol 292: R1977–R1984, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Fulco CS, Rock PB, Cymerman A. Improving athletic performance: is altitude residence or altitude training helpful? Aviat Space Environ Med 71: 162–171, 2000. [PubMed] [Google Scholar]
- 63.Fuller DD, Golder FJ, Olson EB, Jr, Mitchell GS. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol 100: 800–806, 2006. [DOI] [PubMed] [Google Scholar]
- 64.Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci 23: 2993–3000, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuller DD, Zabka AG, Baker TL, Mitchell GS. Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol (1985) 90: 2001–2006, 2001. [DOI] [PubMed] [Google Scholar]
- 66.Garcia N, Hopkins SR, Powell FL. Effects of intermittent hypoxia on the isocapnic hypoxic ventilatory response and erythropoiesis in humans. Respir Physiol 123: 39–49, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Gerst DG, 3rd, Yokhana SS, Carney LM, Lee DS, Badr MS, Qureshi T, Anthouard MN, Mateika JH. The hypoxic ventilatory response and ventilatory long-term facilitation are altered by time of day and repeated daily exposure to intermittent hypoxia. J Appl Physiol (1985) 110: 15–28, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giles TD. Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens 8: 2–16, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Golder FJ, Fuller DD, Davenport PW, Johnson RD, Reier PJ, Bolser DC. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci 23: 2494–2501, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci 25: 2925–2932, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Golder FJ, Ranganathan L, Satriotomo I, Hoffman M, Lovett-Barr MR, Watters JJ, Baker-Herman TL, Mitchell GS. Spinal adenosine A2a receptor activation elicits long-lasting phrenic motor facilitation. J Neurosci 28: 2033–2042, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gonzales GF. Blood levels of 5-hydroxytryptamine in human beings under several physiological situations. Life Sci 27: 647–650, 1980. [DOI] [PubMed] [Google Scholar]
- 73.Gore CJ, Rodriguez FA, Truijens MJ, Townsend NE, Stray-Gundersen J, Levine BD. Increased serum erythropoietin but not red cell production after 4 wk of intermittent hypobaric hypoxia (4,000–5,500 m). J Appl Physiol (1985) 101: 1386–1393, 2006. [DOI] [PubMed] [Google Scholar]
- 74.Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci 21: 2442–2450, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grosfeld A, Zilberfarb V, Turban S, Andre J, Guerre-Millo M, Issad T. Hypoxia increases leptin expression in human PAZ6 adipose cells. Diabetologia 45: 527–530, 2002. [DOI] [PubMed] [Google Scholar]
- 76.Guilleminault C, Kirisoglu C, Ohayon MM. C-reactive protein and sleep-disordered breathing. Sleep 27: 1507–1511, 2004. [DOI] [PubMed] [Google Scholar]
- 77.Guner I, Uzun DD, Yaman MO, Genc H, Gelisgen R, Korkmaz GG, Hallac M, Yelmen N, Sahin G, Karter Y, Simsek G. The effect of chronic long-term intermittent hypobaric hypoxia on bone mineral density in rats: role of nitric oxide. Biol Trace Elem Res 154: 262–267, 2013. [DOI] [PubMed] [Google Scholar]
- 78.Guo HC, Zhang Z, Zhang LN, Xiong C, Feng C, Liu Q, Liu X, Shi XL, Wang YL. Chronic intermittent hypobaric hypoxia protects the heart against ischemia/reperfusion injury through upregulation of antioxidant enzymes in adult guinea pigs. Acta Pharmacol Sin 30: 947–955, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haider T, Casucci G, Linser T, Faulhaber M, Gatterer H, Ott G, Linser A, Ehrenbourg I, Tkatchouk E, Burtscher M, Bernardi L. Interval hypoxic training improves autonomic cardiovascular and respiratory control in patients with mild chronic obstructive pulmonary disease. J Hypertens 27: 1648–1654, 2009. [DOI] [PubMed] [Google Scholar]
- 80.Harris DP, Balasubramaniam A, Badr MS, Mateika JH. Long-term facilitation of ventilation and genioglossus muscle activity is evident in the presence of elevated levels of carbon dioxide in awake humans. Am J Physiol Regul Integr Comp Physiol 291: R1111–R1119, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Hausenloy DJ, Baxter G, Bell R, Botker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM. Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol 105: 677–686, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hayashi F, Coles SK, Bach KB, Mitchell GS, McCrimmon DR. Time-dependent phrenic nerve responses to carotid afferent activation: intact vs. decerebellate rats. Am J Physiol Regul Integr Comp Physiol 265: R811–R819, 1993. [DOI] [PubMed] [Google Scholar]
- 83.Hayes HB, Jayaraman A, Herrmann M, Mitchell GS, Rymer WZ, Trumbower RD. Daily intermittent hypoxia enhances walking after chronic spinal cord injury: A randomized trial. Neurology 82: 104–113, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005. [DOI] [PubMed] [Google Scholar]
- 85.Hoffman MS, Golder FJ, Mahamed S, Mitchell GS. Spinal adenosine A2A receptor inhibition enhances phrenic long-term facilitation following acute intermittent hypoxia. J Physiol 588: 255–266, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptor activation induces long-lasting phrenic motor facilitation. J Physiol 589: 1397–1407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hoffman MS, Mitchell GS. Spinal 5-HT7 receptors and protein kinase A constrain intermittent hypoxia-induced phrenic long-term facilitation. Neuroscience 250: 632–643, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hoffman MS, Nichols NL, Macfarlane PM, Mitchell GS. Phrenic long-term facilitation after acute intermittent hypoxia requires spinal ERK activation but not TrkB synthesis. J Appl Physiol 113: 1184–1193, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hook MA, Huie JR, Grau JW. Peripheral inflammation undermines the plasticity of the isolated spinal cord. Behav Neurosci 122: 233–249, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hughson RL, Yamamoto Y, McCullough RE, Sutton JR, Reeves JT. Sympathetic and parasympathetic indicators of heart rate control at altitude studied by spectral analysis. J Appl Physiol (1985) 77: 2537–2542, 1994. [DOI] [PubMed] [Google Scholar]
- 91.Huxtable AG, Smith SM, Vinit S, Watters JJ, Mitchell GS. Systemic LPS induces spinal inflammatory gene expression and impairs phrenic long-term facilitation following acute intermittent hypoxia. J Appl Physiol 114: 879–887, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irlbeck M, Iwai T, Lerner T, Zimmer HG. Effects of angiotensin II receptor blockade on hypoxia-induced right ventricular hypertrophy in rats. J Mol Cell Cardiol 29: 2931–2939, 1997. [DOI] [PubMed] [Google Scholar]
- 93.Jin K, Mao XO, Sun Y, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest 110: 311–319, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnson M, Bemardi L, VonBargen O, Johnson E, Basnyat B. Sympatho-vagal imbalance during acute cardiovascular complications of high-altitude illness. High Altitude Med Biol 2 (111), 2001. [Google Scholar]
- 95.Jordan AS, Catcheside PG, O'Donoghue FJ, McEvoy RD. Long-term facilitation of ventilation is not present during wakefulness in healthy men or women. J Appl Physiol (1985) 93: 2129–2136, 2002. [DOI] [PubMed] [Google Scholar]
- 96.Julian CG, Gore CJ, Wilber RL, Daniels JT, Fredericson M, Stray-Gundersen J, Hahn AG, Parisotto R, Levine BD. Intermittent normobaric hypoxia does not alter performance or erythropoietic markers in highly trained distance runners. J Appl Physiol (1985) 96: 1800–1807, 2004. [DOI] [PubMed] [Google Scholar]
- 97.Katayama K, Ishida K, Iwasaki K, Miyamura M. Effect of two durations of short-term intermittent hypoxia on ventilatory chemosensitivity in humans. Eur J Appl Physiol 105: 815–821, 2009. [DOI] [PubMed] [Google Scholar]
- 98.Knaupp W, Khilnani S, Sherwood J, Scharf S, Steinberg H. Erythropoietin response to acute normobaric hypoxia in humans. J Appl Physiol (1985) 73: 837–840, 1992. [DOI] [PubMed] [Google Scholar]
- 99.Kofler S, Nickel T, Weis M. Role of cytokines in cardiovascular diseases: a focus on endothelial responses to inflammation. Clin Sci (1979) 108: 205–213, 2005. [DOI] [PubMed] [Google Scholar]
- 100.Kohler M, Ayers L, Pepperell JC, Packwood KL, Ferry B, Crosthwaite N, Craig S, Siccoli MM, Davies RJ, Stradling JR. Effects of continuous positive airway pressure on systemic inflammation in patients with moderate to severe obstructive sleep apnoea: a randomised controlled trial. Thorax 64: 67–73, 2009. [DOI] [PubMed] [Google Scholar]
- 101.Koistinen PO, Rusko H, Irjala K, Rajamaki A, Penttinen K, Sarparanta VP, Karpakka J, Leppaluoto J. EPO, red cells, and serum transferrin receptor in continuous and intermittent hypoxia. Med Sci Sports Exerc 32: 800–804, 2000. [DOI] [PubMed] [Google Scholar]
- 102.Kolar F, Neckar J, Ostadal B. MCC-134, a blocker of mitochondrial and opener of sarcolemmal ATP-sensitive K+ channels, abrogates cardioprotective effects of chronic hypoxia. Physiol Res 54: 467–471, 2005. [PubMed] [Google Scholar]
- 103.Korkushko OV, Shatilo VB, Ishchuk VA. [Effectiveness of intermittent normabaric hypoxic trainings in elderly patients with coronary artery disease] (in Russian). Adv Gerontol 23: 476–482, 2010. [PubMed] [Google Scholar]
- 104.Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol 2011: 609813, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lavie L. Obstructive sleep apnoea syndrome—an oxidative stress disorder. Sleep Med Rev 7: 35–51, 2003. [DOI] [PubMed] [Google Scholar]
- 106.Lavie L. Sleep-disordered breathing and cerebrovascular disease: a mechanistic approach. Neurol Clinics 23: 1059–1075, 2005. [DOI] [PubMed] [Google Scholar]
- 107.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. Br Med J 320: 479–482, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lechtzin N, Rothstein J, Clawson L, Diette GB, Wiener CM. Amyotrophic lateral sclerosis: evaluation and treatment of respiratory impairment. Amyotroph Lateral Scler Other Motor Neuron Disord 3: 5–13, 2002. [DOI] [PubMed] [Google Scholar]
- 109.Lee DS, Badr MS, Mateika JH. Progressive augmentation and ventilatory long-term facilitation are enhanced in sleep apnoea patients and are mitigated by antioxidant administration. J Physiol 587: 5451–5467, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lee TH, Yang JT, Kato H, Wu JH, Chen ST. Expression of brain-derived neurotrophic factor immunoreactivity and mRNA in the hippocampal CA1 and cortical areas after chronic ischemia in rats. J Neurosci Res 76: 705–712, 2004. [DOI] [PubMed] [Google Scholar]
- 111.Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hypertens 15: 1593–1603, 1997. [DOI] [PubMed] [Google Scholar]
- 112.Leuenberger UA, Brubaker D, Quraishi S, Hogeman CS, Imadojemu VA, Gray KS. Effects of intermittent hypoxia on sympathetic activity and blood pressure in humans. Auton Neurosci 121: 87–93, 2005. [DOI] [PubMed] [Google Scholar]
- 113.Libby P. Inflammation in atherosclerosis. Nature 420: 868–874, 2002. [DOI] [PubMed] [Google Scholar]
- 114.Ling L, Bach KB, Kinkead R, Olson EB, Mitchell GS. Enhancement of the hypoxic ventilatory responses following chronic intermittent hypoxia is serotonin dependent. FASEB J 13: 5381–5388,1999. [Google Scholar]
- 115.Ling L, Fuller DD, Bach KB, Kinkead R, Olson EB, Jr, Mitchell GS. Chronic intermittent hypoxia elicits serotonin-dependent plasticity in the central neural control of breathing. J Neurosci 21: 5381–5388, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ling Q, Sailan W, Ran J, Zhi S, Cen L, Yang X, Xiaoqun Q. The effect of intermittent hypoxia on bodyweight, serum glucose and cholesterol in obesity mice. Pak J Biol Sci 11: 869–875, 2008. [DOI] [PubMed] [Google Scholar]
- 117.Lippi G, Franchini M. Intermittent hypoxic training: doping or what? Eur J Appl Physiol 108: 411–412, 2010. [DOI] [PubMed] [Google Scholar]
- 118.Lippi G, Franchini M, Guidi GC. Prohibition of artificial hypoxic environments in sports: health risks rather than ethics. Appl Physiol Nutr Metab 32: 1206–1207; discussion 1208–1209, 2007. [DOI] [PubMed] [Google Scholar]
- 119.Litovka IH. [Alimentary and oxygen deprivation as the modulator of the bone tissue physiological remodelling rate in young rats]. Fiziol Zh 54: 85–93, 2008. [PubMed] [Google Scholar]
- 120.Llado J, Haenggeli C, Pardo A, Wong V, Benson L, Coccia C, Rothstein JD, Shefner JM, Maragakis NJ. Degeneration of respiratory motor neurons in the SOD1 G93A transgenic rat model of ALS. Neurobiol Dis 21: 110–118, 2006. [DOI] [PubMed] [Google Scholar]
- 121.Lovett-Barr MR, Satriotomo I, Muir GD, Wilkerson JE, Hoffman MS, Vinit S, Mitchell GS. Repetitive intermittent hypoxia induces respiratory and somatic motor recovery after chronic cervical spinal injury. J Neurosci 32: 3591–3600, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lu XJ, Chen XQ, Weng J, Zhang HY, Pak DT, Luo JH, Du JZ. Hippocampal spine-associated Rap-specific GTPase-activating protein induces enhancement of learning and memory in postnatally hypoxia-exposed mice. Neuroscience 162: 404–414, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lyamina NP, Lyamina SV, Senchiknin VN, Mallet RT, Downey HF, Manukhina EB. Normobaric hypoxia conditioning reduces blood pressure and normalizes nitric oxide synthesis in patients with arterial hypertension. J Hypertens 29: 2265–2272, 2011. [DOI] [PubMed] [Google Scholar]
- 124.MacFarlane P, Vinit S, Roopra A, Mitchell G. Enhanced phrenic long-term facilitation following repetitive acute intermittent hypoxia: role of glycolytic flux. FASEB J 24: 15, 2010. [Google Scholar]
- 125.MacFarlane PM, Mitchell GS. Respiratory long-term facilitation following intermittent hypoxia requires reactive oxygen species formation. Neuroscience 152: 189–197, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.MacFarlane PM, Satriotomo I, Windelborn JA, Mitchell GS. NADPH oxidase activity is necessary for acute intermittent hypoxia-induced phrenic long-term facilitation. J Physiol 587: 1931–1942, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mackenzie R, Maxwell N, Castle P, Brickley G, Watt P. Acute hypoxia and exercise improve insulin sensitivity (S(I) (2*)) in individuals with type 2 diabetes. Diabetes Metab Res Rev 27: 94–101, 2011. [DOI] [PubMed] [Google Scholar]
- 128.Mahamed S, Mitchell GS. Is there a link between intermittent hypoxia-induced respiratory plasticity and obstructive sleep apnoea? Exp Physiol 92: 27–37, 2007. [DOI] [PubMed] [Google Scholar]
- 129.Mahamed S, Mitchell GS. Respiratory long-term facilitation: too much or too little of a good thing? Adv Exp Med Biol 605: 224–227, 2008. [DOI] [PubMed] [Google Scholar]
- 130.Mallet RT, Ryou MG, Williams AG, Jr, Howard L, Downey HF. β1-Adrenergic receptor antagonism abrogates cardioprotective effects of intermittent hypoxia. Basic Res Cardiol 101: 436–446, 2006. [DOI] [PubMed] [Google Scholar]
- 131.Manukhina EB, Belkina LM, Terekhina OL, Abramochkin DV, Smirnova EA, Budanova OP, Mallet RT, Downey HF. Normobaric, intermittent hypoxia conditioning is cardio- and vasoprotective in rats. Exp Biol Med Maywood 238: 1413–1420, 2013. [DOI] [PubMed] [Google Scholar]
- 132.Manukhina EB, Jasti D, Vanin AF, Downey HF. Intermittent hypoxia conditioning prevents endothelial dysfunction and improves nitric oxide storage in spontaneously hypertensive rats. Exp Biol Med Maywood 236: 867–873, 2011. [DOI] [PubMed] [Google Scholar]
- 133.Marcus ML, Eckberg DL, Braxmeier JL, Abboud FM. Effects of intermittent pressure loading on the development of ventricular hypertrophy in the cat. Circ Res 40: 484–488, 1977. [DOI] [PubMed] [Google Scholar]
- 134.Marti A, Martinez-Gonzalez MA, Martinez JA. Interaction between genes and lifestyle factors on obesity. Proc Nutr Soc 67: 1–8, 2008. [DOI] [PubMed] [Google Scholar]
- 135.Martin N, Pourie G, Bossenmeyer-Pourie C, Jazi R, Gueant JL, Vert P, Daval JL. Conditioning-like brief neonatal hypoxia improves cognitive function and brain tissue properties with marked gender dimorphism in adult rats. Semin Perinatol 34: 193–200, 2010. [DOI] [PubMed] [Google Scholar]
- 136.Martinez-Bello VE, Sanchis-Gomar F, Nascimento AL, Pallardo FV, Ibanez-Sania S, Olaso-Gonzalez G, Calbet JA, Gomez-Cabrera MC, Vina J. Living at high altitude in combination with sea-level sprint training increases hematological parameters but does not improve performance in rats. Eur J Appl Physiol 111: 1147–1156, 2011. [DOI] [PubMed] [Google Scholar]
- 137.Mateika JH, Fregosi RF. Long-term facilitation of upper airway muscle activities in vagotomized and vagally intact cats. J Appl Physiol (1985) 82: 419–425, 1997. [DOI] [PubMed] [Google Scholar]
- 138.Mateika JH, Mendello C, Obeid D, Badr MS. Peripheral chemoreflex responsiveness is increased at elevated levels of carbon dioxide after episodic hypoxia in awake humans. J Appl Physiol (1985) 96: 1197–1205, 2004. [DOI] [PubMed] [Google Scholar]
- 139.Mateika JH, Syed Z. Intermittent hypoxia, respiratory plasticity and sleep apnea in humans: present knowledge and future investigations. Respir Physiol Neurobiol 188: 289–300, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McCallister JW, Adkins EJ, O'Brien JM., Jr Obesity and acute lung injury. Clin Chest Med 30: 495–508, viii, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McDonald JW, Becker D, Sadowsky CL, Jane JA, Sr, Conturo TE, Schultz LM, Conturo TE, Schultz LM. Late recovery following spinal cord injury Case report and review of the literature. J Neurosurg 97: 252–265, 2002. [DOI] [PubMed] [Google Scholar]
- 142.McGuire M, Bradford A. Chronic intermittent hypercapnic hypoxia increases pulmonary arterial pressure and haematocrit in rats. Eur Respir J 18: 279–285, 2001. [DOI] [PubMed] [Google Scholar]
- 143.McGuire M, Bradford A. Chronic intermittent hypoxia increases haematocrit and causes right ventricular hypertrophy in the rat. Respir Physiol 117: 53–58, 1999. [DOI] [PubMed] [Google Scholar]
- 144.McKay LC, Janczewski WA, Feldman JL. Episodic hypoxia evokes long-term facilitation of genioglossus muscle activity in neonatal rats. J Physiol 557: 13–18, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Meerson F, Pozharov V, Minyailenko T. Superresistance against hypoxia after preliminary adaptation to repeated stress. J Appl Physiol (1985) 76: 1856–1861, 1994. [DOI] [PubMed] [Google Scholar]
- 146.Meier-Ewert HK, Ridker PM, Rifai N, Regan MM, Price NJ, Dinges DF, Mullington JM. Effect of sleep loss on C-reactive protein, an inflammatory marker of cardiovascular risk. J Am Coll Cardiol 43: 678–683, 2004. [DOI] [PubMed] [Google Scholar]
- 147.Meissner U, Hanisch C, Ostreicher I, Knerr I, Hofbauer KH, Blum WF, Allabauer I, Rascher W, Dotsch J. Differential regulation of leptin synthesis in rats during short-term hypoxia and short-term carbon monoxide inhalation. Endocrinology 146: 215–220, 2005. [DOI] [PubMed] [Google Scholar]
- 148.Melin A, Fauchier L, Dubuis E, Obert P, Bonnet P. Heart rate variability in rats acclimatized to high altitude. High Alt Med Biol 4: 375–387, 2003. [DOI] [PubMed] [Google Scholar]
- 149.Ment LR, Schwartz M, Makuch RW, Stewart WB. Association of chronic sublethal hypoxia with ventriculomegaly in the developing rat brain. Brain Res Dev Brain Res 111: 197–203, 1998. [DOI] [PubMed] [Google Scholar]
- 150.Minoguchi K, Yokoe T, Tazaki T, Minoguchi H, Tanaka A, Oda N, Okada S, Ohta S, Naito H, Adachi M. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 172: 625–630, 2005. [DOI] [PubMed] [Google Scholar]
- 151.Mitchell G. Respiratory plasticity following intermittent hypoxia: a guide for novel therapeutic approaches to ventilatory control disorders. In: Genetic Basis for Respiratory Control Disorders, edited by Gaultier C. New York: Springer, 2007, p. 291–311. [Google Scholar]
- 152.Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB., Jr Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90: 2466–2475, 2001. [DOI] [PubMed] [Google Scholar]
- 153.Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol 94: 358–374, 2003. [DOI] [PubMed] [Google Scholar]
- 154.Mitchell GS, Powell FL, Hopkins SR, Milsom WK. Time domains of the hypoxic ventilatory response in awake ducks: episodic and continuous hypoxia. Respir Physiol 124: 117–128, 2001. [DOI] [PubMed] [Google Scholar]
- 155.Mitchell GS, Terada J. Should we standardize protocols and preparations used to study respiratory plasticity? Respir Physiol Neurobiol 177: 93–97, 2011. [DOI] [PubMed] [Google Scholar]
- 156.Morelli C, Badr MS, Mateika JH. Ventilatory responses to carbon dioxide at low and high levels of oxygen are elevated after episodic hypoxia in men compared with women. J Appl Physiol (1985) 97: 1673–1680, 2004. [DOI] [PubMed] [Google Scholar]
- 157.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature 443: 289–295, 2006. [DOI] [PubMed] [Google Scholar]
- 158.Mukharliamov F, Smirnova M, Bedritskii S, Liadov K. [Interval hypoxic training in arterial hypertension]. Vopr Kurortol Fizioter Lech Fiz Kult 2: 5–6, 2006. [PubMed] [Google Scholar]
- 159.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 74: 1124–1136, 1986. [DOI] [PubMed] [Google Scholar]
- 160.Nakamura A, Olson EB, Jr, Terada J, Wenninger JM, Bisgard GE, Mitchell GS. Sleep state dependence of ventilatory long-term facilitation following acute intermittent hypoxia in Lewis rats. J Appl Physiol (1985) 109: 323–331, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nashold LJ, Wilkerson JE, Satriotomo I, Dale EA, Svendsen CN, Mitchell GS. Phrenic, but not hypoglossal, motor output is diminished in a rat model of amyotrophic lateral sclerosis (ALS). FASEB J 20: A1212, 2006. [Google Scholar]
- 161a.National Spinal Cord Injury Statistical Center. Annual Report for the Model Spinal Cord Injury Care Systems. Birmingham, AL: National Spinal Cord Injury Statistical Center, 2005. [Google Scholar]
- 162.Nattie EE, Bartlett D, Jr, Johnson K. Pulmonary hypertension and right ventricular hypertrophy caused by intermittent hypoxia and hypercapnia in the rat. Am Rev Respir Dis 118: 653–658, 1978. [DOI] [PubMed] [Google Scholar]
- 163.Neckar J, Markova I, Novak F, Novakova O, Szarszoi O, Ost'adal B, Kolar F. Increased expression and altered subcellular distribution of PKC-delta in chronically hypoxic rat myocardium: involvement in cardioprotection. Am J Physiol Heart Circ Physiol 288: H1566–H1572, 2005. [DOI] [PubMed] [Google Scholar]
- 164.Neckar J, Ostadal B, Kolar F. Myocardial infarct size-limiting effect of chronic hypoxia persists for five weeks of normoxic recovery. Physiol Res 53: 621–628, 2004. [PubMed] [Google Scholar]
- 165.Neckar J, Szarszoi O, Koten L, Papousek F, Ost'adal B, Grover GJ, Kolar F. Effects of mitochondrial KATP modulators on cardioprotection induced by chronic high altitude hypoxia in rats. Cardiovasc Res 55: 567–575, 2002. [DOI] [PubMed] [Google Scholar]
- 166.Neya M, Enoki T, Kumai Y, Sugoh T, Kawahara T. The effects of nightly normobaric hypoxia and high intensity training under intermittent normobaric hypoxia on running economy and hemoglobin mass. J Appl Physiol (1985) 103: 828–834, 2007. [DOI] [PubMed] [Google Scholar]
- 167.Nichols NL, Dale EA, Mitchell GS. Severe acute intermittent hypoxia elicits phrenic long-term facilitation by a novel adenosine-dependent mechanism. J Appl Physiol (1985) 112: 1678–1688, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nichols NL, Gowing G, Satriotomo I, Nashold LJ, Dale EA, Suzuki M, Avalos P, Mulcrone PL, McHugh J, Svendsen CN, Mitchell GS. Intermittent hypoxia and stem cell implants preserve breathing capacity in a rodent model of amyotrophic lateral sclerosis. Am J Respir Crit Care Med 187: 535–542, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Olson EB, Jr, Bohne CJ, Dwinell MR, Podolsky A, Vidruk EH, Fuller DD, Powell FL, Mitchel GS. Ventilatory long-term facilitation in unanesthetized rats. J Appl Physiol (1985) 91: 709–716, 2001. [DOI] [PubMed] [Google Scholar]
- 171.Park AM, Nagase H, Vinod Kumar S, Suzuki YJ. Acute intermittent hypoxia activates myocardial cell survival signaling. Am J Physiol Heart Circ Physiol 292: H751–H757, 2007. [DOI] [PubMed] [Google Scholar]
- 172.Payne RS, Goldbart A, Gozal D, Schurr A. Effect of intermittent hypoxia on long-term potentiation in rat hippocampal slices. Brain Res 1029: 195–199, 2004. [DOI] [PubMed] [Google Scholar]
- 173.Pei JM, Kravtsov GM, Wu S, Das R, Fung ML, Wong TM. Calcium homeostasis in rat cardiomyocytes during chronic hypoxia: a time course study. Am J Physiol Cell Physiol 285: C1420–C1428, 2003. [DOI] [PubMed] [Google Scholar]
- 174.Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci USA 100: 10,073–10,078, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pepperell JC, Davies RJ, Stradling JR. Systemic hypertension and obstructive sleep apnoea. Sleep Med Rev 6: 157–173, 2002. [DOI] [PubMed] [Google Scholar]
- 176.Perry JC, D'Almeida V, Lima MM, Godoi FR, Vital MA, Oliveira MG, Tufik S. Intermittent hypoxia and sleep restriction: motor, cognitive and neurochemical alterations in rats. Behav Brain Res 189: 373–380, 2008. [DOI] [PubMed] [Google Scholar]
- 177.Phillips CL, Yang Q, Williams A, Roth M, Yee BJ, Hedner JA, Berend N, Grunstein RR. The effect of short-term withdrawal from continuous positive airway pressure therapy on sympathetic activity and markers of vascular inflammation in subjects with obstructive sleep apnoea. J Sleep Res 16: 217–225, 2007. [DOI] [PubMed] [Google Scholar]
- 178.Phillips SA, Olson EB, Morgan BJ, Lombard JH. Chronic intermittent hypoxia impairs endothelium-dependent dilation in rat cerebral and skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 286: H388–H393, 2004. [DOI] [PubMed] [Google Scholar]
- 179.Pichiule P, Chavez JC, Boero J, Arregui A. Chronic hypoxia induces modification of the N-methyl-d-aspartate receptor in rat brain. Neurosci Lett 218: 83–86, 1996. [DOI] [PubMed] [Google Scholar]
- 180.Pierchala LA, Mohammed AS, Grullon K, Mateika JH, Badr MS. Ventilatory long-term facilitation in non-snoring subjects during NREM sleep. Respir Physiol Neurobiol 160: 259–266, 2008. [DOI] [PubMed] [Google Scholar]
- 181.Powell FL, Milsom WK, Mitchell GS. Time domains of the hypoxic ventilatory response. Respir Physiol 112: 123–134, 1998. [DOI] [PubMed] [Google Scholar]
- 182.Prabhakar NR, Peng YJ, Jacono FJ, Kumar GK, Dick TE. Cardiovascular alterations by chronic intermittent hypoxia: importance of carotid body chemoreflexes. Clin Exp Pharmacol Physiol 32: 447–449, 2005. [DOI] [PubMed] [Google Scholar]
- 183.Przyklenk K, Whittaker P. Cardioprotection via adaptation to hypoxia: expanding the timeline and targets? Basic Res Cardiol 106: 325–328, 2011. [DOI] [PubMed] [Google Scholar]
- 184.Pshennikova M, Malyshev I, Manukhina E, Meerson F. Distinction between stress resistance and protective effects of adaptation in rats of different genetic strains: role of regulatory systems. In: Adaptation Biology and Medicine, edited by Hargens A, Takeda N, Singal P. New Delhi, India: Narosa, 2005, p. 29–40. [Google Scholar]
- 185.Querido JS, Sheel AW, Cheema R, Van Eeden S, Mulgrew AT, Ayas NT. Effects of 10 days of modest intermittent hypoxia on circulating measures of inflammation in healthy humans. Sleep Breathing 16: 657–662, 2012. [DOI] [PubMed] [Google Scholar]
- 186.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J, and Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176: 532–555, 2007. [DOI] [PubMed] [Google Scholar]
- 187.Rafibekova Z, Dzhumangulova A, Usubaliev N, Abramovich E. Treatment of hypertension disease by hypobaric barochamber hypoxia and middle altitudes. In: IX Congress of Therapeutists. Tashkent, 1987,p. 29–31. [Google Scholar]
- 188.Ralston SH, Ho LP, Helfrich MH, Grabowski PS, Johnston PW, Benjamin N. Nitric oxide: a cytokine-induced regulator of bone resorption. J Bone Miner Res 10: 1040–1049, 1995. [DOI] [PubMed] [Google Scholar]
- 189.Rasche K, Keller T, Tautz B, Hader C, Hergenc G, Antosiewicz J, Di Giulio C, Pokorski M. Obstructive sleep apnea and type 2 diabetes. Eur J Med Res 15 Suppl 2: 152–156, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Reeves J. Sympathetics and hypoxia: a brief overview. In: Hypoxia and Molecular Medicine, edited by Sutton JR, Houston C, Coates G. Burlington, VT: Queen City, 1993, p. 1–6. [Google Scholar]
- 191.Rehn AE, Van Den Buuse M, Copolov D, Briscoe T, Lambert G, Rees S. An animal model of chronic placental insufficiency: relevance to neurodevelopmental disorders including schizophrenia. Neuroscience 129: 381–391, 2004. [DOI] [PubMed] [Google Scholar]
- 192.Rey S, Del Rio R, Alcayaga J, Iturriaga R. Chronic intermittent hypoxia enhances cat chemosensory and ventilatory responses to hypoxia. J Physiol 560: 577–586, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Richard NA, Koehle MS. Differences in cardio-ventilatory responses to hypobaric and normobaric hypoxia: a review. Aviat Space Environ Med 83: 677–684, 2012. [DOI] [PubMed] [Google Scholar]
- 194.Rodriguez FA, Casas H, Casas M, Pages T, Rama R, Ricart A, Ventura JL, Ibanez J, Viscor G. Intermittent hypobaric hypoxia stimulates erythropoiesis and improves aerobic capacity. Med Sci Sports Exerc 31: 264–268, 1999. [DOI] [PubMed] [Google Scholar]
- 195.Roels B, Thomas C, Bentley DJ, Mercier J, Hayot M, Millet G. Effects of intermittent hypoxic training on amino and fatty acid oxidative combustion in human permeabilized muscle fibers. J Appl Physiol 102: 79–86, 2007. [DOI] [PubMed] [Google Scholar]
- 196.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117: e25–e146, 2008. [DOI] [PubMed] [Google Scholar]
- 197.Row BW, Kheirandish L, Cheng Y, Rowell PP, Gozal D. Impaired spatial working memory and altered choline acetyltransferase (CHAT) immunoreactivity and nicotinic receptor binding in rats exposed to intermittent hypoxia during sleep. Behav Brain Res 177: 308–314, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rowley JA, Deebajah I, Parikh S, Najar A, Saha R, Badr MS. The influence of episodic hypoxia on upper airway collapsibility in subjects with obstructive sleep apnea. J Appl Physiol (1985) 103: 911–916, 2007. [DOI] [PubMed] [Google Scholar]
- 199.Ryan S, Nolan P. Episodic hypoxia induces long-term facilitation of upper airway muscle activity in spontaneously breathing anaesthetized rats. J Physiol 587: 3329–3342, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ryan S, Nolan P. Long-term facilitation of upper airway muscle activity induced by episodic upper airway negative pressure and hypoxia in spontaneously breathing anaesthetized rats. J Physiol 587: 3343–3353, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-κB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med 174: 824–830, 2006. [DOI] [PubMed] [Google Scholar]
- 202.Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112: 2660–2667, 2005. [DOI] [PubMed] [Google Scholar]
- 203.Rybnikova E, Mironova V, Pivina S, Tulkova E, Ordyan N, Vataeva L, Vershinina E, Abritalin E, Kolchev A, Nalivaeva N, Turner AJ, Samoilov M. Antidepressant-like effects of mild hypoxia preconditioning in the learned helplessness model in rats. Neurosci Lett 417: 234–239, 2007. [DOI] [PubMed] [Google Scholar]
- 204.Rybnikova EA, Samoilov MO, Mironova VI, Tyul'kova EI, Pivina SG, Vataeva LA, Ordyan NE, Abritalin EY, Kolchev AI. The possible use of hypoxic preconditioning for the prophylaxis of post-stress depressive episodes. Neurosci Behav Physiol 38: 721–726, 2008. [DOI] [PubMed] [Google Scholar]
- 205.Ryou MG, Sun J, Oguayo KN, Manukhina EB, Downey HF, Mallet RT. Hypoxic conditioning suppresses nitric oxide production upon myocardial reperfusion. Exp Biol Med Maywood 233: 766–774, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saito M, Ishimitsu T, Minami J, Ono H, Ohrui M, Matsuoka H. Relations of plasma high-sensitivity C-reactive protein to traditional cardiovascular risk factors. Atherosclerosis 167: 73–79, 2003. [DOI] [PubMed] [Google Scholar]
- 207.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805–809, 2003. [DOI] [PubMed] [Google Scholar]
- 208.Satriotomo I, Dale EA, Dahlberg JM, Mitchell GS. Repetitive acute intermittent hypoxia increases expression of proteins associated with plasticity in the phrenic motor nucleus. Exp Neurol 237: 103–115, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Satriotomo I, Nashold LJ, Svendsen CN, Mitchell G. Enhancement of BDNF and serotonin terminal density in phrenic and hypoglossal motor nuclei in a rat model of amyotrophic lateral sclerosis (ALS). FASEB J 20: A1212, 2006. [Google Scholar]
- 210.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med 175: 1290–1297, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Scheepens A, Wassink G, Piersma MJ, Van de Berg WD, Blanco CE. A delayed increase in hippocampal proliferation following global asphyxia in the neonatal rat. Brain Res Dev Brain Res 142: 67–76, 2003. [DOI] [PubMed] [Google Scholar]
- 212.Selmi C, Montano N, Furlan R, Keen CL, Gershwin ME. Inflammation and oxidative stress in obstructive sleep apnea syndrome. Exp Biol Med Maywood 232: 1409–1413, 2007. [DOI] [PubMed] [Google Scholar]
- 213.Serebrovskaya TV, Manukhina EB, Smith ML, Downey HF, Mallet RT. Intermittent hypoxia: cause of or therapy for systemic hypertension? Exp Biol Med Maywood 233: 627–650, 2008. [DOI] [PubMed] [Google Scholar]
- 214.Serebrovskaya TV, Nikolsky IS, Nikolska VV, Mallet RT, Ishchuk VA. Intermittent hypoxia mobilizes hematopoietic progenitors and augments cellular and humoral elements of innate immunity in adult men. High Alt Med Biol 12: 243–252, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Serebrovskaya TV, Swanson RJ, Kolesnikova EE. Intermittent hypoxia: mechanisms of action and some applications to bronchial asthma treatment. J Physiol Pharmacol 54 Suppl 1: 35–41, 2003. [PubMed] [Google Scholar]
- 216.Shao G, Zhang R, Wang ZL, Gao CY, Huo X, Lu GW. Hypoxic preconditioning improves spatial cognitive ability in mice. Neuro-Signals 15: 314–321, 2006. [DOI] [PubMed] [Google Scholar]
- 217.Shatilo VB, Korkushko OV, Ischuk VA, Downey HF, Serebrovskaya TV. Effects of intermittent hypoxia training on exercise performance, hemodynamics, and ventilation in healthy senior men. High Alt Med Biol 9: 43–52, 2008. [DOI] [PubMed] [Google Scholar]
- 218.Shkoukani M, Babcock MA, Badr MS. Effect of episodic hypoxia on upper airway mechanics in humans during NREM sleep. J Appl Physiol (1985) 92: 2565–2570, 2002. [DOI] [PubMed] [Google Scholar]
- 219.Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121: 173–184, 2000. [DOI] [PubMed] [Google Scholar]
- 220.Smith ML, Niedermaier ON, Hardy SM, Decker MJ, Strohl KP. Role of hypoxemia in sleep apnea-induced sympathoexcitation. J Auton Nerv Syst 56: 184–190, 1996. [DOI] [PubMed] [Google Scholar]
- 221.Stowe AM, Altay T, Freie AB, Gidday JM. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol 69: 975–985, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Stradling JR, Davies Sleep RJ. 1. Obstructive sleep apnoea/hypopnoea syndrome: definitions, epidemiology, and natural history. Thorax 59: 73–78, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Syed Z, Lin HS, Mateika JH. The impact of arousal state, sex, and sleep apnea on the magnitude of progressive augmentation and ventilatory long-term facilitation. J Appl Physiol (1985) 114: 52–65, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tagaito Y, Polotsky VY, Campen MJ, Wilson JA, Balbir A, Smith PL, Schwartz AR, O'Donnell CP. A model of sleep-disordered breathing in the C57BL/6J mouse. J Appl Physiol (1985) 91: 2758–2766, 2001. [DOI] [PubMed] [Google Scholar]
- 225.Tahawi Z, Orolinova N, Joshua IG, Bader M, Fletcher EC. Altered vascular reactivity in arterioles of chronic intermittent hypoxic rats. J Appl Physiol 90: 2007–2013; discussion 2000, 2001. [DOI] [PubMed] [Google Scholar]
- 226.Takahashi H, Asano K, Nakayama H. Effect of endurance training under hypoxic condition on oxidative enzyme activity in rat skeletal muscle. J Physiol Anthropol 15: 111–114, 1996. [DOI] [PubMed] [Google Scholar]
- 227.Tam CS, Wong M, Tam K, Aouad L, Waters KA. The effect of acute intermittent hypercapnic hypoxia treatment on IL-6, TNF-α, and CRP levels in piglets. Sleep 30: 723–727, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37: 119–128, 2011. [DOI] [PubMed] [Google Scholar]
- 229.Tankersley CG, Haenggeli C, Rothstein JD. Respiratory impairment in a mouse model of amyotrophic lateral sclerosis. J Appl Physiol (1985) 102: 926–932, 2007. [DOI] [PubMed] [Google Scholar]
- 230.Tasali E, Van Cauter E. Sleep-disordered breathing and the current epidemic of obesity: consequence or contributing factor? Am J Respir Crit Care Med 165: 562–563, 2002. [DOI] [PubMed] [Google Scholar]
- 231.Tester NJ, Fuller DD, Fromm JS, Spiess MR, Behrman AL, Mateika JH. Long-term fcilitation of ventilation in humans with chronic spinal cord injury. Am J Respir Crit Care Med 189: 57–65, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Townsend NE, Gore CJ, Hahn AG, Aughey RJ, Clark SA, Kinsman TA, McKenna MJ, Hawley JA, Chow CM. Hypoxic ventilatory response is correlated with increased submaximal exercise ventilation after live high, train low. Eur J Appl Physiol 94: 207–215, 2005. [DOI] [PubMed] [Google Scholar]
- 233.Troncoso Brindeiro CM, da Silva AQ, Allahdadi KJ, Youngblood V, Kanagy NL. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol 293: H2971–H2976, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Truijens MJ, Toussaint HM, Dow J, Levine BD. Effect of high-intensity hypoxic training on sea-level swimming performances. J Appl Physiol (1985) 94: 733–743, 2003. [DOI] [PubMed] [Google Scholar]
- 235.Trumbower RD, Jayaraman A, Mitchell GS, Rymer WZ. Exposure to acute intermittent hypoxia augments somatic motor function in humans with incomplete spinal cord injury. Neurorehabil Neural Repair 26: 163–172, 2012. [DOI] [PubMed] [Google Scholar]
- 236.Tsai YW, Yang YR, Chen GH, Chang HC, Wang RY. The time window of intermittent hypoxia intervention after middle cerebral artery occlusion. Chin J Physiol 51: 324–328, 2008. [PubMed] [Google Scholar]
- 237.Tsai YW, Yang YR, Sun SH, Liang KC, Wang RY. Post ischemia intermittent hypoxia induces hippocampal neurogenesis and synaptic alterations and alleviates long-term memory impairment. J Cereb Blood Flow Metab 33: 764–773, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tsai YW, Yang YR, Wang PS, Wang RY. Intermittent hypoxia after transient focal ischemia induces hippocampal neurogenesis and c-Fos expression and reverses spatial memory deficits in rats. PloS One 6: e24001, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Turner DL, Mitchell GS. Long-term facilitation of ventilation following repeated hypoxic episodes in awake goats. J Physiol 499: 543–550, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Urdampilleta A, Gonzalez-Muniesa P, Portillo MP, Martinez JA. Usefulness of combining intermittent hypoxia and physical exercise in the treatment of obesity. J Physiol Biochem 68: 289–304, 2012. [DOI] [PubMed] [Google Scholar]
- 241.Vani R, Reddy CS, Asha Devi S. Oxidative stress in erythrocytes: a study on the effect of antioxidant mixtures during intermittent exposures to high altitude. Int J Biometeorol 54: 553–562, 2010. [DOI] [PubMed] [Google Scholar]
- 242.Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep 27: 194–201, 2004. [DOI] [PubMed] [Google Scholar]
- 243.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab 82: 1313–1316, 1997. [DOI] [PubMed] [Google Scholar]
- 244.Vinit S, Lovett-Barr MR, Mitchell GS. Intermittent hypoxia induces functional recovery following cervical spinal injury. Respir Physiol Neurobiol 169: 210–217, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Vinit S, MacFarlane PM, Satriotomo I, Mitchell GS. Enhanced phrenic long-term facilitation (pLTF) following repetitive acute intermittent hypoxia (Abstract). FASEB J 24: 799.–14., 2010.19897662 [Google Scholar]
- 246.Vinit S, Windelborn JA, Mitchell GS. Lipopolysaccharide attenuates phrenic long-term facilitation following acute intermittent hypoxia. Respir Physiol Neurobiol 176: 130–135, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Wadhwa H, Gradinaru C, Gates GJ, Badr MS, Mateika JH. Impact of intermittent hypoxia on long-term facilitation of minute ventilation and heart rate variability in men and women: do sex differences exist? J Appl Physiol (1985) 104: 1625–1633, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang JS, Chen LY, Fu LL, Chen ML, Wong MK. Effects of moderate and severe intermittent hypoxia on vascular endothelial function and haemodynamic control in sedentary men. Eur J Appl Physiol 100: 127–135, 2007. [DOI] [PubMed] [Google Scholar]
- 249.Wang ZH, Chen YX, Zhang CM, Wu L, Yu Z, Cai XL, Guan Y, Zhou ZN, Yang HT. Intermittent hypobaric hypoxia improves postischemic recovery of myocardial contractile function via redox signaling during early reperfusion. Am J Physiol Heart Circ Physiol 301: H1695–H1705, 2011. [DOI] [PubMed] [Google Scholar]
- 250.White SG, Fletcher EC, Miller CC., 3rd Acute systemic blood pressure elevation in obstructive and nonobstructive breath hold in primates. J Appl Physiol (1985) 79: 324–330, 1995. [DOI] [PubMed] [Google Scholar]
- 252.Wilber RL. Application of altitude/hypoxic training by elite athletes. Med Sci Sports Exerc 39: 1610–1624, 2007. [DOI] [PubMed] [Google Scholar]
- 253.Wilkerson JE, Macfarlane PM, Hoffman MS, Mitchell GS. Respiratory plasticity following intermittent hypoxia: roles of protein phosphatases and reactive oxygen species. Biochem Soc Trans 35: 1269–1272, 2007. [DOI] [PubMed] [Google Scholar]
- 254.Wilkerson JE, Mitchell GS. Daily intermittent hypoxia augments spinal BDNF levels, ERK phosphorylation and respiratory long-term facilitation. Exp Neurol 217: 116–123, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wilkerson JE, Satriotomo I, Baker-Herman TL, Watters JJ, Mitchell GS. Okadaic acid-sensitive protein phosphatases constrain phrenic long-term facilitation after sustained hypoxia. J Neurosci 28: 2949–2958, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255a.World Health Organization. Screening for Type 2 Diabetes. Report of the World Health Organizationa and the International Diabetes Federation Meeting. Geneva, Switzerland: WHO, 1999, p. 32–33. [Google Scholar]
- 256.Xie Y, Zhu WZ, Zhu Y, Chen L, Zhou ZN, Yang HT. Intermittent high altitude hypoxia protects the heart against lethal Ca2+ overload injury. Life Sci 76: 559–572, 2004. [DOI] [PubMed] [Google Scholar]
- 257.Xie Y, Zhu Y, Zhu WZ, Chen L, Zhou ZN, Yuan WJ, Yang HT. Role of dual-site phospholamban phosphorylation in intermittent hypoxia-induced cardioprotection against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 288: H2594–H2602, 2005. [DOI] [PubMed] [Google Scholar]
- 258.Xu WQ, Yu Z, Xie Y, Huang GQ, Shu XH, Zhu Y, Zhou ZN, Yang HT. Therapeutic effect of intermittent hypobaric hypoxia on myocardial infarction in rats. Basic Res Cardiol 106: 329–342, 2011. [DOI] [PubMed] [Google Scholar]
- 259.Yan B, Li L, Harden SW, Gozal D, Lin Y, Wead WB, Wurster RD, Cheng ZJ. Chronic intermittent hypoxia impairs heart rate responses to AMPA and NMDA and induces loss of glutamate receptor neurons in nucleus ambiguous of F344 rats. Am J Physiol Regul Integr Comp Physiol 296: R299–R308, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yu Z, Wang ZH, Yang HT. Calcium/calmodulin-dependent protein kinase II mediates cardioprotection of intermittent hypoxia against ischemic-reperfusion-induced cardiac dysfunction. Am J Physiol Heart Circ Physiol 297: H735–H742, 2009. [DOI] [PubMed] [Google Scholar]
- 261.Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc 5: 226–236, 2008. [DOI] [PubMed] [Google Scholar]
- 262.Zhang JX, Chen XQ, Du JZ, Chen QM, Zhu CY. Neonatal exposure to intermittent hypoxia enhances mice performance in water maze and 8-arm radial maze tasks. J Neurobiol 65: 72–84, 2005. [DOI] [PubMed] [Google Scholar]
- 263.Zhang Y, Zhong N, Zhu HF, Zhou ZN. [Antiarrhythmic and antioxidative effects of intermittent hypoxia exposure on rat myocardium]. Sheng Li Xue Bao 52: 89–92, 2000. [PubMed] [Google Scholar]
- 264.Zhu LL, Zhao T, Li HS, Zhao H, Wu LY, Ding AS, Fan WH, Fan M. Neurogenesis in the adult rat brain after intermittent hypoxia. Brain Res 1055: 1–6, 2005. [DOI] [PubMed] [Google Scholar]
- 265.Zhu XH, Yan HC, Zhang J, Qu HD, Qiu XS, Chen L, Li SJ, Cao X, Bean JC, Chen LH, Qin XH, Liu JH, Bai XC, Mei L, Gao TM. Intermittent hypoxia promotes hippocampal neurogenesis and produces antidepressant-like effects in adult rats. J Neurosci 30: 12653–12663, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Zhuang J, Zhou Z. Protective effects of intermittent hypoxic adaptation on myocardium and its mechanisms. Biol Signals Recept 8: 316–322, 1999. [DOI] [PubMed] [Google Scholar]
- 267.Zinman L, Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol 10: 481–490, 2011. [DOI] [PubMed] [Google Scholar]
- 268.Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol 92: 79–85, 2007. [DOI] [PubMed] [Google Scholar]


