Abstract
Vitamin D is obtained from cutaneous production when 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol) by ultraviolet B radiation or by oral intake of vitamin D2 (ergocalciferol) and D3. An individual's vitamin D status is best evaluated by measuring the circulating 25-hydroxyvitamin D [25(OH)D] concentration. Though controversy surrounds the definition of low vitamin D status, there is increasing agreement that the optimal circulating 25(OH)D level should be ~30-32 ng/ml or above. Using this definition, it has been is estimated that approximately three quarters of all adults in the United States are low. Classically, low vitamin D status has skeletal consequences such as osteomalacia/rickets. More recently, associations between low vitamin D status and increased risk for various non-skeletal morbidities have been recognized; whether all of these associations are causally related to low vitamin D status remains to be determined. To achieve optimal vitamin D status, daily intakes of at least 1000 IU or more of vitamin D are required. The risk of toxicity with “high” amounts of vitamin D intake is low. Substantial between-individual variability exists in response to the same administered vitamin D dose. When to monitor 25(OH)D levels has received little attention. Supplementation with vitamin D3 may be preferable to vitamin D2.
Keywords: Vitamin D, 25-hydroxyvitamin D, supplementation, deficiency, insufficiency
Introduction
Vitamin D is obtained either by ingestion or cutaneous production. When skin is exposed to ultraviolet B radiation, 7-dehydrocholesterol is converted to vitamin D3 (cholecalciferol). Dietary sources may provide either vitamin D3 or vitamin D2 (ergocalciferol),1, 2 however, few foods contain appreciable amounts of vitamin D; as such dietary intake is often low. Combining low intake with indoor lifestyle and sun-avoiding behaviors including sunscreen use, it is not surprising that low vitamin D status is endemic.3-6 The skeletal health consequences of vitamin D deficiency (calcium malabsorption and skeletal fragility) have long been recognized. More recently it has become appreciated that low vitamin D status leads to muscle weakness, falls and potentially a multitude of non-skeletal morbidities.7, 8 This review will consider the definition and prevalence, potential health consequences and approaches to correcting low vitamin D status.
Vitamin D Background and Assessment
Vitamin D must be metabolized to become physiologically active. Specifically, vitamin D (either D2 or D3) is converted to 25 hydroxyvitamin D [25(OH)D] in the liver and subsequently to the active or “hormonal” form, 1, 25-dihydroxyvitamin D [1, 25(OH)2D] in the kidneys.9 Measurement of 25(OH)D is the accepted indicator of an individual's vitamin D status.10 This is not intuitive as it would seem logical that measurement of the active form, 1, 25(OH)2D, would be the appropriate measure of an individual's vitamin D status. Anecdotally, it is not rare to see healthcare providers obtain measurement of 1, 25(OH)2D purportedly to evaluate an individual patient's vitamin D status. However, measurement of circulating 1, 25(OH)2D does not provide a useful assessment of an individual's vitamin D status as vitamin D deficiency leads to parathyroid hormone (PTH) elevation; which enhances renal 1-alpha hydroxylase activity thereby promoting conversion of available 25(OH)D to 1, 25(OH)2D. As 25(OH)D is present in much higher concentration than 1, 25(OH)2D (nanogram/ml vs. picogram/ml) given the enhanced conversion induced by PTH elevation, 1, 25(OH)2D may be normal even in the setting of low vitamin D status.
The clinical measurement of 25(OH)D has been problematic, with substantial variability present between laboratories.11, 12 It is not the purpose of this review to detail approaches to and challenges with vitamin D measurement; this topic is reviewed elsewhere in this volume. Suffice it to say that current evaluations find that clinical 25(OH)D measurement has improved13 allowing healthcare providers to have reasonable confidence in clinical 25(OH)D measurements. Moreover, the recent availability of standard reference materials from the National Institute of Standards and Technology seems destined to further improve between-laboratory agreement. However, despite 25(OH)D assay improvements, healthcare providers must appreciate that assay variability is present for all laboratory results. The analytical imprecision and inaccuracy present in all quantitative medical procedures is due to method, human and instrument limitations which confound application of rigid diagnostic cutpoint approaches. For example, if one were using a 25(OH)D value of 30 ng/ml to differentiate “low” from “optimal” vitamin D status, it must be recognized that a laboratory result of 29 ng/ml does not differ from 31 ng/ml.14
Low Vitamin D Status: Definition and Prevalence
A spectrum of vitamin D status has been proposed wherein individuals whose serum 25(OH)D value below approximately 10 ng/ml are classified as deficient and may sustain impaired bone mineralization (rickets/osteomalacia), while those below approximately 30 ng/ml are identified as insufficient (Figure 1) and may sustain long-term adverse health consequences.15 However, the cutpoint values selected, and even the verbiage to describe low vitamin D status, remain controversial. For example, terminology including deficiency, insufficiency, inadequacy and hypovitaminosis has been variously, and interchangeably, applied to describe low vitamin D status. To avoid what seems to be a non-productive debate, the terminology “low vitamin D status” will be used here. Moreover, as noted above, 25(OH)D assay variability and absence of accepted standards has confounded agreement on a single definition of “low.”
Figure 1. Spectrum of Vitamin D Status.

The spectrum of low vitamin D status is depicted. At very low vitamin D levels, (25[OH]D of approximately 10 ng/ml or below) calcium malabsorption, osteomalacia/rickets and myopathy occur. Less marked vitamin D deficiency (often referred to as inadequacy or insufficiency) has been associated with a variety of adverse health consequences. Consensus regarding an “optimal” 25(OH)D concentration continues to evolve, however there appears to be increasing agreement that values above approximately 30-32 ng/ml are associated with optimal physiologic function.
Recognizing that controversy exists, there appears to be increasing consensus that circulating 25(OH)D values below ~30-32 ng/ml indicate less than ideal vitamin D status.16 These cutpoint values were suggested based upon the long-established role of vitamin D to facilitate calcium and phosphorus absorption with deficiency leading to rickets/osteomalacia.17-19 Thus, less severe vitamin D “deficiency” appears to cause calcium malabsorption, leading to secondary hyperparathyroidism with resulting elevated bone turnover and ultimately bone loss.20 The 25(OH)D to PTH relationship has been extensively reported with various workers finding an apparent inflection point at ~20-30 ng/ml.16, 21 Moreover, some work demonstrates improved calcium absorption at 25(OH)D levels within what had previously been accepted at the “normal” range.22 However, others have challenged this seemingly cardinal tenant of low vitamin D status by reporting that calcium malabsorption does not occur until severe vitamin D deficiency is present, due to PTH mediated maintenance of 1, 25 dihydroxyvitamin D levels.23, 24 Finally, it has recently been suggested by Heaney and others that the point at which hepatic 25(OH)D production becomes zero-order could be used to define the lower end of normal vitamin D status.25 In this work, the authors found serum 25(OH)D to rapidly increase as circulating vitamin D3 (cholecalciferol) increased. When circulating vitamin D3 exceeds approximately 5.8 ng/ml, the hepatic 25-hydroxylase appears to become saturated and this reaction switches from first order to zero order. Taking this approach would define the lower limit of normal at approximately 35 ng/ml;25 obviously quite close to the 30-32 ng/ml suggested by other endpoints such as the relationship with PTH.
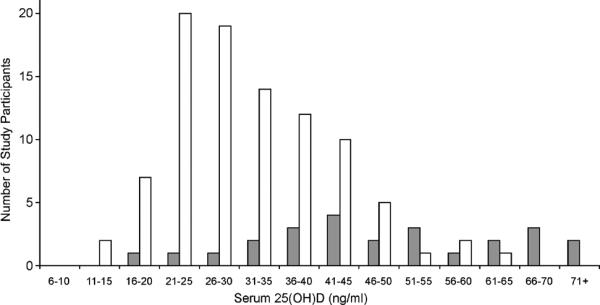
It is plausible that some of the debate surrounding what value defines “optimal” 25(OH)D status is being confounded by different levels for various tissues and endpoints, i.e., the cutpoint for various non-classical targets of vitamin D might vary from that for bone.26 Furthermore, it is possible/likely that the 25(OH)D value for “optimal” physiologic functioning might differ between individuals. Having a range of “normal” for virtually all clinically measured biological parameters is well known by clinicians. As vitamin D is, in essence, an endogenously produced hormone, it is not surprising that between individual variability and regulation would exist. In this regard, the skin of humans,27 and other animals,28 possesses the ability to regulate cholecalciferol production. Moreover, limited data suggest that variation in vitamin D degradation may exist in that differences in 24-hydroxylase capacity between individuals may be based on race.29 Data from our study of adults in Hawaii supports between individual differences despite abundant sun exposure.30 In fact, inspection of serum 25(OH)D concentration in that cohort reveals a virtually normal or Gaussian distribution (Figure 2). Indeed, other studies of highly UV-exposed adults31, 32 find some individuals with “low” 25(OH)D despite high UV exposure and a fairly broad range of what it would seem logical to define as “normal,” as noted in Figure 2. Thus, it seems likely that some of these people with “low” 25(OH)D levels could in fact be “optimal” for them. It is clearly accepted that a range exists for multiple physiologic functions that is considered “normal” for healthy adults; it is not known whether the same should apply to 25(OH)D.
Figure 2. Distribution of Serum 25(OH)D in Highly Sun-exposed Adults.
In these two studies in which the average total body sun exposure was approximately 11 hours per week, a broad, and somewhat Gaussian, distribution of circulating 25(OH)D is apparent. Data adapted from Binkley, et. al.,30 and Barger-Lux, et. al.31 Note that the Barger-Lux, et. al., study utilized a 25(OH)D assay that measures approximately 10% higher than the HPLC assay used in the Binkley, et. al., report.
Given the above, it is not surprising that an exact 25(OH)D cutpoint to define suboptimal vitamin D status remains somewhat controversial.33 Despite this, there is increasing agreement that values below approximately 30-32 ng/ml be identified as “low.”34, 35 When this cutpoint is applied, low vitamin D status is extremely common worldwide. For example, recent reports classify 52-77% of the studied cohorts as “low” using 30 ng/ml as a cutpoint.21, 36-38 Even the more restrictive cutpoint of < 20 ng/ml identifies 18-36% as “low” (Figure 3). It is not surprising that studies report a variable prevalence of low vitamin D status as the studied cohorts differ in age, sex, race, body mass index and dietary vitamin D intake. Though it is often assumed that some of the variable prevalence of low vitamin D reflects limited availability of sun exposure due to living at higher latitudes, it is of interest that a recent meta-analysis involving 394 studies comprising over 32,000 subjects found no influence of latitude on 25(OH)D concentration.39 It seems likely that the absence of an effect based on latitude reflects current human indoor lifestyles, clothing and sun-avoidance behavior. Moreover, it is probable that these factors are contributing to worsening population vitamin D status. Though measurement issues confound data interpretation, recent NHANES data report a decline in mean 25(OH)D concentration from the 1988-1994 data collection to that of 2000-2004.36, 40 In summary, despite variation in 25(OH)D methodology, cutpoint selected and cohort studied, it is clear that low vitamin D status is common worldwide.
Figure 3. Prevalence of Low Vitamin D Status in Various Populations.
In these recent cohort studies, low vitamin D status, whether defined as a 25(OH)D below 20 ng/ml or below 30 ng/ml is extremely common. Data adapted from various sources.21, 36-38
Low Vitamin D Status: Consequences
Bone
Low vitamin D status has long been associated with osteomalacia/rickets and a role in osteoporosis pathogenesis via calcium malabsorption and secondary hyperparathyroidism has more recently been suggested. Consistent with an important role of low vitamin D status in osteoporosis, recent meta-analyses find low 25(OH)D to be associated with higher fracture risk.41-43 Additionally, a dose effect was reported with greater vitamin D intakes and higher achieved 25(OH)D concentrations providing superior fracture reduction benefit.43 In summary, while one can debate the cutpoint, low vitamin D status leads to adverse bone consequences.
Muscle Function/Falls
Both genomic and non-genomic effects of vitamin D on muscle have been proposed.44, 45 Regardless of the mechanism, patients with osteomalacia due to vitamin D deficiency develop muscle pain and weakness that is improved with vitamin D therapy.46-48 Muscle biopsy in such people reveals atrophy of the fast twitch (type II) fibers. As type II fibers are first to be recruited to avoid falling, this observation may explain the increased falls risk in vitamin D deficient people.49 Importantly, randomized prospective studies find vitamin D to reduce falls risk by more than 20%.50 It seems likely that reducing falls contributes in a major way to the fracture reduction efficacy observed with vitamin D.51 Moreover, similar to the relationship observed with fracture, a higher vitamin D dose provides greater reduction in falls risk.52 The 25(OH)D concentration needed to optimize leg function has been explored with various cutpoints (e.g. 16-24 ng/ml) suggested.35, 53 A recent review finds 25(OH)D concentrations below ~16 ng/ml to be associated with substantially poorer leg function, but additionally finds values above ~36-40 ng/ml to be optimal.26
Cancer
Vitamin D has anti-proliferative and pro-differentiating effects on many cell types.54 It has been proposed that these effects are related to local production of 1, 25 dihydroxyvitamin D thus favorably impacting genes affecting cellular proliferation/differentiation and thereby reducing cancer risk.7 Consistent with this, an extensive, albeit largely associational, literature exists relating higher latitude, low vitamin D intake and/or less sunlight exposure to increased risk of, or mortality from, multiple types of cancer.55-59 Prospective trials of vitamin D supplementation with cancer as an endpoint are very limited; the Women's Health Initiative did not demonstrate a reduction in colon cancer risk, perhaps related to the low daily dose (400 IU) of vitamin D used.60 However, a smaller prospective study of postmenopausal women found calcium plus vitamin D3 (1,100 IU daily) to reduce overall cancer risk by ~60%.61 To summarize, physiologically logical hypotheses, observational data and one small randomized trial find low vitamin D status to be associated with higher cancer risk. Additional prospective studies are needed.
Other Conditions
It is likely that vitamin D has immune modulating effects. It has long been recognized that vitamin D deficiency is associated with respiratory infections which perhaps contributed to the use of cod liver oil in anti-tuberculous therapy.62, 63 More recently, it has become appreciated in that calcitriol enhances monocyte mycobacterial killing, likely by facilitating production of the antimicrobial protein, cathelicidin.64 Moreover, helper type 1 and 2 cells are vitamin D targets with vitamin D causing a shift towards an anti-inflammatory profile.65-68 Thus, it is not surprising that low vitamin D status is associated with an increased risk of autoimmune and potentially, infectious diseases.69-71 Additionally, inflammation is increasingly being recognized in as a contributor to the pathogenesis of various diseases and vitamin D modulates inflammatory cytokine production.72-74
It has been suggested that endemic low vitamin D status is contributing to the increased prevalence of diabetes mellitus. Multiple potential mechanisms have been proposed including vitamin D increasing insulin production/secretion.75-77 Additionally, observational studies associate low vitamin D status with diabetes type 1 and type 2.78-80 Prospective studies of vitamin D supplementation are clearly indicated, however on the whole it appears that low vitamin D status impairs glucose metabolism.78
Observational studies report an association between low vitamin D status and cardiovascular disease. 81-85 Potential mechanisms include a vitamin D effect on the endothelium,86 vascular smooth muscle87, 88 and/or cardiomyocytes;89 all of which possess the vitamin D receptor. Prospective studies to further evaluate this reported association are needed.
In summary, low vitamin D status has been associated with a variety of diseases and biologically plausible hypotheses exist to suggest a possible causal role. However, until confirmed by randomized studies, it is wise to be cautious and recognize that association does not prove causation.
When Should Vitamin D Status Be Assessed?
Given the multitude of potential adverse health consequences ascribed to low vitamin D status, it is not surprising that screening 25(OH)D measurement has been advocated.90, 91 Such screening may in fact be appropriate, if it becomes established that low vitamin D status contributes in a causal manner to the multiple adverse health outcomes, e.g., cardiovascular disease, diabetes, hypertension, etc., with which it is currently associated. However, in the absence of randomized trials documenting benefit for these varied outcomes, population based screening seems premature.
At this time, rather than advocating a population screening approach, it seems reasonable to measure 25(OH)D in those identified as being at high risk of vitamin D deficiency and those for whom a prompt musculoskeletal response to optimization of vitamin D status could be expected. Such groups of people include those with osteoporosis, a history of falls or high falls risk, malabsorption (e.g., celiac disease, radiation enteritis, bariatric surgery, etc.), individuals with liver disease and those requiring medications known to alter vitamin D status, e.g., certain anticonvulsants. Given the relationship of low vitamin D status with cancer, it also seems rational to measure 25(OH)D in those with malignancy.92
Alternatively, it could be argued that simple treatment of all individuals with vitamin D should be advocated, thereby making 25(OH)D measurement unnecessary. While this approach is attractive, it is unfortunately problematic in that no expert consensus exists regarding a recommended dose. For example, the National Osteoporosis Foundation recommends 800-1000 IU daily93 while some vitamin D experts suggests values over 2000 IU.94 Moreover, as discussed below, vitamins D2 and D3 appear to not be equally potent in maintaining 25(OH)D.95, 96 As such, daily intake of 1,000 IU of vitamin D2 may well not be equal to 1,000 IU of vitamin D3. Additionally, vitamin D dosing may differ by age in that older adults likely require higher vitamin D intakes due to the lower capability of skin to produce vitamin D with advancing age.97 Similarly, clear differences exist between races with African Americans requiring higher intakes than Caucasian Americans; Hispanic individuals may have intermediate requirements.98 Some of these differences in required intake may reflect differences in cutaneous melanin content,99 however, other less well understood between individual differences in vitamin D absorption and subsequent metabolism may well play a role. In this regard, even among individuals of similar age and race/ethnicity, substantial between individual variability in response to equal vitamin D intake is noted (Figure 4). Thus, if a healthcare provider wishes to assure optimal vitamin D status in an individual patient, it is necessary to obtain a 25(OH)D measurement.
Figure 4. Variable Response to Daily Vitamin D3.
In these seven Caucasian older adults (age 66-88 years), all of whom started the study with a 25(OH)D level less than 30 ng/ml, the variable response to daily administration of 1,600 IU vitamin D3 is apparent.96
Approaches to Vitamin D Repletion/Supplementation
Increasing exposure to sunlight would be an effective and free approach to improving vitamin D status. However, this does not seem to be a viable approach given widespread sun-avoidance campaigns100-103 based on the association of UV exposure with skin cancer.104 Sun avoidance and sunscreen use 55, 100, 105, 106 reduce skin exposure to UV radiation and thereby reduce skin vitamin D production.2, 107 In the face of such pervasive and powerful efforts, advocating sun exposure as a population-based measure to improve vitamin D status faces grave obstacles. Despite this, exposure to sunlight in moderation, perhaps for 15 minutes prior to sunscreen application, seems reasonable and is free. It should be noted that due to differences in skin pigmentation, season, latitude, time of day of sun exposure and amount of body surface exposed, simple recommendations such as “15 minutes of sun on the hands and face” are overly simplistic and will not assure optimal vitamin D status in all people.
Higher dose vitamin D treatment approaches to the clinical correction of vitamin D deficiency, and when to monitor 25(OH)D status during and following vitamin D treatment/supplementation, have received surprisingly little attention. Various “high-dose” repletion approaches, e.g., 50,000 IU three times weekly, weekly or monthly have been evaluated.108-111 A recent evaluation of clinical approaches found vitamin D2 regimens using >600,000 IU administered over an average time of two months achieved 25(OH)D values above 30 ng/ml in 64% of patients with the highest value being 100 ng/ml.108 An additional clinical report of “high-dose” vitamin D2 (50,000 IU once weekly for up to three years) achieved a 25(OH)D level above 30 ng/ml in 23/24 patients with the highest reported value being 100 ng/ml.112
Maintenance of vitamin D status with daily doses from 1,000-4,000 IU have been studied. 96, 113, 114 As noted above, between individual variability exists. However, a reasonable clinical “rule of thumb” is that the addition of 1,000 IU of vitamin D3 daily can be expected to increase circulating 25(OH)D by approximately 10 ng/ml.
Though daily vitamin D supplementation is very inexpensive (approximately one dollar per month), available data finds daily vitamin D supplementation to be less effective than expected at increasing serum 25(OH)D status, simply due to failure to reliably take the supplements.115, 116 This is hardly surprising in that suboptimal adherence to prescribed therapies for a variety of conditions is well known to clinicians.117 However, based upon the increasing calcium intake of the US population over time,118 (perhaps related to widespread educational programs) it seems feasible that similar approaches to informing the public about health benefits of vitamin D supplementation could improve endemic low vitamin D status. An alternative, and highly viable approach, is increased availability of vitamin D fortified foods coupled with higher amounts of vitamin D per serving in such food.
How best to monitor 25(OH)D status in individuals receiving vitamin D therapy has not been systematically evaluated. However, as compliance/adherence with many daily therapies (including vitamins) is often poor, monitoring 25(OH)D status four to six months after initiating treatment in those at high risk (e.g., patients with osteomalacia, fragility fractures or high falls risk) seems reasonable. Repeat evaluation at earlier timepoints seems inappropriate at it takes 3-6 months for serum 25(OH)D to plateau following initiation of supplementation.
What is Vitamin D Toxicity?
Both clinicians and patients may express concern that the “high” amounts of vitamin D noted above will lead to toxicity. Clearly, huge doses of vitamin D do lead to hypercalcemia and hypercalciuria. However, there is no clear-cut definition of which serum 25(OH)D should be considered “toxic.” This has led to variability in the clinical reporting of 25(OH)D results, with some laboratories reporting possibly toxic levels as being >80 ng/ml while others include up to 100 ng/ml as being within the reference range. Such variability is not surprising as recent expert opinion suggests “The serum 25(OH)D concentration that is the threshold for vitamin D toxicity has not been established.”119 However, a review of the published vitamin D toxicity cases finds all reports of hypercalcemia due to vitamin D intoxication to be associated with 25(OH)D concentrations > 88 ng/ml.120
Regarding what constitutes a “high” 25(OH)D values, it seems reasonable that highly sun exposed individuals could be utilized to assist in the determination of “normal” vitamin D status.33 When such individuals are evaluated, it appears that the highest attainable 25(OH)D values from cutaneous production are in the 70-80 ng/ml range.30, 31 Thus, the current approach of reporting 80-100 ng/ml as the upper limit of normal seems appropriate.
Does the Effect of Vitamin D2 Differ From That of Vitamin D3?
Two chemically distinct forms of vitamin D exist; vitamin D3 (cholecalciferol) is a 27 carbon molecule whereas vitamin D2 (ergocalciferol) contains 28 carbons and differs from vitamin D3 by the presence of an additional methyl group and a double bond between carbons 22 and 23. While clear chemical differences exist, whether vitamin D2 and vitamin D3 are equally effective at increasing 25(OH)D and/or have equivalent physiologic effects remains unclear. Currently, these two forms are regarded as equal and interchangeable. However, some data suggest that vitamin D2 is less “potent” at maintaining serum 25(OH)D than is vitamin D3.95, 96, 121 While a recent report challenges this113 and found D2 and D3 to be equally effective, the vast majority of studies find vitamin D3 to be somewhat more potent. It seems probable that this reflects lower affinity of vitamin D2 for vitamin D binding protein in the circulation leading to more rapid clearance. As such, use of supplements containing vitamin D3, rather than vitamin D2, seems appropriate.122 It is unfortunate that vitamin D2 is the only high-dose prescription vitamin D preparation in the United States and some other countries. Despite the lower potency, use of high-dose vitamin D2 does increase circulating 25(OH)D concentration.
Conclusion
Low vitamin D status is extremely common worldwide due to low dietary intake and low skin production. Suboptimal vitamin D status contributes to many conditions, including osteomalacia/rickets, osteoporosis, falls and fractures. It is possible/likely that low vitamin D status increases risk for a multitude of other conditions. Though consensus does not exist, it appears that circulating 25(OH)D concentrations above 30-32 ng/ml are needed for optimal health. To achieve this, daily intakes of at least 1,000 IU of D3 daily are required and it is probable that substantially higher amounts are required to achieve such values on a population basis. It seems premature to recommend widespread screening 25(OH)D measurement. Targeted measurement in those at increased risk for vitamin D deficiency, and those most likely to have a prompt positive response to supplementation, is appropriate. Widespread optimization of vitamin D status likely will lead to prevention of many diseases with attendant reduction of morbidity, mortality and expense.
References
- 1.Holick MF. Environmental factors that influence the cutaneous production of vitamin D. Am J Clin Nutr. 1995;61:638S–645S. doi: 10.1093/ajcn/61.3.638S. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. The photobiology of vitamin D and its consequences for humans. Ann N Y Acad Sci. 1985;453:1–13. doi: 10.1111/j.1749-6632.1985.tb11793.x. [DOI] [PubMed] [Google Scholar]
- 3.Looker AC, Dawson-Hughes B, Calvo MS, et al. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 4.Rucker D, Allan JA, Fick GH, et al. Vitamin D insufficiency in a population of healthy western Canadians. Can Med Assoc J. 2002;166:1517–1524. [PMC free article] [PubMed] [Google Scholar]
- 5.Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988-1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- 6.Calvo MS, Whiting SJ. Prevalence of vitamin D insufficiency in Canada and the United States: Importance to Health status and efficacy of current food fortification and dietary supplement use. Nutr Rev. 2003;61:107–113. doi: 10.1301/nr.2003.marr.107-113. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D Deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Binkley N. Does low vitamin D status contribute to “age-related” morbidity? J Bone Miner Res. 2007;22:V55–V58. doi: 10.1359/jbmr.07s212. [DOI] [PubMed] [Google Scholar]
- 9.DeLuca HF. The vitamin D story: a collaborative effort of basic science and clinical medicine. FASEB J. 1988;2:224–236. [PubMed] [Google Scholar]
- 10.Standing Committee on the Scientific Evaluation of Dietary Reference Intakes Food and Nutrition Board, Institute of Medicine 1997 Dietary Reference Intakes for calcium phosphorus, magnesium, vitamin D and fluoride. National Academy Press; Washington, DC: [Google Scholar]
- 11.Lips P, Chapuy MC, Dawson-Hughes B, et al. An international comparison of serum 25-hydroxyvitamin D measurements. Osteoporos Int. 1999;9:394–397. doi: 10.1007/s001980050162. [DOI] [PubMed] [Google Scholar]
- 12.Binkley N, Krueger D, Cowgill C, et al. Assay variation confounds hypovitaminosis D diagnosis: A call for standardization. J Clin Endocrinol Metab. 2003;89:3152–3157. doi: 10.1210/jc.2003-031979. [DOI] [PubMed] [Google Scholar]
- 13.Binkley N. Vitamin D: Clinical Measurement and use. J Musculoskelet Neuronal Interact. 2006;6:338–340. [PubMed] [Google Scholar]
- 14.Binkley N, Krueger D, Engelke JA, et al. What is your patient's vitamin D status? Clinical consideration of variability in a 25(OH)D measurement. J Bone Miner Res. 2008;23(suppl 1):S351. [Google Scholar]
- 15.Heaney RP. Functional indices of vitamin D status and ramifications of vitamin D deficiency. Am J Clin Nutr. 2004;80(suppl):1706S–1709S. doi: 10.1093/ajcn/80.6.1706S. [DOI] [PubMed] [Google Scholar]
- 16.Dawson-Hughes B, Heaney RP, Holick MF, et al. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 17.McCollum EV, Simmonds N, E BJ, et al. An experimental demonstration of the existence of a vitamin which promotes calcium deposition. J Biol Chem. 1922;53:293–312. [PubMed] [Google Scholar]
- 18.Steenbock H. The induction of growth promoting and calcifying properties in a ration by exposure to light. Science. 1924;60:224–225. doi: 10.1126/science.60.1549.224. [DOI] [PubMed] [Google Scholar]
- 19.DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(suppl):1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- 20.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF, Siris ES, Binkley N, et al. Prevalence of Vitamin D Inadequacy Among Postmenopausal North American Women Receiving Osteoporosis Therapy. J Clin Endocrinol Metab. 2005;90:3215–3224. doi: 10.1210/jc.2004-2364. [DOI] [PubMed] [Google Scholar]
- 22.Heaney RP, Dowell MS, Hale CA, et al. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22:142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 23.Need AG, Nordin BEC. Misconceptions - Vitamin D insufficiency causes malabsorption of calcium. Bone. 2008;42:1021–1024. doi: 10.1016/j.bone.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Need AG, O'Loughlin PD, Morris HA, et al. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008;23:1859–1863. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 25.Heaney RP, Armas LAG, Shary JR, et al. 25-hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am J Clin Nutr. 2008;87:1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- 26.Bischoff-Ferrari HA, Giovannucci E, Willett WC, et al. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 27.Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: Skin pigment is not an essential regulator. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 28.Ferguson GW, Gehrmann WH, Karsten KB, et al. Ultraviolet exposure and vitamin D synthesis in a sun-dwelling and a shade-dwelling species of Anolis: Are there adaptations for lower ultraviolet B and dietary vitamin D3 availability in the shade? Physiol Biochem Zool. 2005;78:193–200. doi: 10.1086/427055. [DOI] [PubMed] [Google Scholar]
- 29.Awumey EMK, Mitra DA, Hollis BW, et al. Vitamin D Metabolism is Altered in Asian Indians in the Southern United States: A Clinical Research Center Study. J Clin Endocrinol Metab. 1998;83:169–173. doi: 10.1210/jcem.83.1.4514. [DOI] [PubMed] [Google Scholar]
- 30.Binkley N, Novotny R, Krueger D, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 31.Barger-Lux MJ, Heaney RP. Effects of above average summer sun exposure on serum 25-hydroxyvitamin D and calcium absorption. J Clin Endocrinol Metab. 2002;87:4952–4956. doi: 10.1210/jc.2002-020636. [DOI] [PubMed] [Google Scholar]
- 32.Tangpricha V, Turner A, Spina C, et al. Tanning is associated with optimal vitamin D status (serum 25-hydroxyvitamin D concentration) and higher bone mineral density. Am J Clin Nutr. 2004;80:1645–1649. doi: 10.1093/ajcn/80.6.1645. [DOI] [PubMed] [Google Scholar]
- 33.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 34.Hollis BW. Assessment of vitamin D status and definition of a normal circulating range of 25-hydroxyvitamin D. Current Opinion in Endocrinology, Diabetes and Obesity. 2008;15:489–494. doi: 10.1097/MED.0b013e328317ca6c. [DOI] [PubMed] [Google Scholar]
- 35.Kuchuk NO, Pluijm SMF, van Schoor NM, et al. Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older adults. J Clin Endocrinol Metab. 2009;94:1244–1250. doi: 10.1210/jc.2008-1832. [DOI] [PubMed] [Google Scholar]
- 36.Ginde AA, Liu MC, Camargo CA. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009;169:626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lips P, Hosking D, Lippuner K, et al. The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Intern Med. 2006;260:245–254. doi: 10.1111/j.1365-2796.2006.01685.x. [DOI] [PubMed] [Google Scholar]
- 38.Orwoll E, Nielson CM, Marshall LM, et al. Vitamin D deficiency in older men. J Clin Endocrinol Metab. 2009;94:1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hagenau T, Vest R, Gissel TN, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic meta-regression analysis. Osteoporos Int. 2009;20:133–140. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- 40.Looker AC, Pfeiffer CM, Lacher DA, et al. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008;88:1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cauley JA, La Croix A, Wu L, et al. Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med. 2008;149:242–250. doi: 10.7326/0003-4819-149-4-200808190-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: A meta-analysis of randomized controlled trials. JAMA. 2005;293:2257–2264. doi: 10.1001/jama.293.18.2257. [DOI] [PubMed] [Google Scholar]
- 43.Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Prevention of nonvertebral fractures with oral vitamin D and dose dependency. Arch Intern Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- 44.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- 45.Janssen HCJP Samson MM, Verhaar HJJ. Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002;75:611–615. doi: 10.1093/ajcn/75.4.611. [DOI] [PubMed] [Google Scholar]
- 46.Mingrone D, Greco AV, Castagneto M, et al. A woman who left her wheelchair. Lancet. 1999;353:806. doi: 10.1016/s0140-6736(98)10206-4. [DOI] [PubMed] [Google Scholar]
- 47.Skaria J, Katiyar BC, Srivastava TP, et al. Myopathy and neuropathy associated with osteomalacia. Acta Neurol Scand. 1975;51:37–58. doi: 10.1111/j.1600-0404.1975.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 48.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–424. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 49.Flicker L, Mead K, MacInnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51:1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 50.Bischoff-Ferrari HA, Dawson-Hughes B, Willett WC, et al. Effect of vitamin D on falls: A meta-analysis. JAMA. 2004;291:1999–2006. doi: 10.1001/jama.291.16.1999. [DOI] [PubMed] [Google Scholar]
- 51.Dawson-Hughes B, Harris SS, Krall EA, et al. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337:670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 52.Broe KE, Chen TC, Weinberg J, et al. A higher dose of vitamin D reduces the risk of falls in nursing home residents: A randomized, multiple-dose study. J Am Geriatr Soc. 2007;55:234–239. doi: 10.1111/j.1532-5415.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 53.Wicherts IS, van Schoor NM, Boeke AJP, et al. Vitamin D status predicts physical performance and its decline in older persons. J Clin Endocrinol Metab. 2007;92:2058–2065. doi: 10.1210/jc.2006-1525. [DOI] [PubMed] [Google Scholar]
- 54.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garland CF, Garland FC, Gorham ED, et al. The Role of Vitamin D in Cancer Prevention. Am J Public Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention A quantitative meta analysis. Am J Prev Med. 2007;32:210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 57.Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98:451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 58.Feskanich D, Ma J, Fuchs CS, et al. Plasma vitamin D metabolites and risk of colorectal cancer in women. Cancer Epidemiol Biomarkers Prev. 2004;13:1502–1508. [PubMed] [Google Scholar]
- 59.Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 60.Wactawski-Wende J, Kotchen JM, Anderson GL, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–696. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 61.Lappe JM, Travers-Gustafson D, Davies KM, et al. Vitamin D and calcium supplementation reduces cancer risk: Results of a randomized trial. Am J Clin Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 62.Martineau AR, Honecker FU, Wilkinson RJ, et al. Vitamin D in the treatment of pulmonary tuberculosis. J Steroid Biochem Mol Biol. 2007;103:793–798. doi: 10.1016/j.jsbmb.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 63.Russell B. The history of lupus vulgaris: Its recognition, nature, treatment and prevention. Proc R Soc Med. 1954;48:127–132. [PMC free article] [PubMed] [Google Scholar]
- 64.Liu PT, Stenger S, Li H, et al. Toll-Like Receptor Triggering of a Vitamin D-Mediated Human Antimicrobial Response. Science. 2006;311:170–173. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 65.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: Basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 66.Bemiss CJ, Mahon BD, Henry A, et al. Interleukin-2 is one of the targets of 1,25-dihydroxyvitamin D3 in the immune system. Arch Biochem Biophys. 2002;402:249–54. doi: 10.1016/S0003-9861(02)00082-6. [DOI] [PubMed] [Google Scholar]
- 67.Mahon BD, Wittke A, Weaver V, et al. The targets of vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;402:922–933. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 68.Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation on interleukin-4 is one mechanism underlying the immunoregulatory effects of 1, 25-dihydroxyvitamin D3. Arch Biochem Biophys. 2000;377:135–138. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 69.Peterlik M, Cross HS. Vitamin D and calcium deficits predispose for multiple chronic diseases. Eur J Clin Invest. 2005;35:290–304. doi: 10.1111/j.1365-2362.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 70.Froicu M, Weaver V, Wynn TA, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 71.Cantorna MT, Hayes CE, DeLuca HF. 1, 25-dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci U S A. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Boonstra A, Barrat FJ, Craine C, et al. 1 alpha 25-dihydroxyvitamin D3 has a direct effect on naive CD4+T cells to enhance the development of TH2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 73.Willheim M, Thien R, Schrattbauer K, et al. Regulatory effects of 1 alpha 25 dihydroxyvitamin D3 on cytokine production of human peripheral blood lymphocytes. J Clin Endocrinol Metab. 1999;84:3739–3744. doi: 10.1210/jcem.84.10.6054. [DOI] [PubMed] [Google Scholar]
- 74.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T-lymphocyte activation by 1, 25 dihydroxyvitamin D3: Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kadowaki S, Norman AW. Demonstration that the vitamin D metabolite 1,25(OH)2-vitamin D3 and not 24R,25(OH)2-vitamin D3 is essential for normal insulin secretion in the perfused rat pancreas. Diabetes. 1985;34:315–320. doi: 10.2337/diab.34.4.315. [DOI] [PubMed] [Google Scholar]
- 76.Norman AW, Frankel JB, Heldt AM, et al. Vitamin D deficiency inhibits pancreatic secretion of insulin. Science. 1980;209:823–825. doi: 10.1126/science.6250216. [DOI] [PubMed] [Google Scholar]
- 77.Rabinovitch A, Suarez-Pinzon WL, Sooy K, et al. Expression of calbindin-D28K in a pancreatic islet b-cell line protects against cytokine-induced apoptosis and necrosis. Endocrinology. 2001;142:3649–3655. doi: 10.1210/endo.142.8.8334. [DOI] [PubMed] [Google Scholar]
- 78.Mathieu C, Gysemans C, Guilietti A, et al. Vitamin D and Diabetes. Diabetologia. 2005;48:1247–1257. doi: 10.1007/s00125-005-1802-7. [DOI] [PubMed] [Google Scholar]
- 79.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 80.Pittas AG, Lau J, Hu FB, et al. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–2029. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. QJM. 1996;89:579–589. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 82.Voors AW, Johnson WD. Altitude and arteriosclerotic heart disease mortality in white residents of 99 of the 100 largest cities in the United States. J Chronic Dis. 1979;32:157–162. doi: 10.1016/0021-9681(79)90044-4. [DOI] [PubMed] [Google Scholar]
- 83.Rostand SG. Ultraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 84.Scragg R, Jackson RD, Holdaway IM, et al. Myocardial infarction is inversely associated with plasma 25-hydroxyvitamin D3 levels: A community based study. Int J Epidemiol. 1990;19:559–563. doi: 10.1093/ije/19.3.559. [DOI] [PubMed] [Google Scholar]
- 85.Poole KE, Loveridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 86.Merke J, Milde P, Lewicka S, et al. Identification and regulation of 1, 25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1, 25-dihydroxyvitamin D3: Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1 alpha hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111:1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 88.Merke J, Hofmann W, Goldschmidt D, et al. Demonstration of 1, 25 (OH)2 vitamin D3 receptors and actions in vascular smooth muscle cells in vitro. Calcif Tissue Int. 1987;41:112–114. doi: 10.1007/BF02555253. [DOI] [PubMed] [Google Scholar]
- 89.Holick MF. High Prevalence of Vitamin D Inadequacy and Implications for Health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 90.Holick MF. Too little vitamin D in premenopausal women: Why should we care? Am J Clin Nutr. 2002;76:3–4. doi: 10.1093/ajcn/76.1.3. [DOI] [PubMed] [Google Scholar]
- 91.Giovannucci E. Can vitamin D reduce total mortality? Arch Intern Med. 2007;167:1709–1710. doi: 10.1001/archinte.167.16.1709. [DOI] [PubMed] [Google Scholar]
- 92.Anonymous . Vitamin D Deficiency: Information for Cancer Patients. The Bone and Cancer Foundation; New York, NY: 2008. [Google Scholar]
- 93.Anonymous . Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; Washington, D.C.: 2008. [Google Scholar]
- 94.Heaney RP. Barriers to optimizing vitamin D3 intake for the elderly. J Nutr. 2006;136:1123–1125. doi: 10.1093/jn/136.4.1123. [DOI] [PubMed] [Google Scholar]
- 95.Armas LAG, Hollis BW, Heaney RP. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab. 2004;89:5387–5391. doi: 10.1210/jc.2004-0360. [DOI] [PubMed] [Google Scholar]
- 96.Binkley N, Gemar D, Woods A, et al. Effect of vitamin D2 or vitamin D3 supplementation on serum 25OHD. J Bone Miner Res. 2008;23(suppl 1):S350. [Google Scholar]
- 97.MacLaughlin JA, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Weaver CM, Fleet JC. Vitamin D requirements: current and future. Am J Clin Nutr. 2004;80:1735S–1739S. doi: 10.1093/ajcn/80.6.1735S. [DOI] [PubMed] [Google Scholar]
- 99.Matsuoka LY, Wortsman J, Haddad JG, et al. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127:536–538. [PubMed] [Google Scholar]
- 100.Anonymous National Coalition for Sun Safety. 2006 www.aad.org/public/sunsafetydb.htm.
- 101.Task Force on Community Preventive Services Recommendations to prevent skin cancer by reducing exposure to ultraviolet radiation. Am J Prev Med. 2004;27:467–470. doi: 10.1016/j.amepre.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 102.Kirsner RS, Parker DF, Brathwaite N, et al. Sun protection policies in Miami-Dade county public schools: Opportunities for skin cancer prevention. Pediatr Dermatol. 2005;22:513–519. doi: 10.1111/j.1525-1470.2005.00130.x. [DOI] [PubMed] [Google Scholar]
- 103.Anonymous Saving your skin from sun damage. Am Fam Physician. 2006;74:815–816. [PubMed] [Google Scholar]
- 104.Randle HW. Suntanning: Differences in Perceptions Throughout History. Mayo Clin Proc. 1997;72:461–466. doi: 10.4065/72.5.461. [DOI] [PubMed] [Google Scholar]
- 105.Skin cancer primary prevention and education initiative . Sun safety at school: What you can do. Center for Disease Control; Guidelines for School; 2006. [Google Scholar]
- 106.US Environmental Protection Agency SunWise Program. 2006 www.epa.gov/sunwise.
- 107.Matsuoka LY, Wortsman J, Hanafin N, et al. Chronic sunscreen use decreases circulating concentrations of 25-hydroxyvitamin D. Arch Dermatol. 1988;124:1802–1804. [PubMed] [Google Scholar]
- 108.Pepper KJ, Judd SE, Nanes MS, et al. Evaluation of vitamin D repletion regimens to correct vitamin D status in adults. Endocrine Practice. 2009;15:95–103. doi: 10.4158/EP.15.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Przybelski R, Agrawal S, Krueger D, et al. Rapid correction of low vitamin D status in nursing home residents. Osteoporos Int. 2008;19:1621–1628. doi: 10.1007/s00198-008-0619-x. [DOI] [PubMed] [Google Scholar]
- 110.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351:805–6. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 111.Geller JL, Adams JS. Vitamin D therapy. Current Osteoporosis Reports. 2008;6:5–11. doi: 10.1007/s11914-008-0002-z. [DOI] [PubMed] [Google Scholar]
- 112.Ramamurthy R, Przybelski R, Gemar D, et al. Long-term high-dose vitamin D supplementation in the elderly is both safe and efficacious. 2009 [Google Scholar]
- 113.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–681. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heaney RP, Davies KM, Chen TC, et al. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr. 2003;77:204–210. doi: 10.1093/ajcn/77.1.204. [DOI] [PubMed] [Google Scholar]
- 115.The Record Trial Reporting Group Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomized Evaluation of Calcium or vitamin D, RECORD): A randomized-placebo-controlled trial. Lancet. 2005;365:1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 116.Wactawski-Wende J, Jackson RD, LaCroix AZ, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354:669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 117.Chesnut CHI. Treating osteoporosis with bisphosphonates and addressing adherence. Drugs. 2006;66:1351–1359. doi: 10.2165/00003495-200666100-00004. [DOI] [PubMed] [Google Scholar]
- 118.Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- 119.Vieth R. Vitamin D toxicity, policy and science. J Bone Miner Res. 2007;22(suppl 2):V64–V68. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 120.Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations and safety. Am J Clin Nutr. 1999;69:842–856. doi: 10.1093/ajcn/69.5.842. [DOI] [PubMed] [Google Scholar]
- 121.Trang HM, Cole DEC, Rubin LA, et al. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr. 1998;68:854–858. doi: 10.1093/ajcn/68.4.854. [DOI] [PubMed] [Google Scholar]
- 122.Houghton LA, Vieth R. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr. 2006;84:694–697. doi: 10.1093/ajcn/84.4.694. [DOI] [PubMed] [Google Scholar]