Abstract
We examined caregiver report of externalizing behavior from 12 to 54 months of age in 102 children randomized to care as usual in institutions or to newly-created high quality foster care. At baseline no differences by group or genotype in externalizing were found. However, changes in externalizing from baseline to 42 months of age were moderated by 5HTTLPR genotype and intervention group, where the slope for s/s individuals differed as a function of intervention group. The slope for individuals carrying the l allele did not significantly differ between groups. At 54 months of age, s/s children in the foster care group had the lowest levels of externalizing behavior, while children with the s/s genotype in the care as usual group demonstrated the highest rates of externalizing behavior. No intervention group differences were found in externalizing behavior among children who carried the l allele. These findings, within a randomized control trial of foster care compared to continued care as usual, indicate that 5HTTLPR genotype moderates the relation between early caregiving environments to predict externalizing behavior in children exposed to early institutional care in a manner most consistent with differential susceptibility.
Keywords: Differential susceptibility, institutionalization, 5httlpr, externalizing
Exposure to early severe psychosocial deprivation as a result of institutional care is associated with persistent externalizing psychopathology (Rutter et al., 2007). The role of the caregiving environment in the development of externalizing psychopathology has previously been established (Shaw, Owens, Giovannelli, & Winslow, 2001). In children who experienced institutional rearing, the elevated risk for externalizing psychopathology is thought to be the consequence of the extreme deviation of the early caregiving relationship from expected norms. The regimented schedule, rotating caregivers, and lack of individualized attention to children’s needs are considered key components of the institutional environment that contribute to the elevated risk, presumably through the impact on the child’s neurobiological development (Sheridan, Drury, McLaughlin, & Almas, 2010; Zeanah et al., 2009). In the English and Romanian Adoptee Study (ERA), signs of hyperactivity positively correlated with the severity of deprivation in post-institutionalized children (Kreppner, O’Connor, & Rutter, 2001). Externalizing behavior, unlike internalizing behavior, appears to be particularly resistant to subsequent improvements in the caregiving environment, suggesting that other factors are involved in recovery (Cicchetti, 2013; Zeanah, 2007). Child specific factors, including genetic variation, likely add to the differential adaptation of children to significant changes in the early caregiving environment (Drury et al., 2012; Drury et al., 2010; Gunnar et al., 2012). In longitudinal studies of less extreme atypical caregiving environments, differential sensitivity to changes in the caregiving environment (Belsky, Hsieh, & Crnic, 1998; Bradley & Corwyn, 2008), and moderation of this sensitivity by genotype(Bakermans-Kranenburg, van IJzendoorn, Mesman, Alink, & Juffer, 2008) have been reported in relation to externalizing psychopathology.
Differential susceptibility
One possible model that explains individual differences in both initial vulnerability and later recovery from negative early experiences is Differential Susceptibility Theory (DST)(Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011b; van IJzendoorn et al., 2011). DST proposes that “susceptibility” or plasticity genes, act in a “for better or worse” manner in response to environmental differences (Belsky et al., 2009; Belsky & Pleuss, 2013; Hankin et al., 2011; Owens et al., 2012; Simons et al., 2011; van IJzendoorn & Bakermans-Kranenburg, 2012; van IJzendoorn, Belsky, & Bakermans-Kranenburg, 2012). In negative environments these putative risk/susceptibility genotypes would be associated with the most negative outcomes. However, divergent from diathesis stress models, these same genotypes, in supportive/enriched environments, are expected to predict the most positive outcomes (Bakermans-Kranenburg & van IJzendoorn, 2011; Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2007; Belsky & Pleuss, 2013; Belsky, Pluess, & Pleuss, 2009). Non-susceptible genotypes, on the other hand, are predicted to be relatively resistant to environmental contexts and demonstrate little difference in outcomes between positive and negative environments.
Consistent with this hypothesis, evidence for DST in response to early caregiving and parent–child relationships continues to grow (Belsky & Beaver, 2011; Knafo, Israel, & Ebstein, 2011; Manuck, Flory, Ferrell, & Muldoon, 2004; Sonuga-Barke et al., 2009). While a significant number of cross-sectional studies have corroborated the DST, randomized controlled trials (RCT) that examine the impact of defined caregiving changes in the same individual, offer the most stringent scientific approach. Three previous studies, to date have examined DST within an RCT framework (Bakermans-Kranenburg, van IJzendoorn, Pijlman, Mesman, & Juffer, 2008; Drury, et al., 2012; Kegel, Bus, & Van Ijzendoorn, 2011). One of these DST within randomized trials of a psychotherapeutic intervention; one study examined DST within an RCT of a parenting intervention. Only one study has explored DST within the context of a RCT of foster care placement (Drury, et al., 2012).
The 5HTTLPR s/s as a Susceptibility Genotype
The serotonin transporter protein, encoded by the 5HTT gene, contributes to the regulation of serotonin in the central nervous system. The variable number tandem repeat length polymorphism located in the 5′ regulatory region (5HTTLPR) has been extensively studied in relation to the interaction between early adversity and the development of psychopathology (Bogdan, Agrawal, Gaffrey, Tillman, & Luby, in press; Cicchetti & Rogosch, 2012; Karg, Burmeister, Shedden, & Sen, 2011). Neurobiological and molecular evidence indicates that this polymorphic variant impacts expression levels of the transporter, affects the developmental trajectory of serotonin neurotransmission, influences underlying neurobiological constructs related to externalizing psychopathology (e.g., negative emotional response to stress), and contributes to individual differences in responsiveness to the caregiving environment (Barr et al., 2003; Canli & Lesch, 2007; Champoux et al., 2002; Hariri & Holmes, 2006; Homberg & Lesch, 2011; Lesch et al., 1994; Philibert et al., 2008; van IJzendoorn & Bakermans-Kranenburg, 2012).
Although the majority of studies have examined this gene in relation to internalizing disorders, a number of studies have also explored the relation between the 5HTTLPR genotype and externalizing disorders (Cicchetti & Rogosch, 2012; Davies & Cicchetti, 2013). Both direct genetic effects of the 5HTTLPR, as well as gene by environment (g × e) interactions, on externalizing behaviors such as impulsivity, aggression, and defiance have been reported (Aslund et al., 2013; Douglas et al., 2011; Nobile et al., 2007; Simons et al., 2012; Vaske, Newsome, & Wright, 2012). In these studies, individuals with the s allele, defined as either s/s individuals or with s/l and s/s individuals grouped together, depending on the specific study, demonstrate lower self-control, higher risk behaviors, higher activity levels, lower attentiveness, increased impulsivity, and elevated disregard for rules (Auerbach, Faroy, Ebstein, Kahana, & Levine, 2001; Brody, Beach, Philibert, Chen, & Murry, 2009; Kendler, Gardner, & Prescott, 2003; Kreek, Nielsen, & LaForge, 2005; Rutter et al., 1997; Suomi, 2004). Complex associations between the s/s genotype, sex, and externalizing behaviors have been seen in other studies that suggest gender differences are also important. In a high-risk subset of children enrolled in the Iowa Adoption cohort (Cadoret et al., 2003), sex of the child resulted in a differential effect of the s/s genotype on externalizing behaviors. Additional risks influencing outcomes that were associated with biological factors in the parent were implicated in this study, highlighting yet another level of complexity in g × e studies. The s/s genotype has also been associated with DST to high risk externalizing behaviors in a randomized controlled trial of a parenting intervention in rural African American youths (Brody, et al., 2009). In that study s/s youths with enhanced caregiving demonstrated the lowest progression to high-risk behavior over the course of 29 months. However, youths with this same genotype, in the non-intervention group, demonstrated the highest progression to high-risk behavior (substance use, sexual intercourse, etc.) over a similar time period.
A recent meta-analysis suggested that the s allele, and the homozygous s/s genotype in particular, is associated with increased responsiveness to the environment, in both a negative and positive manner, dependent on the implied valance of the environment (van IJzendoorn, et al., 2012). However, racial and developmental differences regarding which allele functions as the susceptibility allele have also been suggested (Davies & Cicchetti, 2013; Sulik et al., 2012). As such analyses within specific developmental time periods, well-defined racial groups, and with carefully characterized environmental differences, are needed to disentangle these interactions (van IJzendoorn, et al., 2011).
The Bucharest Early Intervention Project (BEIP)
The Bucharest Early Intervention Project (BEIP) is the first randomized controlled trial of alterations in the caregiving environment for children with a history of early institutional care (Zeanah et al., 2003). Children from six institutions across Romania were randomized to either continued institutional care (care as usual group, CAUG) or placement in high quality foster care (foster care group, FCG) between 6 months and 31 months of age. Consistent with unbiased randomization, no baseline differences in any measures have been found between the CAUG and FCG (Smyke et al., 2007). Unlike adoption studies of children exposed to early institutional care, the BEIP created, within Romania, a new foster care system for study participants. The BEIP foster care placements included considerable support for the social workers overseeing the foster parents, support for the foster care parents themselves including guidance about interventions for problem behaviors and encouragement of parents to commit fully to their foster children. Differing from typical foster care in the United States, 87% placement stability was demonstrated over the course of the intervention within the BEIP (Nelson, Fox, & Zeanah, in press). The positive impact of BEIP foster care, across child developmental domains, including brain structure and function, psychological, attachment, cognitive, and physical growth has been previously reported (Bos, Fox, Zeanah, & Nelson, 2009; Fox, Almas, Degnan, Nelson, & Zeanah, 2011; Johnson et al., 2010; Nelson et al., 2007; Sheridan, et al., 2010; Sheridan, Fox, Zeanah, McLaughlin, & Nelson, 2012; Vanderwert, Marshall, Nelson, Zeanah, & Fox, 2010). The cross domain positive impact, high placement stability, and lack of baseline differences between the two groups indicate that BEIP foster care represents a significant positive change to the caregiving environment.
The impact of the s/s genotype on outcomes in Romanian children with a history of institutionalization has been examined in two previous studies (Drury, et al., 2012; Kumsta et al., 2010). In both studies children with the s/s genotype appeared to demonstrate the greatest susceptibility. In the first study, Kumsta et al. (2010) reported that children with the s/s genotype who were adopted into UK families from Romanian institutions and who experienced no additional adverse life events between ages 11 and 15 years of age, appeared to benefit preferentially from enhanced caregiving, demonstrating decreased symptoms of attention-deficit/hyperactivity disorder (ADHD) during that time period. Children with the same background and the s/s genotype, but exposed to additional adverse life events between 11 and 15 years of age, demonstrated no decrease in ADHD symptoms. In the second study, children who were raised since early infancy in Romanian institutions with the s/s genotype, independently, and in combination with the met allele of Brain Derived Neurotrophic Factor (BDNF), demonstrated differential susceptibility to foster care placement in relation to disinhibited reactive attachment disorder (Drury, et al., 2012). Together, these studies suggest that within this population (i.e., previously institutionalized Romanian orphans), and with the defined exposure of institutional care (as opposed to alternative types of negative early caregiving experiences), the s/s genotype confers heightened susceptibility. The existing evidence from previous studies, coupled with extant molecular and neurobiological data, offer significant support for hypotheses testing differential susceptibility, the 5HTTLPR s/s genotype, and externalizing.
The randomized longitudinal design of the BEIP study permits direct testing of intervention group status as a moderator of externalizing behavior over the course of early development, and provides a unique opportunity for testing of DST in response to well-defined alterations in the early caregiving context. We tested the hypothesis that externalizing scores are responsive, in a “for better or worse” manner, to the interaction between genotype (s/s), time, and intervention group (i.e., FCG or CAUG). We predicted that genotype would not be associated with externalizing behavior at baseline. Subsequently, we hypothesized that, over time, children with the s/s genotype in the CAUG would have the highest externalizing, while children with this same genotype, but randomized to the FCG, would demonstrate significant decreases in externalizing behavior (Aguilera et al., 2009; Nederhof et al., 2010; Wichers et al., 2008).
Methods
Participants
Participants were enrolled in the BEIP (Zeanah, et al., 2003), a RCT of foster care as an alternative to institutional care. The study sample, with inclusion and exclusion criteria, has been described elsewhere (Nelson, et al., 2007; Zeanah, et al., 2009). Briefly, participants included 136 abandoned children living in institutions in Bucharest Romania and at the time of initial assessment between 6 and 30 months of age. Following baseline assessments, 68 of the children (33 males and 35 females) were randomly assigned to the CAUG and 68 (34 males and 34 females) to the FCG. Children were excluded from the study for medical reasons including diagnosed genetic syndromes, significant evidence of fetal alcohol syndrome or microcephaly. The foster care network was created and supported by the project as an intentional alternative to institutional care (Smyke, et al., 2007). Greater detail about the ethical issues and study design are described elsewhere (Millum & Emanuel, 2007; Nelson, et al., in press; Zeanah, Fox, & Nelson, 2012; Zeanah, et al., 2003).
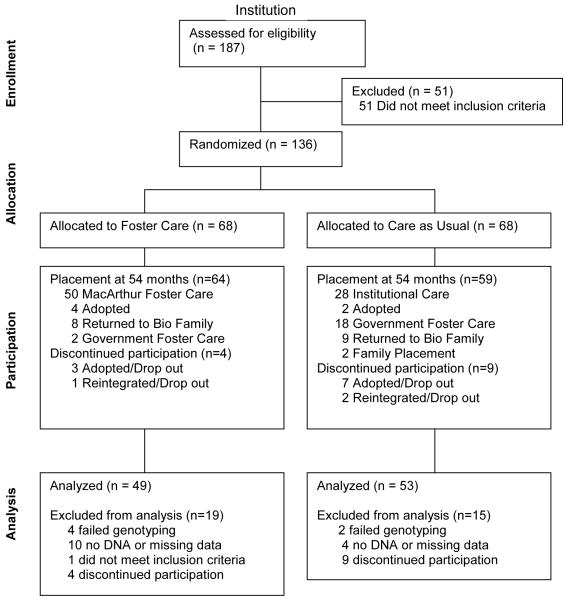
Following randomization, all subsequent decisions regarding placement were made by the Romanian National Authority for Child Protection in accordance with Romanian law, with the agreement that no child removed from an institution and placed in project supported foster care would be returned to an institution. Over the four years of the project, there was considerable movement within the groups. Figure 1 depicts placement at 54 months and flow of participants, including reasons participants were not included in final sample. All analyses follow intent to treat, so that children are analyzed within their originally assigned group regardless of placement at 54 months of age as this is expected to provide the most stringent statistical approach. Psychopathology data and genotype data were available for 102 children.
Figure 1.
Flow of participants through BEIP study
Measures
Externalizing Behavior
Baseline, 30, and 42 months
The Infant Toddler Social Emotional Assessment (ITSEA) (Carter, Briggs-Gowan, Jones, & Little, 2003) is a 195-item caregiver report questionnaire used to assess problem behaviors and competencies in children greater than 12 months of age. The ITSEA was administered to caregivers/parents at baseline, 30, and 42 months of age. For the present study, we used the externalizing domain score, which comprises activity/impulsivity, aggression/defiance, and peer aggression. Both the PAPA (see below) and the ITSEA measures were obtained from foster mothers for children in foster care. For children in institutions, an institutional caregiver, who was considered the child’s favorite caregiver by staff consensus, or, if the child had no favorite, a caregiver who worked with the child regularly, reported on the child’s behavior at each time point.
54 months of age
Psychiatric morbidity at 54 months of age was assessed using the Preschool Age Psychiatric Assessment (PAPA). The PAPA is a semi-structured psychiatric interview assessing caregiver report of child behavior and emotional scores. Test–retest reliability of the PAPA is comparable to structured psychiatric interviews used to assess older children and adults (Egger et al., 2006). The PAPA 1.3 was translated into Romanian, back-translated into English, and assessed for meaning at each step by bilingual research staff. The BEIP lead interviewer was trained in administration of the PAPA by the group who developed the measure, and he subsequently trained other Romanian interviewers. The reliability of the PAPA in this population is described in detail elsewhere (Zeanah, et al., 2009). For this study, total externalizing score, a composite measure of symptoms of conduct disorder, oppositional defiant disorder, and attention-deficit/hyperactivity disorder, was utilized.
Genotyping
DNA was extracted from MasterAmp buccal swabs using Epicentre Biotechnologies MasterAmp DNA extraction solution following manufacturer’s recommendations. 5HTTLPR (rs4795541) allele status was determined using standard PCR methods and gel electrophoresis with careful attention to magnesium concentrations. Variation of Mg levels from 1mM to 2mM did not result in genotype differences as has been previously demonstrated in other studies (Yonan, Palmer, & Gillian, 2006). All samples were run in triplicate, with known controls. Samples with inconsistent triplicates were repeated. Genotype was tested to confirm Hardy-Weinberg Equilibrium (p = 0.96, N=102). 5HTTLPR genotype frequencies were .47 for the s allele and .53 for the l allele. Genotyping failed on 6 individuals for whom DNA was obtained (3 CAUG, 3 FCG) and 7 samples required replication of genotyping for confirmation of genotype. No significant differences in group, ethnicity, or externalizing scores were found between those with and without genotype or DNA.
Human Subjects
The study was approved by Institutional Review Boards at Boston Children’s Hospital/Harvard Medical School, Tulane University, University of Maryland, and by the local commissions on child protection in each sector of Bucharest. The proposed study was reviewed by IRBs in the U.S. institutions of the three principal investigators and additionally, in 2002, by an ad hoc ethics commission appointed by Romanian officials (Zeanah, et al., 2003).
Data Analysis
Bivariate associations were examined between genotype, group, and demographic characteristics (i.e., sex and ethnicity). Linear mixed modeling (LMM) in SPSS (version 20) was used to examine the effect of 5HTTLPR genotype (s/s vs. s/l vs. l/l) on externalizing scores from the ITSEA, across three time points (baseline, 30 months, and 42 months). This method models individual change over time, explores systematic differences in change, and examines the effects of key variables (i.e., 5HTTLPR genotype) on differences in the rate of growth. LMM is an appropriate approach when studying individual change as it creates a two-level hierarchical model that nests time within individuals, and can flexibly accommodate missing data. As such, individuals were included in the analyses even if externalizing scores were missing at one or more time points. LMM specifying a random intercept and random and fixed effects for slope (wave of assessment) was performed. The relationships between 5HTTLPR genotype, intervention group, and model parameters (i.e., initial status and linear growth) were estimated after statistically controlling for the effects of sex and ethnicity.
Univariate ANCOVAs were used to examine the effect of 5HTTLPR group, intervention group, and their interaction on externalizing at 54 months, statistically controlling for sex, ethnicity, and baseline externalizing.
Given that meta-analytic findings indicated the greatest susceptibility with s/s individuals (van IJzendoorn, et al., 2012), all analyses were repeated with a dummy variable (i.e., 5HTTLPR group —s/s genotype vs. s/l genotype vs. l/l genotype) as a predictor.
Results
A total of 102 individuals provided both genetic data and externalizing scores for at least one time point. 5HTTLPR genotype was unrelated to intervention group or demographic variables (including sex and ethnicity) (see Table 1). Additionally, the association between 5HTTLPR genotype and externalizing scores at any time point did not reach statistical significance (i.e., baseline, 30, 42, 54 month, ps ranged from .09 to .94). Genotype frequencies were not significantly different from previously reported genotypes in Romanian children adopted in the United Kingdom from institutions. Specifically, our genotype frequencies were 27% s/s, 51% s/l and 22% l/l, and in the Kumsta et al. (2010) study the reported genotype frequencies were 24% s/s, 48% s/l and 28% l/l, suggesting no differences in overall allele frequencies between these two studies.
Table 1.
Demographics by 5HTTLPR genotype.
| s/s (n = 22) | s/l (n = 51) | l/l (n = 29) | χ 2 | |
|---|---|---|---|---|
| Group (% FCG) | 64% | 51% | 45% | 1.81 ns |
| Sex (% Male) | 55% | 63% | 38% | 4.59 ns |
| Ethnicity (% Romanian) |
55% | 51% | 46% | 1.66 ns |
Note. FCG = foster care group.
Correlations were run to examine whether externalizing scores were associated across time points over time in all individuals as well as within each intervention group (FCG, CAUG) (Table 2). For each time point, both in the complete sample and within intervention group, scores were positively correlated to scores from follow-up periods immediately preceding and following, suggesting developmental continuity between assessments.
Table 2.
Correlation matrix and descriptive statistics for externalizing scores by time point.
| FCG | CAUG | Full Sample | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||
| Baseline | 30 mo | 42 mo | 54 mo | Baseline | 30 mo | 42 mo | 54 mo | Baseline | 30 mo | 42 mo | 54 mo | |
|
|
||||||||||||
| Baseline | 1 | .30* | .17 | .17 | 1 | .28† | .06 | −.12 | 1 | .27* | .12 | .04 |
| 30 mo | 1 | .73*** | .46*** | 1 | .35* | .18 | 1 | .50*** | .32** | |||
| 42 mo | 1 | .64*** | 1 | .31* | 1 | .47*** | ||||||
| 54 mo | 1 | 1 | 1 | |||||||||
| Mean | 0.56 | 0.76 | 0.62 | 7.25 | 0.61 | 0.61 | 0.66 | 8.23 | 0.58 | 0.69 | 0.64 | 7.93 |
| (SD) | (0.39) | (0.43) | (0.35) | (6.88) | (0.41) | (0.41) | (0.42) | (6.99) | (0.40) | (0.44) | (0.39) | (6.86) |
| Range | 0-1.57 | 0-1.91 | 0-1.54 | 0-27 | 0-1.67 | 0-1.68 | 0-1.83 | 0-24 | 0-1.67 | 0-1.91 | 0-1.83 | 0-27 |
Note. FCG = foster care group. CAUG = care as usual group. mo= months of age.
p < .10.
p < .05.
p < .01.
p < .001.
Intervention Group by 5HTTLPR Genotype on Externalizing Trajectory from Baseline to 42 months
5HTTLPR individual genotype analysis (s/s vs. s/l vs. l/l)
Estimates from the unconditional growth model, main effects model, two-way interaction model, and three-way interaction models are shown in Table 3. The only significant predictor of externalizing in the main effects model was sex (F(1,77.80) = 5.10, p = .03), such that boys had higher externalizing scores than girls. No other variable, including genotype was a significant direct predictor of externalizing.
Table 3.
Summary table of the mixed effects model for the three genotype model predicting externalizing scores.
| Estimate | Standard Error | t | p-value | |
|---|---|---|---|---|
| Unconditional Growth Model | ||||
| Intercept | 0.61 | 0.04 | 1.20 | .23 |
| Main Effects Model | ||||
| Intercept | 0.66 | 0.11 | 6.16 | < .001 |
| Time (baseline = 0) | 0.04 | 0.03 | 1.52 | .13 |
| Group (CAUG) | −.01 | 0.07 | −0.19 | .85 |
| 5HTTLPR (l/l) | 0.13 | 0.10 | 1.31 | .19 |
| 5HTTLPR (s/l) | 0.01 | 0.09 | 0.13 | .89 |
| Two-Way Interaction Model | ||||
| Intercept | 0.65 | 0.13 | 4.95 | < .001 |
| Time (baseline = 0) | 0.03 | 0.07 | 0.45 | .66 |
| Group (CAUG) | 0.11 | 0.17 | 0.68 | .50 |
| 5HTTLPR (l/l) | 0.05 | 0.15 | −0.30 | .75 |
| 5HTTLPR (s/l) | 0.14 | 0.13 | 1.05 | .40 |
| Time × Group | 0.02 | 0.06 | 0.32 | .75 |
| Time × 5HTTLPR (l/l) | 0.05 | 0.09 | 0.60 | .55 |
| Time × 5HTTLPR (s/l) | −0.02 | 0.08 | −0.26 | .79 |
| Group × 5HTTLPR (l/l) | 0.00 | 0.20 | 0.02 | .98 |
| Group × 5HTTLPR (s/l) | −0.27 | 0.18 | −1.51 | .14 |
| Three-Way Interaction Model | ||||
| Intercept | 0.74 | 0.14 | 5.48 | < .001 |
| Time (baseline = 0) | −0.07 | 0.08 | −0.92 | .36 |
| Group (CAUG) | −0.19 | 0.21 | −0.95 | .35 |
| 5HTTLPR (l/l) | −0.07 | 0.16 | −0.41 | .68 |
| 5HTTLPR (s/l) | 0.01 | 0.14 | 0.04 | .97 |
| Time × Group | 0.34 | 0.14 | 2.51 | .014 |
| Time × 5HTTLPR (l/l) | 0.17 | 0.11 | 1.60 | .11 |
| Time × 5HTTLPR (s/l) | 0.12 | 0.09 | 1.28 | .21 |
| Group × 5HTTLPR (l/l) | 0.35 | 0.26 | 1.34 | .19 |
| Group × 5HTTLPR (s/l) | 0.12 | 0.24 | 0.49 | .63 |
| Time × Group × 5HTTLPR (l/l) | −0.37 | 0.17 | −2.12 | .037 |
| Time × Group × 5HTTLPR (s/l) | −0.41 | 0.16 | −2.59 | .011 |
Note. Covariates not included in table.
The three-way interaction (5HTTLPR × intervention group × time) significantly predicted externalizing scores (F(2,78.25) = 3.47, p = .04). Post hoc analyses within each genotype revealed that there were no significant intervention group × time interactions for individuals with either the s/l or l/l genotypes. At baseline, among individuals with the s/s genotype, there was no difference between CAUG and FCG on externalizing scores (F(1,13.05) = 0.68, p = .43). However, for individuals with the s/s genotype, there was a significant group × time interaction (F(1,13.64) = 5.89, p = .03), such that over time the trajectory of externalizing scores significantly differed between the two groups. Specifically, in the CAUG the trajectory of externalizing scores increased while within the FCG group externalizing scores declined.
5HTTLPR grouped genotype analysis (s/s vs. s/l + l/l)
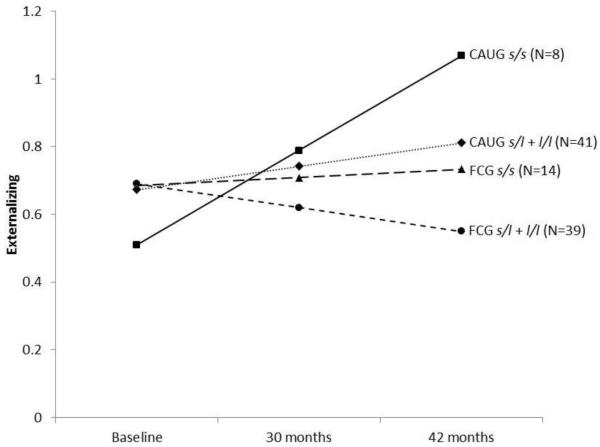
Given the variable categorization of genotype across previous studies, and our initial findings suggesting the effects are driven by individuals with the s/s genotype, we further examined externalizing scores collapsing across other genotypes to test directly whether s/s individuals significantly differed from those with either the s/l or l/l genotype (i.e. s/l + l/l). Findings were largely unchanged. No significant direct effects of genotype, intervention group, and time or two-way interactions were detected. A significant three-way interaction for 5HTTLPR genotype, intervention group, and time predicting externalizing scores was found (F(1,80.40) = 6.75, p = .01). Once again no significant effects were found in children with the l allele. However, in children with the s/s genotype, there were significant differences such that children in the CAUG demonstrated increased trajectory of externalizing scores, while those within the FCG demonstrated decreased scores (see Figure 2).
Figure 2.
Externalizing scores across time by intervention group and 5HTTLPR genotype (s/s vs. s/l and l/l). Note. CAUG = care as usual. FCG = foster care group.
Intervention Group by 5HTTLPR Genotype on Externalizing at 54 months
5HTTLPR individual genotype analysis (s/s vs. s/l vs. l/l)
At 54 months of age a structured psychiatric interview, the PAPA, was used to measure psychopathology. Because a different measure was used to obtain externalizing scores at this time point, the interaction of intervention genotype × intervention group at 54 months of age was directly examined. Neither genotype (F(2,75) = 0.32, p = .72, partial η2 = .01) nor intervention group (F(1,75) = 0.001, p = .97, partial η2 < .001) were significantly associated with externalizing scores. Again, there was a significant effect of sex (F(1,75) = 6.74, p = .01, partial η2 = .08), such that boys had significantly higher externalizing scores than girls.
Univariate ANCOVA analysis testing the interaction of 5HTTLPR genotype × intervention group interaction at 54 months, with sex, ethnicity, and externalizing score at baseline as covariates, revealed a significant interaction effect (F(2,73) = 6.89, p = .002, partial η2 = .16). Post hoc analyses determined that the interaction was driven by intervention group differences within children with the s/s genotype. Within children with the s/l and l/l genotypes, no differences in mean externalizing scores were found between CAUG and FCG. However within children with the s/s genotype those in the CAUG group had significantly higher mean externalizing scores than s/s individuals in the FCG group. Group status predicted 49% of the variance in externalizing scores at 54 months, over and above the effects of sex, ethnicity, and baseline externalizing score (F(1,11) = 15.84, p = .002, partial η2 = .59).
5HTTLPR grouped genotype analysis (s/s vs. s/l + l/l)
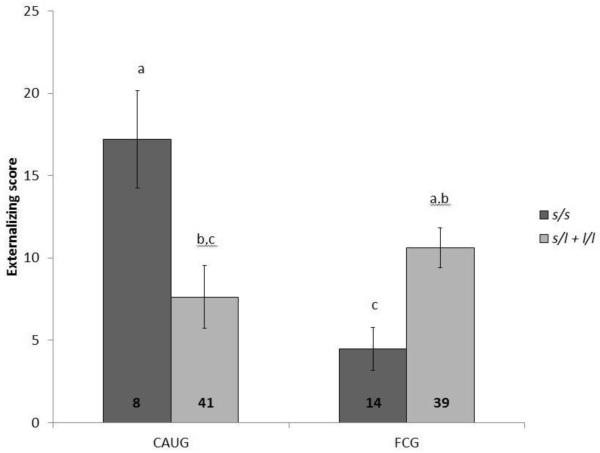
The collapsed l allele genotype produced a significant genotype × intervention group interaction at 54 months of age (F(1,74) = 14.03, p < .001, , partial η2 = .16) (Figure 3). Post hoc tests conducted within genotype indicated that there was no effect of intervention group within s/l + l/l individuals (F(1,60) = 2.56, p = .12). Yet, as noted above, intervention group was a significant predictor of externalizing scores within the s/s individuals. To further establish DST directly, we tested the effects of genotype within each group. There was a significant direct effect of genotype in both the CAUG (F(1,31) = 5.84, p = .02, , partial η2 = .16) and FCG (F(1,40) = 6.62, p = .01, partial η2 = .14). However, these effects were in different directions, i.e., in a “for better or worse” manner. Specifically, s/s genotype was associated with higher externalizing scores at 54 months in the CAUG and lower externalizing scores in the FCG. CAUG individuals with the s/s genotype had an average externalizing score that was 3.97 points higher than the l carriers from the CAUG. Within the FCG, s/s individuals had an average externalizing score that was 4.95 points lower than l carriers within the FCG. The observed interactions between intervention group and genotype remained significant regardless of inclusion of baseline scores.
Figure 3.
Externalizing scores at 54 months by intervention group and 5HTTLPR genotype (s/s vs. s/l and l/l). Note. CAUG = care as usual. FCG = foster care group. Columns with the same letter do not significantly differ from one another using Least Significant Difference post hoc test (p > .05).
Discussion
Our findings, within the RCT design of the BEIP study, indicate that the interaction of 5HTTLPR genotype with intervention group status predicts the trajectory of externalizing scores from baseline to 42 months and externalizing scores at 54 months in a manner consistent with differential susceptibility. Specifically, in the negative environment (i.e., continued institutional care) the s/s genotype predicts the highest externalizing, while in the positive environment (i.e., foster care placement) the s/s genotype is associated with the lowest externalizing scores. In contrast, for individuals with at least one l allele, the interaction of genotype and group status, within and across intervention group, was not a significant predictor of externalizing. As the majority of prior studies of DST have been correlational or cross-sectional, the use of baseline, pre-randomization measures, a RCT design, and longitudinal data analysis add substantial strength to the findings in this study (van IJzendoorn & Bakermans-Kranenburg, 2012). Prior studies of genetic differential susceptibility that focused on the parent-child relationship have also been limited by the potential of genotype-environment correlation (rGE, i.e., the fact that genetic factors related to parenting may exist in the biologically related children, influence directly their response to the parenting environment, and therefore confound the effect of the intervention). The use of non-biologically related individuals as caregivers in both settings (i.e., foster parents and institutional caregivers) permits the isolation of the genetic contribution to differential susceptibility to child specific genetic variation. Alternative approaches to circumvent this confounder (i.e., randomization based on both intervention group and susceptibility marker, i.e. genotype), can also be used to delineate DST in studies examining parenting or caregiving interventions in biologically related individuals (van IJzendoorn & Bakermans-Kranenburg, 2012).
A recent meta-analysis by van IJzendoorn et al. (2012) revealed that the s allele, and particularly the s/s genotype, served as the susceptibility moderator specifically in samples that were predominantly Caucasian. Acknowledging that examples of both s carrier grouping (s/l with s/s), and l carrier grouping (s/l with l/l) can be found in the literature (van IJzendoorn, et al., 2012), our primary analyses did not collapse across genotype groups. Only after s/l and l/l were found to have similar outcomes, that differed significantly from the s/s group, were secondary analyses performed using l carrier grouping (i.e., s/l + l/l). Our results show that the s/s genotype has the greatest susceptibility, consistent with the meta-analytic results.
Our analytic approach was designed to specifically test DST. At baseline, we confirmed no differences, by group or genotype, in externalizing, supporting the effectiveness of the randomization procedure. Next, consistent with DST no effect of the environment (group) or genotype (5HTTLPR) were demonstrated. Third, within the RCT of defined positive and negative environments (i.e. well-supported foster care and institutional care), significant differences in externalizing among children with the s/s genotype, in a “for better and worse manner” were found. Trajectory analyses from baseline to 42 months of age found a significant genotype × intervention group × time interaction driven by differences within in s/s genotype, and culminating in differing trends in externalizing over time based on the group status. At 54 months, s/s children had significantly lower externalizing scores if randomized to the FCG, yet had the highest externalizing scores if randomized to the CAUG. Fourth, we demonstrated that individuals with the s/l or l/l genotypes, presumably the less susceptible or sensitive genotypes, did not differ in externalizing regardless of intervention group. The absence of significant difference in individuals with the non-susceptible genotypes (i.e., s/l and l/l) provides an additional level of evidence for DST (Belsky, Pluess, & Widaman, 2013). Although previous studies, including our own, suggested that externalizing scores were relatively resistant to alterations of the caregiving setting following early institutional care, these current findings demonstrate that a subset of children, defined by the s/s genotype of the 5HTTLPR may be particularly sensitive to early caregiving and demonstrate substantial improvement when provided with enhanced caregiving.
In determining the specific phenotypes and genotypes appropriate for DST analysis, it is critical to consider that direct genetic effects may be absent or minimal and developmental differences may be present. Consistent with this, in both our previous study of differential susceptibility for disinhibited social behavior, and the current study focused on externalizing, no direct effect of genotype was present (Belsky, et al., 2007; Drury, et al., 2012; Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van IJzendoorn, 2011a). In addition, although there is evidence of both increasing (Lavigne et al., 1996) and decreasing (Gilliom & Shaw, 2004) externalizing behavior during this developmental time period (early childhood), little is known about the trajectory of externalizing scores in children who experienced institutional care. Thus, careful attention to the influence of genetic variation across developmental time points is needed. One previous study, of children enrolled in the English and Romanian Adoptee Study (ERA), examined the longitudinal association between the 5HTTLPR, institutional care, and psychopathology (Kumsta, et al., 2010). The interaction between institutional care and the 5HTTLPR genotype was associated with emotional problems, at age 11 and 15, but a differential interaction existed for those children exposed to additional negative life events in the intervening time period. These studies suggest that the interaction between genetic variants and institutional care is relevant for long term child outcomes and the effect of this interaction may vary across development.
Although the mechanism underlying the persistence of externalizing behavior in post-institutionalized children remains poorly defined, our findings suggest that externalizing psychopathology is, in part, the consequence of the adverse early caregiving environment interaction with serotonergic pathways. Higher rates of aggression are found among institutionalized children, and aggressive, or more dominant children, appear to get more adult attention and resources (Sturge-Apple, Davies, Martin, Cicchetti, & Hentges, 2012). In the setting of institutional care, unresponsive caregiving in children is associated with decreased serotonergic turnover (van Goozen, Fairchild, Snoek, & Harold, 2007). As evidence suggests that caregiving influences the development of serotonergic neural pathways underlying aggression and physiologic reactivity to both negative and positive emotions, the early caregiving found in the institutional settings may lead to strengthening of these circuits and persistence of these behaviors. However, in s/s children, where serotonergic neurotransmission is putatively more plastic, the responsive caregiving found in BEIP foster care may lead to the significant decrease in these behaviors as these circuits were less strongly defined, and therefore more responsive to caregiving changes, prior to foster care placement as a consequence of decreased serotonergic tone.
Additional considerations and limitations
Limitations to this study exist. The most significant limitation to this study is sample size and the subsequent challenges associated with statistical power. Although this remains the largest RCT of foster care compared to continued institutional care ever conducted, the issue of sample size is an inescapable limitation. To address sample size issues, we used longitudinal data and a well-defined a priori hypothesis based on the existing cell biology and neuroscience literatures. Notably, our findings overlap significantly with previous studies of this genetic variant in post-institutionalized children, who, while not part of a randomized trial, experienced similarly significant alterations in caregiving (Kumsta, et al., 2010). Differential susceptibility to externalizing behaviors has previously been demonstrated in adopted children and other populations, and, while not exact replications, demonstrate additional convergence with our results (Brody, et al., 2009; Cadoret, et al., 2003; Nobile, et al., 2007; Simons, et al., 2012; Zimmermann, Mohr, & Spangler, 2009). Larger replications in other at-risk populations, with clearly defined environmental changes, are needed. A second prominent limitation is the substantial change in child placements over the course of the study. Although designed as a randomized controlled trial, significant movement between groups occurred over the course of this study as ethical and legal reasons precluded interference with these decisions. While examination of specific movement across caregiving environments (i.e., percent time in institutional care) would be potentially interesting, we believe our approach represents the most statistically stringent analysis. Further, the goal of this analysis was to specifically test DST, and therefore we leveraged the RCT design in our analytic approach. First, we demonstrated that there were no significant differences in children with the non-susceptibility genotype (i.e., s/l and l/l) across environments. Second, we demonstrated that within both the high and low risk environmental groups the effect of the s/s genotype was significant. Most critically, this effect is inverted depending on the environment, i.e., the s/s genotype is significantly more negative in the negative environment and significantly more positive in the positive environment (Belsky, et al., 2013; Roisman et al., 2012). Taken together these findings are most consistent with DST.
We did not correct for potential population stratification, where baseline differences in allele frequencies between ethnic groups may result in type I or type II errors in genetic association studies. However, for population stratification to present a statistical problem, two conditions need to be met: 1) a significant difference in allele frequencies between ethnicities must be present, and 2) differences in outcome between ethnicities must exist (Hutchison, Stallings, McGeary, & Bryan, 2004). In the present study neither of these conditions was present. The AIMS genotype panel, often used in g × e studies to account for genetic admixture, is not expected to be able to distinguish between our two most common ethnicities in this study, Romanian and Roma. As such, ethnicity was included as a covariate in all analyses. Additionally, we examined only the s and l alleles of the 5HTTLPR. Additional polymorphism in the 5HTTLPR (rs25531), as well as methylation status, may further influence the functional aspects of the 5HTTLPR, however these variants are not available in this data (van Ijzendoorn, Caspers, Bakermans-Kranenburg, Beach, & Philibert, 2010). Although we cannot address potential differences due to methylation, the categorization of s/s homozygotes in the secondary analysis minimizes the potential effect of this variant. It is also important to note that other SNPs exist within the 5HTT gene, with varying levels of functional significance, suggesting that the ability to capture the full range of genetically determined variation in the function of the 5HTT remains more complicated than the s vs. l allele or the rs25531 (Nakamura, Ueno, Sano, & Tanabe, 2000; Uher & McGuffin, 2008).
Lastly, different measures of externalizing behavior were used: the ITSEA and the PAPA. Although these measures are different, the independent reliability of both measures is substantial and there was significant correlation between the ITSEA 42 month measurement and 54 month PAPA assessment. In addition, our statistical approach was designed to minimize any effect of incorporating different measures. The consistent finding across two different measures of externalizing behavior could additionally be considered an analytic strength.
Conclusion
In conclusion, our results offer convincing support for a model of genetic differential susceptibility to the caregiving context for externalizing behaviors. The concept that the genetic makeup of an individual child can influence response to changes in the caregiving environment offers an extension to previous theories of risk and resilience and enhanced insight into the biological underpinnings of the developmental trajectory of externalizing in children with a history of early institutional care.
Acknowledgements
This research was supported by the John D. and Catherine T. MacArthur Foundation Research Network on “Early Experience and Brain Development” (Charles A. Nelson, Ph.D., Chair), NIMH grant (1R01MH091363) (Nelson), a NARSAD Young Investigator Award (Drury), NIMH grant (R21 MH094688-01) (Drury) and 2K12HD043451-06 (Drury, scholar). The project described was supported by Award Number K12HD043451 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health & Human Development or the National Institutes of Health. We thank the research staff in Romania and the families and children enrolled in this study.
References
- Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: new evidence of genexenvironment interactions on depressive symptoms in a general population. Psychological Medicine. 2009;39(09):1425–1432. doi: 10.1017/S0033291709005248. [DOI] [PubMed] [Google Scholar]
- Aslund C, Comasco E, Nordquist N, Leppert J, Oreland L, Nilsson K. Self-reported family socioeconomic status, the 5-HTTLPR genotype, and delinquent behavior in a community-based adolescent population. Aggressive Behavior. 2013;39:52–63. doi: 10.1002/ab.21451. [DOI] [PubMed] [Google Scholar]
- Auerbach J, Faroy M, Ebstein R, Kahana M, Levine J. The association of the dopamine D4 gene (DRD4) and the serotonin transporter promoter gene (5httlpr) with temperament in 12-month old infants. Journal of Child Psychology and Pschiatry. 2001;42:777–783. doi: 10.1111/1469-7610.00774. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M, van IJzendoorn M. Differential susceptibility to rearing environment depending on dopamine-related genes: New evidence and a meta-analysis. Development and Psychopathology. 2011;23:39–52. doi: 10.1017/S0954579410000635. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M, van IJzendoorn M, Mesman J, Alink L, Juffer F. Effects of an attachment-based intervention on daily cortisol moderated by dopamine receptor D4: A randomized control trial on 1- to 3-year-olds screened for externalizing behavior. Development and Psychopathology. 2008;20(03):805–820. doi: 10.1017/S0954579408000382. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg M, van IJzendoorn M, Pijlman F, Mesman J, Juffer F. Experimental evidence for differential susceptibility:dopamine D4 receptor polymorphism (DRD4 VNTR) moderates intervention effects on toddler’ externalizing behavior in a randomized controlled trial. Development Psychology. 2008;44:293–300. doi: 10.1037/0012-1649.44.1.293. [DOI] [PubMed] [Google Scholar]
- Barr C, Newman T, Becker M, Champoux M, Lesch K, Suomi S, et al. Serotonin transporter gene variation is associated with alcohol sensitivity in rhesus macapues exposed to early-life stress. Alcoholism: Clinical & Experimental Research. 2003;27(5):812–817. doi: 10.1097/01.ALC.0000067976.62827.ED. [DOI] [PubMed] [Google Scholar]
- Belsky J, Bakermans-Kranenburg M, van IJzendoorn M. For better and for worse: differential susceptibility to environmental influences. Current directions in psychological science. 2007;16:300–304. [Google Scholar]
- Belsky J, Beaver K. Cumulative-genetic plasticity, parenting and adolescent self-regulation. Journal of Child Psychology and Psychiatry. 2011;(5):619–626. doi: 10.1111/j.1469-7610.2010.02327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Hsieh K, Crnic K. Mothering, fathering, and infant negativity as antecedents of boys’ externalizing problems and inhibition at age 3 years: Differential susceptibility to rearing experience? Development and Psychopathology. 1998;10:301–319. doi: 10.1017/s095457949800162x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pleuss M, Stanton M, Brummet B, Williams R. Vulnerability Genes or Plasticity Genes? Molecular Psychiatry. 2009;14(746-754) doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Pleuss M. Beyond risk, resilience, and dysregulation:phenotypic plasticity and human development. Development and Psychopathology. 2013;25:1243–1261. doi: 10.1017/S095457941300059X. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Pleuss M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychological Bulletin. 2009;135(6):885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M, Widaman K. Confirmatory and competitive evaluation of alternative gene-environment interaction hypotheses. Journal of Child Psychology and Psychiatry. 2013;54:1135–1143. doi: 10.1111/jcpp.12075. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Agrawal A, Gaffrey M, Tillman R, Luby J. Serotonin transporter-linked polymorphic region (5-HTTLPR) genotype and stressful life events interact to predict preschool-onset depression: a replication and developmental extension. Journal of Child Psychology and Psychiatry. doi: 10.1111/jcpp.12142. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos K, Fox N, Zeanah C, Nelson C. Effects of early psychosocial deprivation on the development of memory and executive function. Frontiers in Behavioral Neuroscience. 2009;3:1–7. doi: 10.3389/neuro.08.016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF. Infant temperament, parenting, and externalizing behavior in first grade: a test of the differential susceptibility hypothesis. Journal of Child Psychology and Psychiatry. 2008;49:124–131. doi: 10.1111/j.1469-7610.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- Brody G, Beach S, Philibert R, Chen Y, Murry V. Prevention effects moderate the association of 5-HTTLPR and youth risk behavior initiation: Gene Environment hypotheses tested via a randomized prevention design. Child Development. 2009;80:645–661. doi: 10.1111/j.1467-8624.2009.01288.x. [DOI] [PubMed] [Google Scholar]
- Cadoret R, Langbehn D, Caspers K, Troughton E, Yucuis R, Sandhu H, et al. Associations of the serotonin transporter promoter polymorphim with aggressivity, attention deficit, and conduct disorder in an Adoptee Population. Comprehensive Psychiatry. 2003;44(2):88–101. doi: 10.1053/comp.2003.50018. [DOI] [PubMed] [Google Scholar]
- Canli T, Lesch K. Long story short: the serotonin transporter in emotion regulation and social cognition. Nature Neuroscience. 2007;10(9):1103–1108. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carter A, Briggs-Gowan M, Jones S, Little T. The Infant–Toddler Social and Emotional Assessment (ITSEA): Factor structure, reliability, and validity. Journal of Abnormal Child Psychology. 2003;31:495–514. doi: 10.1023/a:1025449031360. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley J, Lesch K, Suomi S. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Cicchetti D. Annual Research Review: Resilient functioning in maltreated children – past, present, and future perspectives. Journal of Child Psychology and Psychiatry. 2013;54(4):402–422. doi: 10.1111/j.1469-7610.2012.02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch F. Gene × Environment interaction and resilience: Effects of child maltreatment and serotonin, corticotropin releasing hormone, dopamine, and oxytocin genes. Development and Psychopathology. 2012;24(2):411–427. doi: 10.1017/S0954579412000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D, Cicchetti D. How and why does the 5-HTTLPR gene moderate associations between maternal unresponsiveness and children’s disruptive problems? Child Dev. 2013 doi: 10.1111/cdev.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas K, Chan G, Gelernter J, Arias A, Anton R, Poling J, et al. 5-HTTLPR as a potential moderator of the effects of adverse childhood experiences on risk of antisocial personality disorder. Psychiatric genetics. 2011;21(5):240–248. doi: 10.1097/YPG.0b013e3283457c15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Gleason M, Theall K, Smyke A, Nelson C, Fox N, et al. Genetic sensitivity to the caregiving context: The influence of 5HTTLPR and BDNF val66met on indiscriminate social behavior. Physiology and Behavior. 2012;106(5):728–735. doi: 10.1016/j.physbeh.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S, Theall K, Smyke A, Keats B, Egger H, Nelson C, et al. Modification of depression by COMT val158met polymorphism in children exposed to early severe psychosocial deprivation. Child Abuse & Neglect. 2010;34:387–395. doi: 10.1016/j.chiabu.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger H, Erkanli A, Keeler G, Potts E, Walter B, Angold A. Test-retest reliability of the Preschool Age Psychiatric Assessment (PAPA) The Journal of the American Academy of Child and Adolesc Psychiatry. 2006;45:538–549. doi: 10.1097/01.chi.0000205705.71194.b8. [DOI] [PubMed] [Google Scholar]
- Ellis B, Boyce W, Belsky J, Bakermans-Kranenburg M, van IJzendoorn M. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Development and Psychopathology. 2011a;23:7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Ellis B, Boyce W, Belsky J, Bakermans-Kranenburg M, van IJzendoorn M. Differential susceptibility to the environment: an evolutionary--neurodevelopmental theory. Development and Psychopathology. 2011b;23(1):7–28. doi: 10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Fox N, Almas A, Degnan K, Nelson C, Zeanah C. The effects of severe psychosocial deprivation and foster care intervention on cognitive development at 8 years of age: findings from the Bucharest Early Intervention Project. Journal of Child Psychology and Psychiatry. 2011;52:919–928. doi: 10.1111/j.1469-7610.2010.02355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliom M, Shaw D. Codevelopment of externalizing and internalizing problems in early childhood. Development and Psychopathology. 2004;16:313–333. doi: 10.1017/s0954579404044530. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wenner JA, Thomas KM, Glatt CE, Mckenna MC, Clark AG. The brain-derived neurotrophic factor Val66Met polymorphism moderates early deprivation effects on attention problems. Development and Psychopathology. 2012;24:1215–1223. doi: 10.1017/S095457941200065X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, et al. Differential susceptibility in youth: evidence that 5-HTTLPR × positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A, Holmes A. Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends in Cognitive Science. 2006;10:182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Homberg J, Lesch K. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Hutchison K, Stallings M, McGeary J, Bryan A. Population stratification in the candidate gene study: Fatal threat or red herring? Psychological Bulletin. 2004;130(1):66–79. doi: 10.1037/0033-2909.130.1.66. [DOI] [PubMed] [Google Scholar]
- Johnson D, Guthrie D, Smyke A, Koga S, Fox N, Zeanah C, et al. Growth and associations between auxology, caregiving environment, and cognition in social deprived Romanian children randomized to foster vs ongoing institutional care. Archives of Pediatric Adolescent Medicine. 2010;164(6) doi: 10.1001/archpediatrics.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel C, Bus A, Van Ijzendoorn M. Differential susceptibility in early literacy instruction through computer games: the role of the dopamine D4 receptor gene (DRD4) Mind Brain and Education. 2011;5:71–78. [Google Scholar]
- Kendler, Kenneth S, Gardner C, Prescott C. Personality and the experience of environmental adversity. Psychological Medicine. 2003;33:1193–1202. doi: 10.1017/s0033291703008298. [DOI] [PubMed] [Google Scholar]
- Knafo A, Israel S, Ebstein RP. Heritability of children’s prosocial behavior and differential susceptibility to parenting by variation in the dopamine receptor D4 gene. Development and Psychopathology. 2011;23(01):53–67. doi: 10.1017/S0954579410000647. [DOI] [PubMed] [Google Scholar]
- Kreek M, Nielsen D, LaForge K. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nature Neuroscience. 2005;8:1450–1457. doi: 10.1038/nn1583. [DOI] [PubMed] [Google Scholar]
- Kreppner J, O’Connor T, Rutter M. The English and Romanian Adoptees Study Team. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. 29. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Stevens S, Brookes K, Schlotz W, Castle J, Beckett C, et al. 5HTT genotype moderates the influence of early institutional deprivation on emotional problems in adolescence: evidence from and English and Romanian Adoptee (ERA) study. The Journal of Child Psychology and Psychiatry. 2010;51:755–762. doi: 10.1111/j.1469-7610.2010.02249.x. [DOI] [PubMed] [Google Scholar]
- Lavigne J, Gibbons R, Christoffel K, Arend R, Rosenbaum D, Binns H, et al. Prevalence rates and correlates of psychiatric disorders among preschool children. Journal of the American Academy of Child & Adolescent Psychiatry. 1996;35:204–214. doi: 10.1097/00004583-199602000-00014. [DOI] [PubMed] [Google Scholar]
- Lesch K, Balling U, Gross J, Strauss K, Wolozin B, Murphy D, et al. Organization of the human serotonin transporter gene. Journal of Neurologic Transmission. 1994;95:157–162. doi: 10.1007/BF01276434. [DOI] [PubMed] [Google Scholar]
- Manuck S, Flory J, Ferrell R, Muldoon M. Socio-economic status covaries with central nervous system serotonergic responsivity as a function of allelic variation in the serotonin transporter gene-linked polymorphic region. Psychoneuroendocrinilogy. 2004;29:651–668. doi: 10.1016/S0306-4530(03)00094-5. [DOI] [PubMed] [Google Scholar]
- Millum J, Emanuel E. The ethics of international research with abandoned children. Science. 2007;318:1874–1875. doi: 10.1126/science.1153822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Ueno S, Sano A, Tanabe H. The human serotonin transporter gene linked polymorphism (5-HTTLPR) shows ten novel allelic variants. Molecular Psychiatry. 2000;5(1):32–38. doi: 10.1038/sj.mp.4000698. [DOI] [PubMed] [Google Scholar]
- Nederhof E, Bouma EMC, Riese H, Laceulle OM, Ormel J, Oldehinkel AJ. Evidence for plasticity genotypes in a gene–gene–environment interaction: the TRAILS study. Genes, Brain and Behavior. 2010;9:968–973. doi: 10.1111/j.1601-183X.2010.00637.x. [DOI] [PubMed] [Google Scholar]
- Nelson C, Fox N, Zeanah C. Romania’s abandoned children: Deprivation, brain development and the struggle for recovery. Harvard Press; Cambridge, MA: (in press) [Google Scholar]
- Nelson C, Zeanah C, Fox N, Marshall P, Smyke A, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Nobile M, Giorda R, Marino C, Carlet O, Pastore V, Vanzin L, et al. Socioeconomic status mediates the genetic contribution of the dopamine receptor D4 and serotonin transporter linked promoter region repeat polymorphisms to externalization in preadolescence. Development and Psychopathology. 2007;19:1147–1160. doi: 10.1017/S0954579407000594. [DOI] [PubMed] [Google Scholar]
- Owens M, Goodyer IM, Wilkinson P, Bhardwaj A, Abbott R, Croudace T, et al. 5-HTTLPR and early childhood adversities moderate cognitive and emotional processing in adolescence. PLoS ONE. 2012;7:e48482. doi: 10.1371/journal.pone.0048482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibert RA, Sandhu H, Hollenbeck N, Gunter T, Adams W, Madan A. The relationship of 5HTT (SLC6A4) methylation and genotype on mRNA expression and liability to major depression and alcohol dependence in subjects from the Iowa Adoption Studies. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2008;147B(5):543–549. doi: 10.1002/ajmg.b.30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roisman G, Newman D, Fraley R, Haltigan J, Groh A, Haydon K. Distinguishing differential susceptibility from diathesis-stress: Recommendations for evaluating interaction effects. Development and Psychopathology. 2012;24:389–409. doi: 10.1017/S0954579412000065. [DOI] [PubMed] [Google Scholar]
- Rutter M, Beckett C, Castle J, Colvert E, Kreppner J, Mehta M, et al. Effects of profound early institutional deprivation: An overview of findings from a UK longitudinal study of Romanian adoptees. European Journal of Developmental Psychology. 2007;4(3):332–350. [Google Scholar]
- Rutter M, Dunn J, Plomin R, Simonoff E, Pickles A, Maughan B, et al. Integrating nature and nurture: Implications of person-environment correlations and interactions for developmental psychopathology. Development and Psychopathology. 1997;9:335–364. doi: 10.1017/s0954579497002083. [DOI] [PubMed] [Google Scholar]
- Shaw D, Owens E, Giovannelli J, Winslow E. Infant and toddler pathways leading to early externalizing disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40:36–43. doi: 10.1097/00004583-200101000-00014. [DOI] [PubMed] [Google Scholar]
- Sheridan M, Drury S, McLaughlin K, Almas A. Early Institutionalization: Neurobiological consequences and genetic modifiers. Neuropsychology Reviews. 2010;20:414–429. doi: 10.1007/s11065-010-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M, Fox N, Zeanah C, McLaughlin K, Nelson C. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences. 2012;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Beach SRH, Brody GH, Philibert RA, Gibbons FX. Social environmental variation, plasticity genes, and aggression: Evidence for the differential susceptibility hypothesis. American Sociological Review. 2011;76:833–912. doi: 10.1177/0003122411427580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons RL, Lei MK, Stewart EA, Beach SRH, Brody GH, Philibert RA, et al. Social adversity, genetic variation, street code, and aggression: A genetically informed model of violent behavior. Youth Violence and Juvenile Justice. 2012;10:3–24. doi: 10.1177/1541204011422087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyke A, Koga S, Johnson D, Fox N, Marshall P, Nelson C, et al. The caregiving context in institution reared and family reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48(2):210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke E, Oades R, Psychogiou L, Chen W, Franke B, Buitelaar J, et al. Dopamine and serotonin transporter genotypes moderated sensitivity to maternal expressed emotion: the case of conduct and emotional problems in attention deficit/hyperactivity disorder. The Journal of Child Psychology and Psychiatry. 2009;50(9):1052–1063. doi: 10.1111/j.1469-7610.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- Sturge-Apple M, Davies P, Martin M, Cicchetti D, Hentges R. An examination of the impact of harsh parenting contexts on children’s adaptation within an evolutionary framework. Developmental Psychology. 2012;48(3):791–805. doi: 10.1037/a0026908. [DOI] [PubMed] [Google Scholar]
- Sulik M, Eisenberg N, Lemery-Chalfant K, Spinrad T, Silva K, Eggum N, et al. Interactions between serotonin transporter gene haplotypes and quality of mothers’ parenting predict the development of children’s noncompliance. Developmental Psychology. 2012;48:740–754. doi: 10.1037/a0025938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi S. How gene-environment interactions shape biobehavioral development: Lessons from the study of rhesus monkes. Research In Human Development. 2004;1:205–222. [Google Scholar]
- Uher R, McGuffin P. The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis. Molecular Psychiatry. 2008;13:131–146. doi: 10.1038/sj.mp.4002067. [DOI] [PubMed] [Google Scholar]
- van Goozen S, Fairchild G, Snoek H, Harold G. The evidence for a neurobiological model of childhood antisocial behavior. Psychological Bulletin. 2007;133:149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn M, Bakermans-Kranenburg M. Differential susceptibility experiments: Going beyond correlational evidence--comment on beyond mental health, differential susceptibility articles. Developmental Psychology. 2012;48:769–774. doi: 10.1037/a0027536. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn M, Bakermans-Kranenburg M, Belsky J, Beach S, Brody G, Dodge K, et al. Gene by environment experiments: a new approach to finding the missing heritability. Nature Reviews Genetics. 2011;12(12):881. doi: 10.1038/nrg2764-c1. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn M, Belsky J, Bakermans-Kranenburg M. Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta-analysis of child and adolescent gene-by-environment studies. Translational Psychiatry. 2012;2:e147. doi: 10.1038/tp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ijzendoorn M, Caspers K, Bakermans-Kranenburg M, Beach S, Philibert R. Methylation matters: interaction between methylation density and serotonin transporter genotype predicts unresolved loss or trauma. Biological Psychiatry. 2010;68(5):405–407. doi: 10.1016/j.biopsych.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderwert R, Marshall P, Nelson C, Zeanah C, Fox N. Timing of intervention affects brain electrical activity in children exposed to severe psychosocial neglect. PLoS ONE. 2010;5:e11415. doi: 10.1371/journal.pone.0011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaske J, Newsome J, Wright JP. Interaction of serotonin transporter linked polymorphic region and childhood neglect on criminal behavior and substance use for males and females. Development and Psychopathology. 2012;24(01):181–193. doi: 10.1017/S0954579411000769. [DOI] [PubMed] [Google Scholar]
- Wichers M, Kenis G, Jacobs N, Mengelers R, Derom C, Vietnick R, et al. The BDNF val(66)met × 5HTTLPR X child adversity interaction on depressive symptoms: an attempt at replication. American Journal of Medical Genetics B. Neuropsychiatric Genetics. 2008;147:120–123. doi: 10.1002/ajmg.b.30576. [DOI] [PubMed] [Google Scholar]
- Yonan A, Palmer A, Gillian T. Hardy-Weinberg disequilibrium identified genotyping error of the serotonin transporter (SLC6a4) promoter polymorphism. Psychiatric genetics. 2006;16(1):31–34. doi: 10.1097/01.ypg.0000174393.79883.05. [DOI] [PubMed] [Google Scholar]
- Zeanah C. Reactive attachment disorder. In: Narrow W, First M, Sirovatka P, Regier D, editors. Age and gender considerations in psychiatric diagnosis: A research agenda for the DSM-V. APA Press; Arlington, VA: 2007. [Google Scholar]
- Zeanah C, Egger H, Smyke A, Nelson C, Fox N, Marshall P, et al. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah C, Fox N, Nelson C. The Bucharest Early Intervention Project: Case study in the ethics of mental health research. Journal of Nervous and Mental Diseases. 2012;200:243–247. doi: 10.1097/NMD.0b013e318247d275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C, Nelson C, Fox N, Smyke A, Marshall P, Parker S, et al. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Mohr C, Spangler G. Genetic and attachment influences on adolescents’ regulation of autonomy and aggressiveness. Journal of Child Psychology and Psychiatry. 2009;50(11):1339–1347. doi: 10.1111/j.1469-7610.2009.02158.x. [DOI] [PubMed] [Google Scholar]