Abstract
The mammalian target of rapamycin (mTOR) senses and incorporates different environmental cues via the two signaling complexes mTORC1 and mTORC2. As a result, mTOR controls cell growth and survival and also shapes different effector functions of the cells including immune cells such as T cells. We demonstrate here that iNKT cell development is controlled by mTORC2 in a cell-intrinsic manner. In mice deficient in mTORC2 signaling due to the conditional deletion of the Rictor gene, iNKT cell numbers were reduced in the thymus and periphery. This is caused by decreased proliferation of stage 1 iNKT cells and poor development through subsequent stages. Functionally, iNKT cells devoid of mTORC2 signaling showed reduced number of IL-4-expressing cells, which correlated with a decrease in the transcription factor GATA-3-expressing cells. However, promyelocytic leukemia zinc-finger (PLZF), a critical transcription factor for iNKT cell development, is expressed at a similar level in mTORC2 deficient iNKT cells compared to that in the wild type iNKT cells. Furthermore, cellular localization of PLZF was not altered in the absence of mTOR2 signaling. Thus, our study reveals the PLZF-independent mechanisms of the development and function of iNKT cells regulated by mTORC2.
INTRODUCTION
The mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase that has a central role in the regulation of cell growth and metabolism (1, 2). mTOR, comprised of two distinct complexes, mTOR complex 1 (mTORC1) and complex 2 (mTORC2), has been studied extensively in variety of biological systems. mTOR integrates a range of different signals such as growth factors, amino acids, nutrients, cytokines and stress factors from the microenvironment in order to ensure not only the delivery of most appropriate immune response during antigen recognition, but also in controlling various other cellular functions involved in cell growth and survival (3, 4). mTORC1 is involved in translation initiation, autophagy inhibition and lipid biosynthesis, whereas mTORC2 promotes actin rearrangement and uptake of nutrients (5). For T cells, antigen recognition together with secondary signals by naïve CD4 and CD8 T cells triggers mTOR activation, which in turn programs their differentiation into functionally distinct lineages (6). Studies have shown a central role of mTOR in determining the effector vs. memory fate of CD8 T cells in infection and tumor immunity (7).
mTORC1 and mTORC2 also regulate TH cell fate (8, 9). TH1 and TH17 cell differentiation requires mTORC1, whereas mTORC2 is essential for TH2 cell generation. However, both the mTOR complexes contribute to the inhibition of Foxp3+ Treg cell differentiation. Although the role of mTOR in T effector cell functions has been studied, little is known about its role in regulating the thymocyte development. A study showed that mTORC2 is essential for proliferation and differentiation of thymic pre T-cells from DN to DP stage, which is driven by Notch signaling through Akt and NF-κB (10).
iNKT cells express a semi-invariant αβ TCR in mice (conserved Vα14-Jα18 paired with a limited repertoire of Vβ chains, mainly Vβ8.2, Vβ7 and Vβ2) and are restricted to or specific for lipids/glycolipids presented by non-polymorphic MHC class I-like CD1d molecule (11). Characteristically, the iNKT cells express promyelocytic leukemia zinc-finger (PLZF), the signature transcription factor of the innate-like T cells, and the natural killer (NK) cell-associated marker NK1.1 (CD161) (12, 13). iNKT cell development and maturation occurs in the thymus, where CD1d-restricted double-positive (CD4+CD8+) thymocytes progress through four different stages - stage 0 (CD24+,CD44−,NK1.1−), stage 1 (CD24−,CD44−,NK1.1−), stage 2 (CD24−,CD44+,NK1.1−) and stage 3 (CD24−,CD44+,NK1.1+) – to develop into mature iNKT cells (14). iNKT cell development requires distinct signaling compared to conventional T cells. Regarding the upstream events of the mTOR signaling (1, 2), it is well established that inappropriate signaling from CD28 (15) and ICOS (16) results in a detrimental effect on NKT cell development. Similar observation has been reported with the potent mTORC1-inducer PI3K and its associated kinase and phosphatase PDK1 and PTEN, respectively (17, 18). In these studies, it is shown that adequate PI3K activity dictates the development and the homeostasis of the iNKT cells. Conversely, two different studies have shown that in mice deficient of TSC1, an mTORC1 suppressor, iNKT population is reduced in size. While one study depicted a massive apoptosis during the iNKT cell lineage expansion (19) in the deficient mice, the other study reported defective terminal iNKT cell differentiation and predominance of NKT-1 effector lineage over NKT-17 (20). TSC1 also promotes iNKT cell anergy in response to antigen stimulation (21). More direct evidence was provided by two recent independent studies that used mice deficient in Raptor that is a component of mTORC1 complex (22, 23). It was clear from both the reports that Raptor deficient mice showed drastically reduced iNKT cell numbers in the thymus and periphery due to defective proliferation of the early iNKT-cell developmental stages. Further, an impaired cytokine production by iNKT cells was also observed (22, 23). These impairments were associated with a defect in the nuclear localization of PLZF. Given the fact that the two mTOR complexes work as a two-signal system and with studies demonstrating a critical role for mTORC1 in iNKT cell development, the question that arises is whether mTORC2 plays a similar role for iNKT cell maturation. It is especially of great interest since at the opposite of mTORC1, upstream events of mTORC2 are yet to be defined. The current study reveals an important role of mTORC2 for iNKT cell development. We demonstrate here that, in the absence of Rictor that is a component of mTORC2, iNKT cell numbers were decreased in a cell intrinsic manner. Unlike mTORC1 deficiency, mTORC2 is required for the proliferation of iNKT cells at the stage 1 to progress to the next stage. Moreover, IL-4 expressing iNKT cells were reduced in Rictor deficient iNKT cells, which is likely caused by a reduction in the expression of transcriptional factor GATA-3. Surprisingly, neither the expression level nor the cellular localization of PLZF was compromised in Rictor deficient iNKT cells. Therefore, we report that mTORC2 regulates iNKT cell development independent of PLZF.
MATERIALS AND METHODS
Mice
C57BL/6NCr were purchased from the NCI (01C55) and mice deficient in Rictor in T cells (RictorCD4Cre fl/fl) have been described earlier (9). B6.SJL-Ptpcra/BoyAiTac mice (CD45.1 congenic, 004007) were purchased from Taconic Farms. β-Catenin transgenic (CATtg) mice described previously (24) express β-catenin in thymocytes and T cells under the control of proximal Lck promotor. β-CAT-Tg mice were bred with RictorCD4Cre fl/fl to generate Rictor-knockout (KO)-β-CAT-Tg mice. All the mice were bred and maintained under specific pathogen-free conditions at the University of Michigan animal facility and used at 8 to 12 weeks of age. All animal experiments were performed under protocols approved by the University of Michigan Institutional Animal Care and Use Committee.
Bone marrow chimera mice
C57BL/6 recipient mice were lethally irradiated (950 rad) 24 hours before receiving bone marrow (BM) transfers. Total BM cells were harvested from the femurs and tibias of donor mice (2–3 months of age) and depleted of mature T cells, B cells, and MHC class II-positive lymphocytes by using a cocktail of antibodies against CD4 (RL172), CD8 (TIB210), CD19 (1D3), and MHC class II (M5/114), followed by complement-mediated lysis. BM cells from RictorCD4Cre fl/fl and B6.SJL-Ptpcra/BoyAiTac donor mice were then mixed at a ratio of 1:1, and each recipient mouse received 5 × 106 cells in 200 µl of 1× PBS via tail vein. Recipient mice were housed in a barrier facility under pathogen-free conditions before and after bone marrow transplantation. After bone marrow transplantation, mice were provided autoclaved acidified water with antibiotics (trimethoprim-sulfamethoxazole) and were fed autoclaved food. Mice were analysed 12 to 16 weeks later.
Cell preparation and flow cytometry
Primary cell suspensions were prepared from thymi, spleens and livers. To prepare liver mononuclear cells, livers were perfused with 1× PBS solution via the portal vein until most of the RBCs were washed out. Tissues were then mechanically disrupted between two frosted microscope slides and resultant homogenates were filtered through a 70-µm filter. Splenic and thymic erythrocytes were lysed in 1.66% NH4Cl solution (at room temperature for 6 minutes). Liver cells were resuspended in a 40% isotonic Percoll solution and a 70% percoll solution was carefully underlain. After centrifugation at 900g at room temperature with no brakes for 30 minutes, liver mononuclear cells were then harvested at the interface of the two layers of Percoll, washed and resuspended.
Up to 10 × 106 cells per sample were resuspended in 200 µl of FACS buffer (2% FBS, 1× PBS) in FACS tubes and preincubated with anti-FcγR mAb 2.4G2 to block nonspecific antibody binding. Cells were then washed and stained for surface molecules expression with FITC-, PE-, PerCP-Cy5.5-, PE-Texas Red-, PeCy-7, APC-, APC-Cy7-, Pacific Blue- or biotin-conjugated antibodies. Following antibodies were purchased from eBioscience: CD4 (GK1.5), CD5 (53-7.3), CD8a (53-6.7), CD44 (IM7), CD45.1 (A20), NK1.1 (PK136), IFN-γ (XMG1.2), IL-4 (BVD6-24G2), Ki-67 (20Raj1), TCR-β (H57-597). Antibodies against CD24 (M1/69), CD45.2 (104), IL-17 (TC11-18H10) were purchased from BD Pharmingen. Conjugated monoclonal antibodies against PLZF (Mags.21F7) were gratefully provided by Prof. Derek Sant’Angelo. APC- or Pacific Blue-conjugated murine CD1d tetramers loaded with PBS-57 were provided by the National Institutes of Health Tetramer Facility.
For intracellular staining, post surface staining, the cells were fixed in 4% paraformaldehyde solution for 10 minutes at room temperature, washed and then permeabilized with 0.2% saponin. Cytokine staining was performed for 30 minutes at 4 °C directly with appropriate antibodies. For intranuclear staining, surface stained cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining Buffer Kit from Ebioscience, according to manufacturer’s recommendation and then stained with corresponding antibodies for one hour at 4°C. Cell fluorescence was assessed using FACSCanto II (BD Bioscience) and data were analysed with FlowJo software (version 9, Tree Star, Ashland, OR). For analysis, forward- and side scatter parameters were used for exclusion of doublets.
In vitro stimulation assay
Freshly isolated cells (10 × 106 cells at a concentration of 5 × 106 cells/ml) were stimulated with 50 ng/ml of phorbol myristyl acetate (PMA; Sigma-Aldrich) and 500 ng/ml of ionomycin (Sigma-Aldrich), in complete RPMI media (RPMI 1640, 10% FBS, antibiotics, β-mercaptoethanol). Stimulation was performed for 5 hours in the CO2 incubator in FACS tubes with loosened caps. Monensin (Sigma-Aldrich) at a final concentration of 3 µM was added during the last 3 hours of stimulation. Cells were then washed twice in FACS buffer (2% FBS, 1× PBS) and stained for flow cytometry analysis as described above.
In vivo BrdU incorporation
Mice were injected intraperitoneally with 0.3 ml of 1 mg/ml BrdU solution (Sigma-Aldrich) every 4 hours for three times. 18 hours after the last injection, mice were euthanized and thymi were processed to obtain primary cell suspension as described earlier. Following surface staining, cells were stained for BrdU incorporation using BrdU flow kit (BD bioscience), as per manufacturer’s instructions.
Apoptosis assay
Apoptosis rate of freshly isolated thymic cells was studied by flow cytometry using a PE-conjugated Annexin V antibody and Apoptosis Detection Kit (eBioscience), following the manufacturer’s recommendation.
Statistical analysis
Data for all experiments were analysed with Prism software (GraphPad, San Diego, CA). Unpaired Student’s t-test was used for comparison of experimental groups. P values < 0.05 (*), < 0.01 (**), < 0.001 (***) were considered statistically significant.
RESULTS
Rictor controls T cell development
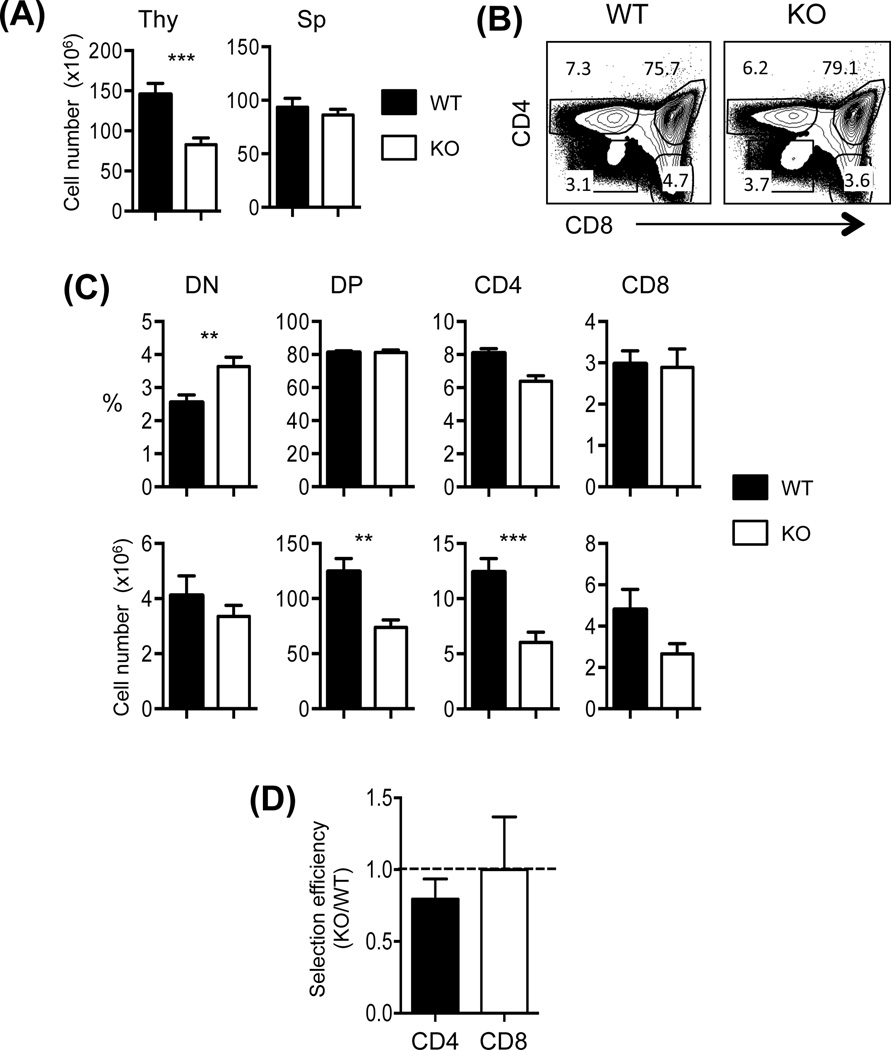
To study the role of mTORC2 in iNKT cell development, we used Rictor conditional knock out mice (RictorCD4Cre fl/fl: designated T-Rictor−/−) that were previously reported (9). CD4 promoter driven Cre was used to study the effect of Rictor deficiency during T cells development. We first measured the total cell numbers in the thymus and the spleen from T-Rictor−/− mice and compared them with the wild type (WT) mice. We observed decreased cell numbers in the thymus but not in the spleen of T-Rictor−/− mice compared to the WT (Fig. 1A). We then assessed and compared the subsets of thymocytes between the T-Rictor−/− and WT mice by analysing the percentages and cell numbers of DN, DP, SPCD4 and SPCD8 cells. Interestingly, while the percentages of thymic DP, SPCD4 and SPCD8 was comparable between the two groups (Figs. 1B and 1C), careful examinations of these sub-populations revealed that the total cell numbers of thymic DP and SPCD4 T cells were significantly lower in the T-Rictor−/− mice (Fig. 1C). Next we calculated and compared the selection efficiency of SPCD4 and SPCD8 cells using the ratio of the percentages of cells of interest to the percentages of DP (Fig. 1D). A ratio of 1 would indicate the equivalent selection efficiency between T-Rictor−/− and WT cells. The data demonstrated that Rictor plays a modest role for SPCD4 T cell development while SPCD8 T cell development was not affected in T-Rictor−/− mice.
Figure 1. Rictor controls T cell development.
(A) The numbers of total thymocytes (Thy) and splenocytes (Sp) from T-Rictor−/− (KO) and wild type (WT) mice were compared. (B) Representative CD4 and CD8 profiles of total thymocytes. Numbers in the dot plots indicate the percentages of gated cells. (C) Summary of each subset of thymocytes in the percentages and numbers as gated in (B). (D) Comparison of selection efficiency SPCD4 and SPCD8 cells. Efficiency of the KO cells to the WT cells was calculated using the formula [% cell type of interest of KO/% DP of KO]/[% cell type of interest of the WT/% DP of the WT]. Dotted line indicates the ratio of 1. Data shown are meanSEM from 12 pairs of mice. **p<0.01; ***p<0.001.
Rictor is important for iNKT cell development and homeostasis
Next we assessed iNKT cell development in Rictor-deficient mice. We compared the iNKT cell compartment in the thymus, spleen and liver between the T-Rictor−/− and WT mice. The percentages as well as the cell numbers of CD1d-tetramer positive iNKT cells were greatly reduced in the T-Rictor−/− thymus (Fig 2A and B). In contrast to the conventional T cells for which the developmental defect is limited to the thymus, both the percentages and the cell numbers of iNKT cells were significantly decreased in the spleen as well as in the liver of the T-Rictor−/− mice (Fig. 2 A and B).
Figure 2. Stage specific defect in NKT cell development in T-Rictor−/− mice.
(A) Representative TCRβ and CD1d tetramers profiles of cells from the thymus, spleen and liver were compared between T-Rictor−/− (KO) and WT mice. Numbers in the dot plots indicate the percentages of iNKT cells. (B) Summary of iNKT cell percentages (top) and numbers (bottom) in indicated tissues. (C) Representative CD1d tetramers and CD24 profiles of total thymocytes derived from KO and WT thymus (left). CD24- cells were further analyzed for Stages 1–3 using CD44 and NK1.1 (right). (D) The percentages and cell numbers of iNKT cells at each stage were shown. (E) Selection efficiency of Stage 0 cells from DP. Numbers of Stage 0 (S0) cells were divided by the numbers of DP from WT and KO mice. (F) Stage specific cell generation. Cell numbers of each stage were divided by the numbers of the previous stage. S0, S1, S2, and S3 indicate Stage 0, Stage 1, Stage 2, and Stage 3. The inset shows S1/S0. Data shown are meanSEM from 12 pairs of mice. *p<0.05; **p <0.01; ***p<0.001; ****p<0.0001.
iNKT cell development occurs as the CD1d-restricted immature progenitor DP cells progress through four different stages defined by the expression of surface molecules CD24, CD44 and NK1.1 (Fig 2C). When we examined the iNKT maturation in the thymus, we observed that the percentages of CD24−CD44+NK1.1− Stage 2 iNKT cells were greatly reduced, whereas CD24−CD44−NK1.1− Stage 1 iNKT cells were increased (Fig 2D, upper panels). CD24+ Stage 0 and CD24−CD44+NK1.1+ Stage 3 iNKT cells did not show significant differences. However, the cell numbers in all stages except Stage 1 were reduced in the T-Rictor−/− thymus, which resulted in the lower number of total thymic iNKT cells in T-Rictor−/− mice as compared to the WT (Fig 2B and 2D). Thus, Rictor participates in iNKT cell development in the thymus.
To further investigate a possible role of Rictor during iNKT cell development, we compared the generation of Stage 0 cells from DP because this step would be equivalent to positive selection of iNKT cells and subsequent stages reflect proliferation and maturation of selected iNKT cells. The results showed that although Stage 0 cell numbers were reduced in T-Rictor−/− mice, the selection efficiency was comparable between WT and T-Rictor−/− mice likely due to the reduced DP in T-Rictor−/− mice (Fig 2E). Next we compared the transition from Stage 0 to 1 (S1/S0), Stage 1 to 2 (S2/S1), and Stage 2 to 3 (S3/S2) using the cell numbers at each Stage (Fig. 2F). As expected, WT iNKT cells showed an increase in cell numbers at each transition period during maturation. In contrast, Stage 1 to 2 transition of T-Rictor−/− iNKT cells was impaired, while Stage 0 to 1 and 2 to 3 were similar to the WT. Together, mTOR is indispensable for Stage 0 iNKT cells to develop into Stage 1 iNKT cells.
Cell-intrinsic defect of Rictor-deficient iNKT cells
To study if the developmental defect of Rictor KO iNKT cells is cell-intrinsic, we performed mixed bone marrow (BM) chimera experiments. Equal proportions of Rictor KO (CD45.2) BM cells were transferred together with WT (CD45.1/CD45.2) BM cells to irradiated hosts (CD45.1) and analysed for T cell development. Rictor KO cells were poorly reconstituted in the thymus as shown by the percentages and the recovery of total cells from each donor (Fig 3A). We then compared the iNKT compartment in the thymus, spleen and liver. Very few iNKT cells from Rictor KO donor were detected in the thymus as well as in the spleen or liver of the chimeric mice (Fig. 3B). Moreover, similar to our observations from Rictor KO mice, the thymic selection efficiency was lowest for iNKT cells followed by CD4 T cells, whereas the efficiency of Rictor KO CD8 T cells was slightly reduced (Fig 3C). Together, our data demonstrate that Rictor controls iNKT development in a cell-intrinsic manner.
Figure 3. Cell intrinsic defect in the development of T-Rictor−/− mice.
(A) Representative CD45.1 and CD45.2 profiles of total thymocytes derived from BM chimeras. BM cells from T-Rictor−/− (KO) mice (CD45.2+) were co-transferred with WT BM (CD45.1+CD45.2+) into B6.SJL hosts (CD45.1+). The graph shows the recovery ratio of KO (CD45.2+) over WT (CD45.1+CD45.2+) total thymocytes. (B) Representative TCRβ and CD1d tetramers profiles of total thymocytes from a chimera. The graph shows the recovery ratio of KO iNKT cells to the WT iNKT cells from indicated tissues, using the formula [% of KO iNKT / % of the WT iNKT]. (C) Representative CD4 and CD8 profiles of total thymocytes from BM chimeras. Numbers in the dot plots indicate the percentages of corresponding quadrants. The graph shows the ratio of KO cell selection efficiency to the WT cell selection efficiency, using the formula [% of KO/ % of the WT] for CD4, CD8 and iNKT cells. Data shown are meanSEM from 5 chimeras.
Rictor is required for optimum proliferation of stage 1 iNKT cells
A reduced thymic iNKT cell output in the absence of Rictor could be explained by at least two independent and non-exclusive possibilities. First, Rictor-deficient cells might undergo a higher rate of spontaneous apoptosis. Alternatively, iNKT lineage expansion is reduced in the absence of Rictor. To test these two possibilities, we first assessed apoptotic cell death by staining cells for annexin V and 7-AAD. In WT mice, the most immature stage 0 iNKT cells showed greater cell proportion that underwent apoptosis (Annexin V+ 7-AAD−) than stages 1–3 (Fig. 4A). Although the percentages of apoptotic immature iNKT cells, namely stage 0 cells, were slightly lower in the T-Rictor−/− mice, the difference was not statistically significant (Fig. 4A). T-Rictor−/− iNKT cells in stages 1 to 3 had comparable fractions of apoptotic cells to the wild type iNKT cells. Furthermore, both WT and T-Rictor−/− iNKT cells showed similar proportions of apoptotic cells after stimulation with anti-CD3 suggesting that cell death was not altered in T-Rictor−/− mice (data not shown). Overall, our data suggest that an increased rate of spontaneous apoptosis in absence of functional mTORC2 is unlikely to explain the reduced iNKT population observed in T-Rictor−/− mice.
Figure 4. Proliferation of Stage 1 iNKT cells requires Rictor.
(A) Representative Annexin-V and 7-AAD profiles of iNKT cells at each stage. Lower panel summarizes the percentages of Annexin-V+ cells from 4 pairs of mice. (B) Ki-67 expression profiles were compared among different stages of iNKT cells. Numbers in the histograms indicate the percentages of Ki-67+ cells. (C) Gradual loss of proliferative capacity was compared between T-Rictor−/− (KO) and WT iNKT cells. (D) BrdU+ thymic NKT cells at each stage are shown. Data shown are meanSEM from total 4 pairs of mice. *p<0.05; **p <0.01
We next measured the proliferation capacity of T-Rictor−/− cells by using two approaches. First, we measured the expression of Ki-67 in each stage of cells to assess the cell cycling activity reflecting proliferation. It is reported that Stage 1 iNKT cells undergo high proliferation while the fully mature Stage 3 cells are quiescent in the absence of stimuli (11, 25). Consistent with the reported studies, our data demonstrated that most of Stage 0 iNKT cells from WT mice were Ki-67 positive indicating that they were in an active phase of their cell cycle (Fig. 4B). As iNKT cells matured, a significant fraction of cells became Ki-67 negative and Stage 3 cells were mostly Ki-67 negative. T-Rictor−/− iNKT cells showed a similar pattern of Ki-67 positive cells except that Stage 1 cells had a significant reduction of Ki-67 positive cells compared to WT (Fig. 4B and 4C), indicating that the stage 1 iNKT cells in T-Rictor−/− mice might undergo decreased rates of proliferation. To further confirm this difference in cell cycling activity, we performed in vivo BrdU incorporation experiments. Consistent with the Ki-67 data, Stage 1 iNKT cells from the T-Rictor−/− thymus showed significantly lower percentage of BrdU+ cells than those from the WT thymus (Fig. 4D). Thus, these results indicate that Rictor is important for Stage 1 iNKT cells to proliferate and progress to the stage 2 during iNKT cell maturation.
It is known that Wnt signaling pathway functions similarly to mTOR and overexpression of active de-phosphorylated form of β-catenin leads to a significant increase in iNKT cells (26). This raises a question as to whether Wnt and mTOR signaling may regulate each other so that constitutively active Wnt signaling may restore the defect of T-Rictor−/− iNKT cell development. To test this, we crossed T-Rictor−/− mice with the transgenic mice expressing active de-phosphorylated form of β-catenin (CATtg) resulting in CATtgxKO. We found that iNKT cell development in CATtgxKO mice was not rescued (Supplemental Fig. 1), suggesting that mTORC2 and Wnt signaling pathway seems to work independent of each other.
Rictor regulates the generation of iNKT cells expressing IL-4 but not IFN-γ or IL-17
Having observed that the iNKT cell maturation is controlled by mTORC2, we next asked if the Rictor deficiency also affects the effector function of iNKT cells. iNKT cells are defined by their ability to secrete various cytokines like IL-4, IFN-γ and IL-17. In addition, previous studies have demonstrated that cytokine production varies with thymic iNKT cell development and these cells produce different cytokines at maximum levels at different developmental stages (27–29). Rictor/mTORC2 has been shown to be important for TH2 cell differentiation but not for TH1 or TH17 phenotypes in conventional CD4 T cells. We therefore asked whether Rictor also regulates the generation of IL-4 expressing iNKT cells. To test this, thymocytes including the thymic iNKT cells were stimulated with PMA and ionomycin and then the expression of IL-4, IFN-γ and IL-17 was measured by intracellular cytokine staining. Assessment of the cytokine expression showed that IL-4- but not IFN- γ– or IL-17-expressing cells were decreased in iNKT cells from T-Rictor−/− mice compared to that in WT iNKT cells (Fig. 5A and 5B).
Figure 5. Rictor controls the generation of IL-4-expressing iNKT cells.
(A) Total thymocytes including thymic iNKT cells were stimulated for 5 hr as described in the Experimental Procedures. IL-4 and IFN-γ (left group) and IL-17 and IFN-γ (right group) expression from total NKT cells are shown. Numbers in the dot plots indicate the percentages of corresponding quadrants. (B) Summary of iNKT cells expressing indicated cytokines. Data shown are meanSEM from 7 pairs of mice.
Considering cytokine expression has been reported to be maturation dependent, we further assessed the cytokine production at each stage of iNKT cells. IL-4 expressing cells were reduced in all the stages of iNKT development in the T-Rictor−/− mice compared to the WT mice, whereas a modest decrease in IFN-γ expressing cells was observed only in stage 2 T-Rictor−/− iNKT cells (Supplemental Fig. 2). Highest levels of IL-17 was produced by stage 2 cells in both the strains and interestingly, and a significantly higher percentage of IL-17+ Stage 2 iNKT cells were observed in T-Rictor−/− mice. However, Stage 2 cells represent a very small fraction of total iNKT cells and therefore overall IL-17 expression pattern was comparable between WT and T-Rictor−/− mice. Together, our data on the cytokine expression indicates that Rictor primarily controls the differentiation of IL-4-expressing iNKT cells.
Rictor deficiency results in decreased GATA-3 expressing cells
It is known that PLZF is the most critical transcription factor for the development as well as IL-4 expression of iNKT cells as illustrated in PLZF−/− mice (12). In addition, PLZF overexpression was shown to induce IL-4 production in CD4 T cells (30). Therefore, we asked whether Rictor controls the expression of PLZF which in turn affects iNKT cell development and IL-4 expression. Expression of PLZF in total iNKT cells as well as at each stage of iNKT cell development showed that the absence of Rictor did not interfere with the expression of PLZF (Fig. 6A). However, it is possible that PLZF function may be compromised in the absence of Rictor. In fact, PLZF localization but not the expression level was found to be affected in mTORC1 deficient iNKT cells (22). We found that the cellular distribution of PLZF was similar between the WT and T-Rictor−/− iNKT cells, which was mostly confined to the nucleus (Fig. 6B). Thus, unlike mTORC1 deficiency, absence of Rictor does not affect either the PLZF expression or its nuclear localization.
Figure 6. Reduction of GATA-3-expressing iNKT cells in the absence of Rictor.
(A) Intracellular PLZF expression in each stage of thymic NKT cells was compared. (B) Intracellular PLZF (red) staining of FACS-sorted thymic iNKT cells from WT and T-Rictor−/− mice. Nuclei were stained with DAPI (blue). (C) PLZF, RORγt, T-bet and GATA-3 expression was analyzed in thymic iNKT cells and (D) summary of cells expressing GATA-3 are shown. Data shown are meanSEM from 3 pairs of mice.
It is known that the transcription factors T-bet, GATA-3 and RORγt control the differentiation of Th1, Th2 and Th17 cells, respectively. Furthermore, the same transcription factors are expressed in iNKT cells generating functionally distinct iNKT cells (31). Therefore, we compared the expression of T-bet, GATA-3 and RORγt. There was no difference in RORγt- or T-bet-expressing cell populations between the two groups (Fig. 6C). However, the frequencies of GATA-3-expressing iNKT cells were significantly lower among the T-Rictor−/− iNKT cells than the WT cells (Figs. 6C and D). Therefore, the reduction in GATA-3 expressing iNKT cells in T-Rictor−/− mice correlated with less IL-4+ iNKT cells observed. Together, Rictor controls the generation of GATA3-expressing iNKT cells, which in turn is responsible for differentiation of IL-4 producing cells.
DISCUSSION
In the current study, we reveal a critical role of mTORC2 in the iNKT development and maturation. We observed that, in the absence of functional mTORC2 due to Rictor deficiency in the T cells, early iNKT cell development is impaired in the thymus as well as in the peripheral organs including the spleen and the liver. Although mTORC1 deficiency also results in reduced iNKT cell numbers, mTORC1 and mTORC2 appear to play a different role during iNKT cell development. In mTORC1 deficient mice, the percentages of Stage 0 and 1 iNKT cells are higher while the percentage of Stage 3 iNKT cells were greatly reduced (22, 23). In contrast, we observed that iNKT cells from T-Rictor−/− mice display a Stage 1 specific defect in proliferation. T-Rictor−/− iNKT cells showed the higher percentage of Stage 1 cells but significantly lower level of Stage 2 cells compared to the wild type. Stage 3 cells were comparable between the two groups of mice.
This indicates that the initial stages of iNKT development require both mTORC1 and mTORC2 and, as cells mature, mTORC2 is especially critical for the transition from Stage 1 to Stage 2. Interestingly, more mature long-term resident thymic Stage 3 iNKT subset seems to depend solely on mTORC1 for their survival. This differential dependence on the two mTOR complexes by the different developmental stages of iNKT cells may be dictated by their different metabolic needs as they proceed through the maturation process thus changing their responsiveness to either mTORC1 or mTORC2. A more striking difference was revealed with PLZF. Unlike mTORC1 deficient NKT cells in which PLZF nuclear localization was impaired, we observed no difference either in the expression level or the cellular localization of PLZF in iNKT cells deficient of Rictor. Thus, mTORC1 and mTORC2 control the iNKT cell development and maturation by mechanisms dependent and independent of PLZF, respectively.
Nevertheless, there appear to be similarities between mTORC1 and mTORC2 mediated iNKT cell development. Both mTORC1 and mTORC2 indeed incorporate upstream proliferation signals and thus promote the cell cycling activity and cell proliferation (Fig. 4) Deficiency in either mTORC1 or mTORC2 would lead to poor proliferation. Interestingly, we report here that lack of functional mTORC2 induces a greater developmental defect in iNKT cells than in conventional CD4 and CD8 T cells. Given the fact that iNKT lineage cells undergo two consecutive rounds of expansion in the thymus (DN to DP transition and then Stage 0 to Stage 2 for iNKT commitment) to become fully mature and functional cells, it is not surprising that iNKT cells rely more on mTORC1 and mTORC2 signaling. Conventional CD4 T cells on the other hand undergo the second round of expansion during differentiation into the helper T cell subsets in the periphery. During this differentiation and expansion stage, the two mTOR complexes have been shown to be requisite regulators for different helper T cell lineage numbers and function.
It is well established that mTORC1 and mTORC2 signals promote TH1 and TH2 responses, respectively, in conventional CD4 T cells (9). Unlike conventional CD4 TH cell differentiation programming that is initiated upon antigen exposure in the periphery, iNKT cells express cytokines during their differentiation and maturation in the thymus (27). It is known that Stage 1 cells express IL-4 and then both IL-4 and IFN-γ are produced by more mature Stage 2 and 3 cells. Therefore, decreased Stage 1 cell proliferation would lead to the reduction of IL-4+ but not IFN-γ+ cells, which we have shown here. In line with this assumption, absence of functional mTORC2 in iNKT cells greatly decreases IL-4+ cells particularly in Stage 2 and 3 (Figure 5 and Supplemental Figure 1). A recent study proposed simultaneous generations of three effector types of NKT cells, which are designated NKT1, NKT2, and NKT17 (31). According to this new model, mTORC2 signaling is important for the differentiation of NKT2 but not NKT1 and NKT17, which mirrors the requirement of mTORC2 signaling for TH2 but not TH1 or TH17 cells. The main difference is that development and effector iNKT cell generation is tightly linked and occurs in the thymus.
In conclusion, mTORC2, together with mTORC1, is an indispensible modulator of iNKT cell development and function by promoting the active proliferation during iNKT cell maturation in the thymus. Further studies to identify signaling molecules and pathways that regulate the mTOR complexes should shed light on the molecular mechanisms to control iNKT cell development.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Derek B. Sant'Angelo (Rutgers, The State University of New Jersey) for Alexa Fluor 488- and PE–conjugated monoclocal antibody to PLZF and critical reading of the manuscript. We also thank Dr. Philip King (U. Michigan) for thoughtful comments on the manuscript. CD1d tetramers were provided by the National Institutes of Health Tetramer Facility.
This work was supported in part by National Institutes of Health Grants AI073677 (to C.-H.C.), AI077610 (to J.P.) and the Intramural Research Program of the National Institute on Aging at the NIH (to J.S.).
REFERENCES
- 1.Chi H. Regulation and function of mTOR signaling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–338. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Waickman AT, Powell JD. mTOR, metabolism, and the regulation of T-cell differentiation and function. Immunological reviews. 2012;249:43–58. doi: 10.1111/j.1600-065X.2012.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delgoffe GM, Powell JD. mTOR: taking cues from the immune microenvironment. Immunology. 2009;127:459–465. doi: 10.1111/j.1365-2567.2009.03125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 6.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Q, Rao RR, Araki K, Pollizzi K, Odunsi K, Powell JD, Shrikant PA. A central role for mTOR kinase in homeostatic proliferation induced CD8+ T cell memory and tumor immunity. Immunity. 2011;34:541–553. doi: 10.1016/j.immuni.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee K, Nam KT, Cho SH, Gudapati P, Hwang Y, Park DS, Potter R, Chen J, Volanakis E, Boothby M. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J Exp Med. 2012;209:713–728. doi: 10.1084/jem.20111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 12.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, Chua K, Eidson M, Kim HJ, Im JS, Pandolfi PP, Sant'Angelo DB. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9:1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, Lantz O, Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202:485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams JA, Lumsden JM, Yu X, Feigenbaum L, Zhang J, Steinberg SM, Hodes RJ. Regulation of thymic NKT cell development by the B7-CD28 costimulatory pathway. J Immunol. 2008;181:907–917. doi: 10.4049/jimmunol.181.2.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbari O, Stock P, Meyer EH, Freeman GJ, Sharpe AH, Umetsu DT, DeKruyff RH. ICOS/ICOSL interaction is required for CD4+ invariant NKT cell function and homeostatic survival. Journal of immunology. 2008;180:5448–5456. doi: 10.4049/jimmunol.180.8.5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finlay DK, Kelly AP, Clarke R, Sinclair LV, Deak M, Alessi DR, Cantrell DA. Temporal differences in the dependency on phosphoinositide-dependent kinase 1 distinguish the development of invariant Valpha14 NKT cells and conventional T cells. J Immunol. 2010;185:5973–5982. doi: 10.4049/jimmunol.1000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishimoto H, Ohteki T, Yajima N, Kawahara K, Natsui M, Kawarasaki S, Hamada K, Horie Y, Kubo Y, Arase S, Taniguchi M, Vanhaesebroeck B, Mak TW, Nakano T, Koyasu S, Sasaki T, Suzuki A. The Pten/PI3K pathway governs the homeostasis of Valpha14iNKT cells. Blood. 2007;109:3316–3324. doi: 10.1182/blood-2006-07-038059. [DOI] [PubMed] [Google Scholar]
- 19.Yang K, Neale G, Green DR, He W, Chi H. The tumor suppressor Tsc1 enforces quiescence of naive T cells to promote immune homeostasis and function. Nat Immunol. 2011;12:888–897. doi: 10.1038/ni.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Yang J, Yang K, Wang H, Gorentla B, Shin J, Qiu Y, Que LG, Foster WM, Xia Z, Chi H, Zhong XP. iNKT cells require TSC1 for terminal maturation and effector lineage fate decisions. J Clin Invest. 2014;124:1685–1698. doi: 10.1172/JCI69780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu J, Shin J, Xie D, Wang H, Gao J, Zhong XP. Tuberous sclerosis 1 promotes invariant NKT cell anergy and inhibits invariant NKT cell-mediated antitumor immunity. J Immunol. 2014;192:2643–2650. doi: 10.4049/jimmunol.1302076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin J, Wang S, Deng W, Wu J, Gao J, Zhong XP. Mechanistic target of rapamycin complex 1 is critical for invariant natural killer T-cell development and effector function. Proc Natl Acad Sci U S A. 2014;111:E776–E783. doi: 10.1073/pnas.1315435111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Tschumi BO, Corgnac S, Ruegg MA, Hall MN, Mach JP, Romero P, Donda A. Mammalian target of rapamycin complex 1 orchestrates invariant NKT cell differentiation and effector function. J Immunol. 2014;193:1759–1765. doi: 10.4049/jimmunol.1400769. [DOI] [PubMed] [Google Scholar]
- 24.Mulroy T, Xu Y, Sen JM. beta-Catenin expression enhances generation of mature thymocytes. International immunology. 2003;15:1485–1494. doi: 10.1093/intimm/dxg146. [DOI] [PubMed] [Google Scholar]
- 25.Godfrey DI, Stankovic S, Baxter AG. Raising the NKT cell family. Nature immunology. 2010;11:197–206. doi: 10.1038/ni.1841. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, Chen Q, Nguyen T, Yu Q, Sen JM. T cell factor-1 and beta-catenin control the development of memory-like CD8 thymocytes. J Immunol. 2012;188:3859–3868. doi: 10.4049/jimmunol.1103729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benlagha K, Kyin T, Beavis A, Teyton L, Bendelac A. A thymic precursor to the NK T cell lineage. Science. 2002;296:553–555. doi: 10.1126/science.1069017. [DOI] [PubMed] [Google Scholar]
- 28.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(−)CD4(+) CD1d-dependent precursor stage. The Journal of experimental medicine. 2002;195:835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE, Gapin L, Kronenberg M, Locksley RM. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. The Journal of experimental medicine. 2003;198:1069–1076. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovalovsky D, Alonzo ES, Uche OU, Eidson M, Nichols KE, Sant'Angelo DB. PLZF induces the spontaneous acquisition of memory/effector functions in T cells independently of NKT cell-related signals. J Immunol. 2010;184:6746–6755. doi: 10.4049/jimmunol.1000776. [DOI] [PubMed] [Google Scholar]
- 31.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.