Abstract
Background
The level of sustained glycemic control in patients with diabetes mellitus (DM) is a major determinant of the occurrence of both acute and chronic complications. However, information about the level of glycemic control among patients in the follow-up care at the University of Gondar Referral Hospital is scanty. The study assessed the degree of glycemic control and associated factors among diabetic patients in the study area.
Method
A hospital-based cross-sectional study was conducted at the University of Gondar Referral Hospital. All diabetic patients aged ≥18 years who visited the Diabetes Clinic in January and February 2013 for follow-up medical evaluation and medication participated in the study. Patients with glycosylated hemoglobin test (HbA1c) of ≥7% were classified as having a poor level of glycemic control. Propensity score was used to estimate the treatment effect. Multivariable logistic regression analysis was applied to determine the associated factors.
Result
Two hundred and fifty three (64.7%) of the 391 diabetic patients included in the study had a poor level of glycemic control, as evidenced by HbA1c ≥7%. Poor glycemic control was much higher among Type 1 patients (82.9%) compared with Type 2 patients (57.5%). Being on insulin treatment (AOR =2.51; 95% CI =1.25, 5.04) and reporting poor medication adherence (AOR =3.19; 95% CI =1.76, 5.80) were found to be associated with poor glycemic control among Type 2 DM patients. High waist circumference was inversely associated with a poor level of glycemic control in Type 1 DM patients (AOR =0.05; 95% CI =0.01, 0.85).
Conclusion
The proportion of diabetic patients with a poor level of glycemic control is high. We recommend a comprehensive intervention to improve the overall treatment adherence with special attention to DM patients receiving insulin.
Keywords: glycemic control, HbA1c, waist circumference, diabetes mellitus, BMI
Background
Developing countries are currently undergoing one of the most rapid epidemiological transitions due to urbanization and changing lifestyles that are associated with the increasing occurrence of diabetes mellitus (DM).1 It is a well-known fact that diabetic patients can live longer with diabetes and that the occurrence of chronic complications that lead to deterioration of the patients’ quality of life and even death can be much delayed through access to comprehensive medical care and achieving a sustained level of good glycemic control.2 The proper use of effective medications over a sustained period and a recommended change of lifestyle have been shown to be crucial for the success of glycemic control in the management of DM.3 Adequate diabetes care has a wider and more comprehensive scope than merely aiming for glycemic control; it also aims at preventing and treating life-threatening end organ damage due to DM. By doing so, adequate diabetes care can prevent premature death.4 However, in routine clinical practice in developing countries, it is very challenging not only to manage end organ damage due to DM but also to achieve sustained glycemic control.3
Studies have shown that sustained poor glycemic control as defined by glycosylated hemoglobin level (HbA1c) ≥7% is a major risk factor for the occurrence of DM-related chronic complications. And HbA1c is a simple blood test that provides useful information on the average blood glucose control over a 6-to-12-week period.3,5–7 Despite the fact that DM is believed to be an emerging and huge health care challenge in developing countries, very little has been studied about the Ethiopian situation to help policy makers and stakeholders initiate evidence-based planning and suitable interventions to combat DM. We conducted a cross-sectional study to assess the level of glycemic control and its associated factors among DM patients receiving diabetes care at the University of Gondar Referral Hospital.
Methods
A hospital-based cross-sectional design was used for the study. The hospital has a Diabetes Illness Follow-up Care Clinic, which was organized 2 decades ago and currently provides free service for more than 8,000 diabetic patients on a follow-up basis. We chose the 2-month follow-up schedule for data collection to avoid repetition of the cases as patients revisit the clinic every 2 months.
Study population
All diabetic patients aged ≥18 years who visited the Clinic in January and February 2013 for control of blood sugar and medication were invited to participate in the study. Also, patients who were on follow-up for ≥12 months were included in the study as adequate time was needed for assessing adherence. On the other hand, those who were critically ill and unable to participate in the interview and also those who were recently diagnosed and had a follow-up of <12 months were excluded. About 1,000 diabetic patients visited the Clinic in the 2 month period. Every second person aged ≥18 years was included. Thus, a total of 407 study subjects were selected through a systematic sampling procedure.
Data collection
Data were collected by interviewing eligible participants using a pretested and structured questionnaire. Patients were given an orientation on the protocol and specific details concerning participation in the study. The questionnaire, which was in the local language (Amharic), included questions that assessed diabetes-related factors (background information, lifestyle factors, clinical history, and medication adherence using the eight-item Morisky medication adherence scale [MMAS-8]).3,4 Anthropometric measurements were taken using standardized techniques and calibrated equipment. Patients were weighed to the nearest 0.1 kg in light indoor clothing and bare feet or with stockings. Height was measured using a stadiometer; waist girth was measured by placing a plastic tape to the nearest 0.5 cm horizontally, midway between the 12th rib and the iliac crest on the midaxillary line. Hip circumference was measured around the widest portion of the buttocks, with the tape parallel to the floor. Finally, biochemical tests (HbA1c, FBG, triglyceride, and total cholesterol test) were carried out.5
Blood samples were collected from each participant by a trained laboratory technician following aseptic techniques. The blood samples were taken to the hospital laboratory for chemistry analyses immediately. Biochemical tests (HbA1c) were carried out using a 902 Automatic Analyzer with Roche/Hitachi kit following a minimum of 8 hours’ fasting. Information about the Type 1 and Type 2 DM was collected from the hospital chart by the study nurse.
Six laboratory technicians, four nurses, and two supervisors were trained by the principal investigator. Every patient was made aware of the fasting requirement for a minimum of 8 hours prior to the lab test. A reminder was sent to them prior to the day of investigation, verbal confirmation of which was obtained prior to the blood test. The study supervisors and the principal investigator conducted regular supervisory checks to ensure data quality. Data entry and cleaning were done using Epi-info version 3.5.3.
Data analysis
Glycemic control was calculated on the basis of test results (HbA1c ≥7.0%) by type of diabetes (Type 1 and 2) and stratified by residence (urban and rural). In the multivariable analysis, diabetic patients with good glycemic control (HbA1c <7.0%) and those with poor glycemic control (HbA1c ≥7.0%)6,7 were compared to identify potential risk factors.8
BMI was used to define underweight (BMI <18.5), normal (18.5≤ BMI <25.0), overweight (25.0≤ BMI <30.0), and obesity (BMI ≥30) adults. On the basis of their waist circumference (WC) measurements, patients were classified, according to cutoffs recommended by the WHO, into three health risk categories: low risk (men, WC =93.9 cm or less; women, WC =79.9 cm or less); increased risk (men, WC =94.0–101.9 cm; women, WC =80.0–87.9 cm); and high risk (men, WC =102.0 cm or more; women, WC =88.0 cm or more) for DM.9 A wealth score was computed, using principal component analysis (PCA), from 16 variables that included monthly income, agricultural productivity, fixed asset, household assets, and utility; and the assumption of PCA was checked.
The propensity score was used for the estimation of treatment effect of nonadherence to poor glycemic control. We stratified the analysis in terms of DM type in the multiple regressions. The results were considered statistically significant at P≤0.05. Multivariable logistic regression analysis was applied to determine the associations of risk factors. The independent variables were included in the model on the basis of prior evidence in the literature and their effect in current analysis. Independent variables with a P-value of 0.20 and less, during the bivariate test, were included.
Ethical statement
The protocol was approved by the IRB of the University of Gondar. In addition, written permission was obtained from the Hospital Director to extract relevant information from the medical record. Participants were recruited on a voluntary basis after full information about the research was provided and a written consent agreement signed.
Results
A total of 407 diabetic patients were invited to participate in this study; 16 declined to participate, yielding a response rate of 96.01% (391 out of 407). The mean age (± SD) of the persons with diabetes was 50.4 (±15.2) years. The mean age (± SD) for Type 1 DM was 35.8 (±13.4) and 56.1 (±11.5) for Type 2. There was a slight preponderance of males (53%) over females (47%). (Sociodemographic characteristics are presented in Tables 1 and 2).
Table 1.
Sociodemographic and clinical characteristics of the study population by type of diabetes attending diabetic follow-up clinic in Gondar Referral Hospital, Ethiopia, 2013
| Variable | n (%) | Type 1 person with diabetes, n (%) | Type 2 person with diabetes, n (%) |
|---|---|---|---|
| Age in years | |||
| ≤24 | 29 (7.4) | 27 (93.1) | 2 (6.9) |
| 25–44 | 98 (25.1) | 59 (60.2) | 39 (39.8) |
| 45–64 | 191 (48.9) | 20 (10.5) | 171 (89.5) |
| ≥65 | 73 (18.7) | 5 (6.9) | 68 (93.2) |
| Sex | |||
| Female | 207 (52.9) | 40 (19.3) | 167 (80.7) |
| Male | 184 (47.1) | 71 (38.6) | 113 (61.4) |
| Residence | |||
| Rural | 44 (11.2) | 33 (75) | 11 (25) |
| Urban | 347 (88.8) | 78 (22.5) | 269 (77.5) |
| Religion | |||
| Orthodox | 332 (84.9) | 101 (30.4) | 231 (69.6) |
| Muslim | 54 (13.8) | 9 (16.7) | 45 (83.3) |
| Other | 5 (1.3) | 1 (20) | 4 (80) |
| Family size | |||
| ≤4 | 165 (42.2) | 54 (32.7) | 111 (67.3) |
| 5–8 | 193 (49.4) | 53 (27.5) | 140 (72.5) |
| ≥9 | 33 (8.4) | 4 (12.1) | 29 (87.9) |
| Duration of diabetes | |||
| <7 years | 204 (52.6) | 54 (26.5) | 150 (73.5) |
| ≥7 years | 184 (47.4) | 56 (30.4) | 128 (69.6) |
| Triglyceride | |||
| Normal (<200) | 240 (61.4) | 92 (38.3) | 148 (61.7) |
| High (200–499) | 145 (37.1) | 16 (11) | 129 (88.9) |
| Very high (≥500) | 6 (1.5) | 3 (50) | 3 (50) |
| Total cholesterol | |||
| Normal (≤250) | 373 (95.4) | 110 (29.5) | 263 (70.5) |
| High (≥251) | 18 (4.6) | 1 (5.6) | 17 (94.5) |
| Wealth index | |||
| Poor | 135 (34.5) | 76 (56.3) | 59 (43.7) |
| Medium | 118 (30.2) | 22 (18.6) | 96 (81.4) |
| Rich | 138 (35.3) | 13 (9.4) | 125 (90.6) |
| Medication adherence | |||
| Low | 99 (25.4) | 31 (31.3) | 68 (68.7) |
| Medium | 112 (28.7) | 33 (29.5) | 79 (70.5) |
| High adherence | 179 (45.9) | 47 (26.3) | 132 (73.4) |
| Waist circumference (WC) | |||
| Low risk | 144 (36.9) | 81 (56.2) | 63 (43.8) |
| Increased risk | 99 (25.4) | 14 (14.1) | 85 (85.9) |
| High risk | 147 (37.7) | 16 (10.9) | 131 (89.1) |
| Insulin therapy | |||
| No | 192 (49.1) | 3 (1.6) | 189 (98.4) |
| Yes | 199 (50.9) | 108 (54.3) | 91 (45.7) |
Table 2.
Multivariate analysis of factors associated with poor glycemic control among persons with type of diabetes in Gondar Referral Hospital of Gondar, North West Ethiopia (2013)
| Variable | n | Poor glycemic control cases, n (%) | Adjusted OR [95% CI]
|
|
|---|---|---|---|---|
| Type 1 diabetes | Type 2 diabetes | |||
| Age in years | ||||
| ≤24 | 29 | 25 (86.2) | 1 | 1 |
| 25–44 | 97 | 76 (78.4) | 1.13 [0.15, 8.32] | 0.74 [0.02, 25.8] |
| 45–64 | 188 | 119 (63.3) | 0.63 [0.05, 8.22] | 0.89 [0.03, 27.9] |
| ≥65 | 73 | 32 (43.8) | 0.28 [0.01, 5.96] | 0.39 [0.01, 12.9] |
| Wealth index | ||||
| Poor | 135 | 106 (78.5) | 1 | 1 |
| Medium | 118 | 61 (51.7) | 0.53 [0.07, 3.91] | 0.54 [0.24, 1.23] |
| Rich | 138 | 86 (62.3) | 0.63 [0.06, 6.81] | 0.87 [0.39, 1.94] |
| Medication adherence | ||||
| Adherence | 179 | 98 (54.8) | 1 | 1 |
| Nonadherence | 211 | 154 (73.0) | 2.1 [0.48, 9.29] | 3.19 [1.76, 5.80] |
| Duration of diabetes | ||||
| <7 years | 230 | 133 (57.8) | 1 | 1 |
| ≥7 years | 158 | 118 (74.7) | 2.71 [0.49, 14.9] | 1.59 [0.82, 3.10] |
| Waist circumference | ||||
| Low risk | 144 | 112 (77.8) | 1 | 1 |
| Increased risk | 99 | 60 (60.6) | 0.60 [0.06, 6.13] | 1.13 [0.50, 2.54] |
| High risk | 147 | 80 (54.4) | 0.05 [0.01, 0.85] | 0.64 [0.28, 1.46] |
| Insulin therapy | ||||
| No | 192 | 99 (51.6) | 1 | 1 |
| Yes | 199 | 154 (77.4) | 0.72 [0.01, 56.6] | 2.51 [1.25, 5.04] |
| Sex | ||||
| Male | 184 | 125 (67.9) | 1 | 1 |
| Female | 207 | 128 (61.8) | 1.39 [0.21, 9.16] | 1.70 [0.87, 3.29] |
| Residence | ||||
| Urban | 347 | 215 (61.9) | 1 | 1 |
| Rural | 44 | 38 (86.4) | 0.45 [0.06, 3.30] | 1.03 [0.19, 5.16] |
| Moderately physically active | ||||
| Yes | 256 | 174 (67.9) | 1 | 1 |
| No | 129 | 75 (58.1) | 2.74 [0.39, 19.12] | 1.16 [0.64, 2.11] |
| Cigarette smoking | ||||
| No | 382 | 245 (64.1) | 1 | – |
| Yes | 9 | 8 (88.9) | 1.46 [0.001, 225] | – |
| Hypertension | ||||
| No | 211 | 155 (73.5) | 1 | 1 |
| Yes | 180 | 98 (54.4) | 5.89 [0.88, 39.5] | 1.02 [0.55, 1.92] |
| Follow-up visit in the last 6 months | ||||
| No visit | 19 | 11 (57.9) | 1 | 1 |
| 2–3 | 315 | 167 (53.0) | 4.58 [0.16, 125] | 0.38 [0.09, 1.44] |
| More than | 45 | 27 (60.0) | 18.9 [0.23, 1,574] | 0.13 [0.03, 0.59] |
| Dyslipidemia | ||||
| No | 322 | 75 (23.3) | – | 1 |
| Yes | 69 | 41 (59.4) | – | 1.64 [0.49, 5.51] |
The mean (± SD) percentage of glycated hemoglobin test (HbA1c) was 7.82 (±1.9) (Table 3). The overall prevalence of poor glycemic control in persons with diabetes was 64.7% (95% CI =59.9, 69.4). The prevalence of poor glycemic control was significantly higher among Type 1 diabetics; the proportion with poor glycemic control was 82.9% (95% CI =75.8, 89.9) among Type 1 and 57.5% (95% CI =51.7, 63.3) among Type 2 DM patients. Poor glycemic control was higher among rural compared with urban dwellers; the proportion with poor glycemic control was 86.4% (95% CI =76.1, 96.6) and 61.9% (95% CI =56.8, 67.1) among rural and urban dwellers, respectively (Figure 1).
Table 3.
Univariate analyses of factors associated with poor glycemic control using the mean HbA1c among patients with DM at Gondar Referral Hospital, Ethiopia (2013)
| Variable | n (%) | Mean (Std Err) | 95% CI | P-value |
|---|---|---|---|---|
| Age in years | ||||
| ≤24 | 29 (7.4) | 9.4 (0.44) | [8.54, 10.26] | |
| 25–44 | 97 (24.8) | 8.4 (0.21) | [7.99, 8.81] | P<0.001 |
| 45–64 | 190 (48.6) | 7.6 (0.12) | [7.36, 7.82] | |
| ≥65 | 73 (18.7) | 7 (0.18) | [6.65, 7.35] | |
| Sex | ||||
| Female | 207 (52.9) | 7.58 (0.12) | [7.35, 7.82] | P=0.010 |
| Male | 184 (47.1) | 8.08 (0.15) | [7.78, 8.38] | |
| Type of diabetes | ||||
| Type 1 | 111 (28.4) | 8.66 (0.20) | [8.26, 9.07] | P<0.001 |
| Type 2 | 280 (71.6) | 7.48 (0.10) | [7.29, 7.68] | |
| Duration of diabetes | ||||
| <7 years | 230 (59.3) | 7.68 (0.14) | [7.41, 7.96] | P=0.136 |
| ≥7 years | 158 (40.7) | 7.97 (0.13) | [7.71, 8.23] | |
Abbreviations: DM, diabetes mellitus; Std Err, standard error; HbA1c, glycosylated hemoglobin level; CI, confidence interval.
Figure 1.
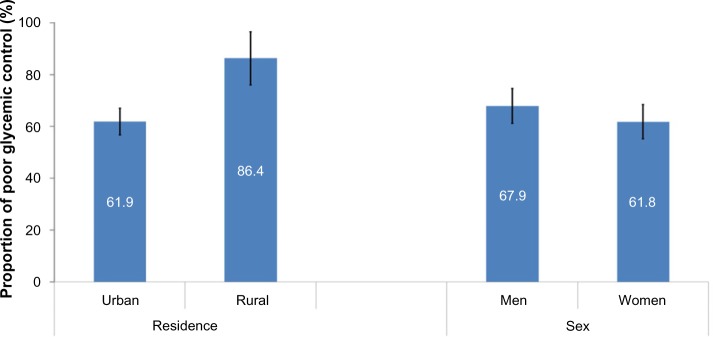
Proportion of glycemic control by residence and sex among person with diabetes, with 95% CI error bar at Gondar Referral Hospital, North West Ethiopia in 2013.
The attributable risk effect estimation of medication adherence using the propensity score showed that medication nonadherence had an effect on poor glycemic control, and the risk difference between nonadherence and good medical adherence for poor glycemic control was high among Type 2 diabetics (23.8%), (95% CI =12, 35) (t=4.14). Similarly, in the multiple regressions analysis, diabetics who reported poor medication adherence were three times more likely to have poor glycemic control (AOR =3.19; 95% CI =1.76, 5.80) among Type 2 diabetic patients (Table 2).
The reported follow-up visit for the last 6 months showed that increased frequency of hospital visits was negatively associated with poor glycemic control (AOR =0.13; 95% CI =0.03, 0.59) among persons with Type 2 diabetes. The occurrence of poor level of glycemic control was 7.6 times more likely in individuals who had no follow-up visits compared with those who had four visits in the last 6 months. Individuals receiving insulin treatment (AOR =2.51; 95% CI =1.25, 5.04) had significant association with poor glycemic control, among subjects with Type 2 DM.
Hundred and forty seven (37.7%) of the diabetic patients had a high-risk score of WC using the threshold of ≥92 cm for women and ≥104 cm for men. The multivariate logistic regression revealed that the high WC was inversely associated with poor glycemic control compared with the low WC among Type 1 persons with diabetes (AOR =0.05; 95% CI =0.01, 0.85). WC had no significant association with poor glycemic control among subjects with Type 2 DM (Table 2). The duration of diabetes was also positively associated with poor glycemic control (AOR =2.17; 95% CI =1.22, 3.85). Those who had been diagnosed with DM for more than 7 years were twice as likely to have poor glycemic control as those who had diabetes for less than 7 years.
Discussion
In this study, we were able to measure objectively the proportion of poor glycemic control and its associated factors in diabetic patients. Poor glycemic control, as defined by HbA1c ≥7%, was higher in persons with Type 1 diabetes, among rural dwellers, and insulin-treated Type 2 diabetics. The risk factors for poor glycemic control in Type 2 DM included poor medication adherence, being on insulin treatment, and having a poor record of follow-up visits during the last 6 months. High WC was found to be inversely associated with poor glycemic control in Type 1 DM patients.
The glycemic control level found in this study was similar to a previous estimation in Africa but lower than (82.7%) that found in studies in Jimma.10–12 The discrepancy between the findings of the study done in Jimma and those of this study can be explained by the fact that our study used the recommended test for glycemic control, the HbA1c test, whereas the Jimma study used the fasting blood sugar (FBS) test for diagnosis.13 Moreover, the Jimma study included only Type 1 DM patients on insulin.
The increased occurrence of poor glycemic control among Type 1 diabetics in rural parts of Ethiopia was consistent with the findings of previous studies.11,14 In our study, a high level of poor glycemic control was noted among Type 2 DM patients on insulin treatment. The findings are consistent with those of other studies.14,15 The use of insulin among Type 2 diabetics often reflects disease severity, and it is also possible that persons with Type 2 diabetes treated with insulin are more difficult to control because of the risk of setting up a vicious cycle of increasing weight, adjusting insulin doses, and increasing insulin resistance, with consequent deterioration in glycemic control.16,17 In our finding, insulin-treated Type 2 diabetics were overweight (a mean BMI of 27.5 kg/m2), which may contribute to the observed rise in poor glycemic levels among the insulin-treated Type 2 persons with diabetes.15
The risk difference for poor glycemic control between good treatment adherence and nonadherence was found to be high; poor glycemic control was common among those in the nonadherence group. This finding is consistent with other reports in Jordan and Malaysia.3,15 Literature shows that low treatment adherence continues to be a considerable barrier that prevents many diabetics from achieving good glycemic control. Increased compliance is associated with substantial improvements in glycemic control in health care.13 The challenge of managing multiple prescriptions and a reduced sense of urgency owing to asymptomatic conditions among Type 2 DM patients, patients’ understanding of their disease condition, and traditional beliefs in the rural community may affect adherence. Similarly, studies have also shown that DM patients with adequate knowledge of their status who regularly attend clinic and receive regular counseling are more likely to have better adherence.18,19 Continuous education is important in motivating patients to cultivate healthy lifestyles and maintain good treatment adherence.15,20 Besides, it is common for patients to improve their medication-taking behavior shortly before and after appointments with health care providers.21
Our study also showed a significant association between low WC and poor level of glycemic control among Type 1 diabetic patients. One of the plausible explanations for such an association is that the strict adherence among insulin-treated persons might have increased body weight and WC. Moreover, individuals who wanted to gain weight were highly likely to be adherent to treatment regimens among Type 1 DM, and this might have contributed to the observed good level of glycemic control among Type 1 DM patients with high WC compared with low WC.22
The limitation of this research was that we used a smaller sample size in view of the high cost of HbA1c, which affected our analysis when we stratified further in terms of diabetes type. Associated factors of poor glycemic control were hospital-based using a cross-sectional design, which might not show temporal relationships with potential risk factors. Longitudinal research is needed to assess the relationship between the variables over time.
Conclusion
The occurrence of poor levels of glycemic control was found to be higher in Type 1 DM patients, insulin-taking Type 2 DM patients, and rural dwellers. Moreover, reported nonadherence to medication regimens was common, and it substantially contributed to the poor level of glycemic control in the setting. We recommend that the health system be equipped with adequate and sustainable diagnostic tools, including the HbA1c test for proper monitoring of glycemic control. Sustainable insulin supply for insulin-taking DM patients and a facilitated referral system for patients with complications could also help improve the situation. Stakeholders should also consider upgrading the capacity of the tertiary hospitals by providing advanced diabetes care for end organ damage that inevitably follows poor glycemic control.
Acknowledgments
We express our deepest gratitude to all of the study participants. We are also very grateful to the University of Gondar (UoG), the World Health Organization (WHO), and Addis Continental Institute of Public Health for funding and supporting the project.
Author contributions
All authors contributed towards data analysis, drafting and revising the paper and agreed to be accountable for all aspects of the work.
Disclosure
The funders had no role in the study design, data collection, analysis, decision to publish, or in the preparation of the manuscript. The authors report no conflicts of interest in this work.
References
- 1.BeLue R, Okoror TA, Iwelunmor J, et al. An overview of cardiovascular risk factor burden in sub-Saharan African countries: a socio-cultural perspective. Global Health. 2009;5:10. doi: 10.1186/1744-8603-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnan PT, MacDonald TM, Morris AD. Adherence to prescribed oral hypoglycaemic medication in a population of patients with Type 2 diabetes: a retrospective cohort study. Diabet Med. 2002;19(4):279–284. doi: 10.1046/j.1464-5491.2002.00689.x. [DOI] [PubMed] [Google Scholar]
- 3.Al-Qazaz H, Hassali MA, Shafie AA, et al. The eight-item Morisky Medication Adherence Scale MMAS: translation and validation of the Malaysian version. Diabetes Res Clin Pract. 2010;90(2):216–221. doi: 10.1016/j.diabres.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abebe SM, Berhane Y, Worku A. Barriers to diabetes medication adherence in North West Ethiopia. Springerplus. 2014;3:195. doi: 10.1186/2193-1801-3-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 6.Moreira ED, Jr, Neves RC, Nunes ZO, et al. Glycemic control and its correlates in patients with diabetes in Venezuela: results from a nationwide survey. Diabetes Res Clin Pract. 2010;87(3):407–414. doi: 10.1016/j.diabres.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Tseng KH. Standards of medical care in diabetes–2006: response to the American Diabetes Association. Diabetes Care. 2006;29(11):2563–2564. doi: 10.2337/dc06-0805. author reply 2564–2565. [DOI] [PubMed] [Google Scholar]
- 8.Keen H. The Diabetes Control and Complications Trial (DCCT) Health Trends. 1994;26(2):41–43. [PubMed] [Google Scholar]
- 9.Jennifer Patry-Parisien MSaSB . Comparison of waist circumference using the World Health Organization and National Institutes of Health protocols. Sep, 2012. [PubMed] [Google Scholar]
- 10.Otieno CF, Kariuki M, Ng’ang’a L. Quality of glycaemic control in ambulatory diabetics at the out-patient clinic of Kenyatta National Hospital, Nairobi. East Afr Med J. 2003;80(8):406–410. doi: 10.4314/eamj.v80i8.8731. [DOI] [PubMed] [Google Scholar]
- 11.Zemlin AE, Matsha TE, Hassan MS, et al. HbA1c of 6.5% to diagnose diabetes mellitus–does it work for us? – the Bellville South Africa study. PloS One. 2011;6(8):e22558. doi: 10.1371/journal.pone.0022558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angamo MT, Melese BH, Ayen WY. Determinants of gly cemic control among insulin treated diabetic patients in Southwest Ethiopia: hospital based cross sectional study. PloS One. 2013;8(4):e61759. doi: 10.1371/journal.pone.0061759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Odegard PS, Gray SL. Barriers to medication adherence in poorly controlled diabetes mellitus. Diabetes Educ. 2008;34(4):692–697. doi: 10.1177/0145721708320558. [DOI] [PubMed] [Google Scholar]
- 14.Benoit SR, Fleming R, Philis-Tsimikas A, et al. Predictors of glycemic control among patients with Type 2 diabetes: a longitudinal study. BMC Public Health. 2005;5:36. doi: 10.1186/1471-2458-5-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khattab M, Khader YS, Al-Khawaldeh A, et al. Factors associated with poor glycemic control among patients with type 2 diabetes. J Diabetes Complications. 2010;24(2):84–89. doi: 10.1016/j.jdiacomp.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Wallace TM, Matthews DR. Poor glycaemic control in type 2 diabetes: a conspiracy of disease, suboptimal therapy and attitude. QJM. 2000;93(6):369–374. doi: 10.1093/qjmed/93.6.369. [DOI] [PubMed] [Google Scholar]
- 17.Peyrot M, Barnett AH, Meneghini LF, et al. Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29(5):682–689. doi: 10.1111/j.1464-5491.2012.03605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baradaran HR, Knill-Jones RP, Wallia S, et al. A controlled trial of the effectiveness of a diabetes education programme in a multi-ethnic community in Glasgow [ISRCTN28317455] BMC public health. 2006;6:134. doi: 10.1186/1471-2458-6-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miras AD, Risstad H, Baqai N, et al. Application of the International Diabetes Federation and American Diabetes Association criteria in the assessment of metabolic control after bariatric surgery. Diabetes, obesity & metabolism. 2013 doi: 10.1111/dom.12177. [DOI] [PubMed] [Google Scholar]
- 20.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 21.Raum E, Kramer HU, Ruter G, et al. Medication non-adherence and poor glycaemic control in patients with type 2 diabetes mellitus. Diabetes Res Clin Pract. 2012;97(3):377–384. doi: 10.1016/j.diabres.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Castaneda C, Janssen I. Ethnic comparisons of sarc`openia and obesity in diabetes. Ethn Dis. 2005;15(4):664–670. [PubMed] [Google Scholar]


