Abstract
Background
Although anal high-risk human papillomavirus (HR-HPV) infection and anal cytological abnormalities are highly prevalent among human immunodeficiency virus (HIV)-infected men who have sex with men (MSM), there are insufficient data on these abnormalities among HIV-infected heterosexual men (HSM) and women. In this study, we evaluated the prevalence of anal HR-HPV, cytological abnormalities, and performance of these screening tests in detecting high-grade anal intraepithelial neoplasia (AIN2+) among our cohort of HIV-infected MSM and non-MSM (HSM and women).
Methods
A single-center, retrospective cohort study was conducted with HIV-infected individuals who underwent anal cancer screening with anal cytology and HR-HPV testing from January 2011 to January 31, 2013.
Results
Screening of 221 HIV-infected individuals for both HR-HPV and anal cytology showed the presence of HR-HPV in 54% (abnormal anal cytology 48%) of MSM, 28% (abnormal anal cytology 28%) of HSM, and 27% (abnormal anal cytology 34%) of women. Among 117 (53%) individuals with abnormal results (HR-HPV-positive and/or cytology was atypical squamous cells of undetermined significance or above), 67 underwent high resolution anoscopy. Of these 67 individuals, 22 individuals had AIN2+ (17 MSM, four women, and one HSM). HR-HPV correlated better with AIN2+ than with anal cytology on biopsy in both MSM (r=0.29 versus r=0.10; P=0.05 versus P=0.49) and non-MSM (r=0.36 versus r=−0.34; P=0.08 versus P=0.09).
Conclusion
Given the presence of AIN2+ in screened HIV-infected HSM and women, routine anal cancer screening in all HIV-infected individuals should be considered. HR-HPV merits further evaluation for anal cancer screening among non-MSM.
Keywords: human immunodeficiency virus, anal human papillomavirus, heterosexual men, women, anal cancer
Introduction
The incidence of anal carcinoma has been increasing in human immunodeficiency virus (HIV)-infected individuals despite the implementation of antiretroviral therapy.1 Among HIV-infected individuals, the incidence of anal cancer is highest in MSM (men who have sex with men), followed by “other” HIV-infected men and then women.2 High-risk human papillomavirus (HR-HPV) is found in the majority of squamous cell anal cancers.3 Routine screening for anal cancer is not yet recommended; however, with its increasing health burden, anal cancer screening among HIV-infected individuals may be beneficial.4
Several studies have evaluated the performance of anal cytology and HR-HPV for anal cancer screening in HIV-infected MSM;5–9 however, an optimal anal cancer screening strategy among MSM is still debatable. A recent study reported that in HIV-infected MSM, direct use of high resolution anoscopy (HRA) is the most cost-effective strategy for detecting high-grade anal intraepithelial neo-plasia (AIN2+= AIN2/3) compared with anal cytology or HR-HPV.10 Although sufficient studies have evaluated the performance of anal cytology and HR-HPV in detecting anal dysplasia among HIV-infected MSM, there are limited data available for HIV-infected women11–14 and heterosexual men (HSM).14,15
At the University of Massachusetts Memorial Medical Center, anal cancer screening is offered routinely for all HIV-infected patients with both anal cytology and HR-HPV since January 1, 2011. If either of the screening tests is found to be abnormal, patients are referred to an on-site HRA clinic. We used this opportunity to determine the prevalence of abnormal anal cytology, HR-HPV infection, and the performance of these screening tests in detecting AIN2+ among our cohort of HIV-infected MSM, HSM, and women.
Materials and methods
This single-center, retrospective cohort study was approved by the University of Massachusetts Medical School institutional review board. All HIV-infected individuals were offered anal cytology and HR-HPV testing irrespective of gender and sexual orientation. Individuals with abnormal anal cytology and/or positive HR-HPV were referred to the HRA clinic. This study was conducted from January 1, 2011 to March 31, 2013. The study period included all patients who underwent routine anal cancer screening between January 1, 2011 and January 31, 2013, and their subsequent HRA clinic visit for an abnormal screening test by March 31, 2013.
Anal cytology specimens were collected by a moistened, nonlubricated polyester swab, which was inserted blindly (without the aid of an anoscope or proctoscope) two inches into the anal canal while rotating the swab to all sides of the anal canal.9 The swab was quickly dipped in ThinPrep (Cytyc, Marlborough, MA, USA), which is a liquid-based cytology solution. Cytology specimens were categorized according to the Bethesda system16 as benign, nondiagnostic due to inadequate sample, atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion, high-grade squamous intraepithelial lesion and atypical squamous cells, and cannot rule out high-grade squamous intraepithelial lesion. Specimen adequacy was determined by the presence of 1–2 nucleated squamous cells per high power field.16 The HR-HPV test was done according to the manufacturer’s instructions on the anal cytology specimen using the Hybrid Capture 2 assay (Qiagen Corporation, Gaithersburg, MD, USA). The Hybrid Capture 2 assay utilizes probes for 13 types of oncogenic HPV for detection of HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68. Low-risk HPV strains were not tested. However, the Hybrid Capture 2 assay has not been validated for detecting HR-HPV in the anus. All HRA procedures were performed in an outpatient clinic by a single infectious diseases physician (MW), who received training in HRA. The HRA was performed as described elsewhere.8 After applying 3% acetic acid, the squamocolumnar junction, distal anal canal, and anal margin were visualized under magnification using a colposcope. Biopsies were performed when there were lesions suggestive of dysplasia with features such as acetowhite positive and punctuated coarse vascularization. Anal histology was graded as negative for dysplasia (benign), AIN1, AIN2, or AIN3.
Demographic and clinical characteristics were collected for all HIV-infected individuals who underwent anal cancer screening from electronic medical records. This information included age, sex, race, sexual orientation, CD4 count, current antiretroviral therapy, HIV viral load, history of acquired immune deficiency syndrome, smoking status, and history of intravenous drug use. For women, cervical cytology and cervical HR-HPV information was collected. Anal histology results were collected for individuals who underwent HRA and subsequent biopsy. In the presence of multiple histology results for a single patient, the highest grade histology result was considered for this study. The demographic and clinical characteristics were grouped for comparison. Categorical variables were compared using the Chi-square test or Fisher’s Exact test as appropriate, and continuous variables were compared using the Wilcoxon rank sum test. The Spearman rank coefficient was used to study the correlation between HR-HPV and anal cytology in detecting AIN2+ on biopsy for the entire cohort, for MSM, and for non-MSM (HSM and women). The correlation coefficient was also calculated for cervical and anal canal cytology and HR-HPV, respectively. A P-value ≤0.05 was considered to be statistically significant. All statistical analyses were performed using Stata IC (Stata Corporation, College Station, TX, USA).
Results
From January 1, 2011 to January 31, 2013, 221 HIV-infected individuals underwent anal cancer screening with both anal cytology and HR-HPV. Baseline characteristics of the HIV-infected individuals were categorized as MSM, HSM, and women (Table 1). The median age for the entire cohort was 50 years (interquartile range 42–56 years) and 51% of the population were Caucasian. Significant differences among the three groups were noted for median age, current antiretroviral therapy, and history of intravenous drug use. These three characteristics were higher for HSM compared with the other two groups. Among 64 women who had information on cervical cytology and cervical high-risk HR-HPV, 17 (27%) had both cervical cytology and positive HR-HPV at one of their previous clinic visits (Table 1).
Table 1.
Baseline demographic and clinical characteristics of HIV-infected individuals by sexual orientation
| Characteristics | All patients (n=221) | MSM (n=107) | HSM (n=40) | Women (n=74) | P-value |
|---|---|---|---|---|---|
| Age (median, IQR) | 50 (42–56) | 49 (41–57) | 55 (49–60) | 49 (42–53) | 0.0006 |
| AIDS diagnosis (%) | 129 (58%) | 65 (61%) | 25 (63%) | 39 (53%) | 0.6 |
| Viral load undetectable (<75 copies/mL) | 188 (85%) | 95 (89%) | 34 (85%) | 59 (80%) | 0.24 |
| CD4 count (median, IQR) | 449 (302–634) | 452 (324–602) | 418 (283–596) | 470 (277–676) | 0.83 |
| CD4 count <200 | 26 (12%) | 13 (12%) | 3 (8%) | 10 (14%) | 0.67 |
| Current ART | 208 (94%) | 103 (96%) | 40 (100%) | 65 (88%) | 0.01 |
| Smoking currently | 112 (51%) | 54 (50%) | 26 (65%) | 32 (43%) | 0.08 |
| IVDU history | 35 (16%) | 3 (3%) | 18 (45%) | 14 (19%) | 0 |
| Race | |||||
| White | 114 (51%) | 86 (81%) | 14 (35%) | 14 (19%) | |
| Black | 30 (14%) | 5 (4%) | 8 (20%) | 17 (23%) | |
| Hispanic | 44 (20%) | 9 (8%) | 11 (28%) | 24 (32%) | |
| American Indian | 2 (1%) | 0 | 1 (2%) | 1 (2%) | |
| Asian | 3 (1%) | 3 (3%) | 0 | 0 | |
| Unknown | 28 (13%) | 4 (4%) | 6 (15%) | 18 (24%) | |
| Cervical HPV+ at any visit | 20 (31%)* | ||||
| Cervical cytology+ at any visit | 30 (47%)* | ||||
| Cervical HPV and cytology+ | 17 (27%)* |
Notes:
Denominator is 64.
Abbreviations: AIDS, acquired immunodeficiency syndrome; ART, antiretroviral therapy; HIV, human immunodeficiency virus; HPV, human papillomavirus; HSM, heterosexual men; IVDU, intravenous drug use; IQR, interquartile range; MSM, men who have sex with men.
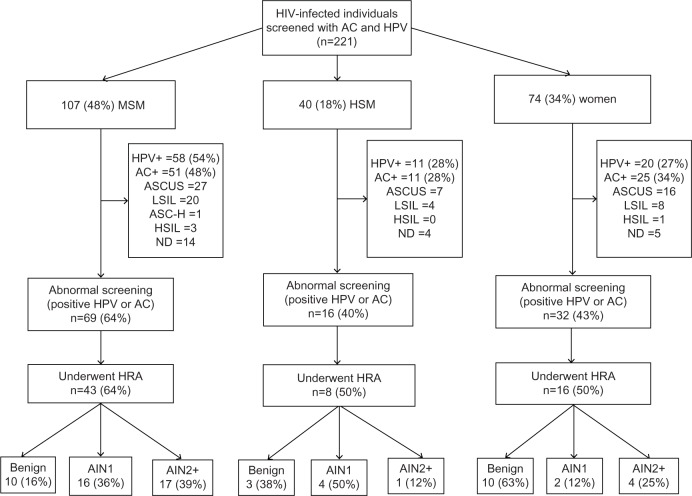
The prevalence of HR-HPV and abnormal cytology in the entire HIV-infected cohort was 40% (89 of 221) and 39% (87 of 221), respectively. The distribution of individuals, screening results, HRA, and biopsy results among the groups is shown in Figure 1. An abnormal screening result (positive HR-HPV and/or abnormal anal cytology) was noted in 64% of MSM, 40% of HSM, and 43% of women. The prevalence of HR-HPV was 54% in MSM, 28% in HSM, and 27% in women (Table 2). Similarly, abnormal anal cytology was positive in 48% of MSM, 28% of HSM, and 34% of women (Table 2). ASCUS was the most common screening abnormality in anal cytology in all three groups. Among 117 individuals who had an abnormal screening result, 67 (57%) underwent HRA by March 31, 2013. Of these 67 individuals, histology showed AIN2+ in 17 (39%) MSM, four women (25%), and one HSM (12%).
Figure 1.
Flow chart showing distribution of HIV-infected individuals, anal cancer screening, and HRA results.
Abbreviations: AC, anal cytology; AIN, anal intraepithelial neoplasia; AIN2+, high-grade anal intraepithelial neoplasia; ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion; HIV, human immunodeficiency virus; HPV, human papilloma virus; HRA, high resolution anoscopy; HSIL, high-grade squamous intraepithelial lesion; HSM, heterosexual men; LSIL, low-grade squamous intraepithelial lesion; MSM, men who have sex with men; ND, nondiagnostic.
Table 2.
High-risk human papillomavirus and anal cytology results among HIV-infected individuals by sexual orientation
| MSM (n=107) | HSM (n=40) | Women (n=74) | P-value | |
|---|---|---|---|---|
| HR-HPV positive | 58 (54%) | 11 (28%) | 20 (27%) | <0.001 |
| Anal cytology abnormal | 51 (48%) | 11 (28%) | 25 (34%) | 0.04 |
| Benign | 42 (39%) | 25 (63%) | 30 (59%) | |
| Nondiagnostic (inadequate specimen) | 14 (13%) | 4 (10%) | 5 (7%) | |
| ASCUS | 27 (25%) | 7 (18%) | 16 (22%) | |
| LSIL | 20 (19%) | 4 (10%) | 8 (11%) | |
| HSIL or ASC-H | 4 (3%) | 0 | 1 (1.3%) |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion; HIV, human immunodeficiency virus; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; HSM, heterosexual men; LSIL, low-grade squamous intraepithelial lesion; MSM, men who have sex with men.
The performance of anal cytology and HR-HPV in detecting AIN2+ was compared among 67 individuals (MSM, HSM, and women) who underwent HRA (Table 3). Among them, 32% (16/50) of individuals with abnormal cytology (ASCUS and above) and 41% (22/54) of those with positive HR-HPV had AIN2+, respectively (P=0.35). HR-HPV correlated much better with AIN2+ on the biopsy compared with anal cytology (r=0.34 versus r=−0.03; P=0.004 versus P=0.80). In the MSM group (n=43), 42% (14/33) of abnormal cytology and 45% (17/43) of positive HR-HPV had AIN2+, respectively (P=0.84). In the non-MSM (n=24) group, 12% (2/17) of abnormal anal cytology had AIN2+, whereas 31% (5/16) of positive HR-HPV had AIN2+ (P=0.22). HR-HPV correlated better with AIN2+ than anal cytology on biopsy in both the MSM group (r=0.29 versus r=0.10; P=0.05 versus P=0.49) and the non-MSM group (r=0.36 versus r=−0.34; P=0.08 versus P=0.09). Cervical and anal HR-HPV correlated well among 64 women who had both cervical and anal HR-HPV results (r=0.59; P=0.0000). Similarly, cervical cytology (benign and abnormal) correlated well with anal cytology (r=0.47; P=0.0001).
Table 3.
Rates of high-grade anal intraepithelial lesion by sexual risk group, Papanicolaou smear cytology, and high-risk human papillomavirus results
| Anal cytology | Positive HR-HPV, n (%) | AIN2+, n (%) |
|---|---|---|
| MSM (n=43) | 38 (88%) | 17 (40%) |
| Benign (n=8) | 8 (100%) | 2 (25%) |
| ND (n=2) | 2 (100%) | 1 (50%) |
| ASCUS (n=15) | 12 (80%) | 7 (47%) |
| ASC-H (n=1) | 1 (100%) | 1 (100%) |
| LSIL (n=15) | 13 (87%) | 6 (40%) |
| HSIL (n=2) | 2 (100%) | 0 |
| HSM (n=8) | 5 (63%) | 1 (13%) |
| Benign (n=3) | 3 (100%) | 1 (33%) |
| ASCUS (n=4) | 2 (50%) | 0 |
| LSIL (n=1) | 0 | 0 |
| Women (n=16) | 11 (69%) | 4 (25%) |
| Benign (n=4) | 4 (100%) | 2 (50%) |
| ASCUS (n=7) | 3 (43%) | 1 (14%) |
| LSIL (n=4) | 3 (75%) | 1 (25%) |
| HSIL (n=1) | 1 (100%) | 0 |
Abbreviations: ASCUS, atypical squamous cells of undetermined significance; ASC-H, atypical squamous cells, cannot rule out high-grade squamous intraepithelial lesion; AIN2+, high-grade anal intraepithelial lesion neoplasia; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; HSM, heterosexual men; LSIL, low-grade squamous intraepithelial lesion; MSM, men who have sex with men; ND, nondiagnostic (inadequate specimen).
Discussion
This is the first study to our knowledge to use and report the relevance of anal cytology and HR-HPV in HIV-infected individuals irrespective of their sexual orientation. There are limited data available on the prevalence of anal HR-HPV among HIV-infected women and HSM. Previous studies have reported an anal HR-HPV prevalence of 14%–85% among HIV-infected women.11–14 In our study, the prevalence of anal HR-HPV was 27% in women. Although slightly higher, these results are close to those of two other studies (16% in the BMC and 14% in the WIHS).11,12 However, the SUN study reported a very high prevalence of 85% and the prevalence in the GRACE study was 44%.13,14 Some possible explanations for the variation in HR-HPV prevalence could be the number of HR-HPV strains included (22 strains in SUN versus 14 in GRACE, 13 in our study and the BMC study, and 12 in the WIHS study), the technique used (polymerase chain reaction versus hybridization), and the underlying differences in the study population. Similarly, the prevalence of HR-HPV among HSM was 28% in our study. Two previous studies in the USA reported a prevalence of 33% and 52%.14,15 Our results were close to those of Wilkin et al, who used the same number of strains, while the SUN study, which included 22 strains, reported a higher prevalence of 52%. Among MSM, the HR-HPV prevalence was 54%, which is lower than previously reported. Three North American studies that utilized the Hybrid Capture 2 assay reported a prevalence of anal HR-HPV of 78%, 83%, and 88%.5,6,15 It is unclear why the prevalence is low in our MSM cohort; however, several risk factors, such as low CD4 count,17,18 lack of HIV suppression,17 frequency of receptive anal intercourse,15,17 and cervical HPV infection12,18 in women are associated with anal HPV infection. Differences in the distribution of these risk factors among study populations could possibly partially explain the variation in HR-HPV prevalence in MSM and non-MSM. For example, in the study by Salit et al,5 the median CD4 count among MSM was lower (390 versus 452) than in our cohort and the proportion of MSM with a CD4 count less than 200 was 18% versus 12% in our cohort.
Several studies have studied the prevalence of abnormal anal cytology among HIV-infected MSM; however, there are limited data on HSM and women. The prevalence of abnormal anal cytology among MSM in our study was 48%, which is slightly low when compared with other North American studies.5,14,15,19–22 Detection of abnormal anal cytology is associated with risk factors14 similar as anal HPV infection mentioned previously. Thus, the low prevalence of abnormal anal cytology in our MSM cohort could be partly explained by differences in the distribution of these risk factors. In the HSM cohort, the prevalence was 28%, which is within the previously reported range of 18%–31% among studies in North America.15,20,21 Similarly, among women, the prevalence was 34%, which is also within the reported range of 11%–42% in other US studies.13,14,20,21,23,24 We noted a good correlation (r=0.59) between cervical and anal HR-HPV as reported previously,12 indicating the importance of screening women with positive cervical HR-HPV for anal cancer.
In our study, we observed AIN2+ in HIV-infected women and HSM, consistent with previous studies demonstrating the need for anal cancer screening in all HIV-infected individuals.12,17,18,21 Studies evaluating the performance of anal cytology and HR-HPV in HIV-infected MSM were found to have high sensitivity but low specificity for detecting AIN2+. In addition, HR-HPV seems to have a high negative predictive value compared with anal cytology, but its high prevalence in HIV-infected MSM compromises its use for screening.5,6 HRA has been found to be the most cost-effective option for detecting AIN2+ among HIV-infected MSM because of these limitations with HR-HPV and anal cytology.10 Conversely, studies evaluating the performance of these two tests, especially HR-HPV, are lacking among HIV-infected HSM and women. In the present study, the prevalence of HR-HPV in non-MSM was low compared with MSM, and correlated much better with AIN2+ when compared with anal cytology in both groups. In addition, AIN2+ was not detected in any individual with negative HR-HPV in our entire cohort (MSM and non-MSM), consistent with its high negative predictive value in previous HIV-infected MSM studies. However, our findings are limited by the fact that HRA-directed biopsies were done in individuals with abnormal screening results and only a small number of individuals in the non-MSM group underwent HRA. The poor correlation of anal cytology in our study with AIN2+ in HIV-infected women and HSM is consistent with other studies.20,21,24 Overall, these findings demonstrate the need for further studies in larger populations to know the true prevalence of anal HR-HPV and its performance in detecting AIN2+ among HIV-infected HSM and women. If the HR-HPV prevalence in HIV-infected non-MSM is low (compared with MSM), as observed in our study, and if found to have a high negative predictive value, then it may have a role in anal cancer screening in HIV-infected non-MSM.
Our study has several limitations. Because of its retrospective nature, all information concerning self-reported sexual behavior was collected from medical records. There is a possibility that certain HSM may not have reported their true sexual behavior. Secondly, only individuals who had abnormal screening tests underwent HRA-directed biopsies, which could underestimate the degree of anal dysplasia in our cohort. In addition, all individuals who had abnormal screening tests did not undergo HRA, and the number of HSM and women who underwent HRA were small. Finally, this was a single-center study, and the results in our cohort may not be representative of the general HIV-infected population.
In conclusion, detection of AIN2+ in screened HIV-infected women and HSM demonstrates the need for routine anal cancer screening in all HIV-infected individuals. The low prevalence (compared with MSM) of HR-HPV and its better correlation with AIN2+ in non-MSM in our study merits further evaluation of HR-HPV as a screening test for histological AIN2+ neoplasia among HIV-infected women and HSM.
Author contributions
SG had full access to all data and takes responsibility for the integrity of the data and the accuracy of the data analysis. AA, MW, and SG were responsible for the study concept and design, and acquisition, analysis, and interpretation of the data. AA and SG drafted the manuscript, and all three authors critically revised it for important intellectual content. The study was supervised by MW. All listed authors approved the final version of the manuscript for publication.
Disclosure
The preliminary data from this study was published in abstract form and presented at the Infectious Disease Society of America annual meeting in San Francisco, CA, USA, on October 5, 2013, and International Anal Neoplasia Society meeting in San Francisco on November 24, 2013. The authors have no conflicts of interest in this work.
References
- 1.Chaturvedi AK, Madeleine MM, Biggar RJ, Engels EA. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst. 2009;101(16):1120–1130. doi: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Amo J, Gonzalez C, Geskus RB, et al. What drives the number of high-risk human papillomavirus types in the anal canal in HIV-positive men who have sex with men? J Infect Dis. 2013;207(8):1235–1241. doi: 10.1093/infdis/jit028. [DOI] [PubMed] [Google Scholar]
- 3.De Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta-analysis. Int J Cancer. 2009;124(7):1626–1636. doi: 10.1002/ijc.24116. [DOI] [PubMed] [Google Scholar]
- 4.Palefsky J, Berry JM, Jay N. Anal cancer screening. Lancet Oncol. 2012;13(7):e279–e280. doi: 10.1016/S1470-2045(12)70215-2. [DOI] [PubMed] [Google Scholar]
- 5.Salit IE, Lytwyn A, Raboud J, et al. The role of cytology (Pap tests) and human papillomavirus testing in anal cancer screening. AIDS. 2010;24(9):1307–1313. doi: 10.1097/QAD.0b013e328339e592. [DOI] [PubMed] [Google Scholar]
- 6.Goldstone SE, Lowe B, Rothmann T, Nazarenko I. Evaluation of the hybrid capture 2 assay for detecting anal high-grade dysplasia. Int J Cancer. 2012;131(7):1641–1648. doi: 10.1002/ijc.27431. [DOI] [PubMed] [Google Scholar]
- 7.Panther LA, Wagner K, Proper J, et al. High resolution anoscopy findings for men who have sex with men: inaccuracy of anal cytology as a predictor of histologic high-grade anal intraepithelial neoplasia and the impact of HIV serostatus. Clin Infect Dis. 2004;38(10):1490–1492. doi: 10.1086/383574. [DOI] [PubMed] [Google Scholar]
- 8.Berry JM, Palefsky JM, Jay N, Cheng SC, Darragh TM, Chin-Hong PV. Performance characteristics of anal cytology and human papillomavirus testing in patients with high-resolution anoscopy-guided biopsy of high-grade anal intraepithelial neoplasia. Dis Colon Rectum. 2009;52(2):239–247. doi: 10.1007/DCR.0b013e31819793d9. [DOI] [PubMed] [Google Scholar]
- 9.Cranston RD, Darragh TM, Holly EA, et al. Self-collected versus clinician-collected anal cytology specimens to diagnose anal intraepithelial neoplasia in HIV-positive men. J Acquir Immune Defic Syndr. 2004;36(4):915–920. doi: 10.1097/00126334-200408010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Lam JM, Hoch JS, Tinmouth J, Sano M, Raboud J, Salit IE. Cost-effectiveness of screening for anal precancers in HIV-positive men. AIDS. 2011;25(5):635–642. doi: 10.1097/QAD.0b013e3283434594. [DOI] [PubMed] [Google Scholar]
- 11.Hessol NA, Holly EA, Efird JT, et al. Anal intraepithelial neoplasia in a multisite study of HIV-infected and high-risk HIV-uninfected women. AIDS. 2009;23(1):59–70. doi: 10.1097/QAD.0b013e32831cc101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baranoski AS, Tandon R, Weinberg J, Huang FF, Stier EA. Risk factors for abnormal anal cytology over time in HIV-infected women. Am J Obstet Gynecol. 2012;207(2):107. e101–e108. doi: 10.1016/j.ajog.2012.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Durante AJ, Williams AB, Da Costa M, Darragh TM, Khoshnood K, Palefsky JM. Incidence of anal cytological abnormalities in a cohort of human immunodeficiency virus-infected women. Cancer Epidemiol Biomarkers Prev. 2003;12(7):638–642. [PubMed] [Google Scholar]
- 14.Conley L, Bush T, Darragh TM, et al. Factors associated with prevalent abnormal anal cytology in a large cohort of HIV-infected adults in the United States. J Infect Dis. 2010;202(10):1567–1576. doi: 10.1086/656775. [DOI] [PubMed] [Google Scholar]
- 15.Wilkin TJ, Palmer S, Brudney KF, Chiasson MA, Wright TC. Anal intraepithelial neoplasia in heterosexual and homosexual HIV-positive men with access to antiretroviral therapy. J Infect Dis. 2004;190(9):1685–1691. doi: 10.1086/424599. [DOI] [PubMed] [Google Scholar]
- 16.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology. JAMA. 2002;287(16):2114–2119. doi: 10.1001/jama.287.16.2114. [DOI] [PubMed] [Google Scholar]
- 17.Wiley DJ, Li X, Hsu H, et al. Factors affecting the prevalence of strongly and weakly carcinogenic and lower-risk human papillomaviruses in anal specimens in a cohort of men who have sex with men (MSM) PLoS One. 2013;8(11):e79492. doi: 10.1371/journal.pone.0079492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palefsky JM, Holly EA, Ralston ML, Da Costa M, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183(3):383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 19.Ciobotaru B, Leiman G, St John T, Hyman N, Ramundo M, Grace C. Prevalence and risk factors for anal cytologic abnormalities and human papillomavirus infection in a rural population of HIV-infected males. Dis Colon Rectum. 2007;50(7):1011–1016. doi: 10.1007/s10350-006-0873-y. [DOI] [PubMed] [Google Scholar]
- 20.Weis SE, Vecino I, Pogoda JM, et al. Prevalence of anal intraepithelial neoplasia defined by anal cytology screening and high-resolution anoscopy in a primary care population of HIV-infected men and women. Dis Colon Rectum. 2011;54(4):433–441. doi: 10.1007/DCR.0b013e318207039a. [DOI] [PubMed] [Google Scholar]
- 21.Gaisa M, Sigel K, Hand J, Goldstone S. High rates of anal dysplasia in HIV-infected men who have sex with men, women, and heterosexual men. AIDS. 2014;28(2):215–222. doi: 10.1097/QAD.0000000000000062. [DOI] [PubMed] [Google Scholar]
- 22.de Pokomandy A, Rouleau D, Ghattas G, et al. HAART and progression to high-grade anal intraepithelial neoplasia in men who have sex with men and are infected with HIV. Clin Infect Dis. 2011;52(9):1174–1181. doi: 10.1093/cid/cir064. [DOI] [PubMed] [Google Scholar]
- 23.Tandon R, Baranoski AS, Huang F, et al. Abnormal anal cytology in HIV-infected women. Am J Obstet Gynecol. 2010;203(1):21. e21–e26. doi: 10.1016/j.ajog.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hou JY, Smotkin D, Grossberg R, et al. High prevalence of high grade anal intraepithelial neoplasia in HIV-infected women screened for anal cancer. J Acquir Immune Defic Syndr. 2012;60(2):169–172. doi: 10.1097/QAI.0b013e318251afd9. [DOI] [PubMed] [Google Scholar]