Abstract
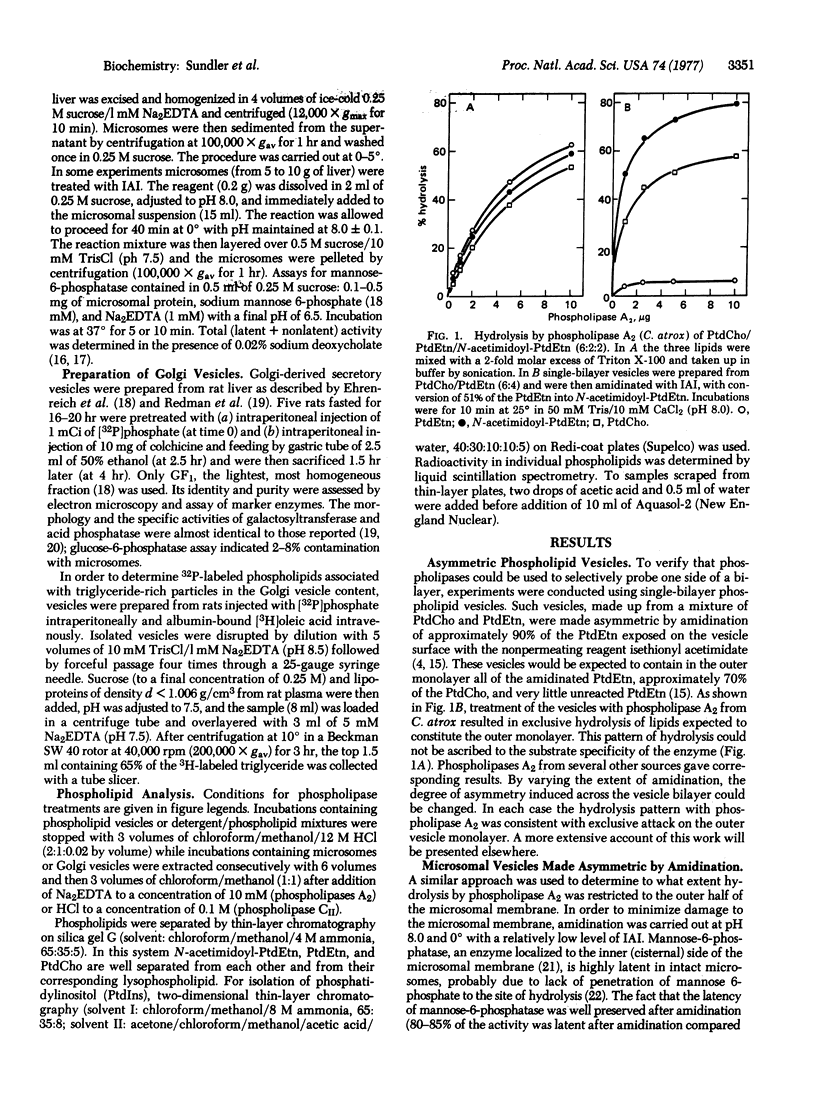
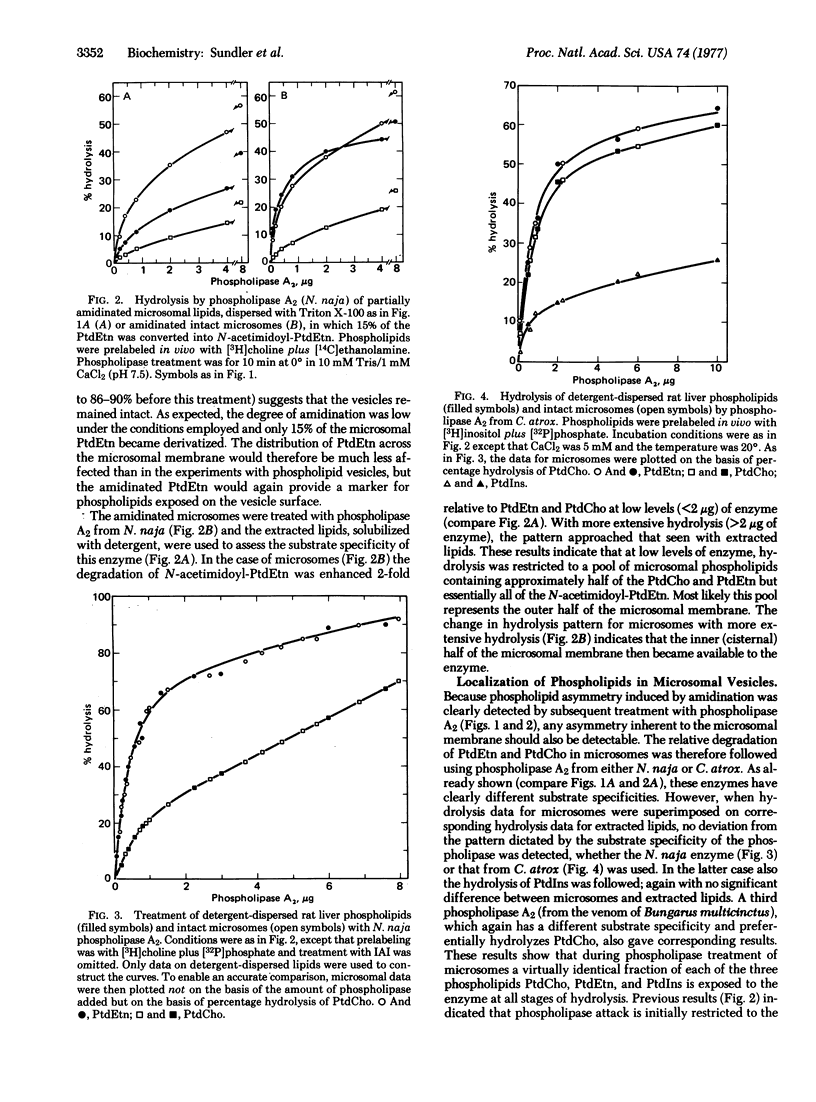
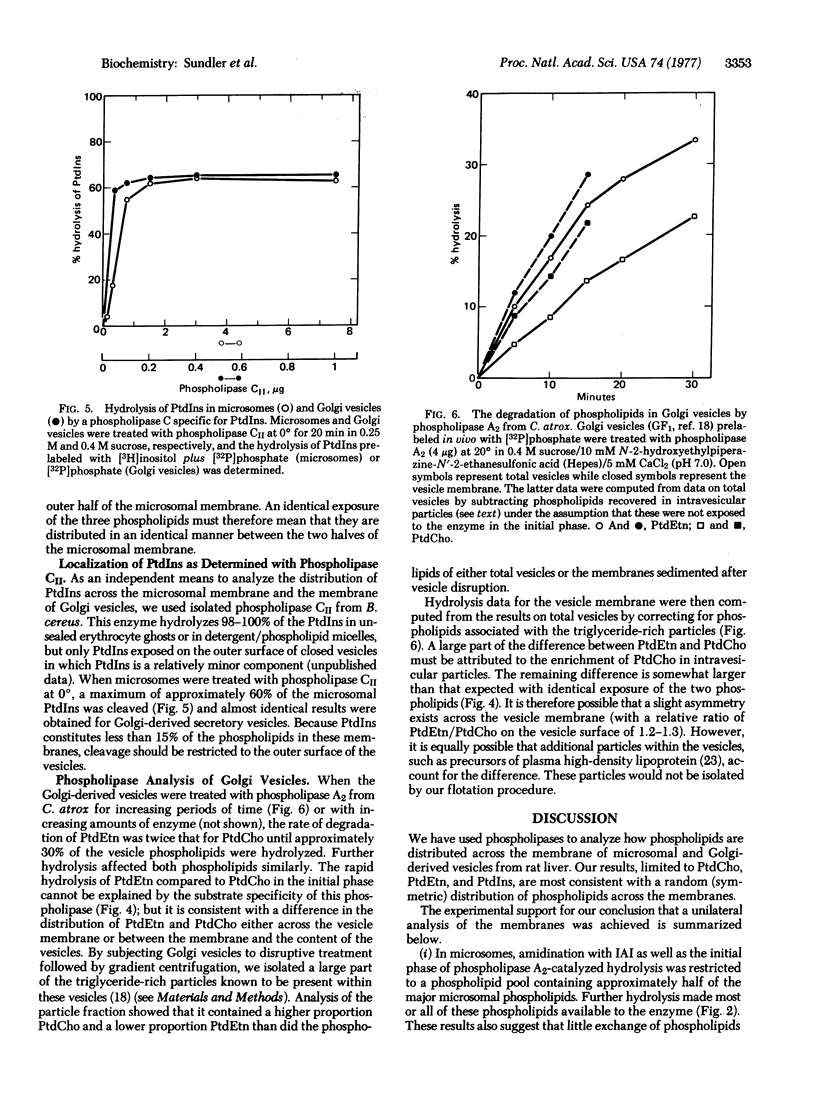
We have studied the distribution of phospholipids across the membrane of microsomal vesicles and Golgi-derived secretory vesicles from rat liver by the use of phospholipases. Model studies on single-bilayer phospholipid vesicles showed that phospholipase A2 (phosphatide 2-acyl-hydrolase, EC 3.1.1.4) cleaved at least 80% of the lipids on the outer surface of such vesicles without significant attack on the inner surface. In microsomal vesicles approximately 40% of the outer surface phospholipids were cleaved before the enzyme gained access to the interior of the vesicles. The same conclusion was reached for Golgi vesicles. By following the degradation of the three major phospholipids in intact microsomes and in extracted lipids we found that the same fraction of each of these phospholipids was exposed on the outer surface of the microsomal vesicles. Corresponding experiments with Golgi vesicles showed that distinctly different fractions of phosphatidylcholine and phosphatidylethanolamine were present on the surface of these vesicles. However, the difference was accounted for by enrichment of phosphatidylcholine in intravesicular particles rather than by asymmetry across the vesicle membrane. The results from specific hydrolysis of phosphatidylinositol confirmed an essentially symmetric distribution of this phospholipid across the microsomal and the Golgi vesicle membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion W. J., Wallin B. K., Carlson P. W., Lange A. J. The specificity of glucose 6-phosphatase of intact liver microsomes. J Biol Chem. 1972 Apr 25;247(8):2558–2565. [PubMed] [Google Scholar]
- Arvidson G. A. Structural and metabolic heterogeneity of rat liver glycerophosphatides. Eur J Biochem. 1968 May;4(4):478–486. doi: 10.1111/j.1432-1033.1968.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Berden J. A., Barker R. W., Radda G. K. NMR studies on phospholipid bilayers. Some factors affecting lipid distribution. Biochim Biophys Acta. 1975 Jan 28;375(2):186–208. doi: 10.1016/0005-2736(75)90188-1. [DOI] [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloj B., Zilversmit D. B. Asymmetry and transposition rates of phosphatidylcholine in rat erythrocyte ghosts. Biochemistry. 1976 Mar 23;15(6):1277–1283. doi: 10.1021/bi00651a017. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S. Phosphatidyl-ethanolamine: differential labelling in intact cells and cell ghosts of human erythrocytes by a membrane-impermeable reagent. J Mol Biol. 1972 Nov 28;71(3):523–528. doi: 10.1016/s0022-2836(72)80020-2. [DOI] [PubMed] [Google Scholar]
- CREMONA T., KEARNEY E. B. STUDIES ON THE RESPIRATORY CHAIN-LINKED REDUCED NICOTINAMIDE ADENINE DINUCLEOTIDE DEHYDROGENASE. VI. FURTHER PURIFICATION AND PROPERTIES OF THE ENZYME FROM BEEF HEART. J Biol Chem. 1964 Jul;239:2328–2334. [PubMed] [Google Scholar]
- Cater B. R., Trivedi P., Hallinan T. Inhibition of glucose 6-phosphatase by pure and impure C-type phospholipases. Reactivation by phospholipid dispersions and protection by serum albumin. Biochem J. 1975 May;148(2):279–294. doi: 10.1042/bj1480279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depierre J. W., Dallner G. Structural aspects of the membrane of the endoplasmic reticulum. Biochim Biophys Acta. 1975 Dec 29;415(4):411–472. doi: 10.1016/0304-4157(75)90006-4. [DOI] [PubMed] [Google Scholar]
- Ehrenreich J. H., Bergeron J. J., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. I. Isolation procedure and morphological characterization. J Cell Biol. 1973 Oct;59(1):45–72. doi: 10.1083/jcb.59.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold G., Widnell C. C. Relationship between microsomal membrane permeability and the inhibition of hepatic glucose-6-phosphatase by pyridoxal phosphate. J Biol Chem. 1976 Feb 25;251(4):1035–1041. [PubMed] [Google Scholar]
- Gordesky S. E., Marinetti G. V. The asymetric arrangement of phospholipids in the human erythrocyte membrane. Biochem Biophys Res Commun. 1973 Feb 20;50(4):1027–1031. doi: 10.1016/0006-291x(73)91509-x. [DOI] [PubMed] [Google Scholar]
- Hamilton R. L., Williams M. C., Fielding C. J., Havel R. J. Discoidal bilayer structure of nascent high density lipoproteins from perfused rat liver. J Clin Invest. 1976 Sep;58(3):667–680. doi: 10.1172/JCI108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa H., Yamanegi M., Taguchi R., Miyashita T., Ohyabu T. Studies on phosphatidylinositol phosphodiesterase (phospholipase C type) of Bacillus cereus. I. purification, properties and phosphatase-releasing activity. Biochim Biophys Acta. 1976 Nov 19;450(2):154–164. [PubMed] [Google Scholar]
- Litman B. J. Determination of molecular asymmetry in the phosphatidylethanolamine surface distribution in mixed phospholipid vesicles. Biochemistry. 1974 Jul 2;13(14):2844–2848. doi: 10.1021/bi00711a010. [DOI] [PubMed] [Google Scholar]
- Litman B. J. Lipid model membranes. Characterization of mixed phospholipid vesicles. Biochemistry. 1973 Jun 19;12(13):2545–2554. doi: 10.1021/bi00737a028. [DOI] [PubMed] [Google Scholar]
- Nilsson O. S., Dallner G. Enzyme and phospholipid asymmetry in liver microsomal membranes. J Cell Biol. 1977 Mar;72(3):568–583. doi: 10.1083/jcb.72.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson O. S., Dallner G. Transverse asymmetry of phospholipids in subcellular membranes of rat liver. Biochim Biophys Acta. 1977 Jan 21;464(2):453–458. doi: 10.1016/0005-2736(77)90019-0. [DOI] [PubMed] [Google Scholar]
- Redman C. M., Banerjee D., Howell K., Palade G. E. Colchicine inhibition of plasma protein release from rat hepatocytes. J Cell Biol. 1975 Jul;66(1):42–59. doi: 10.1083/jcb.66.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renooij W., Van Golde L. M., Zwaal R. F., Van Deenen L. L. Topological asymmetry of phospholipid metabolism in rat erythrocyte membranes. Evidence for flip-flop of lecithin. Eur J Biochem. 1976 Jan 2;61(1):53–58. doi: 10.1111/j.1432-1033.1976.tb09996.x. [DOI] [PubMed] [Google Scholar]
- Roseman M., Litman B. J., Thompson T. E. Transbilayer exchange of phosphatidylethanolamine for phosphatidylcholine and N-acetimidoylphosphatidylethanolamine in single-walled bilayer vesicles. Biochemistry. 1975 Nov 4;14(22):4826–4830. doi: 10.1021/bi00693a008. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Tsai D. K., Dawidowicz E. A., Lenard J. Transbilayer phospholipid asymmetry and its maintenance in the membrane of influenza virus. Biochemistry. 1976 Jun 1;15(11):2361–2370. doi: 10.1021/bi00656a018. [DOI] [PubMed] [Google Scholar]
- Schroeder F., Perlmutter J. F., Glaser M., Vagelos P. R. Isolation and characterization of subcellular membranes with altered phospholipid composition from cultured fibroblasts. J Biol Chem. 1976 Aug 25;251(16):5015–5026. [PubMed] [Google Scholar]
- Sundler R., Arvidson G., Akesson B. Pathways for the incorporation of choline into rat liver phosphatidylcholines in vivo. Biochim Biophys Acta. 1972 Dec 8;280(4):559–568. doi: 10.1016/0005-2760(72)90136-1. [DOI] [PubMed] [Google Scholar]
- Sundler R. Biosynthesis of rat liver phosphatidylethanolamines from intraportally injected ethanolamine. Biochim Biophys Acta. 1973 May 24;306(2):218–226. doi: 10.1016/0005-2760(73)90227-0. [DOI] [PubMed] [Google Scholar]
- Verkleij A. J., Zwaal R. F., Roelofsen B., Comfurius P., Kastelijn D., van Deenen L. L. The asymmetric distribution of phospholipids in the human red cell membrane. A combined study using phospholipases and freeze-etch electron microscopy. Biochim Biophys Acta. 1973 Oct 11;323(2):178–193. doi: 10.1016/0005-2736(73)90143-0. [DOI] [PubMed] [Google Scholar]
- Virtanen I., Brotherus J., Renkonen O., Wartiovaara J. Phospholipids of outer and inner nuclear membranes in rat liver and BHK-21 cells. Biochem Biophys Res Commun. 1977 May 9;76(1):142–149. doi: 10.1016/0006-291x(77)91679-5. [DOI] [PubMed] [Google Scholar]
- Whiteley N. M., Berg H. C. Amidination of the outer and inner surfaces of the human erythrocyte membrane. J Mol Biol. 1974 Aug 15;87(3):541–561. doi: 10.1016/0022-2836(74)90103-x. [DOI] [PubMed] [Google Scholar]
- Zwaal R. F., Roelofsen B., Comfurius P., van Deenen L. L. Organization of phospholipids in human red cell membranes as detected by the action of various purified phospholipases. Biochim Biophys Acta. 1975 Sep 16;406(1):83–96. doi: 10.1016/0005-2736(75)90044-9. [DOI] [PubMed] [Google Scholar]


