Abstract
Purpose
To demonstrate the safety and surgical feasibility of the first-in-man ocular implant of a novel Posterior MicroPump Drug Delivery System (PMP) in patients with diabetic macular edema (DME) and to report on the device capabilities for delivering a programmable microdose.
Methods
This was a single center, single arm, open-label, prospective study. Eleven patients with DME and visual acuity equal to or worse than 20/40 were included. The PMP prefilled with ranibizumab was implanted into the subconjunctival space. After implantation, the PMP was wirelessly controlled to deliver a programmed microdose. Comprehensive ophthalmic exams and optical coherence tomography were performed biweekly for 90 days. At the end of the study, the PMP was explanted and the subjects thereafter received standard of care for DME (i.e., laser or intravitreal injections).
Results
All 11 surgical implantations were without complications and within the skill sets of a retinal surgeon. No serious adverse events occurred during the follow-up period. At no point were visual acuity and central foveal thickness worse than baseline in the implanted eye. The PMP delivered the programmed ranibizumab dosage in seven subjects. The remaining four patients received a lower than target dose, and the treatment was complemented with standard intravitreal injection.
Conclusions
This study demonstrates the first-in-man safety of the Replenish MicroPump implant for a period of 90 days and its capability to deliver a microdose into the vitreous cavity. Further studies to enable longer-term safety and to demonstrate the feasibility of multiple programmable drug delivery are necessary.
Keywords: ocular drug delivery, diabetic macular edema, age-related macular degeneration, macular degeneration, drug delivery
Introduction
The introduction of intraocular therapy for diseases such as age-related macular degeneration (AMD),1–6 diabetic macular edema (DME),7–9 and macular edema secondary to retinal vein occlusion9,10 has changed the routine of the ophthalmic clinic. Intravitreal injection (IVT) drugs such as anti-vascular endothelial growth factor (VEGF), VEGF trap-eye, and triamcinolone can now maintain or improve vision in patients who before did not have treatment. Patients are now followed and treated on a monthly basis. This, however, created a burden for physician, health system, and patients.11,12 Poor compliance became a variable on the treatment outcomes; patients who miss the medical appointment might run the risk of irreversible vision loss. In addition, the need of multiple injections over the years increases the incidence of adverse events (AEs) such as endophthalmitis and retinal detachment.13–15 In an attempt to decrease the total number of IVTs, different therapeutic strategies have been proposed; however, uninterrupted monthly IVTs still provide better outcomes according to different publications.5,9 In additional, recent publications have suggested that a more frequent regime (i.e., weekly or biweekly treatment) could be beneficial in nonresponder patients.16,17
In order to increase patient compliance, potentially decrease side effects from repeated monthly IVTs, and improve outcomes, novel therapeutic strategies have been pursued. Those strategies include the development of drugs with longer treatment interval,6,18 sustained drug delivery system,19,20 and implantable drug delivery systems.21–23
Implantable drug pumps have significant promise in treating ocular disease, if the major challenge of making a miniature pump is achieved. The use of microelectromechanical system (MEMS) technology to fabricate miniaturized and efficient drug delivery systems have already been demonstrated for insulin delivery and delivery of bioactive compounds to neural tissue and are a viable option for ocular use.24,25 Using MEMS technology, our group has developed prototypes of a Posterior MicroPump Drug Delivery System (PMP; Replenish, Inc., Pasadena, CA) that demonstrated the delivery of microdoses in bench top tests and the long-term safety when implanted in an animal model.22,26 Current engineering benchtop data demonstrate that the PMP devices will continue to function reliably for more than 100 programmable injections of intraocular drugs including ranibizumab. At a monthly dosing regimen, this could possibly represent more than 8 years of monthly IVTs.
This study was designed to demonstrate the safety, surgical feasibility, and device capability of the first generation Replenish MicroPump implanted in a small cohort of DME patients over a period of 90 days.
Patients and Methods
Patient Characteristics and Study Design
This was a single center, single arm, open-label, prospective, nonrandomized study in Guadalajara, Mexico. The study adheres to the tenets of the Declaration of Helsinki and the hospital Ethics Committee approvals, and local and federal regulatory approvals including import license from Mexico and export license from the United States Food and Drug Administration for the PMP system. The site in Guadalajara was selected specifically for the principal investigator and research team that have the direct expertise and skill from prior retinal prosthesis implant trials. All 11 patients who underwent implantation had prior informed consent, and all data were collected prospectively on standardized case report forms.
The inclusion criteria were as follows: diagnosis of DME as defined by the Early Treatment Diabetic Retinopathy Study,27 visual acuity equal or worse than 20/40 Snellen equivalent, and central foveal thickness (CFT) equal to or more than 250 μm measured by optical coherence tomography (OCT) Cirrus OCT (Carl Zeiss, Meditec, Dublin, CA). The main exclusion criteria were proliferative diabetic retinopathy, ocular hypertension, glaucoma, previous vitreo-retinal surgery, laser photocoagulation, and/or IVT in the study eye within 3 months prior to being selected.
Preoperative and Postoperative Examinations
Demographic and baseline clinical exams were collected for enrolled patients (day −14 to −1 before implantation). Follow-up visits were scheduled biweekly for a total of 90 days. Each clinical evaluation consisted of best-correct visual acuity (BCVA) using the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart at 4 m, intraocular pressure (IOP), slit-lamp, retinal evaluation with slit lamp and indirect ophthalmoscopy, and CFT measured by OCT. Fluorescein angiography was done at baseline and repeated according to the principal investigator decision during the postoperative period. AEs and interventions were documented at the surgical procedure and at each follow-up visit. The AEs were assigned standard coded terms for the event based upon the MedDRA Coding dictionary version 14.1. Health-related quality of life was measured using the National Eye Institute Visual Function Questionnaire (NEI-VFQ-25) at baseline and at day 90 postoperatively.
MicroPump
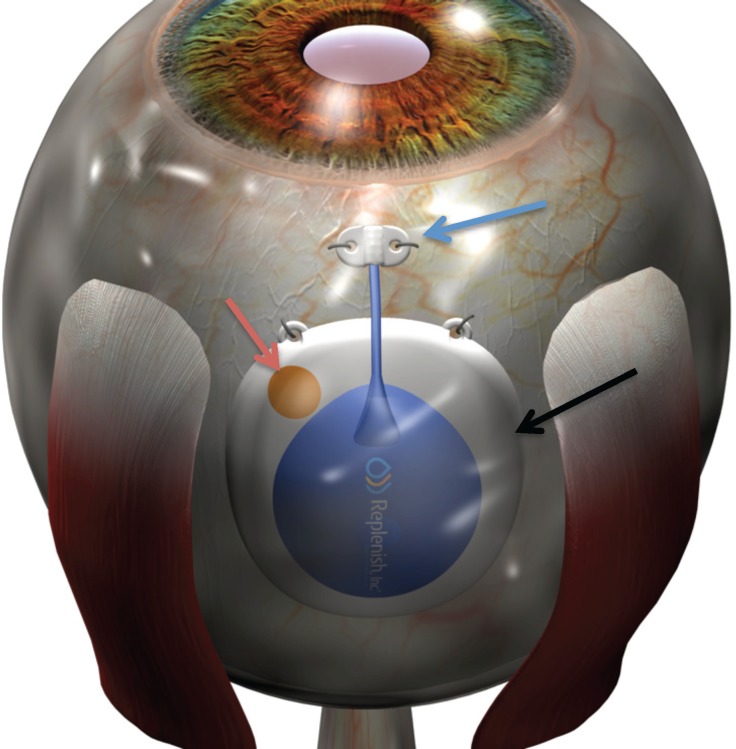
The first generation PMP (Replenish, Inc.; Fig. 1) is a drug delivery system fabricated with MEMS technology using titanium, silicone, and parylene. The dimension and shape of the device were designed taking into consideration the anatomy and curvature of the eye to provide a safe implantation. The PMP is implanted under the conjunctiva and tenons and consists of five subcomponents: (1) electronics contained in a hermetic sealing package responsible to power and control the drug displacement using electrolysis process, (2) drug reservoir chamber capable of storing up to 60 μL of drug (i.e., anti-VEGF, steroids), (3) a check valve (one-way valve) that opens only when the internal reservoir pressure exceeds the check valve cracking pressure and closes after the driving pressure is removed avoiding any back flow, (4) refill port that can be access trans-conjunctiva using a 31-gauge needle, and (5) intraocular cannula that is implanted into the pars plana and responsible to release microdose of drug into the vitreous cavity (Fig. 1). The electrolysis is a low power process in which the electrochemically induced phase change of water to hydrogen and oxygen gas generates pressure in the reservoir forcing the drug throughout the reservoir. The length of operation required to deliver a specific dose is determined by both the magnitude and duration of the applied current, which is correlated to the flow rate. All materials used in the PMP were shown to be biocompatible before implantation.
Figure 1.
Schematic representation of the PMP implanted into the subconjunctival space between the superior and lateral rectus muscles. The blue arrow indicates the intraocular cannula at the pars plana location. The red arrow indicates the refill port. The black arrow indicates the body of the PMP that contains the hermetic sealing package with all electronics, the drug reservoir, and the check valve.
Before the implantation, the PMP reservoir was filled with ranibizumab (1 mg/0.1 mL) and calibrated to deliver a total volume of 0.0085 mL (equivalent of 0.085 mg of drug). The drug was delivered immediately after implantation of the pump to avoid long residence time in the pump (i.e., all drug injected in the subjects within 90 minutes of loading the drug into the pump).
Surgical Technique
The implantation of the PMP is similar to an implantation of a glaucoma drainage device. Under peri-bulbar anesthesia, a peritomy was performed in the superior temporal quadrant. Blunt dissection with scissors was done to create the posterior pocket. A custom-design sterile sizer tool was used to ensure that the dimension of the pocket were adequate to fit the PMP. The sclerotomy site was marked at 3.5 mm posterior to the limbus, and two 5-0 nylon scleral sutures were placed 4 mm posteriorly to the sclerotomy site. Using a custom-design introducer, the PMP was placed into the subconjunctival space and secured using the preplaced scleral sutures. The sclerotomy was then performed at the premarked site using a 20-gauge blade and the cannula placed into the vitreous cavity. The cannula was sutured to the sclera using 8.0 nylon sutures. Conjunctiva was then closed using 6-0 vicryl sutures.
As design in the protocol study that was approved by the regulatory authorities and ethical committee, the PMP was explanted 90 days after implantation. It is not uncommon for such requirement to be imposed on first of a kind implantable device that can be relatively easily removed. For the explantation procedure, a peritomy was performed in the superior temporal quadrant. The sutures that anchored the PMP in place were cut, the device was removed from the subconjunctival space, and the sclerotomy closed using 6-0 vicryl sutures. The conjunctiva was closed using 6-0 vicryl sutures. After the explantation, patients were followed by the primary ophthalmologist and received standard of care treatment (i.e., laser therapy, IVTs) if necessary.
Drug Delivery
Immediately after the implantation, the PMP was connected to a telemetry system (EyeLink SystemTM, Replenish, Inc.). This handheld system is able to wirelessly connect to the PMP, receive pump functionality information, and give the command for the drug delivery. The process to establish a link and deliver the dose in this study took approximately 16 minutes and was done at a single time-point.
Supplementary Treatment
During the follow-up period, after the first 4-week visit was completed, the investigator was allowed to manage the patient with standard of care therapy either with IVT of ranibizumab or focal laser. The treatments were allowed if there was persistent macular edema, a decrease in visual acuity (five letters or more in the ETDRS chart) compared to previous visits, or an increase of the CFT indicating worsening of the macular edema compared to previous visit (50 μm or more measured by the OCT). This intervention was also allowed if the PMP was unable to deliver the target dose of study drug during an allotted time period. The numbers of ranibizumab injections and/or laser treatments were documented.
Endpoints
The endpoints for this study were safety assessment through a 3-month period and feasibility of the surgical implantation of the PMP. In addition, we report the device capabilities for delivering a programmable microdose and evaluate changes on BCVA and CFT in the subjects who received the preprogrammed microdose.
Statistical Analysis
For all changes from baseline endpoints, summary statistics (mean, standard deviation, sample size, minimum, maximum, and median) and shift tables were computed for the intention to treat (ITT) population at each time point. A paired t-test was used to calculate if the mean change from baseline was statistically significantly different from zero. The t-test was chosen because normality of ETDRS scores and CFT is assumed based on previous literature on these endpoints.
For ophthalmic exam and slit lamp exam results, a summary of the counts and proportions of subjects in each category of each question at each time point were computed.
Results
Patient Disposition
A total of 11 patients were implanted with the PMP: seven males and four females. The mean age in years was 65.2 ± 7.5 (range, 49.3–76.5 years). Nine patients had the PMP implanted in the right eye and two in the left eye.
Surgical Technique
All surgical implantation were without complications. The PMP was implanted episclerally in the superior quadrant without damaging ocular structures such as the extra ocular muscles or vortex veins. The surgical procedure time was reduced with each subsequent implant; the first implantation time was 48 minutes, whereas the last implantation time was 28 minutes (mean of 35 minutes). Also, there were no surgical complications during the scheduled explantation procedure at day 90 ± 3 days.
Safety Assessment
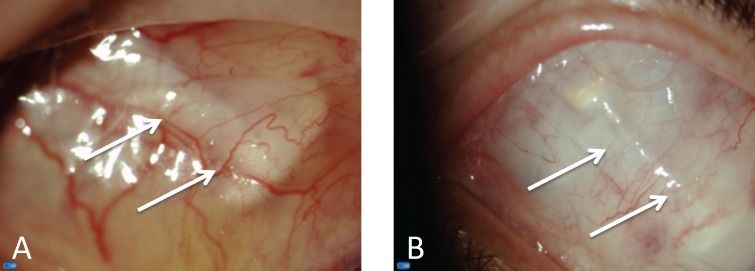
All patients completed the study. No serious AEs, such as endophthalmitis, retinal detachment, or permanent decrease in the visual acuity, were reported for any of the 11 implanted subjects. During the 4 to 6 weeks postoperative period, all patients exhibited normal course of postoperative healing of the conjunctiva, tenons, and cornea (Fig. 2). Ocular motility was normal in primary gaze and all nine cardinal positions in the majority of the exams. Five patients reported transient diplopia on extreme vertical and horizontal duction in the first weeks postoperatively (abduction and elevation gaze), which completely resolved with the resolution of the periorbital edema. When compared with baseline, all eyes had similar or better visual acuity at day 90 before the explantation of the device. At no point was visual acuity worse than baseline in the implanted eye.
Figure 2.
Slit lamp exam of the superior quadrant of the eye of a patient implanted with the PMP at day 28 (A) and day 90 (B). All subjects had a standard healing process after the surgical implantation that improved over time. The white arrows indicate the PMP in the subconjunctival space.
IOP was stable in 10 patients during all 90 days of follow-up. One patient had elevated IOP (26 mm Hg) at day 28 post operatively. This elevation in IOP was deemed to be due to a steroid response, and the steroids were tapered and antiglaucoma medications (Timolol Maleate 0.5% and Brimonidine Tartrate 0.1%, two times a day) were administered. At day 42 postoperatively, the IOP returned to baseline (14 mm Hg) and the antiglaucoma medications were discontinued.
One patient was observed to have a small vitreous hemorrhage (VH) at week 1. This VH was localized at the sclerotomy site and did not lead to decrease in vision. Hence, this VH was attributed to the incision, and with observation the VH resorbed at postoperative week 4.
The scores of the NEI-VFQ-25 showed that the quality of life of the subjects within the duration of the study was similar or better compared to the baseline. Specifically, no ocular pain or discomfort above a slight foreign body sensation was reported at any of the time points.
Assessment of the Drug Delivered by the PMP
The PMP delivered the preprogrammed dosage (0.085 mg ± 20%) in the allotted time in 7 of the 11 subjects. The remaining subjects received a lower than target dose. In this subgroup, the PMP had a slower rate of delivery, and for patient's safety and welfare (i.e., to avoid a more prolonged and complicated procedure), it was deemed by the technical team and the principal investigator to stop the infusion and complete the dose using IVT. Bench evaluation suggested that the four PMPs that did not function properly could have been damaged during surgical implantation due to surgical manipulation. The root causes of each suboptimal dosing issue were investigated by the engineering team and have been resolved for the future generation design of the PMP. All four patients who did not receive the target ranibizumab dosage from the PMP received a 0.5-mg IVT of ranibizumab at day 1 postoperatively.
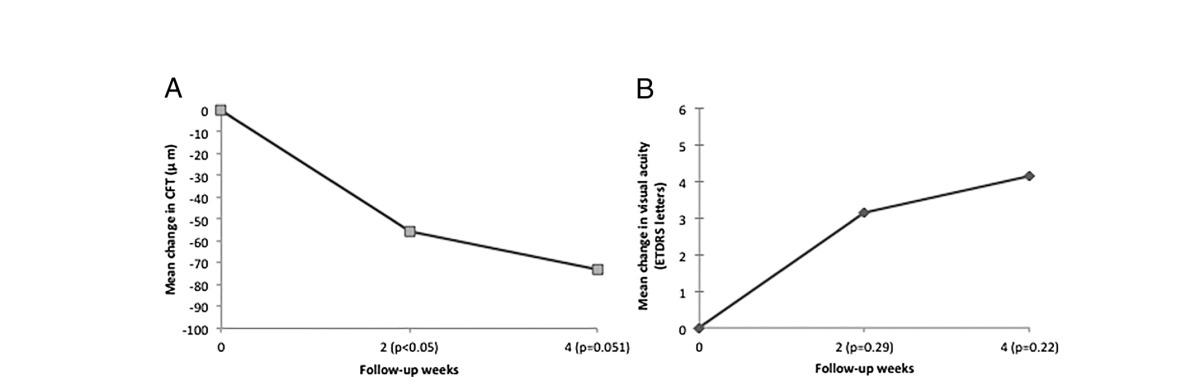
For the safety of the pump, we include follow-up over the entire 90 days of implantation. However, for the efficacy of drug delivered by the PMP, we not only report on the visual acuity over the entire study but have also separated the results into the group of patients who got the drug delivered by the PMP and also show the first month results. The subgroup of patients (n = 7) who received the preprogrammed dose from the PMP had a statistically significant decrease (P ≤ 0.05) in the CFT at week 2 compared to baseline. At week 4, the difference approaches statistical significance (P = 0.0518) with a trend toward an improvement on the CFT (Table 1A). No other statistically significant change in CFT was indicated in the remaining follow-up periods. There was a trend showing a gain of letters in the visual acuity between baseline and follow-up period in the subgroup that received the preprogrammed dose; however, those changes were not statistically significant (Table 1B). None of the patients lost vision during the study.
Table 1.
Changes in CFT (A) and visual acuity (B) from baseline through 4 weeks in the patients (n = 7) who received the preprogrammed ranibizumab dose delivered by the PMP.
Rescue treatment for persistent macular edema was performed in six patients at week 6 using focal laser (modified-ETDRS technique) and in four patients using IVT of 0.5 mg ranibizumab throughout the study. There was no statistical difference in the visual acuity and CFT between the baseline and day 90 for the entire group of patients (n = 11).
Discussion
This article describes the first-in-man implantation of an electronic micropump drug delivery system to infuse drug into the vitreous cavity in patients with retinal disease (i.e., DME). This study, albeit limited in size of patients and also in follow-up period, shows that the PMP implantation was safe and feasible within the skill set of a trained vitreoretinal surgeon, was well tolerated by the subjects, and was able to deliver the preprogramed dose.
Visual acuity and CFT at no point were worse than baseline in implanted eye. Moreover, the unemployed eye had a similar course of changes on OCT. The study design did not include a sham PMP control group, but given the companion unimplanted contralateral eyes of each patient had a similar course of changes on OCT, we can conclude that the PMP had no adverse effect on the macular edema; therefore, the persistence of the DME was due to the natural course of the disease.
The presence of a medical device (i.e., Investigational PMP, scleral buckle, or glaucoma drainage device) into the subconjunctival space always brings a risk of possible complications such as conjunctival erosion, strabismus, and infection, among others.28,29 It's important to state that, differently than a glaucoma drainage device, the PMP does not have a direct connection that allows fluid to drain from the inside of the eye to the extra ocular environment. In our series of cases, no serious AEs, such as infections or clinical complications, were observed and no visual acuity was lost. Safety of the patient is foremost and will be studied over longer terms in future studies.
The benefits of intravitreal therapies in retinal diseases have been well documented. The current standard of care is the injection of a bolus of anti-VEGF into the vitreous cavity on a frequent basis. Such a large bolus of drug has been linked to some of the side effects.13,14 In addition, this administration creates a typical curve of a large peak of drug with rapid decay. The literature supports the fact the half-life of anti-VEFs, such as ranibizumab, is indeed very short (half-life = 2.6 to 4.0 days)30 making it necessary to use a higher initial dose in order to exceed therapeutic levels to allow a longer 28-day treatment interval. Moreover, mathematical modeling demonstrates that the binding activity of 0.5 mg of ranibizumab is fivefold higher if given every 14 days instead of 28 days.16 No comparison between bolus injection and the microdose drug infusion through the PMP was done in this study. However, a device such as the PMP that can be programmed for different posology would provide the ability to study the question of improved safety and efficacy in more detail. Biological effect of the microdose delivered by the PMP was demonstrated by the improvement of the CFT in the patients who received the full-programmed dose. The presence of a device like the PMP can induce the release of proinflammatory and angiogenic cytokines, but those would be transient lasting for only the durations of the healing process. In pars plana glaucoma, drainage devices implanted for many years in life studies, such biological effects, are well tolerated.
Current VEGF inhibitors induce nonselective blockage of all VEGF isoforms, and therefore down regulate VEGF to below physiologic levels; this may, in the short term, help improve the disease but on the long-term could increase neuroretinal apoptosis and capillary dropout in an ischemic macula.31–33 The use of a pulsatile delivery method of smaller amounts of drug by the PMP would have the theoretical advantage over eluting devices because of not continuously eluting anti-VEGF and therefore blocking VEGF all the time to below beneficial physiologic VEGF levels.
Ranibizumab is a humanized monoclonal antibody fragment with a molecular weight of 48 kilo Dalton that inhibits VEGF. One study has demonstrated that ranibizumab is stable even at 40°C having an average monomer percentage of 99.6% observed after 35 days of storage.34 In our study, ranibizumab was initially stored at the recommended temperature (2°C to −8°C) and was only exposed to body temperature for a short period of time (less than 90 minutes) before being administered. Future studies to demonstrate stability and biological activity of monoclonal antibody at body temperature for a long period of time will be necessary.
We have presented for the first time in the literature the successful implant of an electronic first generation drug delivery system in patients with retinal disease. Since the completion of this study, there have been further improvements made to the second generation of the PMP allowing for calibrated drug delivery for many years. Future larger clinical PMP studies to enable even longer-term safety profile and to demonstrate the feasibility of multiple programmable drug delivery as well as in-office refilling of the drug reservoir are necessary.
Acknowledgments
This study was supported by funds provided by Replenish, Inc.
Disclosure: M. Humayun, Replenish, Inc. (C, F), (P); A. Santos, Replenish, Inc. (C, F); J. Altamirano, Replenish, Inc. (C, F); R. Ribeiro, Replenish, Inc. (C, F); R. Gonzalez, Replenish, Inc. (C, F); A. Rosa, Replenish, Inc. (C, F); J. Shih, Replenish, Inc. (E), (P); C. Pang, Replenish, Inc. (E), (P); F. Jiang, Replenish, Inc. (E), (P); P. Calvillo, Replenish, Inc. (E); J. Huculak, (P); J. Zimmerman, None; S. Caffey, Replenish, Inc. (E), (P)
References
- 1.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1432–44. doi: 10.1056/NEJMoa062655. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355:1419–31. doi: 10.1056/NEJMoa054481. [DOI] [PubMed] [Google Scholar]
- 3.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regillo CD, Brown DM, Abraham P, et al. Randomized, double-masked, sham-controlled trial of ranibizumab for neovascular age-related macular degeneration: PIER Study year 1. Am J Ophthalmol. 2008;145:239–48. doi: 10.1016/j.ajo.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Martin DF, Maguire MG, et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012;119:2537–48. doi: 10.1016/j.ophtha.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Lang GE, Berta A, Eldem BM, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology. 2013;120:2004–12. doi: 10.1016/j.ophtha.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 8.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Campochiaro PA, Heier JS, Feiner L, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six-month primary end point results of a phase III study. Ophthalmology. 2010;117:1102–12. doi: 10.1016/j.ophtha.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Cohen SY, Mimoun G, Oubraham H, et al. Changes in visual acuity in patients with wet age-related macular degeneration treated with intravitreal ranibizumab in daily clinical practice: the LUMIERE study. Retina. 2013;33:474–81. doi: 10.1097/IAE.0b013e31827b6324. [DOI] [PubMed] [Google Scholar]
- 12.Droege KM, Muether PS, Hermann MM, et al. Adherence to ranibizumab treatment for neovascular age-related macular degeneration in real life. Graefes Arch Clin Exp Ophthalmol. 2013;251:1281–4. doi: 10.1007/s00417-012-2177-3. [DOI] [PubMed] [Google Scholar]
- 13.Meyer CH, Michels S, Rodrigues EB, et al. Incidence of rhegmatogenous retinal detachments after intravitreal antivascular endothelial factor injections. Acta Ophthalmol. 2011;89:70–5. doi: 10.1111/j.1755-3768.2010.02064.x. [DOI] [PubMed] [Google Scholar]
- 14.Tabandeh H, Boscia F, Sborgia A, et al. Endophthalmitis associated with intravitreal injections: office-based setting and operating room setting. Retina. 2014;34:18–23. doi: 10.1097/IAE.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 15.Storey P, Dollin M, Pitcher J, et al. The role of topical antibiotic prophylaxis to prevent endophthalmitis after intravitreal injection. Ophthalmology. 2014;121:283–9. doi: 10.1016/j.ophtha.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 16.Stewart MW, Rosenfeld PJ, Penha FM, et al. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye) Retina. 2012;32:434–57. doi: 10.1097/IAE.0B013E31822C290F. [DOI] [PubMed] [Google Scholar]
- 17.Otsuji T, Nagai Y, Sho K, et al. Initial non-responders to ranibizumab in the treatment of age-related macular degeneration (AMD) Clin Ophthalmol. 2013;7:1487–90. doi: 10.2147/OPTH.S46317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas M, Mousa SS, Mousa SA. Comparative effectiveness of aflibercept for the treatment of patients with neovascular age-related macular degeneration. Clin Ophthalmol. 2013;7:495–501. doi: 10.2147/OPTH.S29974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pacella E, Vestri AR, Muscella R, et al. Preliminary results of an intravitreal dexamethasone implant (Ozurdex(R)) in patients with persistent diabetic macular edema. Clin Ophthalmol. 2013;7:1423–8. doi: 10.2147/OPTH.S48364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson PA, Comstock TL, Ip M, et al. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology. 2011;118:1580–7. doi: 10.1016/j.ophtha.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 21.Patel SR, Berezovsky DE, McCarey BE, Zarnitsyn V, Edelhauser HF, Prausnitz MR. Targeted administration into the suprachoroidal space using a microneedle for drug delivery to the posterior segment of the eye. Invest Ophthalmol Vis Sci. 2012;53:4433–41. doi: 10.1167/iovs.12-9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saati S, Lo R, Li PY, Meng E, Varma R, Humayun MS. Mini drug pump for ophthalmic use. Trans Am Ophthalmol Soc. 2009;107:60–70. [PMC free article] [PubMed] [Google Scholar]
- 23.Lo R, Li PY, Saati S, Agrawal R, Humayun MS, Meng E. A refillable microfabricated drug delivery device for treatment of ocular diseases. Lab Chip. 2008;8:1027–30. doi: 10.1039/b804690e. [DOI] [PubMed] [Google Scholar]
- 24.Razzacki SZ, Thwar PK, Yang M, Ugaz VM, Burns MA. Integrated microsystems for controlled drug delivery. Adv Drug Deliv Rev. 2004;56:185–98. doi: 10.1016/j.addr.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Lo R, Li PY, Saati S, Agrawal RN, Humayun MS, Meng E. A passive MEMS drug delivery pump for treatment of ocular diseases. Biomed Microdevices. 2009;11:959–70. doi: 10.1007/s10544-009-9313-9. [DOI] [PubMed] [Google Scholar]
- 26.Gutiérrez-Hernándes J-CCS, Abdallah W, et al. Pump for intravitreal drug delivery: a pilot study. Tran Vis Sci Tech. 2014;3:1–13. doi: 10.1167/tvst.3.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kinyoun J, Barton F, Fisher M, Hubbard L, Aiello L, Ferris F., 3rd Detection of diabetic macular edema. Ophthalmoscopy versus photography--Early Treatment Diabetic Retinopathy Study Report Number 5. The ETDRS Research Group. Ophthalmology. 1989;96:746–50. doi: 10.1016/s0161-6420(89)32814-4. discussion 50–51. [DOI] [PubMed] [Google Scholar]
- 28.Tsui I. Scleral buckle removal: indications and outcomes. Surv Ophthalmol. 2012;57:253–63. doi: 10.1016/j.survophthal.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Sarkisian SR., Jr Tube shunt complications and their prevention. Curr Opin Ophthalmol. 2009;20:126–30. doi: 10.1097/ICU.0b013e328323d519. [DOI] [PubMed] [Google Scholar]
- 30.Gaudreault J, Fei D, Rusit J, Suboc P, Shiu V. Preclinical pharmacokinetics of Ranibizumab (rhuFabV2) after a single intravitreal administration. Invest Ophthalmol Vis Sci. 2005;46:726–33. doi: 10.1167/iovs.04-0601. [DOI] [PubMed] [Google Scholar]
- 31.Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012;96:179–84. doi: 10.1136/bjophthalmol-2011-301087. [DOI] [PubMed] [Google Scholar]
- 32.Sabet-Peyman EJ, Heussen FM, Thorne JE, Casparis H, Patel SJ, Do DV. Progression of macular ischemia following intravitreal bevacizumab. Ophthalmic Surg Lasers Imaging. 2009;40:316–8. doi: 10.3928/15428877-20090430-17. [DOI] [PubMed] [Google Scholar]
- 33.Shimura M, Yasuda K. Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br J Ophthalmol. 2010;94:381–3. doi: 10.1136/bjo.2009.160986. [DOI] [PubMed] [Google Scholar]
- 34.Veurink M, Stella C, Tabatabay C, Pournaras CJ, Gurny R. Association of ranibizumab (Lucentis(R)) or bevacizumab (Avastin(R)) with dexamethasone and triamcinolone acetonide: an in vitro stability assessment. Eur J Pharm Biopharm. 2011;78:271–7. doi: 10.1016/j.ejpb.2010.12.018. [DOI] [PubMed] [Google Scholar]