Abstract
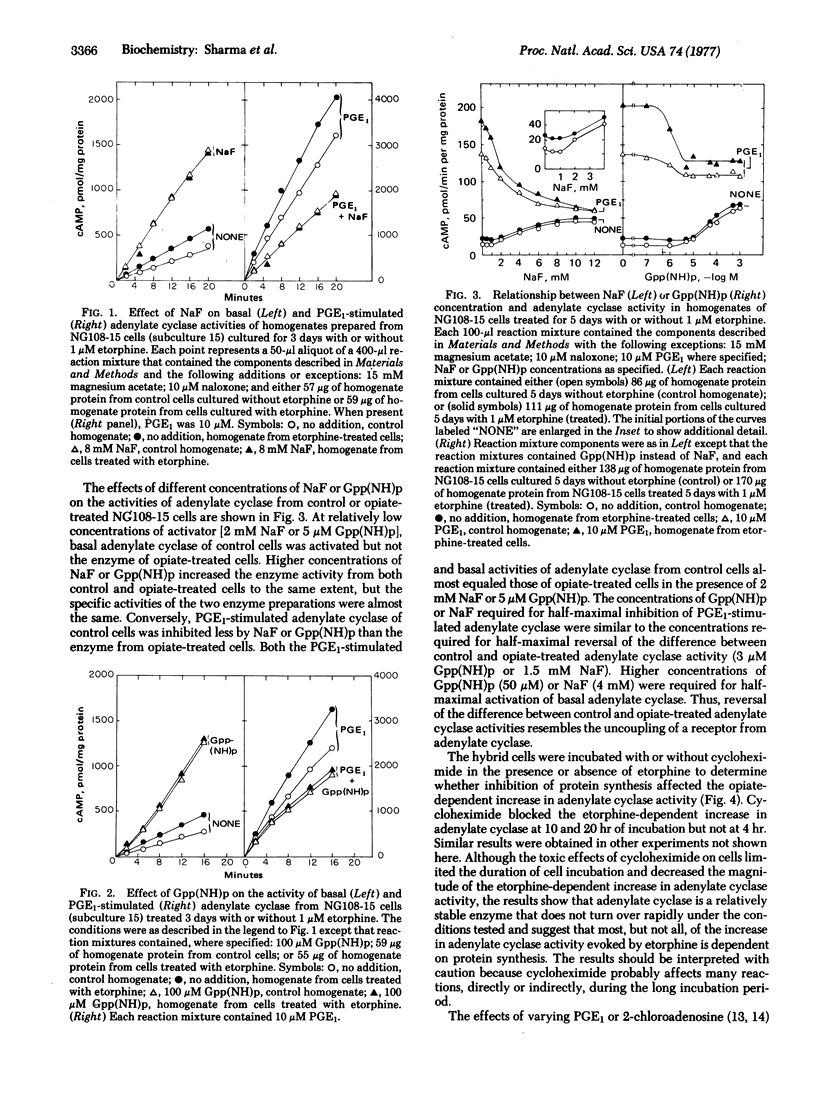
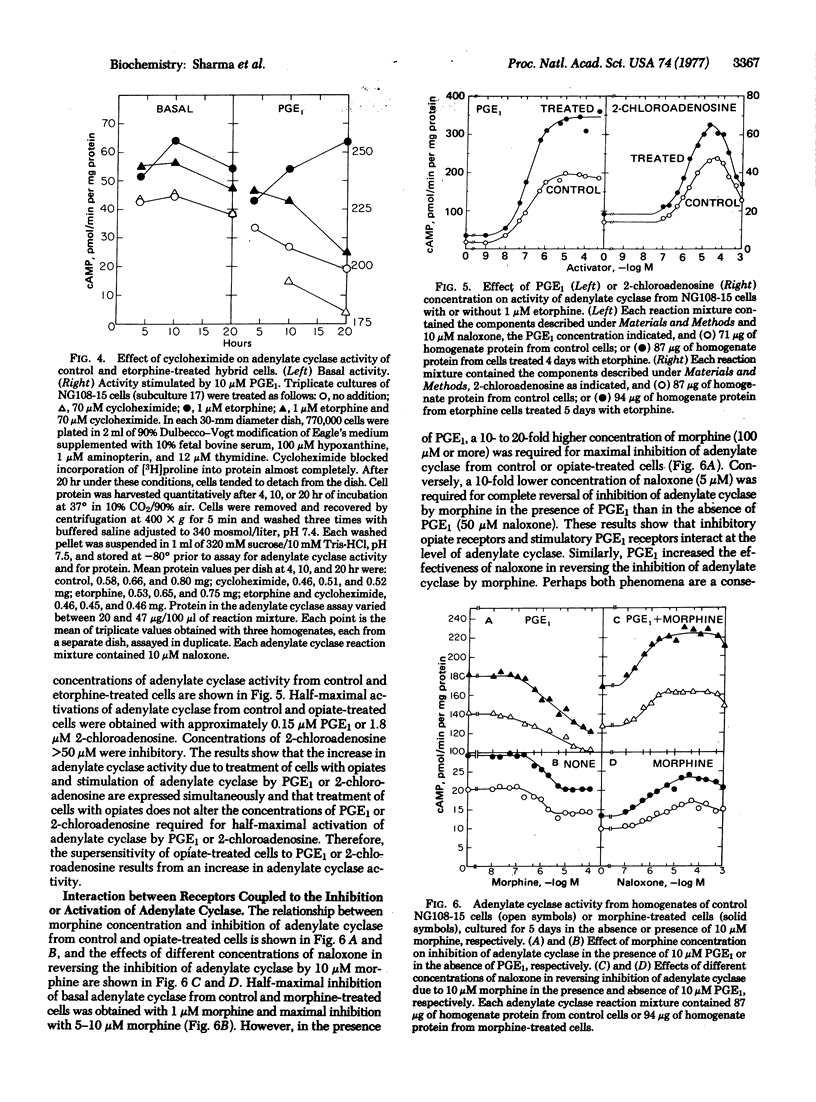
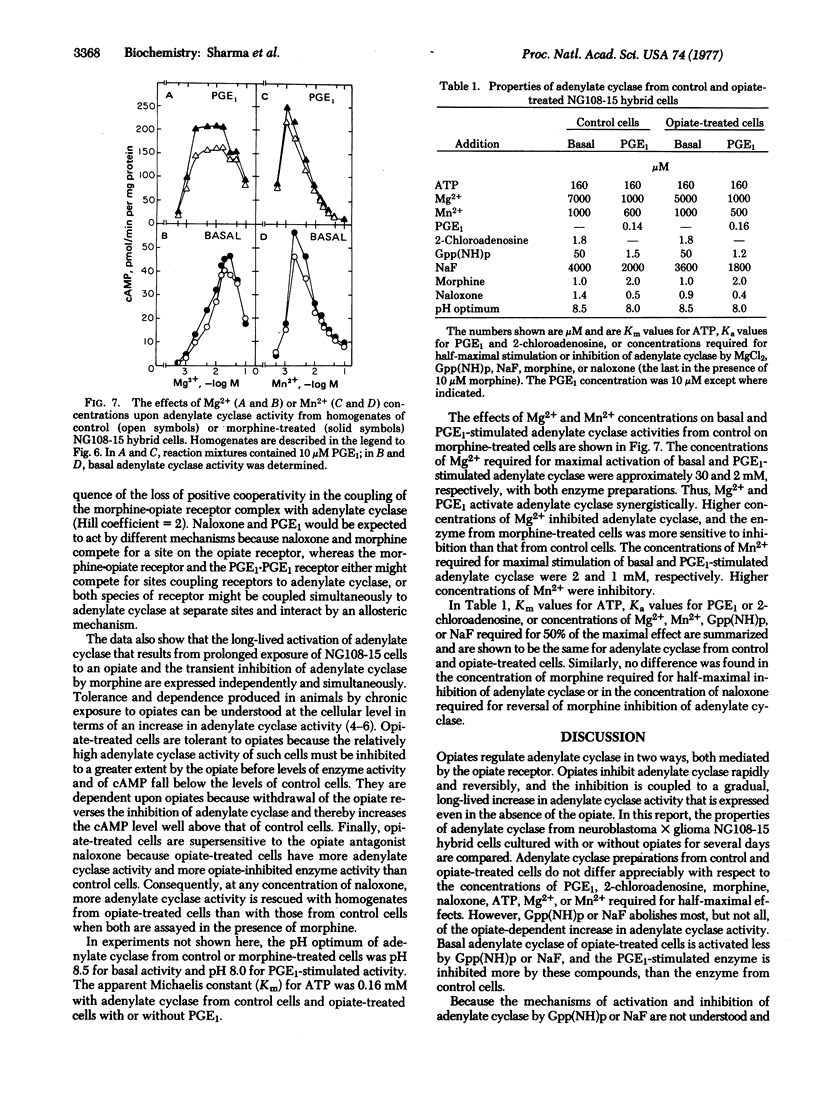
Reactions mediated by the opiate receptors that inhibit adenylate cyclase (EC 4.6.1.1) are closely coupled to subsequent reactions that gradually increase adenylate cyclase activity of neuroblastoma X glioma NG108-15 hybrid cells. Opiate-treated cells have higher basal-, prostaglandin E1-, and 2-chloroadenosine-stimulated activities than do control cells. However, NaF or guanosine 5'-(beta, gamma-imido)triphosphate abolishes most of the differences in adenylate cyclase activity observed with homogenates from control and opiate-treated cells. Cycloheximide blocked some, but not all, of the opiate-dependent increase in adenylate cyclase activity. These results suggest that the opiate-dependent increase in adenylate cyclase is due to conversion of adenylate cyclase to a form with altered activity. Protein synthesis also is required for part of the opiate effect. We propose that activity of adenylate cyclase determines the rate of conversion of the enzyme from one form to the other and that opiates, by inhibiting adenylate cyclase, alter the relative abundance of low- and high-activity forms of the enzyme.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blume A. J., Foster C. J. Neuroblastoma adenylate cyclase. Role of 2-chloroadenosine, prostaglandin E1, and guanine nucleotides in regulation of activity. J Biol Chem. 1976 Jun 10;251(11):3399–3404. [PubMed] [Google Scholar]
- Collier H. O., Francis D. L., McDonald-Gibson W. J., Roy A. C., Saeed S. A. Prostaglandins, cyclic AMP and the mechanism of opiate dependence. Life Sci. 1975 Jul 1;17(1):85–90. doi: 10.1016/0024-3205(75)90242-8. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974 Mar 1;248(5443):24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- Constantopoulos A., Najjar V. A. The activation of adenylate cyclase. II. The postulated presence of (A) adenylate cyclase in a phospho (inhibited) form (B) a dephospho (activated) form with a cyclic adenylate stimulated membrane protein kinase. Biochem Biophys Res Commun. 1973 Aug 6;53(3):794–799. doi: 10.1016/0006-291x(73)90162-9. [DOI] [PubMed] [Google Scholar]
- Engelhard V. H., Esko J. D., Storm D. R., Glaser M. Modification of adenylate cyclase activity in LM cells by manipulation of the membrane phospholipid composition in vivo. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4482–4486. doi: 10.1073/pnas.73.12.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Caron M. G. Characteristics of 5'-guanylyl imidodiphosphate-activated adenylate cyclase. J Biol Chem. 1975 Jun 25;250(12):4418–4422. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganiello V. C., Vaughan M. Activation and inhibition of fat cell adenylate cyclase by fluoride. J Biol Chem. 1976 Oct 25;251(20):6205–6209. [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahyoun N., Cuatrecasas P. Mechanism of activation of adenylate cyclase by cholera toxin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3438–3442. doi: 10.1073/pnas.72.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Klee W. A., Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3092–3096. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallman J. F., Smith C. C., Henneberry R. C. Induction of functional beta-adrenergic receptors in HeLa cells. Proc Natl Acad Sci U S A. 1977 Mar;74(3):873–877. doi: 10.1073/pnas.74.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber J., Fischer K., Latzin S., Hamprecht B. Morphine antagonises action of prostaglandin in neuroblastoma and neuroblastoma times glioma hybrid cells. Nature. 1975 Jan 10;253(5487):120–122. doi: 10.1038/253120a0. [DOI] [PubMed] [Google Scholar]
- Traber J., Gullis R., Hamprecht B. Influence of opiates on the levels of adenosine 3':5'-cyclic monophosphate in neuroblastoma X glioma hybrid cells. Life Sci. 1975 Jun 15;16(12):1863–1868. doi: 10.1016/0024-3205(75)90292-1. [DOI] [PubMed] [Google Scholar]


