Abstract
The kinase PRKD2 (protein kinase D) is a crucial regulator of tumor cell-endothelial cell communication in gastrointestinal tumors and glioblastomas, but its mechanistic contributions to malignant development are not understood. Here, we report that the oncogenic chaperone HSP90 binds to and stabilizes PRKD2 in human cancer cells. Pharmacologic inhibition of HSP90 with structurally divergent small molecules currently in clinical development triggered proteasome-dependent degradation of PRKD2, augmenting apoptosis in human cancer cells of various tissue origins. Conversely, ectopic expression of PRKD2 protected cancer cells from the apoptotic effects of HSP90 abrogation, restoring blood vessel formation in two preclinical models of solid tumors. Mechanistic studies revealed that PRKD2 is essential for hypoxia-induced accumulation of hypoxia-inducible factor-1α (HIF1α) and activation of NF-κB in tumor cells. Notably, ectopic expression of PRKD2 was able to partially restore HIF1α and secreted VEGF-A levels in hypoxic cancer cells treated with HSP90 inhibitors. Taken together, our findings indicate that signals from hypoxia and HSP90 pathways are interconnected and funneled by PRKD2 into the NF-κB/VEGF-A signaling axis to promote tumor angiogenesis and tumor growth.
Introduction
Cancer development is a multistep process characterized by a multitude of genetic and epigenetic changes that induce resistance to proapoptotic stimuli, sustain angiogenesis, and confer insensitivity to antigrowth signals and immune surveillance (1).
Rapid tumor growth often results in hypoxia, which triggers the stabilization of the transcription factor hypoxia-inducible factor-1 (HIF1), an oxygen sensor that controls the expression of multiple target genes implicated in angiogenesis, metabolism, and cell survival (2, 3). A prominent target of HIF1α is VEGF-A, which induces tumor angiogenesis by stimulating proliferation, survival, and migration of endothelial cells (4). HIF1α has been reported to physically interact with HSP90 (5, 6), which can be targeted by small-molecule inhibitors of chaperone, a growing class of clinically utilized antitumori-genic agents. HSP90 is a highly conserved and ubiquitously expressed molecular chaperone involved in the correct folding and final maturation of a plethora of proteins, so-called HSP90 clients, in an effort to maintain cellular homeostasis (7, 8). There are more than 200 HSP90 clients known, including protein kinases, transcription factors, and steroid hormone receptors (9–11). HSP90 is recruited to its kinase clients through interactions with kinase-specific co-chaperone CDC37 (12, 13), which stabilizes the HSP90/kinase (14). In tumor cells, HSP90 aids in folding dysregulated oncoproteins helping to sustain their aberrant activity. Amongst the most known client kinases of HSP90 are SRC (15), AKT (16), PDK-1 (17), and PKC (18). The latter was shown to directly activate protein kinase D (PRKD) family members via phosphorylation at two critical serine residues within the activation loop of the kinase catalytic domain (19). Recently, an affinity-based proteomic screen conducted to identify cancer-specific networks coordinated by HSP90 revealed PRKD2 as a potential client for the chaperone in chronic myelogenous leukemia (CML) cells (20). The serine-threonine kinase PRKD2 and its sister isoforms PRKD1 and PRKD3 belong to the calcium/calmodulin-dependent protein kinase superfamily (21) and are activated by various stimuli, including phorbol esters, reactive oxygen species, receptor tyrosine kinases, and hypoxia (22–24). PRKD2 expression and activity correlate positively with the state of dedifferentiation in lymphoma (25) and were demonstrated to be involved in myeloid leukemia by activating NF-κB transcription factors (26). Furthermore, PRKD2 is involved in migration, invasion, and growth of glioblastoma and pancreatic cancer cells (27–29). We have recently identified PRKD2 as a crucial mediator of hypoxia-induced VEGF-A expression and secretion in pancreatic cancer cells (24).
The aim of this study was to interrogate the contribution of PRKD2 to HSP90-mediated tumor growth and tumor angiogenesis. In addition, the involvement of PRKD2 in the regulation of hypoxia-mediated HIF1α stabilization, NF-κB activation, and VEGF-A production in the context of pharmacologic inhibition of HSP90 represented a major focus of our work. We identified PRKD2 as a novel client of HSP90 and revealed its requirement for tumor viability and tumor angiogenesis during abrogation of chaperone activity in vitro and in vivo. The fact that HSP90 regulates the stability of PRKD2, which acts as a two-pronged protein-mediating tumor blood vessel formation via hypoxia-induced HIF1α stabilization, VEGF-A production and tumor vascularization on one hand and cancer cell viability on the other, makes HSP90 inhibition a strategy to target two cancer characteristics with one drug.
Our work shows that PRKD2 represents a crucial molecule that seems to orchestrate hypoxia/HIF1α and chaperone's molecular signals in epithelial tumors through the activation of NF-κB and their target gene VEGF. Furthermore, our data indicate that HSP90 inhibitors (PU-H71 and STA-9090) currently undergoing clinical evaluation in patients might be used to target cancer growth and blood vessel formation particularly in hypoxic tumors with high expression of PRKD2. Given current efforts to develop PRKD2 kinase inhibitors, we envision the combined use of HSP90 and PRKD2 kinase inhibitors to achieve synergistic effects.
Materials and Methods
For details, see Supplementary Data.
Cell lines and inhibitors
Cancer cell lines originating from ATCC were cultured in early passages in DMEM (Invitrogen) supplemented with 10% FCS (PAA), 1% penicillin/streptomycin, and 5 µg/ml Plasmocin Prophylactic (InvivoGen). HCT-116 colon cancer cells were maintained in McCoy media supplemented with 10% FCS. Cell lines were authenticated using Multiplex Cell Authentication by Multiplexion. MG-132 was obtained from Sigma-Aldrich and bortezomib was purchased from LC Laboratories. PU-H71 was synthesized as reported (30). STA-9090 was purchased from SelleckChem.
Plasmids, transfection, and lentiviral transduction
The Block-IT Pol II miR RNAi sequences (#NM_016457.3-1295 and #NM_016457.3-1295-2019, Invitrogen) targeting human PRKD2 cloned into pLenti6.4CMV/R4R2/V5-DEST vector via Gateway technology (Invitrogen) used for lenti-viral-mediated knockdown were described previously (24) pLKO.1 lentiviral shRNA vectors were obtained from the TRC-Hs 1.0 (Human) shRNA library through Open Biosystem: HSP90A (TRCN0000001025), HSP9090B (TRCN0000008748), AKT1 #1 ((TRCN0000039794), AKT1 #2 (TRCN0000039797), RAF1 (TRCN0000001067). The pLenti6.2-V5-DEST-PRKD2, pLenti6.2-V5-DEST-AKT1, and pLenti6.2-V5-DEST-RAF1 over-expression vectors were generated using the pDONR-223-PRKD2, pDONR-223-AKT1, and pDONR-223-RAF1 entry clones from Addgene (PRKD2, #23490; AKT1, #23752 and RAF1, # 23832). High-titer virus-containing supernatants of 293FT after transient cotransfection of lentiviral vectors with pMD2 and psPAX2 viruses were used for lentiviral-mediated transduction of cancer cells.
CAM assay
HCT-116 or MDA-MB231 cancer cells (1 × 106) were xeno-grafted within 5 mm silicon rings on the surface of the chorionallantoic membrane (CAM) 8 days after fertilization. HSP90 inhibitor was delivered ectopically 24 and 48 hours after tumor xenograft at a concentration of 1 µmol/L in serum-free media in a volume of 20 µL serum-free antibiotics-free DMEM media. Four days after implantation (day 12 after fertilization), tumors were retrieved, fixed in formalin, and further subjected to IHC.
IHC of CAM and mouse tumors
Formalin-fixed tumors were embedded in paraffin using standard procedures. The 5-µm sections were processed and stained with antibodies directed against PRKD2 (1:250; Abcam #ab51250); pan-cytokeratin (1:80; Dako, clone AE1/AE3); desmin (1:80; Dako, clone D33); von Willebrand Factor VIII (1:100; Biocare Medical, #CP039B); and Ki-67 (1:100; Dako, clone MIB-1). Apoptotic cells were detected by TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) using the In Situ Cell Death Detection Kit, POD (Roche, # 11684817910) and quantified by counting >700 cells from at least four microscopic fields.
Mouse xenotransplantation experiments
All animal experiments were conducted according to German Animal Welfare and research protocols were approved by the Animal Care and Use Committee at the Regier-ungspräsidium Tübingen, Germany (TV-1153). MDA-MB231 breast cancer and HCT-116 colon cancer cells (5 × 106 each) were subcutaneously inoculated at the left and right dorsal sides of 6-week-old female athymic mice (NMRI-(nu/nu; Janvier Labs). Each experimental group consisting of 9 animals received either 75 mg per kg body weight PU-H71 intraperitoneally three times/week or PBS as vehicle. Tumor size was monitored and measured for the next 3 weeks. After tumor retrieval, tumor volume was calculated according to the formula 0.5 × L × W × T (L, length; W, width; T, thickness). Tumors were further processed for IHC.
Statistical analysis
Analyses were performed with GraphPad Prims 5.0. Statistical significance was assessed by an unpaired Student t test. P < 0.05 was considered significant.
Results
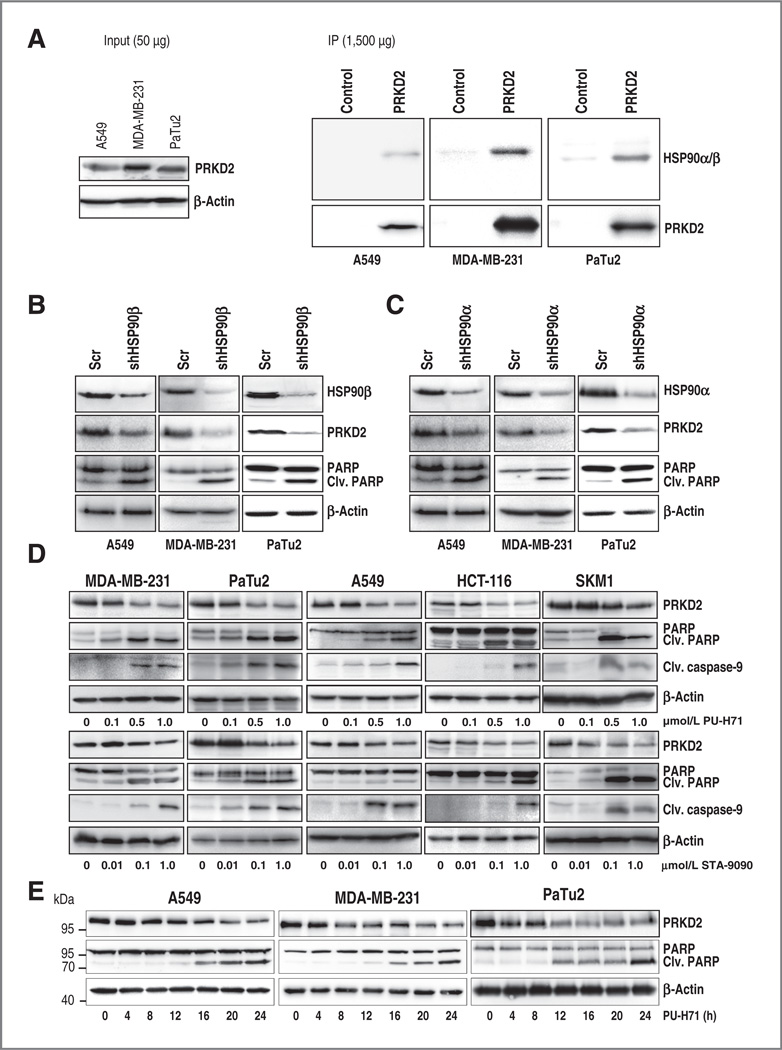
Using an affinity-based proteomic assay followed by chemical precipitation and Western blotting validation, Moulick and colleagues (20) identified PRKD2 as a putative HSP90 client in K562 CML cells. To assess whether HSP90 is able to bind to PRKD2 in solid tumors, coimmunoprecipitation experiments with lung cancer (A549), breast cancer (MDA-MB-231), and pancreatic cancer (PaTu2) cells were performed (Fig. 1A). Although PRKD2 interacted with HSP90 in all three cancer cell lines (Fig. 1A), an interaction between PRKD2 and HSP27 or HSP70 chaperones could not be observed (data not shown). To investigate whether the stability of PRKD2 requires HSP90, we performed knockdown experiments using shRNAs targeting HSP90α (shHSP90α) or HSP90β (shHSP90β), respectively. shRNA-mediated abrogation of both HSP90 isoforms resulted in a decrease of PRKD2 protein levels in A549, MDA-MB-231, and PaTu2 cell lines (Fig. 1B and C) and this was associated with induction of apoptosis as revealed by enhanced PARP cleavage in Western blot analysis (Fig. 1B and C) or TUNEL assay (Supplementary Fig. S1A). Altogether, these data infer PRKD2 as a putative novel HSP90 client in epithelial tumor cells and suggest PRKD2 depletion via HSP90 inhibition as a potential strategy to target cancer cells.
Figure 1.
HSP90 inhibition results in PRKD2 degradation and induces apoptosis. A, immunoprecipitation of endogenous PRKD2 was performed with lysates of PaTu2, A549, and MDA-MB-231 cells (PRKD2) and compared with control beads (Control). Membranes were incubated with HSP90α/β and PRKD2 antibodies. Of note, 50 µg of total lysate was subjected to SDS-PAGE and subsequent incubation with PRKD2 antibody (left). B and C, protein expression of HSP90α, HSP90β, PRKD2, and cleaved PARP was determined in cancer cell lines transduced with a nontargeting control shRNA(Scr) or a shRNA-targeting HSP90α or HSP90β. D, breast cancer (MDA-MB-231), pancreatic cancer (PaTu2), lung cancer (A549), colon cancer HCT-116, and acute myeloid leukemia (SKM1) cell lines were incubated with increasing amounts of PU-H71 and STA-9090 as indicated. Western blot analysis with PRKD2, cleaved PARP, and cleaved caspase-9 antibodies is depicted. E, lysates of cancer cell lines incubated with 1 µmol/L PU-H71 for the indicated time points were subjected to Western blot analysis with PRKD2 and cleaved PARP antibodies.
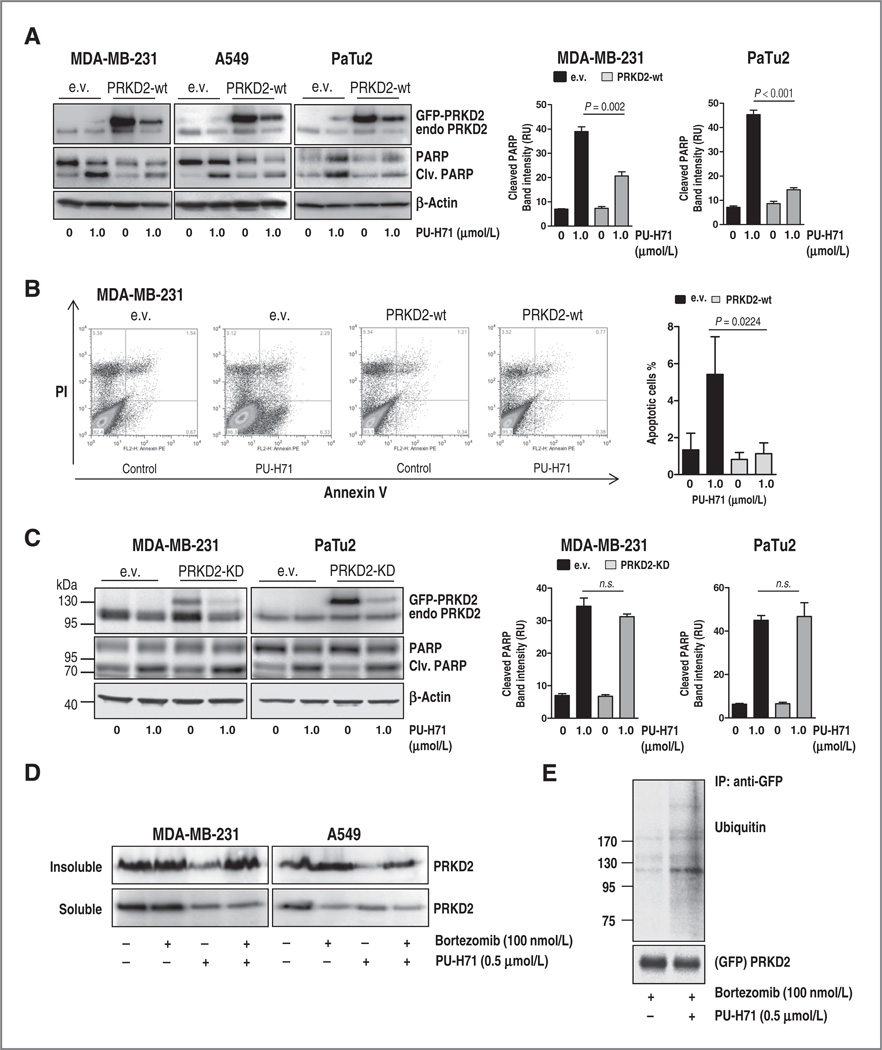
HSP90 is highly expressed in many tumors and allows the activation of tumor-specific signaling pathways and buffering stress conditions in the tumor microenvironment (31). Therefore, several ATP-competitive HSP90 inhibitors targeting a wide range of malignant tumors are currently under clinical investigation (1, 20, 32). To investigate whether PRKD2 stability is affected after pharmacologic HSP90 inhibition, eight human cancer cell lines representing six different tumor types (breast cancer, pancreatic cancer, lung cancer, colon cancer, acute myeloid leukemia, and glioblastoma) were incubated for 24 hours with increasing concentrations of two different compounds: PU-H71, an optimized water soluble member of the purine class of HSP90 inhibitors (20) and STA-9090, a resorcinol-containing triazole molecule with a novel chemical structure, both unrelated to the geldanamycin class of HSP90 inhibitors (1). Both inhibitors caused dose-dependent degradation of PRKD2 in all tumor cell lines (Fig. 1D and Supplementary Fig. S1B). HSP90 inhibition-mediated PRKD2 degradation was associated with increased apoptosis as revealed by augmented PARP and caspase-9 cleavage in all tumor cell lines (Fig. 1D and Supplementary Fig. S1B). The enhanced cleaved caspase-9 indicates apoptosis induction via the mitochondrial pathway. To prove that HSP90 inhibition-mediated depletion of PRKD2 contributes to the induction of cell death in tumor cells, we first sought to investigate whether downregulation of PRKD2 preceded the induction of apoptosis. Pancreatic, breast, and lung cancer cell lines were incubated with PU-H71 for 4, 8, 12, 16, 20, and 24 hours and PRKD2 and cleaved PARP protein levels were assessed by Western blotting (Fig. 1E). Degradation of PRKD2 commenced after 4 hours in PaTu2, 8 hours in MDA-MB-231, and 12 hours in A549 and was followed by PARP cleavage at around 12 hours in all tumor cell lines, indicating causality between the reduction of PRKD2 protein levels and induction of apoptosis (Fig. 1E). To further substantiate that PRKD2 is crucial for killing cancer cells after HSP90 inhibition, we ectopically expressed a GFP-PRKD2 construct in three cancer cell lines (MDA-MB-231, A549, and PaTu2), treated them with PU-H71, and analyzed PRKD2 levels and PARP cleavage by Western blotting. Both endogenous and overexpressed PRKD2 were subject of degradation; however, the higher remaining PRKD2 protein levels partially rescued cell viability after HSP90 inhibition (Fig. 2A). We confirmed these results involving additional approaches such as determination of apoptosis by annexin V staining in MDA-MB-231 cells and MTT assay for PaTu2 and MDA-MB-231 cancer cells after incubation with PU-H71 for 24 hours. Enforced expression of PRKD2 resulted in partial rescue of cell viability (Fig. 2B and Supplementary Fig. S2A and S2B). Conversely, overexpression of a kinase-inactive mutant of PRKD2 (GFP-PRKD2-KD) did not prevent cell death triggered by pharmacologic abrogation of HSP90 activity in MDA-MB-231 and PaTu2 cancer cells, suggesting the involvement of PRKD2 kinase activity in tumor cell viability (Fig. 2C). Furthermore, the examination of the abundance of PRKD2 protein in control or PU-H71-treated A549 and MDA-MB-231 cells after incubation with 5 µmol/L cycloheximide (CHX) showed that HSP90 inhibition accelerated the PRKD2 protein decay after protein translation blockade by CHX (Supplementary Fig. S2C). We next characterized the mechanism of PRKD2 degradation after treatment with PU-H71. To assess whether PRKD2 is degraded via the lysosomal pathway, we treated MDA-MB-231 breast cancer and A549 lung cancer cells with the lysosome inhibitor NH4Cl before PU-H71 incubation. Western blot analysis testing the abundance of PRKD2 protein in the detergent-soluble and detergent-insoluble fractions showed that preincubation with NH4Cl did not result in an increase of PRKD2 levels compared with the HSP90 inhibitor treatment alone, indicating that PRKD2 is not degraded via the lysosomal pathway (Supplementary Fig. S2D). In contrast, pretreatment of A549 and MDA-MB-231 cell lines with two different proteasome inhibitors, bortezomib or MG-132, followed by incubation with PU-H71 rescued PRKD2 levels and resulted in redistribution of PRKD2 to the detergent-insoluble fraction (Fig. 2D and Supplementary Fig. S2E). Consistent with its degradation via the proteasomal pathway, PRKD2 was extensively ubiquitinated in 293T cells transiently overexpressing PRKD2 after treatment with PU-H71 in combination with bortezomib (Fig. 2E). Together, these findings indicated that degradation of PRKD2 upon HSP90 inhibition occurs via the proteasomal pathway. Furthermore, we analyzed whether the effect of HSP90 inhibition might be caused by depletion of other proteins such as serine/threonine kinases AKT1 and RAF1 that have been reported to be HSP90 clients. Pharmacologic inhibition of HSP90 in A549, MDA-MB-231, and HCT-116 cancer cells triggered the abrogation of AKT1 and RAF1 protein expression (Supplementary Fig. S3A and S3B). Interestingly, shRNA-mediated deletion of AKT1 or RAF1 was associated with increased apoptosis in HCT-116 but not in A549 and MDA-MB-231 cancer cells (Supplementary Fig. S3C and S3D). As previously shown, the ectopic expression of either AKT1 or RAF1 was not sufficient to restore the cell viability after HSP90 inhibition (Supplementary Fig. S3E and S3F and 33). Although all these results argue against AKT1 and RAF1 to be responsible for the apoptotic effect of PU-H71 across different tissues, it is of course possible that other clients (33) contribute to the observed effects in addition to PRKD2.
Figure 2.
Destabilization of PRKD2 is essential for HSP90 inhibition-triggered apoptosis in tumor cells. A, lysates of cancer cell lines transiently transfected with empty vector (e.v.) or GFP-PRKD2-wild-type (PRKD2-wt) and incubated with PU-H71 for 24 hours were subjected to Western blot analysis with PRKD2 and cleaved PARP antibodies. Cleaved PARP bands were quantified by densitometric analysis using ImageJ program (right). B, MDA-MB-231 cancer cells transiently transfected with empty vector (e.v.) or PRKD2-wild-type (PRKD2-wt) and incubated with PU-H71 for 24 hours were subjected to Annexin V/propidium iodide staining. The bar graphs represent the mean of AnnexinV+/PI— cells from three independent experiments. C, cancer cell lines transfected with a GFP-tagged kinase dead PRKD2 (PRKD2-KD) mutant and incubated with PU-H71 for 24 hours were subjected to Western blotting. Membranes were ncubated with PRKD2 and cleaved PARP antibodies. Cleaved PARP bands were quantified by densitometric analysis using ImageJ program (right). D, soluble and insoluble protein fractions of breast and lung cancer cell lines pretreated with bortezomib for 2 hours before incubation with PU-H71 were subjected to Western blotting. Membranes were incubated with PRKD2 antibody. E, 293T cells transfected with GFP-PRKD2 were treated as described in D and mmunoprecipitation analysis was performed with anti-GFP antibody. Membranes were incubated with anti-ubiquitin and PRKD2 antibodies.
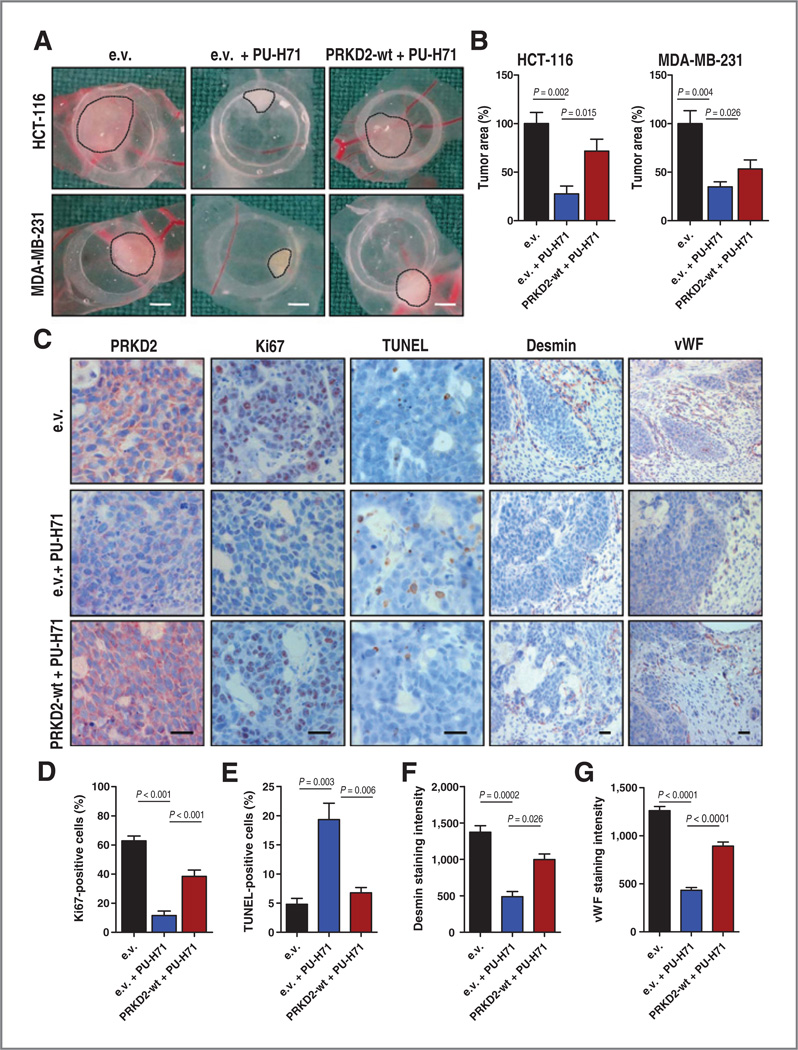
We previously reported a crucial role for PRKD2 in tumor angiogenesis and cancer cell proliferation (24, 27). We therefore sought to investigate whether PRKD2 depletion via HSP90 inhibition might impair tumor growth and blood vessel formation using a CAM xenotransplantation assay. MDA-MB-231 breast cancer and HCT-116 colon cancer cells stably expressing PRKD2 or empty vector were xenografted on the surface of chicken CAM 8 days after egg fertilization. The in vivo efficacy of PU-H71 has been previously tested (33). PU-H71 was ectopically applied 24 and 48 hours after implantation and tumor growth was monitored. Four days after implantation, tumors were excised, photographed, and analyzed by IHC. Treatment with PU-H71 of cancer cell lines expressing empty vector resulted in a significant decrease in tumor size (Fig. 3A and B). IHC analysis showed pronounced PRKD2 degradation upon HSP90 inhibition, which was associated with a significantly reduced proliferation rate as measured by Ki-67 staining, and increased apoptosis as determined by TUNEL analysis (Fig. 3C–E and Supplementary Fig. S4A and S4B). Examination of tumor-driven vascularization in xenografts revealed a marked reduction of blood vessel density, as determined by desmin and von Willebrand Factor (vWF) staining upon PU-H71 treatment compared with tumors treated with vehicle (Fig. 3C, F and G). Overexpression of PRKD2 was able to revert all PU-H71-induced effects as demonstrated by restored tumor formation (Fig. 3A and B), enhanced tumor proliferation (Fig. 3C and D and Supplementary Fig. S4A), impaired apoptosis (Fig. 3C and E and Supplementary Fig. S4B), and restored blood vessel formation (Fig. 3C, F and G).
Figure 3.
HSP90 inhibition impairs tumor growth and tumor blood vessel formation on CAM in a PRKD2-dependent manner. A, MDA-MB-231 or HCT-116 stably expressing PRKD2 (PRKD2-wt) or empty vector (e.v.) was delivered to CAM. Twenty-four hours old tumors were treated with 1 µmol/L PU-H71 for the next 48 hours. Bar, 1.5 mm. B, quantification of tumor area is presented. Error bars represent mean ± SEM of four to seven tumors. C, IHC of HCT-116 cells growing on CAM using specific antibodies directed against PRKD2, Ki-67, desmin, and vWF is presented. Parallel samples were subjected to TUNEL staining. D, quantification of Ki-67-positive HCT-116 cells is shown. E, quantification of TUNEL-positive cells for colon cancer cell xenografted on chicken CAM is displayed. Error bars represent mean ± SEM of at least four microscopic fields with 700 cells. F and G, desmin and vWF immunoreactivity was quantified by subtracting background staining from specific desmin or vWF using Optima software. Scale bar, 125 µm.
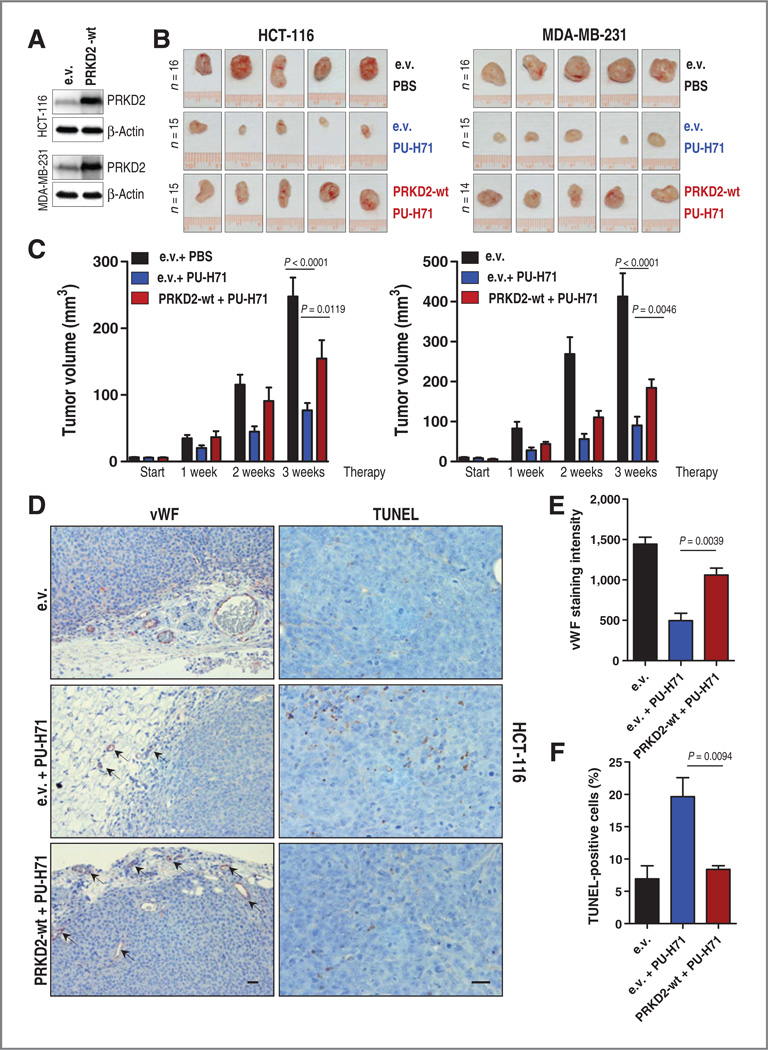
To further substantiate the data obtained in the CAM model, we examined the effects of HSP90 inhibition-mediated PRKD2 degradation in an additional in vivo model. HCT-116 colon carcinoma and MDA-MB-231 breast cancer cells stably overexpressing PRKD2 or empty vector were injected subcutaneously into both flanks of nude mice. One week later when tumors were palpable, mice received either 75 mg PU-H71 per kg body weight or vehicle (PBS) intraperitoneally three times per week. After 3 weeks, mice were sacrificed and tumors were analyzed. In line with the CAM experiments, pharmacologic inhibition of HSP90 resulted in substantially decreased tumor growth, increased apoptosis, and impaired angiogenesis in tumors expressing control vector (Fig. 4A–F and Supplementary Figs. S5A, S5B, and S6A and S6B). Conversely, administration of PU-H71 to mice that received cancer cells overexpressing PRKD2 showed little effect. Tumors from PBS-treated mice and tumors overexpressing PRKD2 from PU-H71-treated mice were associated with less TUNEL-positive cells (Fig. 4D and F and Supplementary Fig. S5B), increased vWF expression (Fig. 4D and E and Supplementary Fig. S5A), augmented VEGF expression (Supplementary Fig. S5A), and higher number of Ki-67-positive tumor cells as compared with tumors transduced with empty vector and treated with PU-H71 (Supplementary Fig. S6A and S6B). These data are in line with our previous finding that PRKD2 plays a major role in tumor growth and tumor angiogenesis and suggest that these properties can be counteracted by HSP90 inhibition-mediated PRKD2 depletion.
Figure 4.
HSP90 inhibition decreases tumor growth and tumor blood vessel formation in nude mice in a PRKD2-dependent manner. A, PRKD2 expression in colon and breast cancer cell lines stably transduced with PRKD2 is presented. B, one week following subcutaneous tumor transplantation, mice were injected with PU-H71 or PBS as control. Three weeks later, animals were sacrificed and tumors were analyzed. Photographs of five representative tumors per experimental group and cell line are depicted. C, the volume of explanted tumors is shown. Graphs represent mean ± SEM of at least 14 tumors per experimental group and cell line as indicated in B. D, IHC analysis of xenografted HCT-116 tumors with antibodies against von vWF and TUNEL are displayed. E, vWF labeling was quantified by subtracting the background staining using Optima software. F, quantification of HCT-116 TUNEL-positive tumor cells is presented. Error bars represent mean ± SEM of at least four microscopic fields with 600 cells. Scale bar, 125 µm.
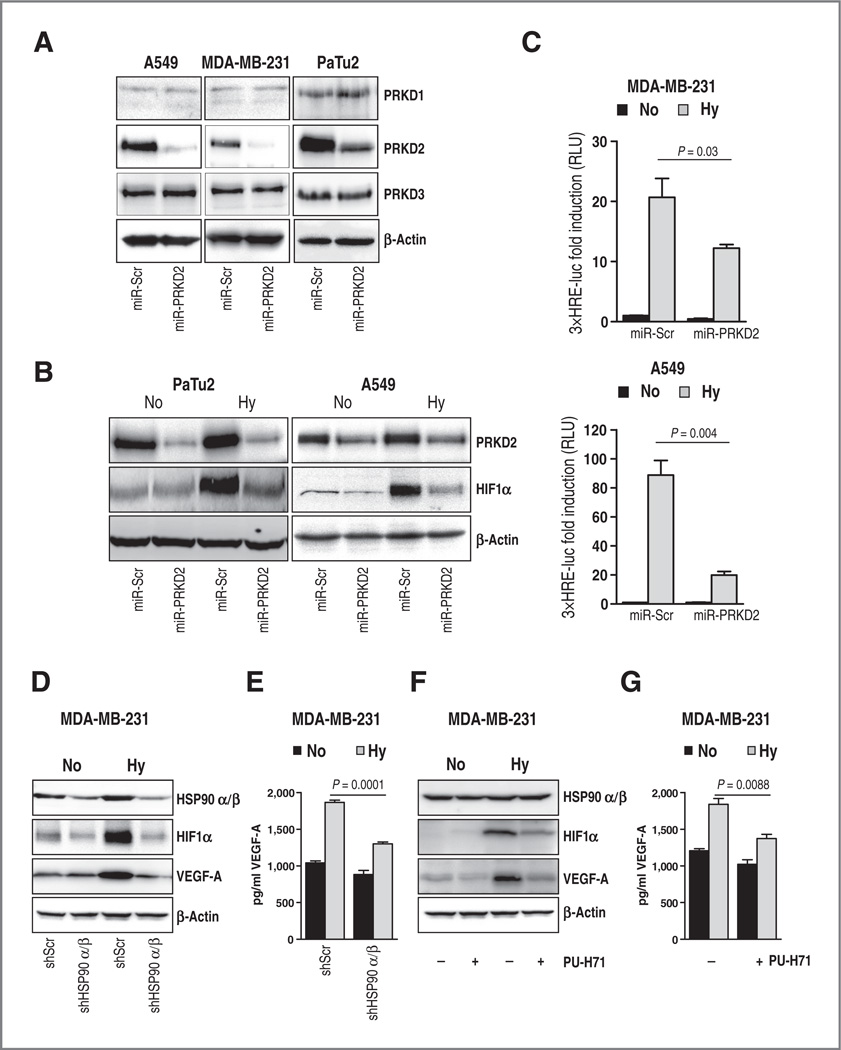
VEGF-A is one of the most potent mediators of the formation of blood vessels both under physiologic and pathologic conditions. We have previously reported that PRKD2 ablation impairs hypoxia-induced VEGF-A expression and secretion in pancreatic cancer cells (24). Because hypoxic upregulation of VEGF-A occurs mainly via the stabilization of HIF1α, we sought to investigate whether PRKD2 might regulate VEGF-A via this sensor protein. Specific PRKD2 suppression via RNAi in pancreatic (PaTu2) and lung (A549) cancer cells abrogated hypoxia-induced accumulation of HIF1α protein (Fig. 5A and B). Accumulation of hypoxia-induced HIF1α was also prevented when A549 and MDA-MB-231 cancer cells were treated with the PRKD inhibitor Gö6976 (Supplementary Fig. S7A). In addition, depletion of PRKD2 in tumor cells was associated with a significant reduction of transcriptional activation of the HIF-response element (HRE), an HIF1α-docking site present in promoters that contain the RCGTG sequence (Fig. 5C). HIF1α has been reported to be an HSP90 client (6, 34). In line with this, shRNA-mediated depletion of HSP90 and pharmacologic HSP90 inhibition in breast cancer cells resulted in impaired hypoxia-induced HIF1α accumulation (Fig. 5D and F). In both cases, abrogation of HIF1α protein accumulation was associated with decreased hypoxia-induced intracellular and secreted VEGF-A levels (Fig. 5D–G).
Figure 5.
PRKD2 mediates hypoxia-induced accumulation and promoter activity of HIF1α. A, lung, breast, and pancreatic cancer cell lines were stably transduced with PRKD2-specific miRNA (miR-PRKD2) or a noncoding miRNA (miR-Scr). Western blot analysis was conducted with antibodies against PRKD1, PRKD2, and PRKD3. B, pancreatic and lung cancer cell lines stably transduced with miR-PRKD2 or miR-Scr were incubated for 24 hours under low oxygen atmosphere. Cell extracts were subjected to Western blot analysis with PRKD2 and HIF1α antibodies. C, breast and lung cancer cell lines with abrogated PRKD2 were transiently transfected with 3xHRE-luc and pTK-Renilla. Four hours after transfection, cells were incubated under normoxic or hypoxic conditions and then cell lysates were subjected to luciferase assay. Bars are the mean ± SEM of at least three independent experiments. D, breast cancer cells transduced with a nontargeting control shRNA or HSP90α and HSP90β-specific shRNAs were incubated under hypoxia or normoxiafor 24 hours and HIF1α and VEGF-A levels were determined using Western blot analysis. E, supernatants of MDA-MB-231 cells with suppressed HSP90α/β and incubated in low oxygen were subjected to VEGF-A-specific ELISA. F, lysates of MDA-MB-231 cancer cells incubated under low oxygen or normoxia in the presence or absence of PU-H71 inhibitor were subjected to Western blot analysis with HIF1 α and VEGF-A antibodies. G, supernatants of MDA-MB-231 cells incubated in hypoxic or normoxic conditions in the presence or absence of PU-H71 were subjected to VEGF-A-specific ELISA. Bars represent the mean ± SEM of two independent experiments in triplicate. No, normoxia; Hy, hypoxia.
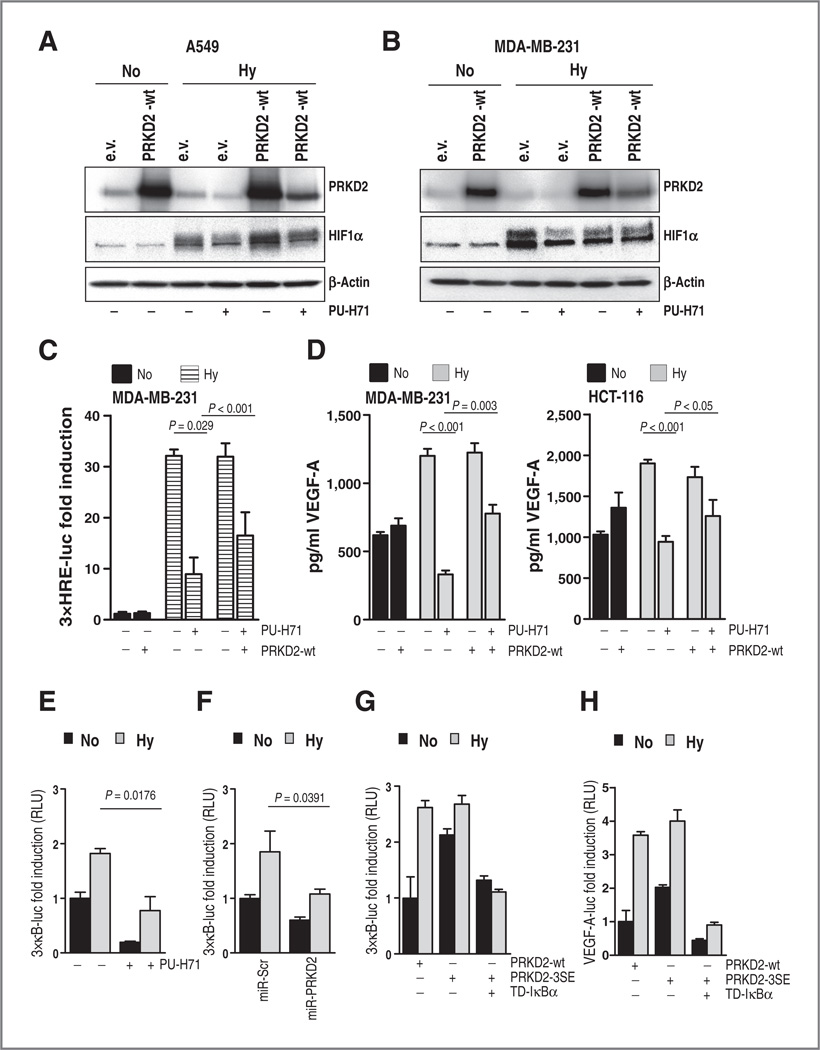
We next asked whether PRKD2 is involved in hypoxia-induced stabilization of HIF1α during HSP90 inhibition. Therefore, cancer cell lines stably transduced with PRKD2 or empty vector were incubated in low-oxygen atmosphere in the presence or absence of HSP90 inhibitor. As expected, treatment of tumor cells containing empty vector with PU-H71 impaired hypoxia-stabilized HIF1α levels (Fig. 6A and B). Overexpression of PRKD2 was able to partially rescue the hypoxia-induced accumulation of HIF1α protein (Fig. 6A and B) and HRE promoter activity (Fig. 6C), resulting in restored VEGF-A levels secreted by MDA-MB-231 and HCT-116 cells (Fig. 6D). Together, these data suggest that hypoxia-induced stabilization of HIF1α protein is mediated by HSP90 directly and through PRKD2, supporting a concept where PRKD2 links chaperone and hypoxia signaling pathways. VEGF-A can be secreted by tumor cells upon activation of HIF1α and NF-κB transcription factors (35). Furthermore, low-oxygen environment was reported to promote not only the accumulation of HIF1α, but also to activate NF-κB transcription factors via TAK1/IKK signaling (36). HSP90 was shown to interact with the kinase domain of IKKα/IKKβ, and inhibition of HSP90 by geldanamycin prevented TNF-induced activation of IKK and NF-κB (37). We therefore wanted to know whether NF-κB signaling might be connected to the hypoxic response regulated by PRKD2 and HSP90. Our experiments show that NF-κB promoter activity is increased upon incubation of MDA-MB-231 cells in low-oxygen atmosphere (Fig. 6E and F). HSP90 inhibition resulted in impaired hypoxia-induced NF-κB promoter and reduced binding activity (Fig. 6E and data not shown). Similarly, shRNA-mediated suppression of PRKD2 resulted in decreased luciferase production of the NF-κB reporter (Fig. 6F). We previously demonstrated that hypoxia-induced VEGF-A promoter activity, and intracellular and secreted VEGF-A levels are also impaired upon PRKD2 knockdown in cancer cells (24). Because hypoxia and HSP90 mediate their signals through the IKK complex toward NF-κB, which is also activated by PRKD2 (26, 46), we asked whether PRKD2 plays any role in this scenario. A triple active mutant of PRKD2 (PRKD2-S244/706/710E, PRKD2-3SE) was sufficient to enhance both, the NF-κB and VEGF-A promoter activity (Fig. 6G and H). Conversely, coexpression of a dominant negative IκBα (TD-IκBα) mutant was associated with the blockade of hypoxia and hypoxia/PRKD2-3SE-induced NF-κB and VEGF-A transcriptional activity (Fig. 6G and H). Altogether, these data suggest that activation of NF-κB by PRKD2 involves the phosphorylation and proteasomal degradation of IκBα. Notably, the activation of VEGF-A promoter by hypoxia and/or active PRKD2 is suppressed upon blockade of NF-κB signaling, which would suggest a hypoxia → PRKD2 → NF-κB → VEGF-A signaling axis. However, enforced expression of PRKD2 was able to marginally restore the hypoxia-induced NF-κB promoter activity affected by the inhibition of HSP90 (Supplementary Fig. S7B). Taken together, these results favor PRKD2 as a kinase acting in both the NF-κB and HIF1α pathways, thereby connecting hypoxic signals and HSP90 chaperone function to promote tumor growth and tumor angiogenesis.
Figure 6.
Ectopic PRKD2 restores hypoxia-induced stabilization of HIF1 α after HSP90 inhibition. A and B, lung and breast cancer cells transduced with control vector (e.v.) or PRKD2 (PRKD2-wt) were incubated under hypoxic conditions or normoxia for 24 hours in the presence or absence of HSP90 inhibitor. Western blot analysis with HIF1α and PRKD2-specific antibodies is presented. C, breast cancer cells overexpressing PRKD2 were transfected with 3xHRE-luc reporter and then incubated in hypoxic or normoxic environment in the presence or absence of HSP90 inhibitor. Luciferase production was assayed. Bars are the mean ± SEM of three independent experiments. D, supernatants of breast and colon cancer cells stably overexpressing PRKD2 or empty vector and incubated in low oxygen in the presence or absence of PU-H71 were subjected to VEGF-A-specific ELISA. E, breast cancer cells transfected with 3x κB-luc reporter were incubated in hypoxic or normoxic environment in the presence or absence of HSP90 inhibitor and cell lysates were subjected to luciferase assays. F, MDA-MB-231 cancer cells transduced with PRKD2-specific miR transfected with 3x-κB-luc and pTK-Renilla were incubated under normoxic or hypoxic conditions. Cell lysates were subjected to luciferase assay. G, lysates of MDA-MB-231 cells transfected with PRKD2-wt, constitutive active PRKD2 (PRKD2-3SE), and trans-dominant lκBα mutant (TD-lκBα) and incubated in normoxic or hypoxic atmosphere were subjected to luciferase assay. H, MDA-MB-231 cells were transfected with PRKD2-wt, constitutive active PRKD2 (PRKD2-3SE), and trans-dominant lκBα mutant (TD-IκBα) subsequent to incubation in normoxic or low oxygen atmosphere. Lysates were subjected to luciferase reporter assay. For all experiments, bars represent the mean ± SEM of at least three independent experiments.
Discussion
HSP90 serves as an ATP-dependent stabilizer of diverse signaling proteins, including many kinases that are involved in cell proliferation and survival. Chaperone inhibitors were recently shown to effectively inhibit tumor cell growth and angiogenesis in hematologic and solid-organ malignancies. However, it remains elusive whether cancer cell killing or disruption of vasculature network supplying tumor cells is mediated by depletion of a single molecule or simultaneous degradation of multiple client proteins that are overexpressed and/or mutated in cancer (38).
In this study, we have identified PRKD2 as a novel client of the HSP90 chaperone. We could demonstrate that PRKD2 interacts with HSP90 in several cancer cell lines. Depletion of PRKD2 protein following pharmacologic inhibition of HSP90 was associated with tumor cell death in vitro in various human cancer cell lines, as well as in two in vivo xenograft models. These data not only confirm the role of PRKD2 as an anti-apoptotic signaling molecule (39, 40), but also implicate PRKD2 in the cell death evoked by HSP90 inhibition. Our earlier findings demonstrated that PRKD2 is a crucial mediator of tumor angiogenesis involving upregulation and secretion of VEGF-A (24). This prompted us to investigate whether HSP90 might contribute to tumor angiogenesis through PRKD2 protein stabilization. We demonstrated that pharmacologic inhibition of HSP90 impaired blood vessel formation in vivo. The fact that PRKD2 overexpression restored vascularization and cell viability after HSP90 inhibition points to the involvement of PRKD2 in these HSP90 inhibitor-induced effects. Our data support PRKD2 degradation through HSP90 inhibition as a putative strategy to hit two important cancer characteristics, angiogenesis and cell viability, with one drug.
HSP90 inhibitors have been reported to indirectly regulate HIF1α (41–44). Furthermore, the HSP90 inhibitor geldanamycin reduced hypoxia-mediated HIF1α activation, indicating that chaperone activity is needed for this activation (6). The fact that HSP90 interacts both with HIF1α (6) and PRKD2 (this study) prompted us to evaluate the contribution of PRKD2 with respect to HIF1α stabilization in hypoxic tumors. We found that abrogation of PRKD2 in cancer cells prevented hypoxia-mediated HIF1α accumulation and HIF1α promoter activity. In line with several reports, reduced HSP90 expression and/or activity resulted in impaired hypoxia-triggered HIF1α accumulation and decreased VEGF-A expression. Notably, ectopically expressed PRKD2 was able to partially restore HIF1α protein levels, HIF1α transcriptional activity, and secreted VEGF-A levels after pharmacologic HSP90 inhibition. Together, these findings suggest that PRKD2 is required for hypoxia-induced HIF1α accumulation and that HSP90-supported angiogenesis is modulated by PRKD2 in hypoxic tumors by regulating HIF1α protein levels and subsequent VEGF-A secretion.
VEGF-A can be produced by tumor cells upon activation of HIF1α and NF-κB (35). Hypoxia drives the accumulation of HIF1α but also activates NF-κB transcription factors (36). Furthermore, inhibition of HSP90 was shown to promote apoptosis through suppression of AKT/NF-κB signaling (45). Thus, NF-κB represents a downstream effector of two major signaling routes: the hypoxia-induced HIF1α and HSP90 pathways. Because PRKD2 was reported to mediate stress-induced NF-κB activation and cell survival (46), we reasoned that PRKD2 acts upstream of NF-κB and might be a possible important molecule involved in hypoxia/HIF1α and HSP90 signaling down to NF-κB and subsequently to VEGF-A expression/secretion. We found that hypoxia-induced NF-κB activation was blocked by HSP90 inhibition and shRNA-mediated suppression of PRKD2. The finding that PRKD2 was able to just marginally restore hypoxia-induced NF-κB promoter activity affected by the inhibition of HSP90 suggests that other factors might be required as well for the HSP90 angiogenic signals through the NF-κB pathway.
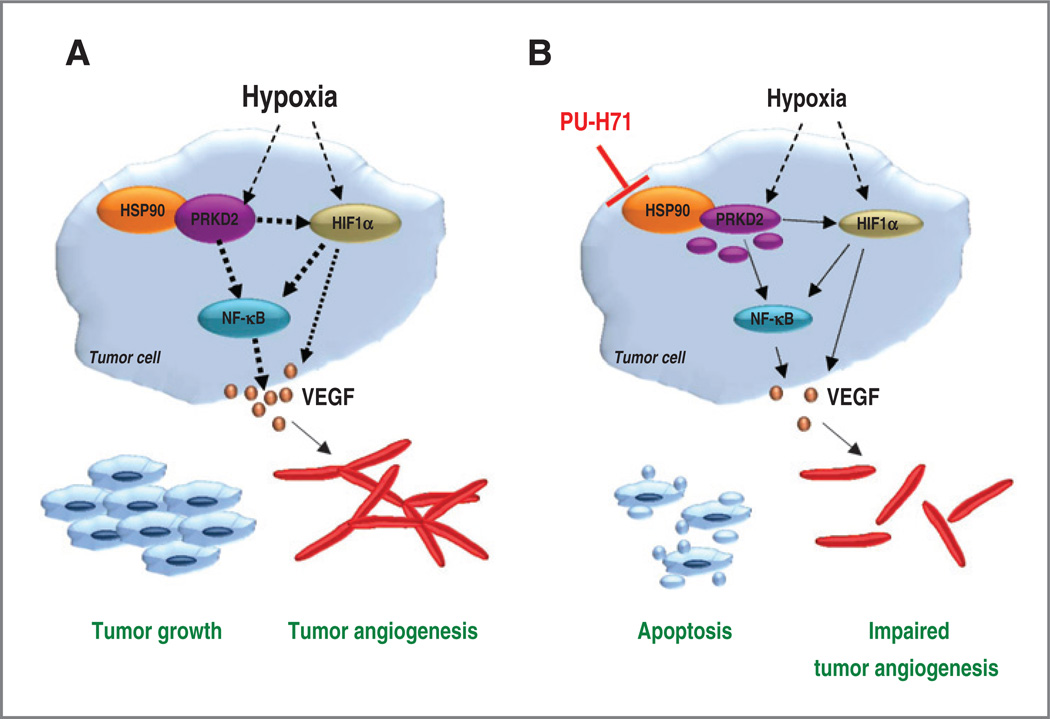
In conclusion, our data suggest a central role for PRKD2 to enhance HIF1α accumulation in low-oxygen environment. Stabilization of PRKD2 by HSP90 also results in the activation of NF-κB and its target VEGF-A, which promotes cancer cell growth and increases blood vessel formation in hypoxic tumors (Fig. 7). Whether and how PRKD2 activates NF-κB/VEGF-A via upregulation of HIF1α in hypoxic tumors or whether and how it contributes to the parallel activation of distinct HIF1α/ VEGF-A or NF-κB/VEGF-A pathways remains to be elucidated. This study may also have clinical implications because several HSP90 and PRKD2 inhibitors are currently in clinical trials or under development. The combination of HSP90 and PRKD2 inhibitors might have synergistic effects in patients with hypoxic tumors expressing high levels of PRKD2.
Figure 7.
PRKD2 modulates HSP90-driven tumor growth and tumor angiogenic by regulating hypoxia-mediated HIF1α accumulation and inducing VEGF-Asecretion via activation of NF-κB. A, stabilization of PRKD2 by HSP90 contributes to enhanced HIF1 α accumulation in low oxygen environment. In this scenario, activation of NF-κB and its target VEGF-A is associated with augmented tumor growth and increased blood vessel formation. B, degradation of PRKD2 following HSP90 inhibition affects HIF1α/ VEGF-A and/or HIF1α/NF-κB/ VEGF-A signaling pathways and triggers enhanced cancer cell apoptosis and impaired tumor vascularization. Dotted bold lines, basal signaling in hypoxic tumors; dotted thin lines, impaired signaling.
Supplementary Material
Acknowledgments
The authors thank Susanne Bobrovich for excellent technical assistance.
Grant Support
This work was supported by German Research Foundation (DFG; grant AZ.96/1-1; N. Azoitei), Deutsche Krebshilfe #109373 and DFG SE.676/10-1 (T. Seufferlein), and R01 CA172546 and R01 CA155226 grants (G. Chiosis). N. Azoitei, and K. Diepold were supported by the DFG grant to N. Azoitei, and C. Scholl was supported by an Emmy Noether Fellowship from the DFG.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
G. Chiosis is director of Samus Therapeutics. No potential conflicts of interest were disclosed by the other authors.
Authors’ Contributions
Conception and design: N. Azoitei, S. Fröhling C. Scholl
Development of methodology: N. Azoitei, K. Diepold, A. Rouhi, F. Genze, A. Becher
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): N. Azoitei, K. Diepold, C. Brunner, A. Rouhi, F. Genze, J. Koren III, T. Seufferlein
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): N. Azoitei, C. Brunner, A. Rouhi, H. Kestler, T. Seufferlein
Writing, review, and/or revision of the manuscript: N. Azoitei, C. Brunner, A. Becher, J. van Lint, G. Chiosis, S. Fröhling, C. Scholl, T. Seufferlein
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): N. Azoitei, S. Fröhling, C. Scholl
Study supervision: N. Azoitei, T. Seufferlein
Other (provided reagents and information on their use): G. Chiosis, T. Seufferlein
References
- 1.Wang Y, Trepel JB, Neckers LM, Giaccone G. STA-9090, a small-molecule HSP90 inhibitor for the potential treatment of cancer. Curr Opin Investig Drugs. 2010;11:1466–1476. [PubMed] [Google Scholar]
- 2.Semenza G. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol. 2002;64:993–998. doi: 10.1016/s0006-2952(02)01168-1. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL. Regulation of cancer metabolism by hypoxia-inducible factor 1. Semin Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 5.Gradin K, McGuire J, Wenger RH, Kvietikova I, Whitelaw ML, Toftgard R, et al. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Bid. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minet E, Mottet D, Michel G, Roland I, Raes M, Remade J, et al. Hypoxia-induced activation of HIF1: role of HIF1alpha-Hsp90 interaction. FEBS Lett. 1999;460:251–256. doi: 10.1016/s0014-5793(99)01359-9. [DOI] [PubMed] [Google Scholar]
- 7.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 8.Caplan AJ, Mandal AK, Theodoraki MA. Molecular chaperones and protein kinase quality control. Trends Cell Biol. 2007;17:87–92. doi: 10.1016/j.tcb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Zhao R, Houry WA. Hsp90: a chaperone for protein folding and gene regulation. Biochem Cell Biol. 2005;83:703–710. doi: 10.1139/o05-158. [DOI] [PubMed] [Google Scholar]
- 10.McClellan AJ, Xia Y, Deutschbauer AM, Davis RW, Gerstein M, Fryd-man J. Diverse cellular functions of the Hsp90 molecular chaperone uncovered using systems approaches. Cell. 2007;131:121–135. doi: 10.1016/j.cell.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 11.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59:1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearl LH. Hsp90 and Cdc37 – a chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15:55–61. doi: 10.1016/j.gde.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 13.Karnitz LM, Felts SJ. Cdc37 regulation of the kinome: when to hold 'em and when to fold 'em. Sci STKE. 2007;2007:e22. doi: 10.1126/stke.3852007pe22. [DOI] [PubMed] [Google Scholar]
- 14.Lee P, Shabbir A, Cardozo C, Caplan AJ. Sti1 and Cdc37 can stabilize Hsp90 in chaperone complexes with a protein kinase. Mol Biol Cell. 2004;15:1785–1792. doi: 10.1091/mbc.E03-07-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci U S A. 1999;96:109–114. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Basso AD, Solit DB, Chiosis G, Giri B, Tsichlis P, Rosen N. Akt forms an intracellular complex with heat shock protein 90 (Hsp90) and Cdc37 and is destabilized by inhibitors of Hsp90 function. J Biol Chem. 2002;277:39858–39866. doi: 10.1074/jbc.M206322200. [DOI] [PubMed] [Google Scholar]
- 17.Fujita N, Sato S, Ishida A, Tsuruo T. Involvement of Hsp90 in signaling and stability of 3-phosphoinositide-dependent kinase-1. J Biol Chem. 2002;277:10346–10353. doi: 10.1074/jbc.M106736200. [DOI] [PubMed] [Google Scholar]
- 18.Gould CM, Kannan N, Taylor SS, Newton AC. The chaperones Hsp90 and Cdc37 mediate the maturation and stabilization of protein kinase C through a conserved PXXP motif in the C-terminal tail. J Biol Chem. 2009;284:4921–4935. doi: 10.1074/jbc.M808436200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zugaza JL, Sinnett-Smith J, Van Lint J, Rozengurt E. Protein kinase D (PKD) activation in intact cells through a protein kinase C-dependent signal transduction pathway. EMBO J. 1996;15:6220–6230. [PMC free article] [PubMed] [Google Scholar]
- 20.Moulick K, Ahn JH, Zong H, Rodina A, Cerchietti L, Gomes DaGama EM, et al. Affinity-based proteomics reveal cancer-specific networks coordinated by Hsp90. Nat Chem Biol. 2011;7:818–826. doi: 10.1038/nchembio.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 22.Rykx A, DeKimpe L, Mikhalap S, Vantus T, Seufferlein T, Vandenheede JR, et al. Protein kinase D: a family affair. FEBS Lett. 2003;546:81–86. doi: 10.1016/s0014-5793(03)00487-3. [DOI] [PubMed] [Google Scholar]
- 23.Storz P. Mitochondrial ROS eradical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Azoitei N, Pusapati GV, Kleger A, Möller P, Küfer R, Genze F, et al. Protein kinase D2 is a crucial regulator of tumor cell -endothelial cell communication in gastrointestinal tumors. Gut. 2010;59:1316–1330. doi: 10.1136/gut.2009.206813. [DOI] [PubMed] [Google Scholar]
- 25.Kovalevska LM, Yurchenko OV, Shlapatska LM, Berdova GG, Mikha-lap SV, Van Lint J, et al. Immunohistochemical studies of protein kinase D (PKD) 2 expression in malignant human lymphomas. Exp Oncol. 2006;28:225–230. [PubMed] [Google Scholar]
- 26.Mihailovic T, Marx M, Auer A, Van Lint J, Schmid M, Weber C, et al. Protein kinase D2 mediates activation of nuclearfactor kappaB by Bcr-Abl in Bcr-Abl+ human myeloid leukemia cells. Cancer Res. 2004;64:8939–8944. doi: 10.1158/0008-5472.CAN-04-0981. [DOI] [PubMed] [Google Scholar]
- 27.Azoitei N, Kleger A, Schoo N, Thal DR, Brunner C, Pusapati GV, et al. Protein kinase D2 is a novel regulator of glioblastoma growth and tumor formation. Neuro Oncol. 2011;13:710–724. doi: 10.1093/neuonc/nor084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhart E, Damm S, Wintersperger A, DeVaney T, Zimmer A, Raynham T, et al. Protein kinase D2 regulates migration and invasion of U87MG glioblastoma cells in vitro. Exp Cell Res. 2013;319:2037–2048. doi: 10.1016/j.yexcr.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wille C, Köhler C, Armacki M, Jamali A, Gössele U, Pfizenmaier K, et al. Protein kinase D2 induces invasion of pancreatic cancer cells by regulating matrix metalloproteinases. Mol Biol Cell. 2014;25:324–336. doi: 10.1091/mbc.E13-06-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caldas-Lopes E, Cerchietti L, Ahn JH, Clement CC, Robles Al, Rodina A, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–8373. doi: 10.1073/pnas.0903392106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taipale M, Jarosz DF, Lindquist S. HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol. 2010;11:515–528. doi: 10.1038/nrm2918. [DOI] [PubMed] [Google Scholar]
- 32.Whitesell L, Bagatell R, Falsey R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- 33.Azoitei N, Hoffmann CM, Ellegast JM, Ball CR, Obermayer K, Gößele U, et al. Targeting of KRAS mutant tumors by HSP90 inhibitors involves degradation of STK33. J Exp Med. 2012;209:697–711. doi: 10.1084/jem.20111910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Schmid T, Frank R, Brüne B. PI3K/Akt is required for heat shock proteins to protect hypoxia-inducible factor 1 alpha from pVHL-independent degradation. J Biol Chem. 2004;279:13506–13513. doi: 10.1074/jbc.M310164200. [DOI] [PubMed] [Google Scholar]
- 35.Ono M. Molecular links between tumor angiogenesis and inflammation: inflammatory stimuli of macrophages and cancer cells as targets for therapeutic strategy. Cancer Sci. 2008;99:1501–1506. doi: 10.1111/j.1349-7006.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Culver C, Sundqvist A, Mudie S, Melvin A, Xirodimas D, Rocha S. Mechanism of hypoxia-induced NF-kappaB. Mol Cell Biol. 2010;30:4901–4921. doi: 10.1128/MCB.00409-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Cao P, Goeddel DV. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol Cell. 2002;9:401–410. doi: 10.1016/s1097-2765(02)00450-1. [DOI] [PubMed] [Google Scholar]
- 38.Whitesell L, Mimnaugh EG, De Costa B, Myers CE, Neckers LM. Inhibition of heat shock protein HSP90-pp60v-src heteroprotein complex formation by benzoquinone ansamycins: essential role for stress proteins in oncogenic transformation. Proc Natl Acad Sci USA. 1994;91:8324–8328. doi: 10.1073/pnas.91.18.8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang QJ. PKD at the crossroads of DAG and PKC signaling. Trends Pharmacol Sci. 2006;27:317–323. doi: 10.1016/j.tips.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 40.Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–13208. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- 41.Thomas GV, Tran C, Mellinghoff IK, Welsbie DS, Chan E, Fueger B, et al. Hypoxia-inducible factor determines sensitivity to inhibitors of mTOR in kidney cancer. Nat Med. 2006;12:122–127. doi: 10.1038/nm1337. [DOI] [PubMed] [Google Scholar]
- 42.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7:437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 43.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1a degradative pathway. J Biol Chem. 2002;277:29936–29944. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 44.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/s1471-4914(02)02316-x. [DOI] [PubMed] [Google Scholar]
- 45.Wang R, Shao F, Liu Z, Zhang J, Wang S, Liu J, et al. The Hsp90 inhibitor SNX-2112, induces apoptosis in multidrug resistant K562/ ADR cells through suppression of Akt/NF-κB and disruption of mitochondria-dependent pathways. Chem Biol Interact. 2013;205:1–10. doi: 10.1016/j.cbi.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Storz P, Toker A. Protein kinase D mediates a stress-induced NF-kappaB activation and survival pathway. EMBO J. 2003;22:109–120. doi: 10.1093/emboj/cdg009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.