Abstract
Background
In this study we investigated the potential of neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), mean platelet volume (MPV), and red cell width distribution (RDW) as new inflammatory markers to identify chronic inflammations during symptom-free periods in children diagnosed with Familial Mediterranean Fever (FMF).
Material/Methods
The study included 153 children diagnosed with FMF based on the Tel-Hashomer Criteria, and 90 healthy volunteers. Hospital records were obtained to collect NLR, PLR, MPV, RDW, and FMF scores and the FMF mutation analyses of the patients enrolled in the study. Data on proteinuria were also collected and defined as a protein/creatinine ratio >0.2.
Results
NLR, PLR, MPV, and RDW were significantly higher in symptom-free FMF patients than in the control group. C-reactive protein values also weakly correlated with NLR, PLR, MPV, and RDW, but the correlation was not statistically significant. NLR had the strongest correlation with CRP. The NLR cut-off point to indicate subclinical inflammation in symptom-free FMF patients was calculated to be 1.65.
Conclusions
NLR, PLR, MPV, and RDW are potential subclinical inflammation markers in patients with FMF. NLR, PLR, MPV, and RDW values are higher in patients with FMF during symptom-free periods. NLR was found to be the most reliable marker for subclinical inflammation when compared to PLR, MPV, and RDW. We also found that these markers are not significantly higher in proteinuric patients when compared with levels in non-proteinuric patients.
MeSH Keywords: Child, Familial Mediterranean Fever, Inflammation
Background
Familial Mediterranean fever (FMF) is an autosomal recessively inherited disease characterized by recurrent self-limited attacks of fever accompanied by aseptic inflammation of serosal spaces, joints, and skin. Although acute attacks of this disease are self-limited, some patients develop AA type amyloidosis, leading to renal failure, which is responsible for severe morbidity in FMF patients [1,2].
In FMF, an abnormal form of pyrin, which is a protein encoded by the MEFV gene, causes inflammation. These inflammatory episodes are then mediated by a massive influx of neutrophils into the serous cavities and are accompanied by an elevation in the levels of acute-phase proteins and cytokines [3]. The levels of many blood cytokines and acute phase reactants were studied in FMF patients and contributed to our understanding of the pathogenesis of FMF [4,5]. Further studies demonstrated an activation of the cytokine network in the disease course of FMF. Increased levels of interleukin-6 (IL-6), interleukin-10 (IL-10), serum-soluble interleukin-2 receptor (sIL-2r), and tumor necrosis factor alpha (TNF-α) have been detected during or between acute attacks of FMF in different studies [6,7]. These cytokines are primarily expressed by macrophages and monocytes at inflammatory sites [8]. Recent studies have shown that subclinical inflammation may continue in FMF, even during symptom-free periods [9]. The erythrocyte sedimentation rate (ESR) and acute-phase proteins such as C-reactive protein (CRP), serum amyloid A (SAA), and fibrinogen increase during attack periods and usually return to normal during symptom-free periods [10]. However, amyloidosis can develop in patients during a subclinical period of FMF. NLR, PLR, MPV, and RDW may be indicators of systemic subclinical inflammation [11–13]. NLR, PLR, RDW, and MPV have been associated with conditions such as chronic inflammation in cardiovascular diseases, malignancies, ulcerative colitis, hepatic cirrhosis, and systemic lupus erythematosus. Recent studies have suggested that NLR and MPV are significantly higher in FMF patients [11–15]. The current study was also planned to determine if this association exists between RDW levels and FMF, and to determine which marker – NLR, PLR, MPV, or RDW – best indicates subclinical inflammation in FMF patients. We also aimed to determine if subclinical inflammation markers could act as predictors of amyloidosis.
Material and Methods
This study was conducted retrospectively and included 153 pediatric patients diagnosed with FMF from the pediatric clinic at Gaziosmanpaşa University Medical Practice and Research Center between May 2009 and May 2014. The diagnosis of FMF was made according to the Tel-Hashomer criteria: the presence of at least 1 of 4 major criteria, 2 of 5 minor criteria, 1 minor criterion plus 5 of 10 supportive criteria, or 4 of 5 specific supportive criteria [16]. The control group included 90 age- and sex-matched healthy subjects. Both the study group and the control group subjects had no acute infection, pneumonia, diabetes mellitus, systemic hypertension, acute or chronic renal failure, chronic liver disease, chronic obstructive pulmonary disease, obstructive sleep apnea, coronary artery disease, connective tissue disease, inflammatory bowel disease, allergic rhinitis, asthma history, or any inflammatory disease.
We used an automated blood cell counter and the urine protein/creatinine ratio. A complete blood count (CBC) and biochemical analyses were obtained from hospital records at the clinic. Laboratory findings were collected during symptom-free periods. NLR was noted as a simple ratio between the absolute neutrophil and the absolute lymphocyte counts. NLR, PLR, MPV, and RDW were derived from the CBC.
Subjects were screened for the presence of all 12 known FMF mutations. Genomic DNA was extracted from 5-mL amounts of whole blood obtained via standard procedures. FMF mutations were detected using a reverse-hybridization test strip-based assay (FMF StripAssay, ViennaLab Labordiagnostika, Vienna, Austria), allowing detection of the 12 most common MEFV mutations: p.E148Q (c.442G>C) in exon 2; p.P369S (c.1105C>T) in exon 3; p.F479L (c.1437C>G) in exon 5; and p.M680I (c.2040G>C), p.M680I (c.2040G>A), I692del (c.2076>2078del), p.M694V (c.2080A>G), p.M694I (c.2082G>A), p.K695R (c.2084A>G), p.V726A (c.2177T>C), p.A744S (c.2230G>T), and p.R761H (c.2282G>A) in exon 10.
Continuous variables are presented as mean ±SD and categorical variables are expressed as numbers and percentages. To test the significance between 2 means, we used a comparison of continuous variables between groups. The t test was used for continuous variables with normal distribution and the Mann-Whitney U test was used for continuous variables without normal distribution. The chi-square test was used for categorical variables. Pearson correlation analysis was used to assess the relationships. A p value <0.05 was accepted as statistically significant. A region of conversion (ROC) analysis was used to determine the association of NLR with disease. The relationship between disease and variables was determined with multivariate logistic regression. For statistical calculations, SPSS Statistical Software (SPSS for Windows, version 17.0; SPSS Inc. Chicago, IL, USA) was used.
The Ethics Committee for Clinical Research of Gaziosmanpasa University School of Medicine approved this study. Our study was conducted in accordance with the ethical principles described by the Declaration of Helsinki. The study was supported by Gaziosmanpasa University Health Research and Practice Center.
Results
The study group consisted of 78 boys (51.0%) and 75 girls (49.0%), while the control group had 49 boys (54.4%) and 41 girls (45.6%). The mean age was 12.33±4.41 in the FMF study group and 10.96±3.45 in the control group. Mean follow-up time was 47 months and mean disease onset was 8.41 years. All FMF patients were using colchicine 1–3 times a day. The mean disease severity score was 6.46. The primary characteristics of FMF patients in the study were abdominal pain 96.1% (n=148), fever 84.7% (n=128), arthritis 34% (n=52), pleuritis 14.4% (n=34), erysipelas 4.6% (n=7), appendectomy 17.6% (n=27), and family history 52% (n=79). All of the identified mutations are summarized in Table 1. Thirty-four patients (22.2%) were homozygous, 43 (28.1%) were compound heterozygous, 68 (44.4%) were heterozygous, 5 (3.3%) were wild type, and 3 (1.9%) were not analyzed. The most commonly encountered MEFV gene mutation among the patients was heterozygous.
Table 1.
MEFV gene mutation analysis.
| Mutation | Mutation type | N | % |
|---|---|---|---|
| Homozygous | M694V, R202Q, M680I(G/C), E148Q | 34 | 21.6 |
| Heterozygous | M694V,R202Q,E148Q,V726A,A744S, M680I(G/C), K695R, R761H, P369S | 68 | 44.6 |
| Compound heterozygous | M694V/R202Q, E148Q/V726A, M694V/M680IG/C), M694V/E148Q, M680I(G/C)/V726A, R202Q/V726A, M694V/V726A, M694V/R761H, M694V/P369S, R202Q/E148Q | 43 | 28.3 |
| Wild type | 8 | 5.3 |
The 2 groups were evaluated in terms of NLR, PLR, MPV, and RDW values. We found that NLR, PLR, MPV, and RDW levels were significantly higher in the FMF study group than the control group. CRP values in symptom-free patients were significantly higher than in the control group (p<0.001), despite the role of CRP as an acute-phase reactant. NLR, PLR, MPV, RDW, and CRP values of FMF patients with proteinuria and without proteinuria were compared. There was no significant difference between these 2 groups. All laboratory data are summarized in Table 2.
Table 2.
Comparison of laboratory parameters among the groups.
| Variables | Control (n=90) | FMF group (n=153) | P1 value | P2 value |
|---|---|---|---|---|
| Hgb (gr/dl) | 12.84±1.42 | 13.25±8.16 | 0.636 | – |
| Wbc (×103/mm3) | 6.82±1.61 | 7.66±2.44 | 0.001 | |
| Plt (×103/mm3) | 271.93±57.32 | 284.98±77.27 | 0.165 | – |
| Mon (×103/mm3) | 0.52±0.14 | 0.57±0.21 | 0.023 | – |
| RDW | 13.68±2.35 | 14.89±2.56 | <0.001 | 0.420 |
| MPV | 7.37±0.78 | 7.76±1.12 | 0.002 | 0.879 |
| NLR | 1.26±0.45 | 1.99±1.7 | <0.001 | 0.488 |
| PLR | 104.14±34.06 | 121.75±52.30 | 0.005 | 0.341 |
| CRP (mg/L) | 3.37±0.21 | 7.5±8.19 | <0.001 | 0.602 |
Values are given as mean ±SD; p<0.05 is significant. P1 value comparison between FMF and control group. P2 value comparison between FMF with proteinuria and FMF without proteinuria.
Patients using colchicine 1 mg and under were compared with patients using colchicine over 1 mg. It is seen that higher NLR values required higher colchicine dosage for controlling symptoms (p=0.008).
Patients carrying M694V (either heterozygous or homozygous) mutation were compared with patients carrying other mutations. NLR values of patients with M694V were higher than NLR values in patients with other mutations (p=0.017).
The relationship between disease severity score and NLR was investigated in patients. There was no correlation between these 2 parameters (p=0.343).
There was a positive weak correlation between CRP and either NLR, PLR, MPV, or RDW. NLR had the strongest correlation with CRP levels (Table 3).
Table 3.
Comparison of effect values of markers.
| Variables | B | P | Odds ratio | 95% C.I. | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| MPV | 0.445 | 0.006 | 1.561 | 1.138 | 2.141 |
| RDW | 0.236 | <0.001 | 1.266 | 1.118 | 1.433 |
| NLR | 0.896 | <0.001 | 2.449 | 1.438 | 4.170 |
| PLR | 0.004 | 0.420 | 1.004 | 0.995 | 1.013 |
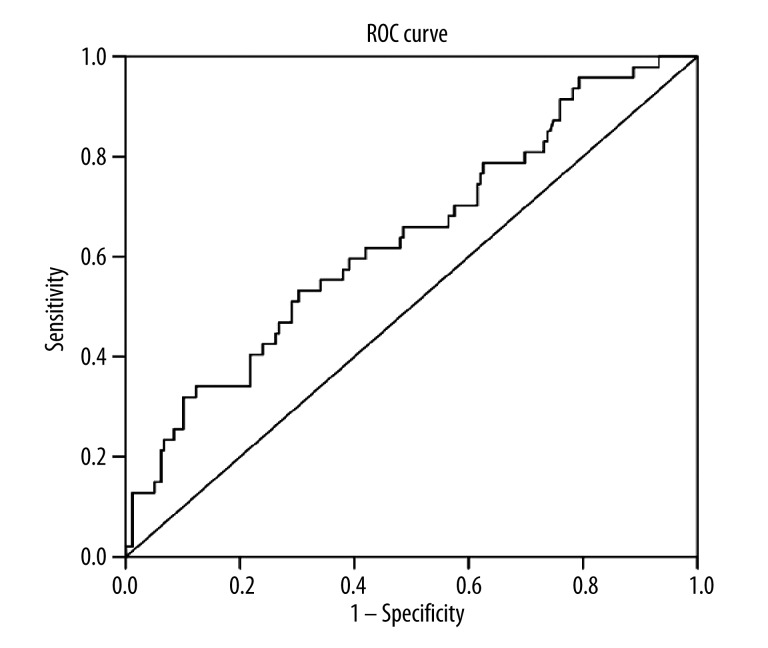
NLR showed an area under the curve (AUC) of 0.636 (95% CI, 0.569–0.698) with a cut-off value of 1.65 based on ROC analysis. This was found to be a reliable but simple marker for subclinical inflammation, as shown in Figure 1.
Figure 1.
The ROC curve showing the performance of NLR in patients with FMF.
Discussion
The main result of our study was determining the best marker for subclinical inflammation in children with FMF by comparing NLR, PLR, MPV, and RDW. Although PLR, MPV, and RDW demonstrated changes with subclinical inflammation in FMF, NLR had the strongest correlation with subclinical inflammation within these markers. Based on these data, the cut-off value for NLR can be used to detect subclinical inflammation.
In FMF, characteristic features of an attack include high fever lasting from several hours to 3–4 days and serositis involving the peritoneum (90%), fever (90%), arthritis (33%), pleuritis (31%), scrotum (5%), and pericardium (1%). Erysipelas-like erythema, which is usually associated with arthritis, tends to involve the distal end of the lower limbs, usually between the knee and the ankle, and on the dorsum of the proximal foot adjacent to the ankle [17–19]. The most devastating complication of FMF is amyloidosis. The main pathogenesis of amyloidosis is subclinical inflammation despite colchicine treatment. To date, many inflammatory markers have been studied in FMF. The erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), fibrinogen, serum amyloid A protein, and white blood cell levels are all being used as markers of acute phase response (APR) in FMF [20]. These markers increase during the attack periods and usually return to normal in attack-free periods [21]. Due to the risk of amyloidosis with subclinical inflammation, several studies have aimed to discover new markers to determine subclinical inflammation in FMF patients. Subclinical inflammation in FMF increases the risk of developing complications such as anemia, splenomegaly, decreased bone mineral density, heart disease, and especially amyloidosis, which can be fatal [21,22]. Inflammation usually occurs through the secretion of inflammatory cytokines by macrophages and monocytes. IL-1, sIL-2r, IL-6, and TNF-a play a major role during acute attacks in FMF [23]. In contrast, IL-1β levels are increased in FMF patients during the attack-free period, and are correlated with CRP levels. IL-1β levels may be important to show subclinical inflammation during the attack-free period in FMF patients. Also, IL-6, IL-8, and TNF-α levels were observed in attack-free FMF patients [24,25]. In this study, NLR was found to be a reliable inflammatory marker in FMF. In addition, NLR was significantly higher in patients with chronic renal failure than in healthy individuals, and levels were increased in proportion to the degree of chronic renal failure [26,27]. As in end-stage renal disease, NLR can used to detect inflammation in patients who receive hemodialysis or peritoneal dialysis, have cardiac disorders (especially myocardial infarction), endometriosis, and type 2 diabetes mellitus [21,28,29]. Ahsen et al. found that NLR is related to subclinical inflammation in FMF patients and that NLR was also correlated with CRP values [9]. NLR is a simple marker to determine subclinical inflammation because it can be achieved from CBC easily and does not require additional cost.
Many investigators have suggested that MPV reflects disease activity, inflammatory load, and/or systemic inflammation in many diseases such as hypertension, myocardial infarction, rheumatoid arthritis, and acute pancreatitis. Many studies have indicated higher MPV levels in FMF patients [30–33]. In this study, we found that PLR and MPV levels are higher in patients with FMF and, furthermore, MPV was also correlated with CRP. However, the link between MPV and CRP is still weaker than the link between MPV and NLR.
RDW has been proposed as a chronic inflammatory marker and proteinuria has been associated with a chronic inflammatory state and implicated as the origin of early renal damage [34–36]. In our study, we found that RDW levels are associated with subclinical inflammation, but only weakly correlated with CRP. Moreover, we found that RDW levels are not associated with proteinuria in children with FMF. In this study, we found no link between proteinuria and levels of the inflammatory markers, NLR, PLR, MPV, and RDW.
Many studies have reported that M694V mutation is closely related with the development of amyloidosis. Another major finding of our study was that FMF patients with M694V mutation had higher NLR values than in patients with other mutations.
Limitation of the study
It would have been useful to investigate the NLR values in patients during the attack period, but we could not compare patients in attack and attack-free periods because all of our included patients in this study were in the attack-free period. We would have compared the values of patients with amyloidosis, but only 1 of our patients was followed-up with amyloidosis secondary to FMF.
Conclusions
Although NLR, PLR, MPV, and RDW cannot be used to detect early signs of proteinuria, NLR, PLR, MPV, and RDW levels can be used to determine subclinical inflammation. However, we found that NLR had the strongest correlation with subclinical inflammation with CRP among these parameters. In this relationship we found that the cut-off value of NLR is 1.65 as a numerical marker for subclinical inflammation. Subclinical inflammation goes on in attack-free periods and causes amyloidosis. We suggest that NLR may be useful as a predictor of development of amyloidosis.
Footnotes
Source of support: Departmental sources
References
- 1.Acar BÇ, Yalçınkaya F, Mesiha E. Ailevi akdeniz ateşi patogenezi. Turkiye Klinikleri Journal of Pediatrics. 2006;15(4):151–55. [Google Scholar]
- 2.Ozen S, Demirkaya E, Duzova A, et al. FMF50: a score for assessing outcome in familial Mediterranean fever. Ann Rheum Dis. 2014;73(5):897–901. doi: 10.1136/annrheumdis-2013-204719. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Zvi I, Livneh A. Chronic inflammation in FMF: markers, risk factors, outcomes and therapy. Nat Rev Rheumatol. 2011;7(2):105–12. doi: 10.1038/nrrheum.2010.181. [DOI] [PubMed] [Google Scholar]
- 4.Yalçınkaya F, Cakar N, Acar B, et al. The value of the levels of acute phase reactants for the prediction of familial Mediterranean fever associated amyloidosis: a case control study. Rheumatol Int. 2007;27(6):517–22. doi: 10.1007/s00296-006-0265-6. [DOI] [PubMed] [Google Scholar]
- 5.Fassbender K, Dempfle CE, Mielke O, et al. Proinflammatory cytokines: indicators of infection in high-risk patients. J Lab Clin Med. 1997;130(5):535–39. doi: 10.1016/s0022-2143(97)90131-1. [DOI] [PubMed] [Google Scholar]
- 6.Schattner A, Lachmi M, Livneh A, et al. Tumor necrosis factor in familial Mediterranean fever. Am J Med. 1991;90(4):434–38. [PubMed] [Google Scholar]
- 7.Gang N, Drenth JP, Langevitz P, et al. Activation of the cytokine network in familial Mediterranean fever. J Rheumatol. 1999;26(4):890–97. [PubMed] [Google Scholar]
- 8.Manukyan GP, Ghazaryan KA, Ktsoyan Zh A, et al. Cytokine profile of Armenian patients with Familial Mediterranean fever. Clin Biochem. 2008;41(10–11):920–22. doi: 10.1016/j.clinbiochem.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Ahsen A, Ulu MS, Yuksel S, et al. As a new inflammatory marker for familial Mediterranean fever: neutrophil-to-lymphocyte ratio. Inflammation. 2013;36(6):1357–62. doi: 10.1007/s10753-013-9675-2. [DOI] [PubMed] [Google Scholar]
- 10.Tunca M, Kirkali G, Soyturk M, et al. Acute phase response and evolution of familial Mediterranean fever. Lancet. 1999;353(9162):1415. doi: 10.1016/S0140-6736(99)00990-3. [DOI] [PubMed] [Google Scholar]
- 11.Sakalli H, Kal O. Mean platelet volume as a potential predictor of proteinuria and amyloidosis in familial Mediterranean fever. Clin Rheumatol. 2013;32(8):1185–90. doi: 10.1007/s10067-013-2257-8. [DOI] [PubMed] [Google Scholar]
- 12.Hu ZD, Chen Y, Zhang L, et al. Red blood cell distribution width is a potential index to assess the disease activity of systemic lupus erythematosus. Clin Chim Acta. 2013;425:202–5. doi: 10.1016/j.cca.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Akbas EM, Demirtas L, Ozcicek A, et al. Association of epicardial adipose tissue, neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio with diabetic nephropathy. Int J Clin Exp Med. 2014;7(7):1794–801. [PMC free article] [PubMed] [Google Scholar]
- 14.Taşoğlu İ, Sert D, Colak N, et al. Neutrophil-Lymphocyte Ratio and the Platelet–Lymphocyte Ratio Predict the Limb Survival in Critical Limb Ischemia. Clin Appl Thromb Hemost. 2013;20(6):645–50. doi: 10.1177/1076029613475474. [DOI] [PubMed] [Google Scholar]
- 15.Zahorec R. Ratio of neutrophil to lymphocyte counts – rapid and simple parameter of systemic inflammation and stress in critically ill. Bratisl Lek Listy. 2001;102(1):5–14. [PubMed] [Google Scholar]
- 16.Livneh A, Langevitz P, Zemer D, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. 1997;40(10):1879–85. doi: 10.1002/art.1780401023. [DOI] [PubMed] [Google Scholar]
- 17.Savic S, Dickie LJ, Wittmann M, McDermott MF. Autoinflammatory syndromes and cellular responses to stress: pathophysiology, diagnosis and new treatment perspectives. Best Pract Res Clin Rheumatol. 2012;26(4):505–33. doi: 10.1016/j.berh.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 18.Yilmaz R, Ozer S, Ozyurt H, et al. Familial Mediterranean fever gene mutations in the inner northern region of Turkey and genotype-phenotype correlation in children. J Paediatr Child Health. 2009;45(11):641–45. doi: 10.1111/j.1440-1754.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- 19.Savran Y, Sari I, Kozaci DL, et al. Increased levels of macrophage migration inhibitory factor in patients with familial mediterranean Fever. Int J Med Sci. 2013;10(7):836–39. doi: 10.7150/ijms.6116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guzel S, Andican G, Seven A, et al. Acute phase response and oxidative stress status in familial Mediterranean fever (FMF) Mod Rheumatol. 2012;22(3):431–37. doi: 10.1007/s10165-011-0517-5. [DOI] [PubMed] [Google Scholar]
- 21.Uslu AU, Deveci K, Korkmaz S, et al. Is neutrophil/lymphocyte ratio associated with subclinical inflammation and amyloidosis in patients with familial Mediterranean fever? Biomed Res Int. 2013;2013:185317. doi: 10.1155/2013/185317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bilginer Y, Akpolat T, Ozen S. Renal amyloidosis in children. Pediatr Nephrol. 2011;26(8):1215–27. doi: 10.1007/s00467-011-1797-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baykal Y, Saglam K, Yilmaz MI, et al. Serum sIL-2r, IL-6, IL-10 and TNF-alpha level in familial Mediterranean fever patients. Clin Rheumatol. 2003;22(2):99–101. doi: 10.1007/s10067-002-0682-1. [DOI] [PubMed] [Google Scholar]
- 24.Yildirim K, Uzkeser H, Keles M, et al. Relationship between serum interleukin-1beta levels and acute phase response proteins in patients with familial Mediterranean fever. Biochem Med. 2012;22(1):109–13. doi: 10.11613/bm.2012.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiraz S, Ertenli I, Arici M, et al. Effects of colchicine on inflammatory cytokines and selectins in familial Mediterranean fever. Clin Exp Rheumatol. 1998;16(6):721–24. [PubMed] [Google Scholar]
- 26.Turkmen K, Guney I, Yerlikaya FH, Tonbul HZ. The relationship between neutrophil-to-lymphocyte ratio and inflammation in end-stage renal disease patients. Ren Fail. 2012;34(2):155–59. doi: 10.3109/0886022X.2011.641514. [DOI] [PubMed] [Google Scholar]
- 27.Okyay GU, Inal S, Onec K, et al. Neutrophil to lymphocyte ratio in evaluation of inflammation in patients with chronic kidney disease. Ren Fail. 2013;35(1):29–36. doi: 10.3109/0886022X.2012.734429. [DOI] [PubMed] [Google Scholar]
- 28.Turkmen K. Platelet-to-Lymphocyte Ratio: One of the novel and valuable platelet indices in hemodialysis patients. Hemodial Int. 2013;17(4):670. doi: 10.1111/hdi.12095. [DOI] [PubMed] [Google Scholar]
- 29.Celikbilek M, Dogan S, Akyol L, et al. Neutrophil-Lymphocyte Ratio in Patients With Familial Mediterranean Fever. J Clin Lab Anal. 2014 doi: 10.1002/jcla.21732. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gasparyan AY, Sandoo A, Stavropoulos-Kalinoglou A, Kitas GD. Mean platelet volume in patients with rheumatoid arthritis: the effect of anti-TNF-alpha therapy. Rheumatol Int. 2010;30(8):1125–29. doi: 10.1007/s00296-009-1345-1. [DOI] [PubMed] [Google Scholar]
- 31.Kapsoritakis AN, Koukourakis MI, Sfiridaki A, et al. Mean platelet volume: a useful marker of inflammatory bowel disease activity. Am J Gastroenterol. 2001;96(3):776–81. doi: 10.1111/j.1572-0241.2001.03621.x. [DOI] [PubMed] [Google Scholar]
- 32.Karakurt Ariturk O, Ureten K, Sari M, et al. Relationship of paraoxonase-1, malondialdehyde and mean platelet volume with markers of atherosclerosis in familial Mediterranean fever: an observational study. Anadolu Kardiyol Derg. 2013;13(4):357–62. doi: 10.5152/akd.2013.103. [DOI] [PubMed] [Google Scholar]
- 33.Dursun I, Gok F, Babacan O, Sarı E, Sakallıoglu O. Are mean platelet volume and splenomegaly subclinical inflammatory marker in children with familial mediterranean fever? Health. 2010;2:692. [Google Scholar]
- 34.Semba RD, Patel KV, Ferrucci L, et al. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr. 2010;29(5):600–4. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen LA, Felker GM, Mehra MR, et al. Validation and potential mechanisms of red cell distribution width as a prognostic marker in heart failure. J Card Fail. 2010;16(3):230–38. doi: 10.1016/j.cardfail.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shantha GP, Kumar AA, Bharadhi MK, Arthur P. Role of gender in the associations of microalbuminuria with inflammatory markers in hypertensive subjects: a cross-sectional study. Kidney Blood Press Res. 2009;32(6):434–39. doi: 10.1159/000266477. [DOI] [PubMed] [Google Scholar]