Abstract
Background
The clinical efficacy of furosemide administration in preventing contrast-induced nephropathy (CIN) remains uncertain. This meta-analysis was designed to update data on the incidence of CIN with additional furosemide treatment beyond saline hydration in comparison with hydration alone in patients undergoing percutaneous coronary intervention (PCI).
Material/Methods
A computerized literature search of MEDLINE, EMBASE, and Cochrane databases was performed. Trials were eligible if they enrolled patients undergoing coronary angiography and randomly allocated participants to receive furosemide administration in addition to saline hydration or saline hydration alone. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) for combinations of studies.
Results
Five trials involving 1294 patients (640 for additional furosemide treatment and 654 for hydration alone) were included in the meta-analysis. In the synthesis of data, additional furosemide administration had little impact on the incidence of CIN post-PCI compared with peri-procedural saline hydration alone (OR=0.96; 95% CI 0.33–2.84, p=0.95). Moreover, as for the subsequent need for dialysis, there was no statistical significant difference between the 2 groups (OR=1.01; 95% CI 0.38–2.67, p=0.99). Sensitivity analyses did not show any relevant influence on the overall results. There was no publication bias in the meta-analysis.
Conclusions
Furosemide administration did not achieve additional benefit beyond saline hydration in reducing the incidence of CIN in patients undergoing PCI.
MeSH Keywords: Balkan Nephropathy, Meta-Analysis, Percutaneous Coronary Intervention
Background
Contrast-induced nephropathy (CIN) is characterized by acute deterioration of renal function that occurs after parenteral administration of contrast media in the absence of other causes [1]. It is a frequent complication of percutaneous coronary intervention (PCI) and is associated with prolonged hospital stay, potential need for dialysis, increased health care costs, and mortality [2–4]. Targeting the potential risk factors of CIN (including chronic renal dysfunction) reduced effective circulating volume, and preventive intravenous hydration with isotonic saline solution remains the cornerstone of preventing hospital-acquired renal insufficiency [5,6]. However, due to the potentially increased risk of overhydration and pulmonary edema, particularly in patients with impaired left ventricular function, saline hydration might partially offset the protective effect on kidney injury after exposure to contrast media following a PCI procedure, which limits to some extent clinical utility of preventive hydration treatment. Furosemide has been verified to have the potential to protect the injured kidney [7]. In theory, the use of furosemide, a loop diuretic, can reduce the risk of fluid overload and protect the kidney in the setting of CIN prevention by volume expansion [7]. However, previous clinical studies gave contradictory results in clinical feasibility of furosemide. The PRINCE [8] study and MYTHOS study [9] revealed that the preventive effect of additional furosemide administration beyond hydration against CN was modest. In contrast, several small-scale studies suggested that furosemide might result in the deterioration of renal function after the use of radiocontrast media [10,11]. In this context, uncertainty remains regarding the true effect afforded by additional furosemide administration beyond saline hydration on preventing CIN post-coronary interventional procedures. In an attempt to resolve this issue, we performed a meta-analysis of previous randomized trials to examine the prophylactic effect of furosemide treatment in addition to saline hydration on CIN in these subjects undergoing PCI.
Material and Methods
Search strategy
The eligible studies were identified through a computerized literature search of MEDLINE, EMBASE, and Cochrane databases from 1970 through February 2014 using the following keywords: renal dysfunction, renal failure, kidney failure, acute kidney injury, nephropathy, contrast, contrast-induced nephropathy, CIN, furosemide, diuretics, saline, sodium chloride, hydration, coronary angiography, angioplasty, percutaneous coronary intervention, PCI, stent, random. In addition, extensive manual searching was performed using cross-references from original articles and reviews.
Inclusion of studies
Trials were included if they enrolled patients undergoing coronary angiography and randomly allocated these participants to receive additional furosemide administration beyond saline hydration or saline hydration alone. Moreover, they reported clinical data on the incidence of CIN or further dialysis. CIN was defined as serum creatinine levels (SCr) >25% of baseline, or >0.5 mg/dl, within 48–72 h after administration of a contrast agent. We excluded studies that compared other diuretics beyond furosemide versus saline hydration alone for the prevention of CIN, and studies where patients with non-coronary artery diseases receiving radiologic procedures were randomized to diuretics versus hydration treatment.
Data extraction and quality assessment
We systematically recorded study characteristics (publication year and number of patients randomized), patient demographics, risk factors for CIN (hypertension, diabetes, chronic renal dysfunction, and contrast volume), and strategy of furosemide administration. Moreover, we also recorded the number of events of CIN and/or further dialysis. Data extraction from each of the eligible studies was performed independently by 2 investigators. We reviewed the methodologic quality of randomized controlled trials by using a scoring system developed by Jadad et al. [12].
Statistical analysis
We calculated odds ratios (ORs) and 95% confidence intervals (CIs) for combinations of studies according to fixed-effects models on the basis of the Mantel-Haenszel method. We used the I2 statistic to assess heterogeneity across the included studies. In the presence of statistical homogeneity, defined as p value less than 0.10, we analyzed the data using random-effects models. Sensitivity analyses were conducted to examine the robustness of the effect by omitting each trial one at a time from analysis and computing meta-analysis estimates for the remaining studies. We also generated a funnel plot with estimable ORs for considered end-point to assess the presence of publication bias. All statistical analyses were conducted in STATA 10.0 (StataCorp, College Station, TX). P<0.05 was set as the level of significance.
Results
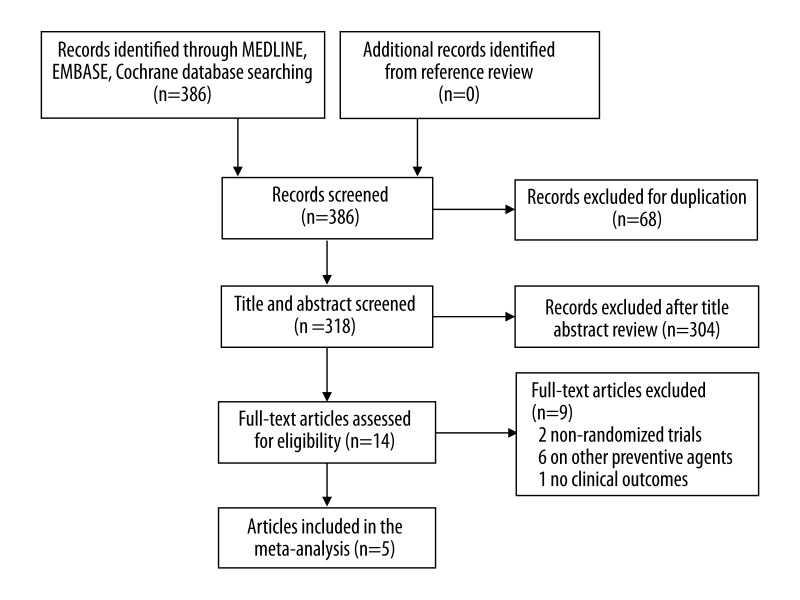
Detailed search steps are described in Figure 1. Briefly, from the initial literature search we identified 318 citations. After initial screening of study titles and abstracts, 304 articles were considered of interest and full text was retrieved for detailed evaluation. References of all 14 articles were reviewed, and no additional relevant study was identified. Nine of these 14 articles were subsequently excluded from the meta-analysis. Five trials [5,9,11,13,14] involving 1294 patients (640 additional intravenous furosemide treatment and 654 intravenous saline hydration alone) met the inclusion criteria and were included in the analysis (Table 1).
Figure 1.
Flow diagram of trial selection.
Table 1.
Demographic characteristics of the enrolled studies.
| First author | Publication year | No. enrolled | Age | Male, % | Hypertension, % | Diabetes, % | Chronic renal dysfunction, % | Contrast volume (F/C), ml | Furosemide administration | CIN definition | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gu GQ, et al. | 2013 | 859 | 59 | 72 | 72 | 21 | 25.2 | 100/100 | IV, 20 mg before procedure | SCr >25% of baseline, or >0.5 mg/dl, at 48 h post-procedure | 3 |
| Majumdar SR, et al. | 2009 | 92 | 65 | 77 | 77 | 37 | 100 | 152/126 | IV drip, 100 mg before procedure | SCr >25% of baseline, or >0.5 mg/dl, at 48 h post-procedure | 5 |
| Marenzi G, et al. | 2012 | 170 | 74 | 78 | 83 | 40 | 100 | 181/158 | IV, 0.5 mg/kg before procedure | SCr >25% of baseline, or >0.5 mg/dl, at 72 h post-procedure | 4 |
| Shemirani H, et al. | 2012 | 120 | 65 | 51 | – | – | – | 119/133 | Oral | SCr >0.5 mg/dl, at 48 h post-procedure | 3 |
| Solomon R, et al. | 1994 | 53 | 65 | 66 | – | 53 | 100 | 132/125 | IV, 80 mg before procedure | SCr >0.5 mg/dl, at 48 h post-procedure | 3 |
CIN – contrast-induced nephropathy; F/C – furosemide/control; IV – intravenous injection; SCr – serum creatinine.
All of the 5 trials reported the incidence of CIN after PCI and 4 provided information on the subsequent need for dialysis at admission. CIN was consistently defined as SCr >25% of baseline, or >0.5 mg/dl, within 48–72 h of administration of a contrast agent. Patient age was 59–74 years and participants were mostly male (66–78%). Moreover, there was a high prevalence of hypertension among the participants (72–83%). Of the 5 included trials, 3 recruited patients with coronary heart disease accompanying chronic renal deficiency [5,9,14]. All patients underwent PCI with exposure to intracoronary contrast media of 100–181 ml. Methodological quality of each clinical study was assessed using Jadad scores (Table 1).
The summary of events of the selected outcomes across the studies is presented in Table 2. Among the 5 trials, 2 reported the beneficial effect of additional furosemide on lowering the occurrence of CIN compared with saline hydration alone [9,11], and 1 did not find an intergroup difference [13], whereas the other 2 found an unfavorable effect of the diuresis strategy [5,14]. Notably, all of them except the study by Shemirani et al. [13], which did not report the data on dialysis, consistently showed negative results regarding the end-point of the need for dialysis due to CIN. Across all 5 trials, 72 (11.3%) and 95 (14.5%) patients had a CIN, and 8 (1.4%) and 8 (1.3%) needed dialysis in the furosemide and saline treatment groups, respectively (Table 2).
Table 2.
Clinical events reported by included studies.
| First author | CIN (event/total) | Need for dialysis (event/total) | ||||
|---|---|---|---|---|---|---|
| Furosemide | Control | p | Furosemide | Control | p | |
| Gu GQ | 34/422 | 62/437 | 0.005 | 1/422 | 1/437 | 0.98 |
| Majumdar SR | 23/46 | 13/46 | 0.03 | 546 | 4/46 | 0.73 |
| Marenzi G | 4/87 | 15/83 | 0.009 | 1/87 | 3/83 | 0.32 |
| Shemirani H | 1/60 | 2/60 | 0.55 | – | – | – |
| Solomon R | 10/25 | 3/28 | 0.02 | 1/25 | 0/28 | 0.45 |
CIN – contrast-induced nephropathy.
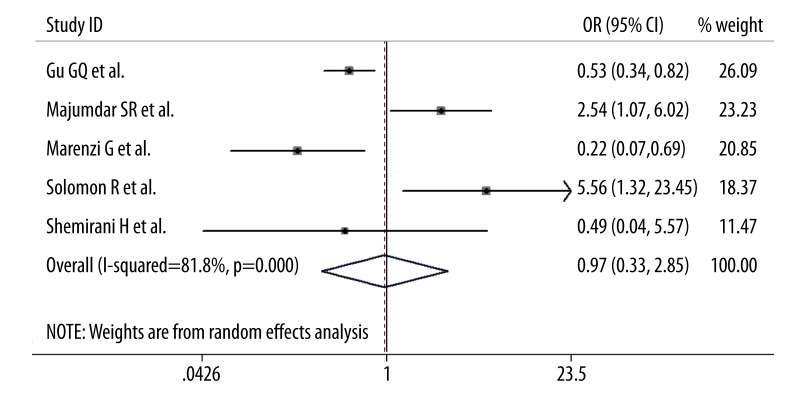
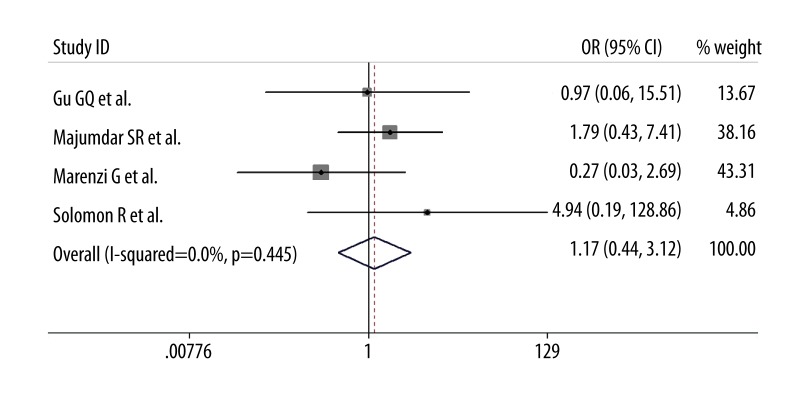
In the synthesis of data, additional furosemide administration had little impact on the incidence of CIN after PCI compared with intravenous saline hydration alone (OR=0.96; 95% CI 0.33–2.84, p=0.95; I2=82%; Figure 2). Moreover, as for the need for subsequent dialysis, there was no statistical significant difference between the 2 groups (OR=1.01; 95% CI 0.38–2.67, p=0.99; I2=0%; Figure 3). Omission of each trial one at a time from the analysis did not have any relevant influence on the 2 overall results. Additionally, publication bias was not found by funnel plot method on the basis of data on the incidence of CIN.
Figure 2.
Pooled odds ratio of CIN following additional furosemide treatment versus hydration alone. CI – confidence intervals.
Figure 3.
Pooled odds ratio of the need for dialysis following additional furosemide treatment versus hydration alone. Abbreviations as in Figure 2.
Discussion
This meta-analysis investigated whether furosemide administration in addition to intravenous saline hydration attenuated renal injure after PCI with exposure to contrast media following PCI in comparison with hydration alone. The results failed to reveal an additional benefit of furosemide in reducing the incidence of CIN and subsequent dialysis in patients undergoing a coronary interventional procedure.
Hydration of high-risk patients for CIN before contrast administration is a universally accepted, appropriate, and safe measure to prevent CIN [15]. The effect of hydration on reducing development of CIN was shown in several clinical trials focusing on patients receiving PCI [16,17]. Indeed, the use of diuretics clearly reduced the potential adverse events associated with peri-procedural overhydration. However, additional effect of diuretic treatment on preventing CIN beyond saline hydration remains unclear. In addition to increasing urine flow, diuretics blocked tubular sodium reabsorption, resulted in greater contrast dilution within the renal tubules, and reduced direct kidney toxicity and medullary ischemia [9]. These beneficial pharmacological functions might be associated with a renal protective effect against CIN [18]. Thus, as a diuretic, furosemide might help to reduce the occurrence of CIN after exposure to contrast agents following PCI. In the present meta-analysis, additional benefits of furosemide treatment beyond saline hydration in the prevention of CIN and subsequent dialysis were not demonstrated and to date there is no solid evidence to confirm the preventive effect of the loop diuretic on CIN post-PCI. Nevertheless, it was notable that 2 of the 5 included studies indicated a significant benefit of this diuretic agent in reducing the incidence of CIN [9,11]. The different study designs in the 2 studies might contribute to the different study findings from other studies included in the meta-analysis. The study by Gu et al. [11], the largest-scale RCT in this field, recruited patients with low percentage (approximately 25%) of chronic renal dysfunction, used the lowest-volume contrast agents (mean volume of 100 ml) during the PCI procedure, and prescribed low-dose furosemide (mean dose of 20 mg), which carries relatively low risk of CIN. Based on the unique study design, we easily considered that, in subjects at low risk for CIN, the additional benefit of furosemide might be achieved more easily than with saline hydration alone. Additionally, in the study by Marenzi et al. (1 of the 2 studies with positive results) the 2 study groups differed with respect to the fluids infused. The furosemide group had a net positive fluid balance (the cumulative saline hydration was more than urine output), whereas the hydration group had a net negative fluid balance. Moreover, 48 of 83 (58%) of patients in the hydration group were on diuretics and there was no protocol to stop these medications before the intervention [19]. The differences in fluid administered and fluid balance achieved likely influenced the results of the study in the 2 groups [19], which might be an important reason for the positive results in the individual study.
A limitation of this meta-analysis deserves comment. We did not consider the impacts of furosemide dose on clinical end-points due to limited study size; therefore, we still could not confirm whether diuretic therapy had dose-specific effect on the incidence of CIN. In addition, in the pooling analysis on CIN, there was a considerable statistical heterogeneity among the individual studies and we performed the combination of studies using a random-effects model. Thus, the conclusion on CIN in the present study was prone to be conservative due to the use of this statistical model. Nevertheless, sensitivity analysis further confirmed reliability and stability of the overall results.
Conclusions
Furosemide treatment appears to have no additional influence beyond saline hydration on the incidence of CIN and subsequent dialysis after a coronary interventional procedure. Nevertheless, some specific patients receiving PCI still likely benefitted from additional diuretic therapy. Thus, it is critical to identify these subjects and further elucidate some uncertainties concerning the effectiveness of the therapeutic strategies in future clinical trials.
Footnotes
Disclaimers
No conflicts of interest to be noted. No relationships with industry to be noted.
Source of support: Self financing
References
- 1.Palevsky PM. Defining contrast-induced nephropathy. Clin J Am Soc Nephrol. 2009;4:1151–53. doi: 10.2215/CJN.03410509. [DOI] [PubMed] [Google Scholar]
- 2.Bagshaw SM, Culleton BF. Contrast-induced nephropathy: epidemiology and prevention. Minerva Cardioangiol. 2006;54:109–29. [PubMed] [Google Scholar]
- 3.Best PJ, Holmes DR., Jr Prevention and management of contrast-induced acute kidney injury. Curr Treat Options Cardiovasc Med. 2012;14:1–7. doi: 10.1007/s11936-011-0162-5. [DOI] [PubMed] [Google Scholar]
- 4.Kelly AM, Dwamena B, Cronin P, et al. Meta-analysis: effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–94. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 5.Majumdar SR, Kjellstrand CM, Tymchak WJ, et al. Forced euvolemic diuresis with mannitol and furosemide for prevention of contrast-induced nephropathy in patients with CKD undergoing coronary angiography: a randomized controlled trial. Am J Kidney Dis. 2009;54:602–9. doi: 10.1053/j.ajkd.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 6.Hiremath S, Akbari A, Shabana W, et al. Prevention of contrast-induced acute kidney injury: is simple oral hydration similar to intravenous? A systematic review of the evidence. PLoS One. 2013;8:e60009. doi: 10.1371/journal.pone.0060009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia. 2010;65:283–93. doi: 10.1111/j.1365-2044.2009.06228.x. [DOI] [PubMed] [Google Scholar]
- 8.Stevens MA, McCullough PA, Tobin KJ, et al. A prospective randomized trial of prevention measures in patients at high risk for contrast nephropathy: results of the P.R.I.N.C.E. Study. Prevention of Radiocontrast Induced Nephropathy Clinical Evaluation. J Am Coll Cardiol. 1999;33:403–11. doi: 10.1016/s0735-1097(98)00574-9. [DOI] [PubMed] [Google Scholar]
- 9.Marenzi G, Ferrari C, Marana I, et al. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovas Interv. 2012;5:90–97. doi: 10.1016/j.jcin.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Dussol B, Morange S, Loundoun A, et al. A randomized trial of saline hydration to prevent contrast nephropathy in chronic renal failure patients. Nephrol Dial Transplant. 2006;21:2120–26. doi: 10.1093/ndt/gfl133. [DOI] [PubMed] [Google Scholar]
- 11.Gu GQ, Lu R, Cui W, et al. Low-dose furosemide administered with adequate hydration reduces contrast-induced nephropathy in patients undergoing coronary angiography. Cardiology. 2013;125:69–73. doi: 10.1159/000350648. [DOI] [PubMed] [Google Scholar]
- 12.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 13.Shemirani H, Pourrmoghaddas M. A randomized trial of saline hydration to prevent contrast-induced nephropathy in patients on regular captopril or furosemide therapy undergoing percutaneous coronary intervention. Saudi J Kidney Dis Transpl. 2012;23:280–85. [PubMed] [Google Scholar]
- 14.Solomon R, Werner C, Mann D, et al. Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med. 1994;331:1416–20. doi: 10.1056/NEJM199411243312104. [DOI] [PubMed] [Google Scholar]
- 15.Schilp J, de Blok C, Langelaan M, et al. Guideline adherence for identification and hydration of high-risk hospital patients for contrast-induced nephropathy. BMC Nephrol. 2014;15:2. doi: 10.1186/1471-2369-15-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SL, Zhang J, Yei F, et al. Clinical outcomes of contrast-induced nephropathy in patients undergoing percutaneous coronary intervention: a prospective, multicenter, randomized study to analyze the effect of hydration and acetylcysteine. Int J Cardiol. 2008;126:407–13. doi: 10.1016/j.ijcard.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Trivedi HS, Moore H, Nasr S, et al. A randomized prospective trial to assess the role of saline hydration on the development of contrast nephrotoxicity. Nephron Clin Pract. 2003;93:C29–34. doi: 10.1159/000066641. [DOI] [PubMed] [Google Scholar]
- 18.Pisani A, Sabbatini M, Riccio E, et al. Effect of a recombinant manganese superoxide dismutase on prevention of contrast-induced acute kidney injury. Clin Exp Nephrol. 2014;18(3):424–31. doi: 10.1007/s10157-013-0828-2. [DOI] [PubMed] [Google Scholar]
- 19.Sharma D. Hydration is critical for prevention of contrast-induced nephropathy. JACC Cardiovas Interv. 2012;5:454–55. doi: 10.1016/j.jcin.2012.02.007. [DOI] [PubMed] [Google Scholar]