Abstract
Previous functional neuroimaging studies demonstrated that different neural networks underlie different types of cognitive processing by engaging participants in particular tasks, such as verbal or spatial working memory (WM) tasks. However, we report here that even when a WM task is defined as verbal or spatial, different types of memory strategies may be used to complete it, with concomitant variations in brain activity. We developed a questionnaire to characterize the type of strategy used by individual members in a group of 28 young healthy participants (18–25 years) during a spatial WM task. A cluster analysis was performed to differentiate groups. We acquired functional magnetoencephalography and structural diffusion tensor imaging measures to characterize the brain networks associated with the use of different strategies. We found two types of strategies were used during the spatial WM task, a visuospatial and a verbal strategy, and brain regions and time courses of activation differed between participants who used each. Task performance also varied by type of strategy used with verbal strategies showing an advantage. In addition, performance on neuropsychological tests (indices from Wechsler Adult Intelligence Scale‐IV, Rey Complex Figure Test) correlated significantly with fractional anisotropy measures for the visuospatial strategy group in white matter tracts implicated in other WM and attention studies. We conclude that differences in memory strategy can have a pronounced effect on the locations and timing of brain activation and that these differences need further investigation as a possible confounding factor for studies using group averaging as a means for summarizing results. Hum Brain Mapp 35:5127–5140, 2014. © 2014 Wiley Periodicals, Inc.
Keywords: MEG, magnetoencephalography, brain networks, verbal memory, visual memory, memory strategies
INTRODUCTION
If “healthy” participants of the same age group are asked to perform an identical task, it is often assumed that similar brain areas will be activated across all members of the group. Consequently, it is common in neuroimaging disciplines (e.g., fMRI, positron emission tomography (PET), and evoked related potentials (ERPs)) to average data over large numbers of subjects in order to make inferences about general phenomena that apply to an entire population [Faigman, 2010]. However, many studies have concluded that the results of group analysis do not accurately represent the individuals that make up the group [Aine et al., 2011; Feredoes and Postle, 2007; Heun et al., 2000; Machielsen et al., 2000; Miller et al., 2002, 2009; Parasuraman and Jiang, 2012; Seghier and Price, 2009; Seghier et al., 2008]. For example, Kherif et al. [2009] found that age and reading strategy were prominent sources of variability in fMRI activation for reading familiar words aloud.
Our research program has identified several potential confounds in studies of aging and memory when group averaging is used to compare young participants to a group of elders [Aine et al., 2011]. For one, we find more variability within the elder group. This can in part be attributed to cardiovascular risk factors which are not typically used as exclusion criteria in aging studies, such as high blood pressure and type 2 diabetes. Thus, elder individuals with these risk factors are still considered “healthy” elderly in many aging studies.
We reported previously on the variability witnessed in data acquired from elders due to the types of health differences described above [Aine et al., 2010, 2014], and noted the additional potential confound that elders may be using different memory strategies than young individuals. Here, we focus on strategy differences in a young group who are unlikely to have health issues. We reasoned that if we demonstrate the natural use of different strategies in this young homogeneous group, and corroborate that these strategies are mediated by different brain patterns (regions and time courses of activity), then we would have strong evidence that the use of different strategies should be considered as a potential confound by neuroimaging studies of aging, and for neuroimaging studies in general that use group averaging techniques.
In this study, we used magnetoencephalography (MEG) to record functional brain activity of young healthy participants (18–25 years of age) while they were engaged in a modified Sternberg spatial working memory (WM) task. MEG is a noninvasive imaging method that measures the weak magnetic fields induced by synchronous neuronal activity. It is used to study many areas of brain function and disorders such as aging [Aine et al., 2005, 2006, 2010, 2014; Fernandez et al., 2013], schizophrenia [Chen et al., 2013; Hanlon et al., 2012], and epilepsy [Agirre‐Arrizubieta et al., 2014, Velez‐Ruiz and Klein, 2012] with excellent temporal resolution (ms) and good spatial resolution (mm for simple activations, cm for complex activations) [Aine et al., 2012; Sanfratello et al., 2010, in press; Supek and Aine, 1993, 1997]. We hypothesized that if participants used different memory strategies to conduct the same task, they would show different brain activation patterns at the time of recognition. For example, a verbal strategy may be used to complete a spatial location task by verbalizing to oneself “upper right hand corner” versus simply holding the location image in mind (a visuospatial strategy). Individual strategies were assessed by administering a questionnaire after the MEG session was completed. A cluster analysis was then applied to the questionnaire responses to determine the type of strategy used by each participant and to form groups, i.e., no a priori assumptions were made as to how many groups would be formed. Then, functional (MEG), behavioral (task performance and neuropsychological tests), and anatomical (diffusion tensor imaging (DTI)) measures were evaluated according to the cluster analysis groupings for independent corroboration that different brain networks were used.
METHODS
Participants
A group of 28 healthy subjects (age 18–25 years, 12 male) were recruited from flyers posted at the University of New Mexico and the surrounding community. To assess health, neuropsychological tests and a neurological exam were performed for each participant. In addition, a lipid panel (i.e., high‐density lipoprotein, low‐density lipoprotein, total cholesterol, and triglycerides), hbA1c test (average blood glucose level across 3 months), and blood pressure measurements were obtained (note: these individuals participated in a larger study on aging). The Human Research Review Committee at the University of New Mexico Health Sciences Center approved the use of human participants for this study (HRRC#06–267) and written informed consent was obtained from all subjects participating in the study.
Spatial Working Memory Task, MEG Acquisition, and Preprocessing
Because the ultimate goal was to determine if we could differentiate between different strategies used during a spatial task, we tested this young, homogeneous group using the spatial Sternberg WM task illustrated in Figure 1. Displays (16‐cell arrays) subtended 4.0° in the central field and appeared sequentially every 1.2 s with a 2 s inter‐stimulus interval and a 3.6 s delay interval. Stimulus duration was 266 ms. Some of the digits in the matrix were presented backward to avoid using 2‐digit numerals. However, the digits to be remembered were always presented normally. The task had two memory set items. Participants were to respond “yes” (right index finger press) or “no” (left index finger press) if the last display (probe) contained one of the to‐be‐remembered items (the locations of the red digits). The total number of trials for each condition was 120 (e.g., 120 trials of “yes” it matched and 120 trials of “no” it did not match). Only the “yes” trials were used for this study.
Figure 1.
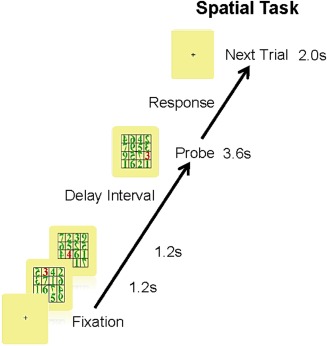
Spatial working memory task. A modified Sternberg task was used to test memory performance of young healthy participants. The participants were instructed to remember the location of a red digit within an array of green digits, for two different arrays, presented sequentially.
Participants were seated in an Elekta Neuromag 306 MEG system (306 channels, sampling rate = 1,000 Hz, high pass filter = 1 Hz, low pass filter = 300 Hz) while completing the WM task described above. The task was explained and participants were given a short practice session before beginning. Tasks were performed on the same day with short rest breaks every 7–8 min. Those who needed corrective lenses were fitted with MEG compatible frames and lenses for the duration of the task. The MEG data were later filtered (Maxfilter, Elekta) to minimize signals coming from outside a sphere around the brain using signal space separation (e.g., heartbeat artifacts). Eyeblinks were suppressed separately using signal space projection and the trials were averaged together within subject for each condition.
Immediately after the completion of the spatial WM task within the MEG scanner, a structured questionnaire was administered (Likert scale). Eleven of these statements were used in a cluster analysis to determine the type of strategy each participant used during the task. This subset was specific to this paradigm (i.e., because this study was a part of a larger aging study, only questions related to this particular paradigm and the young group of participants were used).
MRI/Diffusion Tensor Imaging Data Acquisition and Processing
MRI scans of the individual subjects were acquired in order to combine this structural information with the MEG results, and thus determine the locations of brain activity during the spatial WM task described above. The Siemens 3T Tim Trio was used for T 1 structural and T 2 scans (MPRAGE, Turbo Spin Echo (TSE), and FLAIR). T 1‐weighted MPRAGE sequence: 1.0 mm sagittal slices, 7° Flip angle, Repetition Time (TR) = 2,530 ms, Echo Time, TE1 = 1.64 ms, TE2 = 3.5 ms, TE3 = 5.36 ms, TE4 = 7.22 ms, TE5 = 9.08 ms, Field of view (FOV) was 256 × 256, 6 min; T 2‐weighted TSE sequence: 1.5 mm axial slices, 155° Flip angle, TR = 13,500 ms, TE1 = 77 ms, FOV was 220 × 220 with 1.5 mm slice thickness, acquisition time of ∼3 min; and T 2‐weighted FLAIR sequence: 1.5 mm sagittal slices, TR = 6,000 ms, TE = 412 ms, FOV was 256 × 256, acquisition time of ∼5 min.
A DTI sequence was also acquired to determine white matter tract integrity via fractional anisotropy (FA) values. The DTI sequence had 60 directions, b = 800 s/mm2, and 10 measurements of b = 0, for 12 min of acquisition time. The b = 0 measurements were interleaved after every six non‐zero b‐value measurements. DTI was obtained in the axial direction along the anterior commissure (AC)–posterior commissure (PC) line. The FOV was 256 × 256 mm with a 2 mm slice thickness, 72 slices, 128 × 128 matrix size, voxel size = 2 mm × 2 mm × 2 mm, TE = 84 ms, TR = 9,000 ms, NEX = 1, partial Fourier encoding of 3/4, and with a GRAPPA acceleration factor of 2. Tract‐based spatial statistics (TBSS) was used for the analysis [Smith et al., 2006], available in the FSL software package. DTI preprocessing consisted of the following steps: (1) gradient directions with more than 10% signal dropouts caused by subject motion were not included in further analysis; (2) motion and eddy current correction (FSL); and (3) correction of gradient directions for any image rotation done during the previous motion correction step. The scalar diffusion parameter FA was calculated using dtifit (FSL). The FA image was aligned to an FA template (normalized to the Montreal Neurological Institute brain atlas space) with a nonlinear registration algorithm, FNIRT (FMRIB's Nonlinear Image Registration Tool; FSL). A mean FA image was calculated from the set of spatially normalized images. The TBSS algorithm was applied to the mean FA image to calculate a mean white matter tract skeleton. The FA data of each subject was then projected onto this mean skeleton to obtain a skeletonized image corresponding to each subject. To examine group differences, mean FA values were calculated from the FA skeleton for the 50 white matter regions defined in the JHU‐ICBM 50 region white‐matter atlas included in the FSL software package [Mori, 2008]. Between group t‐tests were computed for each of the 50 white matter regions and were then adjusted using Benjamini and Hochberg's false discovery rate (FDR) procedure [Benjamini and Hochberg, 1995]. FDR is the expected fraction of tests declared significant in a study in which the null hypothesis is true. FDR explicitly controls the error rate of test conclusions among significant results [Benjamini and Hochberg, 1995] and is often more appropriate than FWER corrections such as Bonferroni while retaining more power in the results [Genovese et al., 2002].
MEG Data Analysis
The structural MPRAGE or FLAIR MRI scan (depending on data quality) was processed within MRIVIEW [Ranken, 1993] software to calculate a best‐fitting sphere for each individuals' head model to use with the multidipole, spatiotemporal inverse procedure calibrated‐start spatio‐temporal (CSST). CSST determines the best‐fitting source locations for a given dataset, as well as their corresponding time courses [Ranken et al., 2002, 2004]. CSST begins with random combinations of MR‐derived starting locations from within the cortical volume and uses the Nelder–Mead nonlinear downhill simplex procedure to perform a spatial search [Nelder and Mead, 1965]. Information based on a singular value decomposition of the data matrix is used for determining a range for the number of sources to be localized. Fits to the data were conducted for each model order included in this range. Model adequacy was determined by assessing the reduced chi‐square values associated with each model order [Supek and Aine, 1993, 1997], along with an examination of the dipole clusters to assess scatter (typically associated with overmodeling or fitting of noise) and the residual waveforms to assess whether additional signal remained (an indication of undermodeling).
After CSST was used to calculate the dipole locations and their time courses to probe stimuli (the recognition aspect of the task) for each individual participant, the locations were converted to Talairach coordinates using a Matlab script [COORDTRANS, Uutela, 2000, personal communication], to normalize the locations for each individual to the same brain space. COORDTRANS uses a 9‐point affine transformation to convert from the Neuromag coordinate system to the Talairach coordinate system. The anatomical locations and distances necessary for the code are determined from individual MRIs. The required locations are the anterior commissure (AC), posterior commissure (PC), and midline; the required distances are AC to right edge of brain, AC to left edge of brain, AC to front of brain, PC to back of brain, AC to top of brain, and AC to bottom of brain. The applet at http://Talairach.org [Lancaster et al., 1997, 2000] was then used to identify the boundaries of medial temporal and occipital brain areas. These boundaries (±5 mm) were then used to identify which participants revealed activities in these brain areas. Finally, the sources found in medial temporal lobe (MTL) and occipital cortex (OCC) were plotted on the “adult brain mesh” [Fang and Boas, 2009] and were color coded to reflect the type of strategy the participants used to remember spatial locations, as discussed above. Chi‐squared was used to evaluate group differences in presence/absence of sources. T‐tests (1‐tailed, unequal variance) were conducted to determine significant differences between groups on peak amplitudes and onset latencies for the source time courses.
Neuropsychological Tests
The neuropsychological tests administered were the Rey Complex Figure Test (REY‐D), California Verbal Learning Test (CVLT), and the Wechsler Adult Intelligence Scale‐IV (WAIS‐IV). We were interested in whether the 4 indices derived from the WAIS‐IV would differ between strategy groups: working memory index (WMI—digit span and arithmetic), verbal comprehension index (VCI—similarities and vocabulary), perceptual reasoning index (PRI—block design, matrix reasoning, and visual puzzles), and processing speed index (symbol search and coding). We discuss only those that showed significant differences between groups.
Statistics for Behavioral Tasks
Strategy cluster groups were formed using average linkage, cluster analysis with Euclidean distances from 11 relevant Likert scale statements taken from the questionnaire which participants completed after the Sternberg WM task/MEG scan (Fig. 2). These statistics were computed by a biostatistician using SAS 9.3 (http://www.sas.com, Cary, NC).
Figure 2.
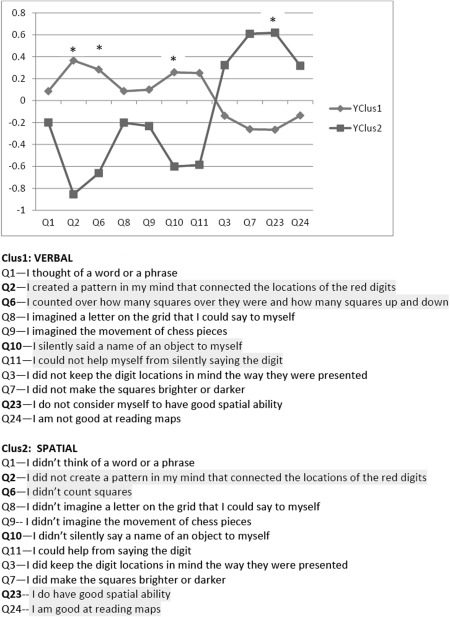
Cluster groups based on responses to 11 items of the questionnaire. The statements are paraphrased below, participants were asked how strongly they agreed with each statement. Highlighted items are examples that contributed to significant differences between groups.
T‐tests (1‐tailed, unequal variance) were computed between groups to determine if there were significant differences in: performance on the neuropsychological tests, performance on the spatial WM task (total correct and RTs), and FA values. Pearson correlations were computed between FA in white matter tracts and performance on the neuropsychological tests, as well as performance on the spatial WM task (total correct and RTs). Correlations were adjusted for Type I errors using FDR.
RESULTS
Behavioral Results
Cluster analysis
Two groups were identified by cluster analysis, based on the questionnaire data (Fig. 2), a verbal strategy group and a visuospatial strategy group. Participants were asked how strongly they agreed or disagreed (Likert scale) with particular statements. Examples of the statements used to differentiate the groups were, “I did keep the digit locations in mind the way they were presented” for the visuospatial group and for the verbal group, “To help me remember the locations of the red digits I thought of a word or phrase.” For example, I thought to myself “upper left corner” or “2 o'clock.” To be assured that our groups were homogeneous, we present the age, health, and IQ data of the two groups in Table 1. No significant differences were found between groups. These results also confirm our premise that these groups of young participants were healthy individuals.
Table 1.
Characteristics of the two strategy groups identified using the strategy Likert scale
| Strategy group | Age | Gender (M/F) | IQ Full WAIS | Trig (mg/dL) | Total Chol (mg/dL) | HDL (mg/dL) | LDL (mg/dL) | A1c (%) | BP (Sys/Dia) (mm Hg) |
|---|---|---|---|---|---|---|---|---|---|
| Verbal | 21.9 (1.9) | 5/11 | 109.1 (9.4) | 84.3 (30.4) | 161.6 (31.8) | 52.3 (16.2) | 92.3 (27.0) | 5.4 (0.4) | 110.2/67.6 (11.4/8.1) |
| Spatial | 21.4 (2.2) | 5/4 | 104.8 (8.2) | 98.6 (52.2) | 154.3 (27.4) | 43.3 (15.9) | 91.3 (22.7) | 5.5 (0.3) | 114.0/68.2 (9.9/8.1) |
Means and standard deviations are shown. IQ is based on full scale score of WAIS‐IV. There were no significant differences between groups on any of these variables. Trig = triglycerides (<150 is normal); Total Chol = total cholesterol (<200 is desirable); HDL = high density lipoprotein (>60 is ideal); LDL = low density lipoprotein (<100 is optimal); A1c = average blood glucose level across 3 months (<6.0 is normal); BP = blood pressure (<120 systolic/<80 diastolic is normal). All blood tests were conducted after 12 h fasting. Some young in both groups had lower HDL than ideal and 1 young from each group had higher systolic BP levels than normal.
Spatial WM task
There was a significant difference in WM performance on the spatial Sternberg WM task depending on strategy type (verbal vs. visuospatial). Performance was determined by calculating the proportion of trials the participant responded to correctly. Those participants who used a verbal strategy did better in terms of total correct on the spatial WM task (M = 95%, SD = 3.6), than those who used a visuospatial strategy (M = 87%, SD = 10), P = 0.005. Reaction times on the spatial WM task showed no significant difference between the verbal (M = 745 ms, SD = 122) and visuospatial groups (M = 767 ms, SD = 109), with P = 0.33.
Neuropsychological tests
The verbal group also performed significantly better than the visuospatial group on the CVLT (P = 0.046), supporting the premise that they are better at verbal tests than the group who used a visuospatial strategy for the WM task. No significant difference in performance on any of the other neuropsychological tests was observed between the verbal and visuospatial groups.
MEG Results
Figure 3 shows representative samples of averaged evoked fields for two participants when (1) using a verbal strategy to accomplish the spatial WM task (left column) versus (2) using a spatial strategy to conduct the spatial WM task (right column). The signals measured at the sensor level are shown in the top row for both participants. The forward model determined from the average of the 10 best‐fitting CSST solution is shown in the middle row. The residual variance, the difference between the measured and the modeled data are shown in the third row. Note that the residual error is small in both instances, indicating that the multidipole, spatiotemporal solutions did a good job at reconstructing the magnetic signals measured at the sensor level. Additional procedural information on the use of the CSST method can be found in Aine et al. [2010].
Figure 3.
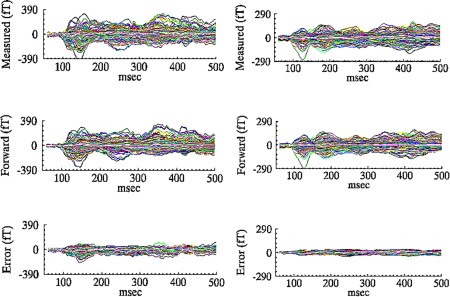
Top: Magnetic fields recorded at the sensor level (Measured). Middle: Magnetic fields reconstructed from CSST dipole solution (Forward solution). Bottom: Difference between the measured and forward fields, indicating how well the model explains the original data. Shown for 2 subjects, one from the verbal group (Left) and one from the visuospatial group (Right).
Distinct brain activation patterns were found that were dependent on the general memory strategy used by participants in the study, verbal or visuospatial. There were two distinct brain areas preferentially used whose location and time courses depended on the strategy employed. When examining the MTL and OCC areas, we determined that many more participants using a verbal strategy evoked activity in right MTL than those who used a visuospatial strategy (Fig. 4). Eleven of 17 verbal strategists (65%) showed right MTL activity, whereas only 11% of visuospatial strategists did (χ 2 (1, N = 26) = 4.25, P = 0.039). Six of the 11 verbal participants who exhibited right MTL activity also showed activity in left MTL (i.e., bilateral MTL activity). There was no significant difference in the number of participants revealing activity in left MTL between the two strategy groups. The trend was reversed for right OCC activation, with many more visuospatial strategists showing activity in right OCC, 78%, versus 29% of those using a verbal strategy (χ 2 (1, N = 26) 5.13, P = 0.024). As can be seen in Figure 4, occasionally a participant showed 2 areas of activation (dipoles within the same brain region, e.g., MTL). We examined the locations and time courses to determine that (1) one was not a noise source (i.e., low amplitude random time course activity or scatter seen across the best‐fitting dipole solutions) and (2) the two sources were not actually the same source with antiparallel orientations (typically resulting in very high amplitude signals). It was determined that these additional sources were reasonable activations. For example, both anterior and posterior MTL activity was found for subject 290. Even if a subject showed two areas of activation within the same brain region, they were only counted once for the chi‐squared test. We found no significant difference in age between the verbal (M = 21.9 years, SD = 1.8) and visuospatial (M = 21.4 years; SD = 2.2) groups, with P = 0.29 (also shown in Table 1).
Figure 4.
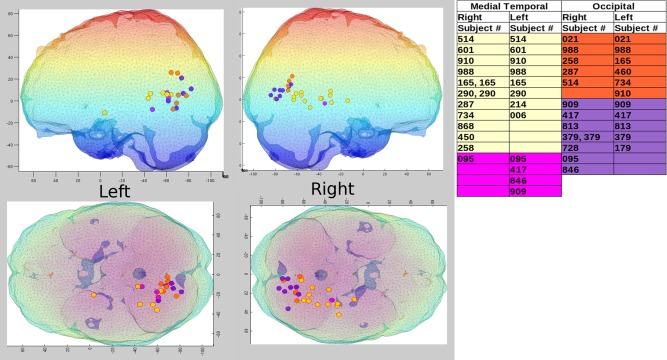
Locations of brain activity for verbal and visuospatial groups. Yellow = verbal MTL; orange = verbal OCC; pink = visuospatial MTL; purple = visuospatial OCC. Left side of mesh brain plot includes only left hemisphere sources (Top = sagittal view, Bottom = axial view, top‐down). Right side of mesh brain plot includes only right hemisphere sources (Top = sagittal view, Bottom = axial view, top‐down). Participants showing left/right MTL and OCC sources are included in the table at right of mesh brain plot, using the same color coding shown above. Note, color shading of the mesh brain simply reflects differing depths.
Differences between the strategy groups were also seen in the time courses in cases where the same brain areas were activated (Fig. 5). For example, for the visuospatial group, the onset of left MTL activation (green tracing in the bottom panel; M = 58 ms, SD = 12) was delayed relative to both left OCC activation (blue tracing in the bottom panel; M = 50 ms, SD = 20) within the visuospatial group (P = 0.05), as well as relative to left MTL activation (green tracing in top panel; M = 36 ms, SD = 9) of the verbal group (P = 0.004). We define onset latency of activity as the time at which the time course is greater than 3 SD of the baseline, for a minimum of 10 ms to eliminate noise‐related onsets. We also show in Figure 5 source locations and their associated time courses for two representative individuals, one from each group (verbal and visuospatial).
Figure 5.
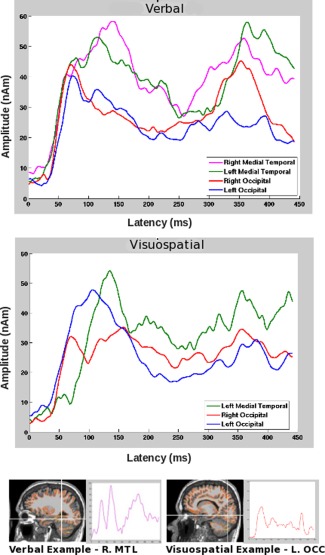
Top: Averaged time courses across common locations for subjects who used each strategy. Bottom: MRIs (radiological convention) displaying locations of activation (white crosshairs) are shown for two representative individuals, one who used a verbal strategy (left) and the other who used a visuospatial strategy (right), along with the associated time courses for these cortical regions. Note these are the same two individuals for which measured, modeled, and residual waveforms were shown in Figure 3.
Skeletal FA Results
Between group results
The only significant difference in FA values between the visuospatial and verbal strategy groups, after controlling for multiple comparisons, was for the right superior cerebellar peduncle tract (SCP‐R), with the visuospatial group (M = 0.68; SD = 0.01) showing higher FA values than the verbal group (M = 0.65; SD = 0.02, P = 0.001).
FA and behavioral performance correlations
We additionally examined the relationship between FA of the participants and behavioral performance results on neuropsychological tests and on our Sternberg spatial WM task (total correct and RTs). We discuss FDR corrected results at a significance level of α < 0.1 (N = 50) (Table 2, *).
Table 2.
Correlations between FA in white matter regions and neuropsychological task performance
| Visuospatial | Verbal | ||||||
|---|---|---|---|---|---|---|---|
| FDR (0.1) | REY | WMI | PRI | REY | WMI | PRI | |
| PCT | 0.283 | 0.824* | 0.430 | PCT | 0.068 | 0.023 | 0.065 |
| BCC | 0.368 | 0.291 | 0.880* | BCC | −0.220 | 0.206 | 0.122 |
| PCR‐R | 0.645 | 0.749 | 0.857* | PCR‐R | −0.044 | 0.391 | 0.264 |
| SLF‐R | 0.725 | 0.345 | 0.811* | SLF‐R | 0.070 | 0.507 | 0.388 |
| SFO‐L | 0.562 | 0.935* | 0.494 | SFO‐L | 0.043 | 0.069 | 0.094 |
| UNC‐R | 0.918* | 0.516 | 0.716 | UNC‐R | −0.091 | 0.448 | 0.232 |
| TAP‐R | 0.595 | 0.531 | 0.799* | TAP‐R | 0.025 | 0.250 | 0.321 |
Asterisks denote significant correlations between white matter tracts and behavioral performance.
First, FA of white matter tracts correlated significantly with performance on neuropsychological tests for the visuospatial group only (Table 2). REY‐D, a visuospatial memory test, correlated significantly with UNC‐R. In contrast, CVLT, a verbal memory test, showed no significant correlations with FA for either the visuospatial or verbal groups (not shown in the table).
WMI, an index of WM (weighted by verbal working memory subscales on the WAIS‐IV), was correlated positively with FA in the pontine crossing tract (PCT, a part of the middle cerebellar peduncle) and with the left superior fronto‐occipital fasciculus (SFO‐L) for the visuospatial group.
Perceptual reasoning ability is indexed by PRI. Correlations between performance on this index and FA of white matter tracts showed the greatest difference between groups, with the visuospatial group having a number of brain areas strongly correlated with performance, whereas the verbal group again showed no significant correlation between performance on this index and FA in any region. The FA tracts that correlated significantly with PRI for the visuospatial group were the body of corpus callosum (BCC), posterior corona radiata (PCR‐R), superior longitudinal fasciculus (SLF‐R), and the tapetum (TAP‐R).
There was a strong correlation between BCC and RTs solely for the visuospatial group (r = 0.88, FDR corrected), recall that BCC was also strongly correlated with this groups' performance on PRI (Table 2). There were no significant correlations between total correct and any white matter tracts, for either group.
The most interesting result is the number of white matter tracts that were significantly correlated with the visuospatial groups' performance on a variety of neuropsychological tests and the spatial WM task, whereas the verbal group showed no such correlations. To provide a visual corroboration of these differences (and to determine that outliers were not responsible), we plotted behavioral performance values versus FA values for the visuospatial group where significant correlations were observed, along with the corresponding verbal group's results. An example is shown in Figure 6, supporting the assertion that meaningful correlation differences between the strategy groups were identified.
Figure 6.
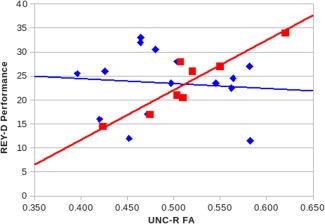
Correlations between FA values in UNC‐R white matter tract and performance on REY‐D complex figure. Squares = visuospatial group, r = 0.92. Diamonds = verbal group, r = −0.09. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Strategy Differences and Memory Performance
Young healthy participants were objectively divided into groups using a cluster analysis based on questionnaire responses provided immediately after completing a Sternberg spatial WM task. Two cluster groups resulted which were labeled as verbal and visuospatial, given the nature of the responses. The verbal group performed significantly better than the visuospatial group on both our Sternberg spatial WM task and the CVLT, a test of verbal memory. Their performance on the CVLT provides independent verification that the verbal group was better at verbal memory tasks, than the visuospatial group. Furthermore, their better performance on the spatial task suggests that a verbal strategy was more effective for this particular WM task. Although we found no statistically significant difference in age between the two groups it is possible that the cognitive maturity of the groups differed, in the sense that as individuals develop in adolescence they rely less on rote spatial rehearsal and begin to use more verbally mediated strategies [Aine et al., 2011; Scherf et al., 2006; Schweinsburg et al., 2005]. It has also been suggested that as we age we develop complex mnemonic strategies such as chunking to lessen cognitive load [Bor et al., 2003]. In this case, using a verbal strategy may simply have reduced cognitive load sufficiently to improve task performance.
MEG Activity
We predicted that we would find converging functional imaging results to support the clustering of participants based on the type of memory strategy individuals used. We found that both the visuospatial and verbal groups showed activation of MTL. MTL and portions of the hippocampus, in particular, have been shown to be active during WM tasks such as during a letter WM task with and without distractors [Sakai and Passingham, 2004] and during a 15‐word learning test including tasks of immediate and delayed recall in nondemented elderly [Hackert et al., 2002]. MTL has also been shown to be responsive during spatial WM tasks. Olson et al. [2006] conducted two experiments in which patients with hippocampal lesions and controls were required to remember the location of a line‐drawn object within each of a series of three 3 × 3 matrices. The patients with hippocampal lesions showed a deficit in both studies. Furthermore, Prince et al. [2005] provided evidence that cortical activity related to successful encoding and retrieval of relational items was associated with MTL structures, where encoding activated anterior hippocampus and retrieval activated posterior hippocampus/parahippocampal gyrus as well as prefrontal cortices. The findings of Schacter et al. [1997] and Giovanello et al. [2009] support the notion that posterior hippocampus may mediate perceptual matching or exact reinstatement of events between study and test phases. MTL activity has also been observed during performance of standard neuropsychological verbal WM subtests and in visuospatial WM tasks [Doucet et al., 2013; Travis et al., 2013].
In this study, however, we found that the verbal group showed preferential activation in right MTL, which was not seen in participants who used a visuospatial strategy. Specifically, more participants showed bilateral MTL activity for the verbal group while left MTL activity was found for the visuospatial group. We reiterate that the task was a designed to be a spatial WM task, in light of these results. We also found a distinctive time course difference between the two groups in MTL, even when the same brain area was active—the onset of left MTL activity was delayed by ∼50 ms in the visuospatial group relative to the verbal group. We speculate that this delay in left MTL activity could reflect the poorer task accuracy for the visuospatial group in the spatial WM task. For example, research has shown that verbal abstraction can lead to better and faster performance by conserving attentional resources [Tun et al., 1998].
As predicted, we found independent convergence of results with the imaging data showing that different functional networks mediated the different strategies each group utilized. In addition, differences in time courses in left MTL further corroborate that different brain networks mediated these different strategies.
FA, Behavioral Task Performance, and Memory Strategy
We have already shown that there are distinct differences in how the verbal group processes visuospatial and verbal information, reflected in brain activation patterns and superior performance on both the spatial WM task and CVLT, in comparison with the visuospatial group. Interestingly, only the visuospatial group showed significant correlations between anatomical locations (differences in FA in certain white matter regions) and performance on the behavioral tasks/tests. Many of these white matter regions have previously been associated with WM and/or attention.
One of these white matter tracts is the uncinate fasciculus (UNC), a band of long association fibers connecting the frontal and temporal lobes of the cerebrum. The UNC connects parts of the limbic system such as the hippocampus and amygdala in the temporal lobe with frontal cortex (e.g., orbitofrontal cortex). It is the last white matter tract to mature in the human brain and continues to mature past 30 years of age [Lebel et al., 2008]. Typically, UNC‐L has been shown to have greater FA than UNC‐R in studies due to left hemisphere specialization of language [Rodrigo et al., 2007]. In the research presented here, we find a high correlation between performance on the REY‐D Complex Figure and UNC‐R, for a group of individuals who have shown a preference for using a visuospatial strategy to complete memory tasks.
The high correlation between PCT and WMI for the visuospatial group is also worth elaborating, because only recent evidence has shown a role for the cerebellum in WM (recall that we also found a significant difference in FA between the verbal and visuospatial groups in SCP‐R (P = 0.001), with the visuospatial group having higher FA values). For example, the cerebellar peduncle, which includes PCT, has recently been correlated with accuracy on a verbal 2‐back task indicating a role for this white matter tract in sustained attention and WM [Takahashi et al., 2010]. Furthermore, efferents from the cerebellar nuclei project to multiple subdivisions of the ventrolateral thalamus [Percheron et al., 1996], which, in turn, project to many cortical areas, including regions of frontal, prefrontal, and posterior parietal cortex [Jones et al., 1985]. In fact, it has been argued that the functional map of the cerebellar cortex is likely to be as rich and complex as that in the cerebral cortex [Kelly and Strick, 2003]. It is also now apparent that a significant portion of the output of the cerebellum projects to nonmotor areas of the cerebral cortex, including regions of prefrontal and posterior parietal cortex. Thus, the anatomy exists for cerebellar output to influence the cognitive and visuospatial computations performed in prefrontal and posterior parietal cortex [Clower et al., 2001, 2005; Middleton and Strick, 2001; Strick et al., 2009].
WMI performance also correlated significantly with FA in SFO‐L. The inferior and superior fronto‐occipital fasciculi (IFO/SFO) are part of the dorsal visual stream linking parieto‐occipital regions with dorsolateral and frontal areas. This area has been implicated in attention and visual processing in a number of studies [Doricchi et al., 2008; Rudrauf et al., 2008].
A number of tracts also correlated with the visuospatial groups' performance on the PRI, including BCC, PCR‐R, SLF‐R, and TAP‐R. Interestingly, all of these tracts, except BCC, were in the right hemisphere. We discuss each of these areas below.
BCC, in addition to significantly correlating with PRI, also significantly correlated with RTs on the Sternberg WM task for the visuospatial group. The BCC, and related cortical regions (GCC, SCC, anterior, and posterior cingulate), are often tagged as a network mediating memory functions [Burgess et al., 2001; Kraus et al., 2007; Torta and Cauda, 2011]. The GCC connects medial and lateral surfaces of the frontal lobes while FX provides hippocampal and parahippocampal output to the mammillary bodies [Aggleton et al., 2005; Vann et al., 2011]. FX is the largest efferent pathway from the hippocampus [Koenig et al., 2013], while TAP is the continuation of the fiber tract from the corpus callosum into the cerebral white matter of the occipital lobe.
The corona radiata is a white matter sheet that contains both descending and ascending axons that carry nearly all of the neural traffic to and from the cerebral cortex. Children with higher estimates of white matter integrity in PCR were more accurate during a task of cognitive control, where cognitive control is defined as the ability to pay attention and suppress interference [Chaddock‐Heyman et al., 2013], indicating a role for PCR in attentional processes.
Lastly, SLF is a major tract that connects regions of the temporal (posterior and superior) and parietal lobes with prefrontal cortex [Croxson et al., 2005]. Several DTI studies have associated portions of the SLF with verbal processing and memory [Gold et al., 2007; Peters et al., 2012]. For example, Karlsgodt et al. [2008] found a positive correlation between performance on a Sternberg verbal WM task and FA in the SLF in recent‐onset schizophrenics. Similarly, Peters et al. [2012] showed a significant bilateral increase in FA in the SLF with development, which correlated positively with verbal WM performance. Kamali et al. [2014] demonstrated the trajectory and connectivity of the SLF fibers in relation to other language pathways using high resolution DTI. However, there may also be a role for the SLF in visuospatial attention. Thiebaut de Schotten et al. [2011] showed evidence that hemispheric specialization of part of the SLF is associated with an unbalanced speed of visuospatial processing and the amount of anatomical lateralization and degree of asymmetry of the SLF correlated with performance of visuospatial tasks. Vestergaard et al. [2011] observed a significant association between higher FA in the left fronto‐parietal network and better spatial WM skills, independent of age, for a group of adolescents. The left fronto‐parietal network is composed of the SLF, the regional white matter underlying the dorsolateral PFC, and the posterior parietal cortex. Finally, there is also evidence of attention orienting being dependent upon SLF function [Ge et al., 2013].
To summarize, we found significant correlations between FA and neuropsychological tests (e.g., REY‐D and WMI) solely for the visuospatial group, with many of the significantly correlated white matter regions previously shown to be involved in WM/attentional tasks. In contrast, the verbal group showed no significant correlations. Therefore, our anatomical results also provides converging evidence that these two groups, verbal and visuospatial, use different brain regions to conduct memory tasks.
CONCLUSIONS
This study provides strong evidence that different memory strategies may be employed by healthy individuals within the same age cohort, and that these strategy groups use different brain networks for completing WM tasks. Our results also indicate that when a verbal memory strategy is used for a spatial WM task better task performance is attained. We believe this is the first demonstration of the effect of strategies on task performance and brain activity based on a cluster analysis to initially separate strategy types. The existence of strategy differences was independently corroborated by converging results obtained from behavioral task measures, in addition to functional and anatomical measures.
As pointed out by Miller et al. [2012], if neuroimaging is to be used to make inferences about an individual, then multiple dimensions on which an individual may vary from one to another must be considered [Aine et al., 2011; Miller et al., 2012]. Type of memory strategy utilized certainly appears to be one of them. We are not arguing against the use of averaged data. Clearly averaging data across individuals can and has been very useful for neuroimaging research. Yet, the importance of taking into account individual differences in strategy is highlighted by our current results and, for example, by results from a study conducted by Kherif et al. [2009]. Kherif and colleagues discuss how in normal populations activation in the posterior cingulate and precuneus is not consistently reported in fMRI studies of reading aloud. But they show that it is activated by a subset of subjects in their study and can be linked to a particular reading strategy [Kherif et al., 2009]. Therefore, the use of averaging to make inferences about a group should be done carefully, with for example, task selection chosen such that alternative strategies to perform the task are less likely to be utilized.
There is also a tendency in the literature to describe the use of atypical brain areas as “compensatory.” For example, elders are typically proclaimed as somehow deficient when they are shown to recruit additional and/or different brain areas than young to perform the same task [Cabeza, 2002]. However, an alternative explanation is that different neural activation patterns sometimes seen between young and elderly groups may result from the different strategies invoked to complete a task which may evolve with age and the maturity of, for example, white matter tracts [Aine et al., 2006, 2010, 2011].
Future work in this area should include a study of individual trials and how they differ within the same individual. For example, although we looked at the tendency for an individual to use a particular strategy there is no reason to assume that this strategy was always used throughout the entire task. Therefore, perhaps sorting the individual trials into categories and looking on a trial by trial basis to determine differences between those trials where it was easy to verbalize the location (e.g., “upper right hand corner”) and those where it was not easy to verbalize the location may provide insight into, e.g., how consistently a strategy is used. In addition, further elaboration of an analysis of functional connectivity can provide additional insight into the uniqueness of strategies, and would be particularly interesting when individuals use different strategies with some overlap in areas of activity.
Finally, analysis of intersubject variability may be complementary to conventional analysis that averages data across individuals. Recent efforts have been made in this regard, which take into account both intersubject variability and take advantage of the power of averaging. For example, group independent component analysis in fMRI data [Beckmann and Smith, 2005; Calhoun et al., 2001], which identifies group components and reconstructs activations at the individual level. Studies using this newer approach have shown promise when tested with simulated data [Allen et al., 2014]. Regardless of the type of imaging technique and analysis used, individual variability should not be ignored, as evidence continues to emerge that individuals may exhibit varying brain activity even within a “homogeneous” healthy group completing an identical task.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes on Aging or the National Institutes of Health.
REFERENCES
- Aggleton JP, Vann SD, Saunders RC (2005): Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci 22:2519–2530. [DOI] [PubMed] [Google Scholar]
- Agirre‐Arrizubieta Z, Thai NJ, Valentín A, Furlong PL, Seri S, Selway RP, Elwes RD, Alarcon G (2014): The value of magnetoencephalography to guide electrode implantation in epilepsy. Brain Topogr 27:197–207. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Adair JC, Knoefel JE, Hudson D, Qualls C, Kovacevic S, Woodruff CC, Cobb W, Padilla D, Lee RR, Stephen JM (2005): Temporal dynamics of age‐related differences in auditory incidental verbal learning. Brain Res Cogn Brain Res 24:1–18. [DOI] [PubMed] [Google Scholar]
- Aine CJ, Woodruff CC, Knoefel JE, Adair JC, Hudson D, Qualls C, Bockholt J, Best E, Kovacevic S, Cobb W, Padilla D, Hart B, Stephen JM (2006): Aging: Compensation or maturation? Neuroimage 32:1891‐1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Bryant JE, Knoefel JE, Adair JC, Hart B, Donahue CH, Montano R, Hayek R, Qualls C, Ranken D, Stephen JM (2010): Different strategies for auditory word recognition in healthy versus normal aging. Neuroimage 49:3319–3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Sanfratello L, Adair JC, Knoefel JE, Caprihan A, Stephen JM (2011): Development and decline of memory functions in normal, pathological and healthy successful aging. Brain Topogr 24:323–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Sanfratello L, Ranken D, Best E, MacArthur JA, Wallace T, Gilliam K, Donahue CH, Montano R, Bryant JE, Scott A, Stephen JM (2012): MEG‐SIM: A web portal for testing MEG analysis methods using realistic simulated and empirical data. Neuroinformatics 10:141–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aine CJ, Sanfratello L, Adair JC, Knoefel JE, Qualls C, Lundy SL, Caprihan A, Stone D, Stephen JM (2014): Characterization of a normal control group: Are they healthy? Neuroimage 84:796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD (2014): Tracking whole‐brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM (2005): Tensorial extensions of independent component analysis for multisubject fMRI analysis. Neuroimage 25:294–311. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995): Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Ser B 57:289–300. [Google Scholar]
- Bor D, Duncan J, Wiseman RJ, Owen AM (2003): Encoding strategies dissociate prefrontal activity from working memory demand. Neuron 37:361–367. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O'Keefe J (2001): A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14:439–453. [DOI] [PubMed] [Google Scholar]
- Cabeza R (2002): Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol Aging 17:85–100. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ (2001): A method for making group inferences from functional MRI data using independent component analysis. Hum Brain Mapp 14:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaddock‐Heyman L, Erickson KI, Voss MW, Powers JP, Knecht AM, Pontifex MB, Drollette ES, Moore RD, Raine LB, Scudder MR, Hillman CH, Kramer AF (2013): White matter microstructure is associated with cognitive control in children. Biol Psychol 94:109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Edgar JC, Huang M, Hunter MA, Epstein E, Howell B, Lu BY, Bustillo J, Miller GA, Canive JM (2013): Frontal and superior temporal auditory processing abnormalities in schizophrenia. Neuroimage Clin 2:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, West RA, Lynch JC, Strick PL (2001): The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21:6283–6291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clower DM, Dum RP, Strick PL (2005): Basal ganglia and cerebellar inputs to ‘AIP.' Cereb Cortex 15:913–920. [DOI] [PubMed] [Google Scholar]
- Croxson PL, Johansen‐Berg H, Behrens TE, Robson MD, Pinsk MA, Gross CG, Richter W, Richter MC, Kastner S, Rushworth MF (2005): Quantitative investigation of connections of the prefrontal cortex in the human and macaque using probabilistic diffusion tractography. J Neurosci 25:8854–8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P (2008): White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex 44:983–995. [DOI] [PubMed] [Google Scholar]
- Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI (2013): Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connect 3:398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faigman D (2010): Evidentiary incommensurability: A preliminary exploration of the problem of reasoning from general scientific data to individualized legal decision‐making. Brooklyn L Rev 75:1115–1136. [Google Scholar]
- Fang Q, Boas D (2009): Tetrahedral mesh generation from volumetric binary and gray‐scale images In: Proceedings of IEEE International Symposium on Biomedical Imaging, Boston, MA, USA: pp 1142–1145. [Google Scholar]
- Feredoes E, Postle BR (2007): Localization of load sensitivity of working memory storage: Quantitatively and qualitatively discrepant results yielded by single‐subject and group‐averaged approaches to fMRI group analysis. Neuroimage 35:881–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez A, Turrero A, Zuluaga P, Gil‐Gregorio P, del Pozo F, Maestu F, Moratti S (2013): MEG delta mapping along the healthy aging‐Alzheimer's disease continuum: Diagnostic implications. J Alzheimers Dis 35:495–507. [DOI] [PubMed] [Google Scholar]
- Ge H, Yin X, Xu J, Tang Y, Han Y, Xu W, Pang Z, Meng H, Liu S (2013): Fiber pathways of attention subnetworks revealed with tract‐based spatial statistics (TBSS) and probabilistic tractography. PLoS One 8:e78831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T (2002): Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage 15:870–878. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer D, Verfaellie M (2009): Distinct hippocampal regions make unique contributions to relational memory. Hippocampus 19:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Powell DK, Xuan L, Jiang Y, Hardy PA (2007): Speed of lexical decision correlates with diffusion anisotropy in left parietal and frontal white matter: Evidence from diffusion tensor imaging. Neuropsychologia 45:2439–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackert VH, den Heijer T, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MM (2002): Hippocampal head size associated with verbal memory performance in nondemented elderly. Neuroimage 17:1365–1372. [DOI] [PubMed] [Google Scholar]
- Hanlon FM, Houck JM, Klimaj SD, Caprihan A, Mayer AR, Weisend MP, Bustillo JR, Hamilton DA, Tesche CD (2012): Frontotemporal anatomical connectivity and working‐relational memory performance predict everyday functioning in schizophrenia. Psychophysiology 49:1340–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heun R, Jessen F, Klose U, Erb M, Granath D, Freymann N, Grodd W (2000): Interindividual variation of cerebral activation during encoding and retrieval of words. Eur Psychiatry 15:470–479. [DOI] [PubMed] [Google Scholar]
- Jones LS, Gauger LL, Davis JN (1985): Anatomy of brain alpha 1‐adrenergic receptors: In vitro autoradiography with [125I]‐heat. J Comp Neurol 231:190–208. [DOI] [PubMed] [Google Scholar]
- Kamali A, Flanders AE, Brody J, Hunter JV, Hasan KM (2014): Tracing superior longitudinal fasciculus connectivity in the human brain using high resolution diffusion tensor tractography. Brain Struct Funct 219:269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, van Erp TG, Poldrack RA, Bearden CE, Nuechterlein KH, Cannon TD (2008): Diffusion tensor imaging of the superior longitudinal fasciculus and working memory in recent‐onset schizophrenia. Biol Psychiatry 63:512–518. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif F, Josse G, Seghier ML, Price CJ (2009): The main sources of intersubject variability in neuronal activation for reading aloud. J Cogn Neurosci 21:654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig KA, Sakaie KE, Lowe MJ, Lin J, Stone L, Bermel RA, Beall EB, Rao SM, Trapp BD, Phillips MD (2013): High spatial and angular resolution diffusion‐weighted imaging reveals forniceal damage related to memory impairment. Magn Reson Imaging 31:695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM (2007): White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain 130:2508–2519. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, Toga AW, Mazziotta JC (1997): Automated labeling of the human brain: A preliminary report on the development and evaluation of a forward‐transform method. Hum Brain Mapp 5:238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT (2000): Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp 10:120–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C, Walker L, Leemans A, Phillips L, Beaulieu C (2008): Microstructural maturation of the human brain from childhood to adulthood. Neuroimage 40:1044–1055. [DOI] [PubMed] [Google Scholar]
- Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP (2000): fMRI of visual encoding: reproducibility of activation. Hum Brain Mapp 9:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2001): Cerebellar projections to the prefrontal cortex of the primate. J Neurosci 21:700–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Van Horn JD, Wolford GL, Handy TC, Valsangkar‐Smyth M, Inati S, Grafton S, Gazzaniga MS (2002): Extensive individual differences in brain activations associated with episodic retrieval are reliable over time. J Cogn Neurosci 14:1200–1214. [DOI] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Van Horn JD, German E, Sokol‐Hessner P, Wolford GL (2009): Unique and persistent individual patterns of brain activity across different memory retrieval tasks. Neuroimage 48:625–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Donovan CL, Bennett CM, Aminoff EM, Mayer RE (2012): Individual differences in cognitive style and strategy predict similarities in the patterns of brain activity between individuals. Neuroimage 59:83–93. [DOI] [PubMed] [Google Scholar]
- Mori H (2008): [Diffusion tensor imaging]. Rinsho Shinkeigaku 48:945–946. [DOI] [PubMed] [Google Scholar]
- Nelder J, Mead R (1965): A simplex method for function minimization. Comput J 7:308–313. [Google Scholar]
- Olson IR, Moore KS, Stark M, Chatterjee A (2006): Visual working memory is impaired when the medial temporal lobe is damaged. J Cogn Neurosci 18:1087–1097. [DOI] [PubMed] [Google Scholar]
- Parasuraman R, Jiang Y (2012): Individual differences in cognition, affect, and performance: Behavioral, neuroimaging, and molecular genetic approaches. Neuroimage 59:70–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Funelon G (1996): The primate motor thalamus. Brain Res Brain Res Rev 22:93–181. [PubMed] [Google Scholar]
- Peters BD, Szeszko PR, Radua J, Ikuta T, Gruner P, DeRosse P, Zhang JP, Giorgio A, Qiu D, Tapert SF, Brauer J, Asato MR, Khong PL, James AC, Gallego JA, Malhotra AK (2012): White matter development in adolescence: Diffusion tensor imaging and meta‐analytic results. Schizophr Bull 38:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R (2005): Neural correlates of relational memory: Successful encoding and retrieval of semantic and perceptual associations. J Neurosci 25:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranken D, George J (1993): MRIVIEW: An Interactive Computational Tool for Investigation of Brain Structure and Function. IEEE Visualization: 324–332. [Google Scholar]
- Ranken D, Best E, Schmidt D, George J, Wood C, Huang M (2002): MEG/EEG forward and inverse modeling using MRIVIEW In: Proceedings of the 13th international conference on biomagnetism. Nowak H, Jaueisen J, Giebler F, Huonker R, editors. pp 785–787. [Google Scholar]
- Ranken D, Stephen JM, George JS (2004): MUSIC seeded multi‐dipole MEG modeling using the Constrained Start Spatio‐Temporal modeling procedure. Neurology and Clinical Neurophysiology, 80. [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Golestani N, Cointepas Y, Poupon C, Semah F, Mangin JF, Le Bihan D, Meder JF (2007): Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol 17:1663–1668. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Grabowski TJ (2008): Disconnection's renaissance takes shape: Formal incorporation in group‐level lesion studies. Cortex 44:1084–1096. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE (2004): Prefrontal selection and medial temporal lobe reactivation in retrieval of short‐term verbal information. Cereb Cortex 14:914–921. [DOI] [PubMed] [Google Scholar]
- Sanfratello L, Stephen J, Ranken D, Best E, Wallace T, MacArthur J, Gilliam K, Aine C (2010): MEG‐SIM portal: Reconstructions from realistic simulations of sensory and cognitive processing In: Supek S, Sušac A, editors. IFMBE Proc 28:132–135. [Google Scholar]
- Sanfratello L, Stephen J, Best E, Ranken D, Aine C: MEG‐SIM web portal: A database of realistic simulated and empirical MEG data for testing algorithms In: Supek S, Aine C, editors. Magnetoencephalography: From signals to dynamic cortical networks. Heidelberg: Springer‐Verlag; (in press). [Google Scholar]
- Schacter DL, Uecker A, Reiman E, Yun LS, Bandy D, Chen K, Cooper LA, Curran T (1997): Effects of size and orientation change on hippocampal activation during episodic recognition: a PET study. Neuroreport 8:3993–3998. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, Luna B (2006): Brain basis of developmental change in visuospatial working memory. J Cogn Neurosci 18:1045–1058. [DOI] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Tapert SF (2005): fMRI reveals alteration of spatial working memory networks across adolescence. J Int Neuropsychol Soc 11:631–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Lazeyras F, Pegna AJ, Annoni JM, Khateb A (2008): Group analysis and the subject factor in functional magnetic resonance imaging: Analysis of fifty right‐handed healthy subjects in a semantic language task. Hum Brain Mapp 29:461–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier ML, Price CJ (2009): Dissociating functional brain networks by decoding the between‐subject variability. Neuroimage 45:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Strick PL, Dum RP, Fiez JA (2009): Cerebellum and nonmotor function. Annu Rev Neurosci 32:413–434. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine CJ (1993): Simulation studies of multiple dipole neuromagnetic source localization: Model order and limits of source resolution. IEEE Trans Biomed Eng 40:529–540. [DOI] [PubMed] [Google Scholar]
- Supek S, Aine CJ (1997): Spatio‐temporal modeling of neuromagnetic data: I. Multi‐source location versus time‐course estimation accuracy. Hum Brain Mapp 5:139–153. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Iwamoto K, Fukatsu H, Naganawa S, Iidaka T, Ozaki N (2010): White matter microstructure of the cingulum and cerebellar peduncle is related to sustained attention and working memory: A diffusion tensor imaging study. Neurosci Lett 477:72–76. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Dell'Acqua F, Forkel SJ, Simmons A, Vergani F, Murphy DG, Catani M (2011): A lateralized brain network for visuospatial attention. Nat Neurosci 14:1245–1246. [DOI] [PubMed] [Google Scholar]
- Torta DM, Cauda F (2011): Different functions in the cingulate cortex, a meta‐analytic connectivity modeling study. Neuroimage 56:2157–2172. [DOI] [PubMed] [Google Scholar]
- Travis SG, Huang Y, Fujiwara E, Radomski A, Olsen F, Carter R, Seres P, Malykhin NV (2013): High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia 53C:233–245. [DOI] [PubMed] [Google Scholar]
- Tun PA (1998): Fast noisy speech: age differences in processing rapid speech with background noise. Psychol Aging 13:424–434. [DOI] [PubMed] [Google Scholar]
- Vann SD, Erichsen JT, O'Mara SM, Aggleton JP (2011): Selective disconnection of the hippocampal formation projections to the mammillary bodies produces only mild deficits on spatial memory tasks: implications for fornix function. Hippocampus 21:945–957. [DOI] [PubMed] [Google Scholar]
- Velez‐Ruiz NJ, Klein JP (2012): Neuroimaging in the evaluation of epilepsy. Semin Neurol 32:361–373. [DOI] [PubMed] [Google Scholar]
- Vestergaard M, Madsen KS, Baare WF, Skimminge A, Ejersbo LR, Ramsoy TZ, Gerlach C, Akeson P, Paulson OB, Jernigan TL (2011): White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. J Cogn Neurosci 23:2135–2146. [DOI] [PubMed] [Google Scholar]


