Abstract
Background
Optimal clinical trial endpoints for irritable bowel syndrome with constipation (IBS-C) are uncertain.
Objective
The objective of this article is to compare adequate relief (AR) to abdominal/bowel symptoms, global endpoints, and FDA and EMA responder criteria; and to use AR as an anchor to assess clinically meaningful change (CMC) in IBS-C symptoms.
Methods
Using pooled 12-week data from two phase 3 linaclotide clinical trials, daily abdominal/bowel symptoms and weekly global assessments were correlated with AR. Symptom CMC thresholds were estimated using AR as an anchor. Agreement between AR and FDA/EMA responder criteria was assessed.
Results
Correlations of AR with percentage change in abdominal symptoms, bowel symptoms, and global endpoints ranged from 0.48–0.54, 0.32–0.39, and 0.61–0.71, respectively. Using AR as an anchor, CMC thresholds were 29% improvement in abdominal pain, 29% improvement in abdominal discomfort, and 0.7/week increase in CSBMs, similar to thresholds for IBS-C responder endpoints recommended by the FDA and EMA. There was considerable agreement of weekly responder rates between AR and the FDA and EMA endpoints (on average, 70%–76% and 71%–82% of weeks with agreement, respectively).
Conclusions
AR bridges IBS-C clinical trials, putting into perspective the disparate primary endpoints recommended by professional societies and regulatory authorities, and allowing researchers, practitioners, and regulators to compare trial results.
Keywords: Linaclotide, adequate relief, clinically meaningful change, responder endpoints, GC-C, IBS-C
Introduction
Irritable bowel syndrome (IBS) is a chronic functional gastrointestinal disorder, the hallmark symptoms of which are abdominal pain or discomfort associated with altered bowel function.1 IBS with constipation (IBS-C), a subtype of IBS, is associated with patient reports of hard stools, straining, a sensation of incomplete evacuation, and bloating, in addition to the characteristic abdominal pain or discomfort and reduced stool frequency.2
Patient-reported outcomes (PROs) play a pivotal role in evaluating IBS symptoms in clinical trials; however, the multifaceted nature of IBS can lead to difficulties in appropriately portraying the full patient experience. Traditionally, IBS clinical trials have utilized global assessment endpoints (ratings of overall change in condition; for example, “adequate relief” or “satisfactory relief”) as the primary efficacy endpoint.3 Adequate relief (AR) is measured as a dichotomous endpoint (e.g. “Overall, have you had adequate relief of your IBS symptoms during the past 7 days?” (yes/no)). AR of IBS symptoms as a global endpoint has been compared to multiple secondary endpoints and symptoms,4–6 and has been shown to demonstrate excellent construct validity and ability to detect clinically meaningful changes (CMC) in key abdominal and bowel symptoms associated with IBS illness severity. However, it has been suggested that more sensitive endpoints might be needed to best distinguish between responders and nonresponders in an IBS-C population.5 Likewise, the Food and Drug Administration (FDA) and, more recently, the European Medicines Agency (EMA), have expressed concerns with the use of global measures (including AR) as primary endpoints because they neither specifically indicate improvement of individual symptoms nor provide relative degree of improvement or worsening of symptoms.7,8
The recommended FDA primary endpoint is a composite responder endpoint incorporating abdominal pain and abnormal bowel function, the hallmark symptoms of IBS considered important and bothersome to patients.7 The change in bowel function is based on specific definitions: a spontaneous bowel movement (SBM) is a bowel movement (BM) occurring in the absence of any laxative, enema, or suppository use during the preceding 24 hours, and a complete spontaneous bowel movement (CSBM) is an SBM that is associated with a sensation of complete emptying. According to the FDA criteria, an IBS-C responder must report (in the same week for at least 50% of the treatment period weeks) both an improvement from baseline of ≥30% in the weekly average of daily scores for worst abdominal pain and an increase of ≥1 CSBM per week from baseline.
The current EMA recommendations for primary efficacy endpoints in IBS clinical trials differ from the FDA recommendations; the EMA recommends a patient global assessment of symptoms and abdominal discomfort/pain as co-primary endpoints.9 However, it should be noted that a recent draft update of the EMA Guidelines on the Evaluation of Medicinal Products for the Treatment of Irritable Bowel Syndrome (released for public comment in 2013) advocates the use of a combined endpoint similar to that recommended by the FDA. The FDA and current and draft EMA recommendations specify that responders should be based on CMC in the measures for a specified time interval (suggested ≥50% of the time on treatment in a study).
Neither the FDA-recommended responder endpoint nor the current EMA co-primary endpoints have been examined relative to traditional global assessment endpoints, such as AR.
Linaclotide is a first-in-class guanylate cyclase C (GC-C) agonist that has been approved in the United States and Europe for the treatment of IBS-C in adult men and women. In animal models, linaclotide has also been shown to reduce visceral hypersensitivity; this effect may be related to cGMP modulating afferent nerve activity in the extracellular space.10–12 In humans, linaclotide accelerated colonic transit in a pharmacodynamics study13 and improved abdominal pain and constipation associated with IBS-C in two large phase 3, double-blind, placebo-controlled trials.14,15 The phase 3 trials used primary responder endpoints according to both the current FDA and EMA guidelines (primary endpoints were specified for each territory) and also examined global endpoints, including AR, to assess overall IBS symptom improvement.
The objectives of the analyses conducted in the current study were: first, to examine the relationship between AR and abdominal and bowel symptoms, other global endpoints, and the current FDA and EMA responder criteria for IBS; and, second, to use AR as an anchor for assessing CMC in abdominal and bowel symptoms in IBS-C.
Methods
Linaclotide phase 3 trial design
Two phase 3 trials were conducted to evaluate the efficacy and safety of linaclotide in patients with IBS-C (published elsewhere—including entry criteria, methodology, and results).14,15 Both trials included a screening period and a two-week baseline period, after which eligible patients were randomized to receive an oral capsule of linaclotide 290 µg or matching placebo daily during a 12- or 26-week treatment period. The current pooled analyses utilize data from the first 12 weeks of treatment, the pre-specified treatment duration for assessment of the primary endpoints. Baseline and treatment period efficacy data were captured using an interactive voice response system (IVRS) for daily and weekly symptom assessments.
Patients
In total, 1602 patients were included in the pooled phase 3 IBS-C intent-to-treat (ITT) population, defined as patients who received ≥1 dose of double-blind trial medication during the treatment period and had ≥1 post-randomization assessment of abdominal pain or BM frequency.
For symptom-specific analyses, the analysis populations were limited to patients in the pooled phase 3 IBS-C ITT population who were experiencing specified symptom scores at baseline, as outlined in Table 1. These populations were established to ensure that patients included in the analyses were experiencing at least mild symptoms at baseline. These restrictions on the populations were applied to the correlations of AR with symptom-specific endpoints (Table 2), estimations of CMC thresholds (Table 3), and responder rates based on CMC thresholds (Table 3).
Table 1.
Symptom-level analysis population restrictions
| Symptom | Criteria for inclusion in analysis population | % of Patients meeting criteria |
|---|---|---|
| Abdominal symptoms (0- to 10-point NRS) | ||
| Abdominal pain | Baseline score ≥ 3a | 100% (1602/1602) |
| Abdominal discomfort | Baseline score ≥ 3 | 99.2% (1589/1602) |
| Abdominal bloating | Baseline score ≥ 3 | 98.3% (1574/1602) |
| Abdominal fullness | Baseline score ≥ 3 | 98.8% (1583/1602) |
| Abdominal cramping | Baseline score ≥ 3 | 90.9% (1457/1602) |
| Bowel symptoms | ||
| CSBMs/week | Baseline < 3a | 100% (1602/1602) |
| SBMs/week | Baseline ≤ 5a | 100% (1602/1602) |
| Straining (five-point ordinal scale) | Baseline score ≥ 2 | 97.8% (1362/1392)b |
| Stool consistency (seven-point BSFS) | Baseline score ≤ 3 | 79.5% (1107/1392)b |
BSFS: Bristol Stool Form Scale; CSBM: complete spontaneous bowel movement; NRS: numerical rating scale; SBM: spontaneous bowel movement. aPatients needed to meet these criteria during the baseline period in order to be eligible for randomization (average of daily abdominal pain scores and average per week for CSBMs/SBMs). bA total of 210 patients did not have an SBM during the baseline period and, therefore, were missing baseline straining and stool consistency scores.
Table 2.
Correlation of adequate relief with symptom-specific and global endpoints at week 12
| Symptom | Correlation with adequate relief |
|---|---|
| Abdominal symptoms (percentage change from baseline) | |
| Abdominal pain | 0.50 |
| Abdominal discomfort | 0.54 |
| Abdominal bloating | 0.52 |
| Abdominal fullness | 0.54 |
| Abdominal cramping | 0.48 |
| Bowel symptoms (change from baseline) | |
| CSBMs/week | 0.38 |
| SBMs/week | 0.34 |
| Straining | 0.39 |
| Stool consistency | 0.32 |
| Global endpoints | |
| IBS degree of relief | 0.71 |
| IBS severity | 0.62 |
| Constipation severity | 0.61 |
| Treatment satisfaction | 0.70 |
Absolute values are given for the correlations; p < 0.0001 for all correlations. CSBM: complete spontaneous bowel movement; IBS: irritable bowel syndrome; ITT: intent to treat; NRS: numerical rating scale; SBM: spontaneous bowel movement. Week 12 last observation carried forward (LOCF), pooled phase 3 IBS-C ITT population; patients with a baseline score of <3 (on the 0- to 10-point NRS) for an abdominal symptom were excluded from the analysis of that symptom; patients with a baseline score of <2 for straining, >3 for stool consistency, or a missing stool consistency or straining score (i.e. due to 0 SBMs) were excluded from the analysis of that parameter.
Table 3.
Responder rates based on thresholds for clinically meaningful change anchored by adequate relief
| Symptom | Threshold | 12-Week responder rate |
NNT | |
|---|---|---|---|---|
| Linaclotide % (n/N) | Placebo % (n/N) | |||
| Abdominal symptoms (% improvement from baseline) | ||||
| Pain | 29.3% | 56.3% (453/805) | 38.6% (308/797) | 5.7 |
| Discomfort | 29.3% | 55.6% (444/798) | 35.8% (283/791) | 5.0 |
| Bloating | 20.0% | 61.1% (483/791) | 41.5% (325/783) | 5.1 |
| Fullness | 23.5% | 57.0% (454/796) | 36.2% (285/787) | 4.8 |
| Cramping | 26.5% | 57.3% (423/738) | 42.8% (308/719) | 6.9 |
| Bowel symptoms (change from baseline) | ||||
| SBMs/week | 1.9 | 65.2% (525/805) | 30.7% (245/797) | 2.9 |
| CSBMs/week | 0.7 | 58.4% (470/805) | 31.0% (247/797) | 3.6 |
| Straining (five-point ordinal scale) | –0.8 | 72.3% (498/689) | 39.7% (267/673) | 3.1 |
| Stool consistency (seven-point BSFS) | 1.6 | 70.7% (396/560) | 22.3% (122/547) | 2.1 |
BSFS: Bristol Stool Form Scale; CMH: Cochran-Mantel-Haenszel; CSBM: complete spontaneous bowel movement; ITT: intent to treat; NNT: number needed to treat; NRS: numerical rating scale; SBM: spontaneous bowel movement. For all symptoms, p < 0.0001 for linaclotide vs placebo responder rates; p values obtained from a CMH test controlling for geographic region and clinical trial. Pooled phase 3 IBS-C ITT population; patients with a baseline score of <3 (on the 0-10-point NRS) for an abdominal symptom were excluded from the analysis of that symptom; patients with a baseline score of <2 for straining, >3 for stool consistency, or a missing stool consistency or straining score (i.e. due to 0 SBMs) were excluded from the analysis of that parameter.
Clinical assessments
Clinical assessments (daily and weekly assessments, as well as assessments conducted at trial visits) have been published in detail elsewhere14,15 and are summarized in the online Appendix.
Endpoints
The FDA responder endpoint for IBS-C required that, during the same week for at least 50% of treatment period weeks (i.e. at least six of the first 12 weeks), the patient reported: (i) an improvement of ≥30% from baseline in the average of the daily worst abdominal pain scores and (ii) an increase of ≥1 CSBM from baseline. The co-primary endpoints used in the linaclotide phase 3 trials for EMA submission required that, for at least six of the first 12 weeks of the treatment period, the patient reported: (i) an improvement of ≥30% from baseline in either the mean abdominal pain or abdominal discomfort score for that week, with neither of the scores worsening from baseline for that week (weekly abdominal pain/abdominal discomfort responder) and (ii) a response of “considerably relieved” or “completely relieved” (corresponding to a score ≤2) to the degree of relief of IBS symptoms question (weekly IBS degree of relief responder). For the purposes of comparing the FDA and EMA responder criteria with the AR criteria, the current analysis focuses on patients meeting the FDA responder criteria for a particular week (to be referred to hereafter as weekly FDA responders) and each of the EMA co-primary criteria for a particular week (to be referred to hereafter as weekly EMA responders).
Abdominal symptom endpoints included percentage change from baseline in abdominal pain, discomfort, bloating, fullness, and cramping. Bowel symptom endpoints included change from baseline in CSBMs, SBMs, stool consistency, and straining. Global endpoints included AR, IBS degree of relief, IBS severity, constipation severity, and treatment satisfaction.
Statistical methods and data analysis
Relationship of AR and symptom-specific and global endpoints
To examine the relationship of AR of IBS with abdominal and bowel symptom endpoints, as well as with global endpoints, correlations between AR and the percentage change from baseline in abdominal symptoms, the change from baseline in bowel habits, and the global endpoints at Week 12 are presented (using a last-observation-carried-forward (LOCF) approach). For the purposes of interpretation of the correlations, r = 0.3 was considered a medium effect size and r = 0.5 was considered a large effect size.16
In addition, to further examine the relationship between AR and other global endpoints (i.e. degree of relief, IBS severity, constipation severity, and treatment satisfaction), as well as symptom-specific patient-rating-of-change (PRC) assessments (i.e. abdominal pain and CSBMs), the percentage of patients reporting AR is presented by response category for each assessment (using an LOCF approach).
CMC threshold determination and analysis
CMC thresholds for the abdominal and bowel symptoms were estimated using receiver-operating characteristics (ROC) methods.17 A repeated-measures mixed model (RMMM) was applied to the observed-case data from the ITT population to account for the within-patient correlation due to weekly reporting of AR over the 12-week treatment period. The CMC thresholds were then used to determine 12-week responder rates (i.e. the average across Weeks 1 through 12) for each of the abdominal and bowel endpoints; p values for linaclotide versus placebo were determined using a Cochran-Mantel-Haenszel (CMH) test controlling for geographic region and clinical trial. The online Appendix provides additional statistical methods for CMC threshold determination and analysis.
Agreement of AR with FDA and EMA responder criteria
To compare AR with the FDA responder criteria for IBS-C, the agreement between the weekly AR and weekly FDA responder criteria was determined. For each patient, response pairs for the AR and FDA responder criteria were determined at each week and the agreement (agreement = percentage of times a patient had AR and was a weekly FDA responder or did not have AR and was not a weekly FDA responder) was calculated for the patient’s 12 weekly response pairs. Then, an average across patients was taken to provide an estimate of the percentage of weeks across the 12-week treatment period that the weekly AR and weekly FDA responder criteria agreed.
The same method was used to compare the weekly responder rates for AR with each of the weekly EMA criteria for IBS-C (weekly abdominal pain/abdominal discomfort responder and weekly IBS degree of relief responder).
Results
Correlation of AR to symptom-specific and global endpoints
The correlations of AR with abdominal symptoms ranged from 0.48 to 0.54. In contrast, the correlations of AR with change in bowel symptoms were slightly lower, ranging from 0.32 to 0.39. As would be expected, correlations of AR with other global endpoints were higher, 0.61 to 0.71 (Table 2).
Relationship between AR and other global responses based on categories of change (relief, improvement, severity, or satisfaction)
In general, AR strongly corresponded to degree of relief and PRC questions (Table 4). For example, >90% of patients who reported being “completely relieved/improved” and >80% of patients who reported being “considerably relieved/improved” on the IBS degree of relief and abdominal pain and CSBM PRC questions also reported AR of their IBS at Week 12. Approximately half (46% to 55%) of patients who reported being “somewhat relieved/improved” reported AR. In contrast, relatively few patients (≤17%) who reported their relief/improvement as “unchanged” or “worse” reported AR. Patient ratings of IBS severity and constipation severity revealed a similar trend, with lesser severity corresponding to higher rates of AR. Likewise, patients reporting greater treatment satisfaction also had higher rates of AR.
Table 4.
Percentage of patients reporting adequate relief by other global response category at week 12
| Relief/improvement category |
|||||
|---|---|---|---|---|---|
| Global Assessment | Completely relieved/improved | Considerably relieved/improved | Somewhat relieved/improved | Unchanged | Worsea |
| IBS degree of relief | |||||
| N | 131 | 411 | 424 | 467 | 136 |
| Patients with AR, n (%) | 128 (98) | 367 (89) | 208 (49) | 20 (4) | 1 (1) |
| Abdominal pain (PRC) | |||||
| N | 137 | 436 | 382 | 448 | 156 |
| Patients with AR, n (%)b | 129 (94) | 366 (84) | 175 (46) | 32 (7) | 17 (11) |
| CSBMs (PRC) | |||||
| N | 142 | 357 | 326 | 581 | 153 |
| Patients with AR, n (%)b | 134 (94) | 294 (82) | 180 (55) | 96 (17) | 15 (10) |
| Severity category |
|||||
| |
None | Mild | Moderate | Severe | Very severe |
| IBS severity | |||||
| N | 125 | 472 | 585 | 308 | 79 |
| Patients with AR, n (%)c | 120 (96) | 389 (82) | 192 (33) | 19 (6) | 4 (5) |
| Constipation severity | |||||
| N | 170 | 440 | 545 | 322 | 92 |
| Patients with AR, n (%)c | 154 (91) | 350 (80) | 197 (36) | 18 (6) | 5 (5) |
| Satisfaction category |
|||||
| |
Very satisfied | Quite satisfied | Moderately satisfied | A little satisfied | Not at all satisfied |
| Treatment satisfaction | |||||
| N | 259 | 327 | 277 | 253 | 441 |
| Patients with AR, n (%) | 238 (92) | 270 (83) | 143 (52) | 49 (19) | 19 (4) |
AR: adequate relief; CSBM: complete spontaneous bowel movement; IBS: irritable bowel syndrome; PRC: patient rating of change; ITT: intent to treat. Week 12 last observation carried forward (LOCF), pooled phase 3 IBS-C ITT population. aRelief/improvement category “Worse” includes patient ratings of “somewhat worse,” “considerably worse,” and “as bad as I can imagine.” bImprovement was defined as patient rating of “somewhat improved,” “considerably improved,” or “completely improved.” A total of 70% (670 of 955 patients) and 74% (608 of 825 patients) of patients who reported improvement in abdominal pain and CSBMs, respectively, also reported AR. c85% (509 of 597 patients) and 83% (504 of 610 patients) of patients who reported their IBS severity and constipation severity, respectively, as “none” or “mild” also reported AR; 6% (23 of 387 patients) and 6% (23 of 414 patients) of patients who reported their IBS severity and constipation severity, respectively, as “severe” or “very severe” also reported AR.
CMC relative to thresholds based on AR
Thresholds for CMC in abdominal and bowel symptoms using AR as an anchor are displayed in Table 3. Thresholds for related abdominal symptoms, abdominal pain, discomfort, and cramping, were similar (27% to 29%), and those for fullness and bloating were also similar but lower (20% to 24%).
The percentage of patients reporting CMC for abdominal and bowel symptoms was significantly greater for linaclotide-treated patients compared with placebo-treated patients (p < 0.0001 for all endpoints). The numbers needed to treat (NNT) ranged from 4.8 to 6.9 for abdominal symptoms and 2.1 to 3.6 for bowel symptoms.
Agreement of weekly AR and current FDA and EMA responder criteria
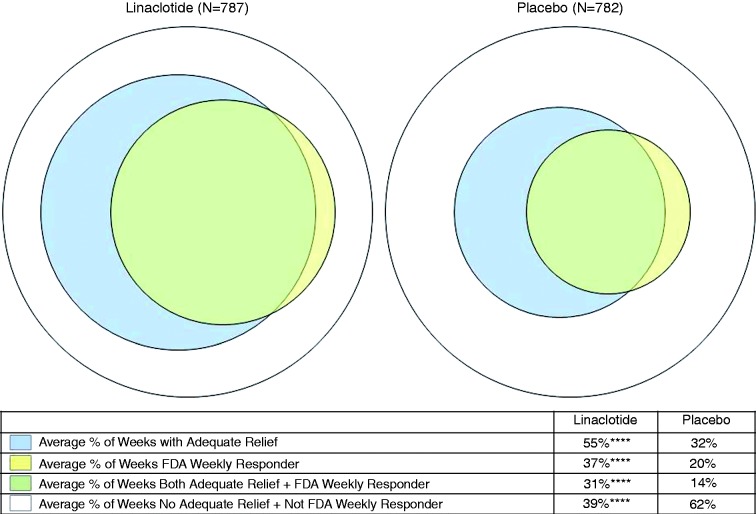
An analysis of the within-patient agreement between weekly AR and the FDA weekly responder criteria (i.e. AR and weekly FDA responder + no AR and not a weekly FDA responder) revealed considerable agreement between AR and the weekly FDA responder criteria (on average, 70% and 76% of weeks with agreement in the linaclotide and placebo groups, respectively; Figure 1). The weekly AR response criteria appear to be less stringent than the weekly FDA responder criteria, as patients reported AR but were not FDA responders for an average of 24% and 18% of weeks in the linaclotide and placebo groups, respectively; conversely, patients were FDA responders but did not report AR for an average of only 6% of weeks on either linaclotide or placebo.
Figure 1.
Within-patient agreement between weekly adequate relief and weekly FDA responder criteria. Pooled phase 3 IBS-C ITT population, Weeks 1–12. ****p < 0.0001 for linaclotide vs placebo; p values were obtained from an ANOVA model with treatment group, geographic region, and trial as factors. Agreement (average % of weeks with AR and FDA weekly responder + no AR and not FDA weekly responder) = 70.2% for linaclotide and 76.4% for placebo; average % of weeks with AR and not FDA weekly responder = 24.0% for linaclotide and 18.2% for placebo; average % of weeks no AR and FDA weekly responder = 5.7% for linaclotide and 5.5% for placebo.
FDA: Food and Drug Administration; IBS-C: irritable bowel syndrome with constipation; ITT: intent to treat; ANOVA: analysis of variance; AR: adequate relief.
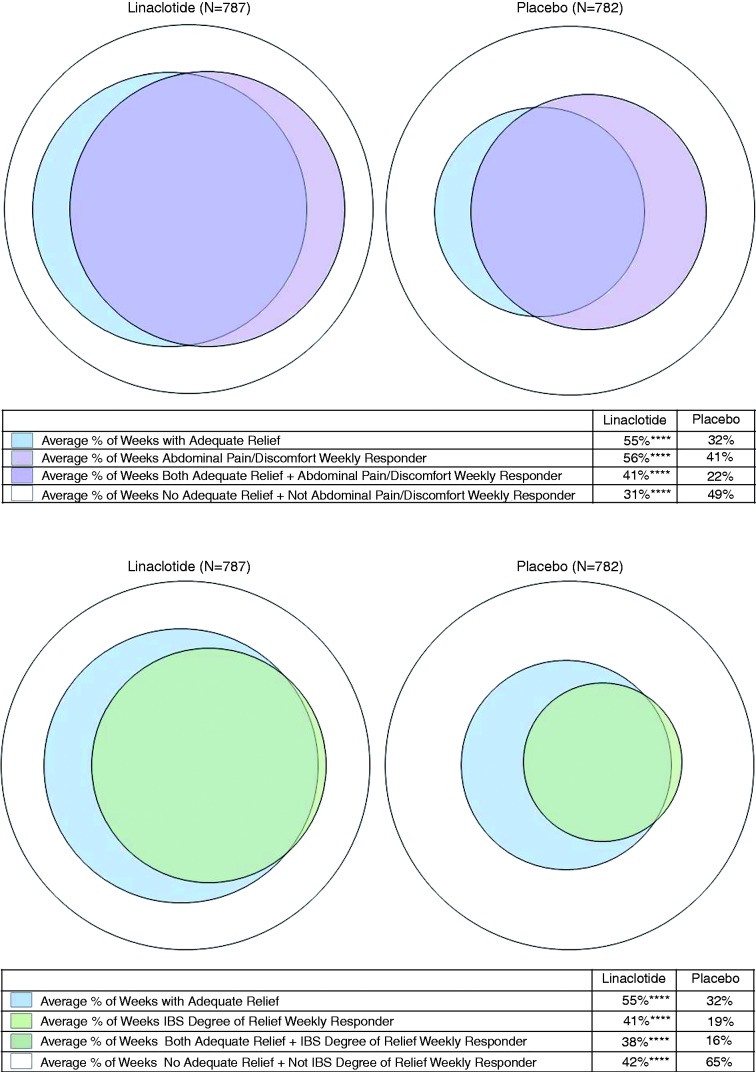
Similarly, the within-patient agreement between AR and the current EMA responder criteria averaged 72% and 71% of weeks in the linaclotide and placebo groups, respectively, for weekly abdominal pain/discomfort responder (Figure 2(a)), and 80% and 82% of weeks, respectively, for weekly IBS degree of relief responder (Figure 2(b)). The EMA weekly abdominal pain/discomfort responders and weekly AR responders appear to overlap significantly. The weekly AR response criteria appear to be less stringent than the EMA weekly IBS degree of relief responder criteria, as patients reported AR but were not IBS degree of relief responders for an average of 18% and 16% of weeks in the linaclotide and placebo groups, respectively; conversely, patients were IBS degree of relief responders but did not report AR for an average of only 3% and 2% of weeks in the linaclotide and placebo groups, respectively.
Figure 2.
Within-patient agreement between weekly adequate relief and weekly EMA responder criteria. (a) Weekly abdominal pain/abdominal discomfort responder. Pooled phase 3 IBS-C ITT population, Weeks 1–12. ****p < 0.0001 for linaclotide vs placebo; p values were obtained from an ANOVA model with treatment group, geographic region, and trial as factors. Agreement (average % of weeks with AR and abdominal pain/discomfort weekly responder + no AR and not abdominal pain/discomfort weekly responder) = 71.8% for linaclotide and 71.2% for placebo; average % of weeks with AR and not abdominal pain/discomfort weekly responder = 13.9% for linaclotide and 10.0% for placebo; average % of weeks no AR and abdominal pain/discomfort weekly responder = 14.3% for linaclotide and 18.7% for placebo. (b) Weekly IBS degree of relief responder. Pooled phase 3 IBS-C ITT population, Weeks 1–12. ****p < 0.0001 for linaclotide vs placebo; p values were obtained from an ANOVA model with treatment group, geographic region, and trial as factors. Agreement (average % of weeks with AR and IBS degree of relief weekly responder + no AR and not IBS degree of relief weekly responder) = 79.8% for linaclotide and 81.5% for placebo; average % of weeks with AR and not IBS degree of relief weekly responder = 17.5% for linaclotide and 16.0% for placebo; average % of weeks no AR and IBS degree of relief weekly responder = 2.7% for linaclotide and 2.4% for placebo.
EMA: European Medicines Agency; IBS-C: irritable bowel syndrome with constipation; ITT: intent to treat; ANOVA: analysis of variance; AR: adequate relief.
Discussion
This paper shows that there is substantial agreement of weekly AR with weekly FDA and EMA responder criteria. When anchored by AR, CMC thresholds for abdominal and bowel symptoms correspond well with thresholds required to meet FDA and EMA endpoints.
AR, as a patient-reported outcome assessment for IBS, has historical, regulatory, and clinical relevance. It is recommended by the Rome III committee for clinical trials as the primary outcome assessment in IBS treatment trials.18 AR has been used as an endpoint in numerous upper and lower functional gastrointestinal diseases6,19 and was accepted by the FDA as the primary endpoint for the approval of alosetron.20 It is consistently able to distinguish efficacious drugs from placebo, with treatment effects that are at least as large as other measured endpoints.5,6 AR has shown excellent quantitative measurement properties, including construct validity, reproducibility, and responsiveness.4–6,21
The linaclotide pooled phase 3 IBS-C database, which included more than 1600 patients, provided an opportunity to examine the relationship between AR and individual symptoms, other global assessments, and the current FDA- and EMA-recommended responder criteria for IBS-C in the context of randomized, controlled trials using uniform endpoints.
These analyses provide data supporting the use of AR as an endpoint in IBS-C trials. Using AR as an anchor, the CMC thresholds for abdominal pain and CSBMs were similar to the FDA responder endpoint criteria. Interestingly, the CMC thresholds for bloating and fullness, both important and bothersome symptoms in patients with IBS-C, were lower than the threshold for abdominal pain, suggesting that patients may value smaller improvement in these symptoms compared with abdominal pain.
Assessment of AR and individual symptoms in IBS-C
AR demonstrated medium to large,16 and statistically significant, correlations with the individual symptoms of IBS-C, particularly for abdominal symptoms. In addition, the majority of patients who reported improvement (i.e. responses of “somewhat improved,” “considerably improved,” or “completely improved” on PRC questions) on individual symptoms such as abdominal pain and CSBMs also reported AR (70% for abdominal pain and 74% for CSBMs); and few patients who reported worsening of these individual symptoms reported AR (≤11%). Similarly, lesser IBS severity and constipation severity corresponded to higher rates of AR. These data suggest that AR is effectively demonstrating patients’ current symptom intensity.
Assessment of AR with satisfaction and improvement
AR is strongly associated with treatment satisfaction, in that patients who reported being more satisfied with treatment also had higher rates of AR. As a dichotomous (yes/no), integrative (of all relevant symptoms to a particular patient) endpoint, based on the patient’s own reference system, AR appears to be measuring a degree of relief that is somewhat more stringent than only “somewhat relieved/improved” for general or symptom-specific PRC assessments. Therefore, this analysis shows that AR may be measuring clinically meaningful improvement that is slightly beyond the minimal important difference perceptible to a patient.
Assessment of AR in comparison to current FDA and EMA endpoints
AR was used to anchor estimates of CMC in the current analysis. This analysis revealed thresholds for abdominal pain and CSBM frequency that are similar to the values chosen by the FDA for its recommended responder endpoint and consistent with thresholds determined by studies in neuropathic pain (i.e. approximately 30% improvement over baseline).7,22 These findings provide support for the FDA IBS-C composite endpoint. Weekly AR and the weekly FDA responder criteria showed considerable agreement (at least 70% of weeks across all patients).
Similarly, using AR as an anchor, the CMC threshold for abdominal discomfort coincides very closely with the threshold required for the current EMA endpoint, and provides support for this component of the EMA co-primary endpoint. Analysis of agreement between weekly AR and each of the EMA’s two weekly responder parameters revealed agreement for more than 70% of weeks for the weekly abdominal pain/discomfort responder and for at least 80% of weeks for the weekly global responder. These data suggest that the dichotomous endpoint of AR behaves similarly to the current EMA co-primary endpoints and could be considered for the global assessment requirement for one of the two primary endpoints currently recommended by the EMA. These findings also suggest that the FDA composite IBS-C responder endpoint and EMA co-primary IBS-C endpoints overlap with a similar proportion of AR responders and provide strong quantitative support for the regulatory agencies’ currently approved endpoints.
Assessment of AR as a PRO endpoint
These post-hoc analyses demonstrate that AR has statistical significance and clinical meaningfulness as a PRO measure for clinical trials in IBS-C, and extends prior analyses that validated AR as a relevant outcome in IBS with diarrhea (IBS-D)5 by showing similar properties in IBS-C. AR and other dichotomous outcome measures (e.g. satisfactory relief, considerable relief) have been used as primary or secondary endpoints in many IBS clinical trials,6 and as additional endpoints in the most recent linaclotide trials. Therefore, AR serves as a bridge across clinical trials to put into perspective the disparate and evolving primary endpoints recommended by professional societies and regulatory authorities,7,9,18 allowing researchers, practitioners, and regulators to compare the results of these trials. IBS patients experience multiple symptoms and different patients may not have the same symptoms; additionally, the importance or relative contribution of each symptom, including the most bothersome symptom, to a patient’s overall disease experience differs among patients. The dichotomous AR endpoint reflects, in a single outcome measure, the range of symptoms of the syndrome, and thereby represents a generalized improvement in the individual symptom and impact parameters. Asking the patient if he or she has experienced AR of IBS symptoms allows the patient to integrate all symptoms relevant to that patient and judge the assessment of improvement against that patient’s own reference system.6 If used in combination with individual symptom measures, AR is a useful benchmark in clinical trials to compare across treatments, and may be relevant as a supportive endpoint for regulatory approvals. Moreover, AR has utility in the clinic (in conjunction with individual symptom measures), where more complex responder endpoints (such as the FDA’s newly recommended endpoint and the EMA’s older co-primary endpoints) may not, as AR is easily understood by the IBS patient and easily administered and interpreted by the practitioner.
In conclusion, AR has utility as a measure of clinical meaningfulness and, when AR is used as an anchor to estimate the thresholds for CMC in abdominal and bowel symptoms suffered by IBS-C patients, thresholds correspond well with the levels of improvement required to meet the FDA and EMA endpoints. Each of these endpoints is acceptable for use in clinical trials, but AR may be more useful in the clinical setting.
Acknowledgement
Jacquelyn Cronin of Ironwood Pharmaceuticals provided medical writing support for this manuscript.
Author contribution
Analyses were designed by JEM, VSLW, and LMN. Data were interpreted by MC, AJL, JEM, VSLW, LMN, BJL, RTC, SJS, MGC, CBK, and JMJ. MC and JMJ wrote the initial draft of the manuscript. All authors provided critical review of the manuscript. All authors approved the submitted version of the manuscript.
Funding
These trials were funded by Forest Research Institute and Ironwood Pharmaceuticals. MC’s research in irritable bowel syndrome is supported by DK92179 from the National Institutes of Health.
Conflict of interest
MC and AJL have served as paid consultants to Forest Research Institute and Ironwood Pharmaceuticals. JMJ, BJL, JEM, MGC, and CBK are employees of Ironwood Pharmaceuticals and own stock/stock options in Ironwood Pharmaceuticals. RTC and SJS are employees of Forest Research Institute and own stock/stock options in Forest Research Institute. VSLW and LMN are paid consultants to Forest Research Institute and Ironwood Pharmaceuticals.
References
- 1.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology 2006; 130: 1480–1491. [DOI] [PubMed] [Google Scholar]
- 2.Mayer EA. Clinical practice: Irritable bowel syndrome. New Engl J Med 2008; 358: 1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trentacosti AM, He R, Burke LB, et al. Evolution of clinical trials for irritable bowel syndrome: Issues in end points and study design. Am J Gastroenterol 2010; 105: 731–735. [DOI] [PubMed] [Google Scholar]
- 4.Mangel AW, Hahn BA, Heath AT, et al. Adequate relief as an endpoint in clinical trials in irritable bowel syndrome. J Int Med Res 1998; 26: 76–81. [DOI] [PubMed] [Google Scholar]
- 5.Spiegel B, Camilleri M, Bolus R, et al. Psychometric evaluation of patient-reported outcomes in irritable bowel syndrome randomized controlled trials: A Rome Foundation report. Gastroenterology 2009; 137: 1944–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camilleri M, Mangel AW, Fehnel SE, et al. Primary endpoints for irritable bowel syndrome trials: A review of performance of endpoints. Clin Gastroenterol Hepatol 2007; 5: 534–540. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER). Guidance for industry: Irritable bowel syndrome—clinical evaluation of drugs for treatment, 2012. Available at: http://www.fda.gov/downloads/Drugs/Guidances/UCM205269.pdf.
- 8.Committee for Medicinal Products for Human Use. Guideline on the evaluation of medicinal products for the treatment of irritable bowel syndrome. CPMP/EWP/785/97 2014; Rev. 1: 1–18. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2013/07/WC500146176.pdf.
- 9.Committee for Proprietary Medicinal Products. Points to consider on the evaluation of medicinal products for the treatment of irritable bowel syndrome, 2003. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003187.pdf.
- 10.Bryant AP, Busby RW, Bartolini WP, et al. Linaclotide is a potent and selective guanylate cyclase C agonist that elicits pharmacological effects locally in the gastrointestinal tract. Life Sci 2010; 86: 760–765. [DOI] [PubMed] [Google Scholar]
- 11.Busby RW, Bryant AP, Bartolini WP, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol 2010; 649: 328–335. [DOI] [PubMed] [Google Scholar]
- 12.Eutamene H, Bradesi S, Larauche M, et al. Guanylate cyclase C-mediated antinociceptive effects of linaclotide in rodent models of visceral pain. Neurogastroenterol Motil 2010; 22: 312–322. [DOI] [PubMed] [Google Scholar]
- 13.Andresen V, Camilleri M, Busciglio IA, et al. Effect of 5 days linaclotide on transit and bowel function in females with constipation-predominant irritable bowel syndrome. Gastroenterology 2007; 133: 761–768. [DOI] [PubMed] [Google Scholar]
- 14.Chey WD, Lembo AJ, Lavins BJ, et al. Linaclotide for irritable bowel syndrome with constipation: A 26-week, randomized, double-blind, placebo-controlled trial to evaluate efficacy and safety. Am J Gastroenterol 2012; 107: 1702–1712. [DOI] [PubMed] [Google Scholar]
- 15.Rao S, Lembo AJ, Shiff SJ, et al. A 12-week, randomized, controlled trial with a 4-week randomized withdrawal period to evaluate the efficacy and safety of linaclotide in irritable bowel syndrome with constipation. Am J Gastroenterol 2012; 107: 1714–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates, 1998, 1988, pp. 77–83. [Google Scholar]
- 17.de Vet HC, Terluin B, Knol DL, et al. Three ways to quantify uncertainty in individually applied “minimally important change” values. J Clin Epidemiol 2009; 63: 37–45. [DOI] [PubMed] [Google Scholar]
- 18.Irvine EJ, Whitehead WE, Chey WD, et al. Design of treatment trials for functional gastrointestinal disorders. Gastroenterology 2006; 130: 1538–1551. [DOI] [PubMed] [Google Scholar]
- 19.Mangel AW. Personal view: Adequate relief as a primary endpoint in irritable bowel syndrome. Aliment Pharmacol Ther 2006; 23: 879–881. [DOI] [PubMed] [Google Scholar]
- 20.Lotronex briefing document. Briefing document for the Joint Gastrointestinal Drugs Advisory Committee and Drug Safety and Risk Management Subcommittee. Lotronex (alosetron hydrochloride tablets). FDA online (2002). Available at: http://www.fda.gov/ohrms/dockets/AC/02/briefing/3848B1_01_GSK%20Briefing%20Pkg.pdf (accessed 23 November 2010).
- 21.Mangel AW. Study design issues in irritable bowel syndrome. Aliment Pharmacol Ther 2004; 19: 141–142. [DOI] [PubMed] [Google Scholar]
- 22.Farrar JT, Young JP, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158. [DOI] [PubMed] [Google Scholar]
- 23.Robinson GK. That BLUP is a good thing: The estimation of random effects. Stat Sci 1991; 6: 15–32. [Google Scholar]