Abstract
Background
There is evidence for post-infectious irritable bowel syndrome (PI-IBS) in adults, but little is known about PI-IBS in children. The nationwide representative German Health Interview and Examination Survey for Children and Adolescents (KiGGS) assessed children’s health.
Objective and methods
We identified 643 children (50.1% males) in the KiGGS cohort (N = 15,878, 51% males) with a history of Salmonella infection. The number was validated comparing this group with the known infection statistics from the Robert Koch-Institute registry. We compared this group to the remaining KiGGS cohort (n = 12,951) with respect to sociodemographic characteristics, pain and quality of life. To check for specificity, we repeated the comparisons with a group with a history of scarlet fever.
Results
Infection statistics predicted 504 cases of Salmonella infection in the KiGGS cohort, indicating high validity of the data. In children between 3 and 10 years with a history of Salmonella infection, significantly more abdominal pain (31.7% versus 21.9%, p < 0.001) and headache (27.2% versus 15.1%, p < 0.001) were reported. This group showed lower quality of life (p < 0.001). Comparison to a group of scarlet fever-infected children revealed poor specificity of the data.
Conclusion
Differences found between children with and without Salmonella infection reveal the role of gastrointestinal infection in the development of post-infectious abdominal problems, but poor specificity may point toward a psychosocial (“somatization”) rather than a Salmonella-specific mechanism.
Keywords: Abdominal pain, prevalence, children, salmonella infection, post-infectious irritable bowel syndrome
Introduction
Salmonella infections in children are frequent and substantially more frequent than in adults.1 According to the German registry of infectious disorders collected by the Robert Koch-Institute (Berlin, Germany) (RKI), the annual incidence of Salmonella infections was 400 to 500 cases per 100,000 children at age 1 to 4, but less than 100 cases/100.000 at age 15 or higher in 2001; these numbers decreased to 70–100 and less than 50, respectively, in 2013.1 Accordingly, children have a five-fold higher risk for Salmonella infection as compared to adolescents and adults. However, the long-term consequences of such infections during childhood are unknown owing to missing long-term epidemiological surveys.
A large number of studies have been performed to shed light on the connection between such gastrointestinal (GI) infections in the medical history (Hx) and the development of functional bowel disorders such as irritable bowel syndrome (IBS) later in life.2–4 It is well established that GI infections can lead to chronic functional abdominal complaints.5,6 A causal relationship between a GI infection and IBS was postulated by Chaudhary and Truelove in 1962 for the first time.7 Ever since, many studies have been published that report post-infectious irritable bowel syndrome (PI-IBS) to range between 5% and over 30% of those affected by a bacterial or viral infection during their adult life.8,9 There is increasing evidence for PI-IBS to exist not only in adults but also in children and adolescents. A first report stated a high risk of more than 30% for PI-IBS following infectious enteritis.10 Another paper could substantiate the connection between GI infection and PI-IBS in children but reported a lower incidence of only 10%.11 The authors postulate that risk factors for developing PI-IBS are nearly the same in children and in adults, e.g. female gender and severe GI infection. A recently published paper could underline the role of GI infection in childhood as a risk factor for PI-IBS.12 The authors reported an odds ratio of 1.92 for having IBS after a Salmonella gastroenteritis in childhood. Interestingly Salmonella was a PI-IBS risk factor only in participants exposed in childhood, but not in adulthood. A Chinese study found gastroenteritis in childhood as a risk factor for PI-IBS too; the authors reported an odds ratio of 1.29.13
Except for those four studies,10–13 there is a lack of data to characterize PI-IBS-affected children and to describe similarities and differences between PI-IBS in children and adults.
KiGGS is a nationwide community-based and representative survey carried out in Germany between May 2003 and May 2006. It intended to map health and life circumstances of children and adolescents aged 0 to 17 years living in Germany.14,15 Therefore, the RKI collected information from more than 17,000 children/adolescents and/or their parents.
The present analysis pursues three aims: First we wanted to assess the validity of the KiGGS data with respect to the number of parents reporting Salmonella infections in their children. Second we aimed to compare children with and without Salmonella gastroenteritis in the past to check for a possible association of GI infection and abdominal pain. We furthermore assessed if children and adolescents with and without a Salmonella infection in the past are different concerning sociodemographic characteristics, prevalence of other pain localizations and quality of life. Since in adults it had been shown that patients with PI-IBS report a significant impact of the disease on quality of life,16 we expected differences in health-related quality of life (HR-QoL) following a Salmonella infection during childhood. Third, we compared a scarlet fever-affected group with the whole sample to check for the specificity of our findings in the Salmonella versus non-Salmonella comparison.
Methods
The methodology of the KiGGS survey has been described elsewhere.14 In brief, a two-stage sampling procedure was used for KiGGS. First, 167 representative study locations (sample points) were selected by the Centre for Survey Design and Methodology (Mannheim, Germany). Using local population registries, a random sample of individuals stratified by age and sex were then drawn in proportion to the age and gender structure of Germany’s child population. Altogether 17,641 children and adolescents (8,656 females and 8,985 males) participated in KiGGS.
KiGGS questionnaire
Among others, KiGGS assessed a number of infections in the medical history of each child by asking parents whether their child had suffered from pertussis, measles, mumps, rubella, varicella, scarlet fever, infectious mononucleosis, herpes, salmonellosis, and hepatitis during childhood or adolescence.
A parent questionnaire in children (3 to 10 years, in the following referred to as children) and self-reports in adolescents (11 to 17 years, referred to as adolescents) were used to assess pain.15 Parents of children between 0 and 2 years were not asked questions concerning pain, therefore our analysis included only children and adolescents aged 3 to 17 years (n = 13,240, unweighted). An initial filtering question was posed: Did you/your child suffer from any type of pain during the last three months? Participants with pain only once per three months were considered to be pain free.
If answered with “yes,” the following pain localizations were offered: headache, back pain, ear and eye pain, upper and lower abdominal pain, limb pain (arms and legs), chest pain, sore throat, and toothache.
To assess HR-QoL, the German KINDL-R was used.17 The KINDL-R consists of 24 items covering six dimensions: Physical and emotional well-being, well-being concerning family and friends/peers, well-being at school, and self-esteem. The item scores per dimension were added and transformed into values between 0 and 100, with 100 representing the highest HR-QoL.
Data analysis
KiGGS collected socioeconomic data such as age, gender, place of residency (eastern/western part of Germany), migration background, education, parents’ job position, and net household income.18 A validated weighting factor was developed and used for all analyses of the whole sample to achieve representativeness for the German population. All data reported are weighted.
In a first analysis step we extracted children and adolescents with a history of a Salmonella infection according to their parents’ report from the total sample of children between 3 and 17 years (n = 13,581, weighted). Parents did not indicate at what age this infection might have occurred.
To validate the number of cases of Salmonella infections in the KiGGS cohort concerning a potential reporting bias, we computed the number of Salmonella cases per birth cohort and year in Germany for the entire KiGGS cohort using the RKI database for infectious diseases.1,19 Based on the number of registered childhood Salmonella cases, we projected the number of KiGGS cases with a history of Salmonella infection in our sample.
In a second step we extracted children and adolescents with a history of Salmonella infection and compared them with the group of children and adolescents without Salmonellosis in the past.
To control for the specificity of any findings in step 2, we finally drew a sample of children and adolescents with a history of scarlet fever from the KiGGS cohort and compared this subgroup to the remaining KiGGS cohort.
Chi2-test (Fisher’s exact test for n < 5) and t-test were used to compare the Salmonella group and the scarlet fever group to the KiGGS cohort with respect to pain and HR-QoL. In case of inequality of variance the use of t-tests is not permitted, and Welch tests were performed. The level of significance was set to be α = 0.05. All data were analyzed using SPSS 19.0 (SPSS Inc, Chicago, IL, USA).
Results
Sample validation
In n = 630 children (314 males and 316 females) parents had reported a history of Salmonella infection during childhood. To estimate the accuracy of this number, we used the infection reporting statistics of the RKI1,19 to tabulate the likelihood of Salmonella cases per year for each annual birth cohort, allowing independence of infectious events from year to year. Table 1 shows the example of a single year (2005). Table 2 summarizes the assumed cases for every birth year (1986 to 2005) across the entire KiGGS cohort and for each year separately.
Table 1.
Estimated number of Salmonella cases in the KiGGS cohort for the year 2005
| Age groupa |
Salmonella incidenceb in 2005 |
Birth yearc | KiGGS participantsd |
Estimated KiGGS Salmonella casese |
|||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | ||
| 00..00 | 176 | 181 | 2005 | 155 | 154 | 0.2728 | 0.2784 |
| 01..01 | 349 | 369 | 2004 | 457 | 466 | 1.5949 | 1.7195 |
| 02..02 | 356 | 376 | 2003 | 315 | 305 | 1.1214 | 1.1468 |
| 03..03 | 312 | 311 | 2002 | 479 | 435 | 1.4944 | 1.3528 |
| 04..04 | 277 | 267 | 2001 | 477 | 485 | 1.3212 | 1.2949 |
| 05..05 | 173 | 164 | 2000 | 496 | 507 | 0.8580 | 0.8314 |
| 06..06 | 173 | 164 | 1999 | 464 | 482 | 0.8027 | 0.7904 |
| 07..07 | 173 | 164 | 1998 | 513 | 481 | 0.8874 | 0.7888 |
| 08..08 | 173 | 164 | 1997 | 508 | 512 | 0.8788 | 0.8396 |
| 09..09 | 173 | 164 | 1996 | 545 | 509 | 0.9428 | 0.8298 |
| 10..10 | 100 | 83 | 1995 | 527 | 506 | 0.5270 | 0.4199 |
| 11..11 | 100 | 83 | 1994 | 562 | 486 | 0.5620 | 0.4033 |
| 12..12 | 100 | 83 | 1993 | 508 | 499 | 0.5080 | 0.4141 |
| 13..13 | 100 | 83 | 1992 | 522 | 478 | 0.5220 | 0.3967 |
| 14..14 | 100 | 83 | 1991 | 557 | 478 | 0.5570 | 0.3967 |
| 15..15 | 67 | 64 | 1990 | 503 | 514 | 0.3370 | 0.3289 |
| 16..16 | 67 | 64 | 1989 | 485 | 431 | 0.3249 | 0.2758 |
| 17..17 | 67 | 64 | 1988 | 456 | 446 | 0.3055 | 0.2854 |
| Total number of cases | 13,817 | 13,189 | |||||
Age grouping in 2005. bNumber of Salmonella cases per 100,000 for the year 2005 as reported by the Robert Koch-Institute registry.1 cBirth year of KiGGS participants. dNumber of males in females in the KiGGS cohort. eEstimated Salmonella cases by multiplying the incidence (column 2 or 3) with the number of cases in each birth group (column 5 or 6, respectively). KiGGS: German Health Interview and Examination Survey for Children and Adolescents.
Table 2.
Estimated total number of Salmonella infections for male and female KiGGS participants. Note that the calculation allows (independent) infections to occur every year for each participant
| Infection yeara | Birth yearsb | Estimated total Salmonella casesc |
|
|---|---|---|---|
| Male | Female | ||
| 1986 | 1986 | 0.2958 | 0.2958 |
| 1987 | 1986–1987 | 1.3936 | 1.4304 |
| 1988 | 1986–1988 | 3.3591 | 3.3961 |
| 1989 | 1986–1989 | 5.8683 | 5.6817 |
| 1990 | 1986–1990 | 8.3371 | 7.8721 |
| 1991 | 1998–1991 | 10.498 | 9.7663 |
| 1992 | 1986–1992 | 12.451 | 10.632 |
| 1993 | 1986–1993 | 11.828 | 11.385 |
| 1994 | 1986–1994 | 15.215 | 13.644 |
| 1995 | 1986–1995 | 15.669 | 14.783 |
| 1996 | 1986–1996 | 17.831 | 15.849 |
| 1997 | 1986–1997 | 17.908 | 16.737 |
| 1998 | 1986–1998 | 19.515 | 17.369 |
| 1999 | 1986–1999 | 19.994 | 17.925 |
| 2000 | 1986–2000 | 20.435 | 17.960 |
| 2001 | 1986–2001 | 17.018 | 15.116 |
| 2002 | 1986–2002 | 18.937 | 17.816 |
| 2003 | 1986–2003 | 17.836 | 15.784 |
| 2004 | 1986–2004 | 15.180 | 14.080 |
| 2005 | 1986–2005 | 13.817 | 13.189 |
| Total number of cases | 263.38 | 240.71 | |
Year used from the infection statistics of the Robert Koch-Institute (see text for details). bCumulative birth years used to estimate the likelihood of a Salmonella infection per year. cEstimated Salmonella cases per infection year in male and female KiGGS participants. KiGGS: German Health Interview and Examination Survey for Children and Adolescents.
In comparison to 630 reported by the parents, the RKI statistics predicts a total number of 504 Salmonella infection cases (80%) in the entire KiGGS cohort. These data allow us to conclude that there is no further under-reporting of Salmonella infections beyond the degree that is known for all infections registries, and that the reported Salmonella infection rates represent valid data for further comparison.
Salmonella cohort versus total sample
Comparing the Salmonella sample (N = 630) with the entire KiGGS cohort without infection (N = 12,951), we found both groups to be similar with respect to gender and social class (Table 3). As expected, the proportion of participants with life-time Salmonella infection is significantly increasing with age (r = −0.071; p < 0.01) (Figure 1).
Table 3.
Sociographic characteristics (in %) of children and adolescents with and without a history of Salmonella infection in the KIGGS cohort
| KiGGS participants | With salmonellosis |
Without salmonellosis |
|---|---|---|
| N = 630 | N = 12,951 | |
| Gender (N = 13,581) | ||
| Male | 49.9 | 51.1 |
| Female | 50.1 | 48.9 |
| Age (N = 13,581) | ||
| 3 to 6 years | 11.0 | 25.7 |
| 7 to 10 years | 24.4 | 25.6 |
| 11 to 13 years | 28.0 | 19.4 |
| 14 to 17 years | 36.5 | 29.2 |
| Migration background (N = 13,551) | ||
| Yes | 8.3 | 15.3 |
| No | 91.7 | 84.7 |
| Social class (N = 13,519) | ||
| Lower class | 22.4 | 26.3 |
| Middle class | 50.8 | 45.8 |
| Upper class | 26.7 | 27.8 |
| Place of residence (N = 13,581) | ||
| East Germany | 21.4 | 16.5 |
| West Germany | 78.6 | 83.5 |
KiGGS: German Health Interview and Examination Survey for Children and Adolescents.
Figure 1.
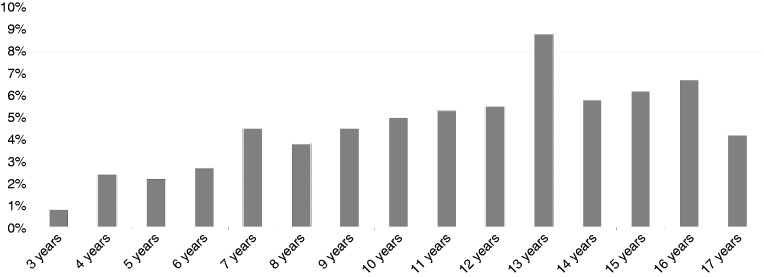
Portion of children and adolescents with a history of Salmonellosis (%) depending on age.
We found Salmonella infection to be twice as common in children and adolescents without (2.6%) than in peers with (5.0%) a migration background (Chi2 = 23.0; p < 0.001). Furthermore, there was a significant difference between participants living in the eastern (6.0%) and the western (4.4%) part of Germany (Chi2 = 10.6; p = 0.001).
Pain after Salmonella infection
Children up to the age of 10 years with a Salmonella infection in the past were significantly more often affected by pain during the last three months than peers without infection: A total of 31.7% of children after Salmonellosis have abdominal pain in contrast to 21.9% in the Salmonella-free group (Chi2 = 11.0; p < 0.001). There was no difference in adolescents. Children with Salmonella infection in the past also showed a significantly higher prevalence of headache (27.2% versus 15.1%; Chi2 = 22.1; p < 0.001), back pain (5.9% versus 2.4%; p = 0.007), lower abdominal pain (5.9% versus 2.1%; p = 0.007), limb pain (arm) (3.2% versus 1.3%; p = 0.037), chest pain (3.7% versus 1.5%; p = 0.029), and sore throat (16.6% versus 9.1%; Chi2 = 12.4; p < 0.001) (Figure 2).
Figure 2.
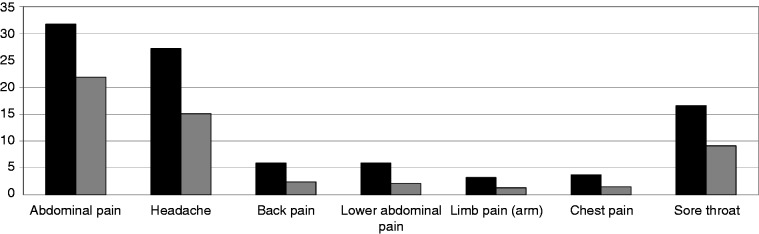
Pain within the last three months in children with (black bars) and without (gray bars) Salmonella infection. Differences are significant either in Chi2 or Fisher’s exact test.
Comparing the Salmonella group to a selected subset of non-infected children matched for age and sex, we found abdominal pain as the most frequent pain localization more commonly in the Salmonella group than in Salmonella-free controls (15,4% versus 9,4%, Chi2 = 4.4; p = 0.035).
HR-QoL after Salmonellosis (KINDL-R)
Asking the parents of all children and adolescents, we found a reduced HR-QoL in children after Salmonella infection concerning physical well-being (75.6 versus 77.6; t = 2.87, p = 0.004), mental well-being (79.7 versus 81.1; t = 2.74, p = 0.006), friends (76.7 versus 78.2; t = 2.73, p = 0.006) and school (75.4 versus 77.4; t = 3.0, p = 0.003). Also the total HR-QoL of life is significantly reduced after Salmonella infection (75.6 versus 77.1; t = 3.8, p < 0.001). For adolescents there were no differences between the Salmonella and non-Salmonella group.
Scarlet fever cohort versus total sample
Pain after scarlet fever
To check our results from the Salmonella versus non-Salmonella comparison, we conducted a similar comparison between a group with a history of scarlet fever (n = 3,350, 51.2% males, age 10.9 ± 4.0 years) and the remaining scarlet fever-free group of the KiGGS sample (n = 9,501, 50.9% males, age 10.0 ± 4.4 years). We found a significant positive association between a history of scarlet fever and abdominal pain (27.4% versus 20.6%; Chi2 = 29.7; p < 0.001), headache (21.7% versus 13.5%; Chi2 = 55.3; p < 0.001), back pain (3.8% versus 2.1%; Chi2 = 11.9; p < 0.001), pain in the eyes (2.3% versus 1.3%; Chi2 = 7.2; p = 0.007), limb pain (legs) (14.9% versus 11.5%; Chi2 = 11.2; p < 0.001), sore throat (12.5% versus 8.3%; Chi2 = 23.0; p < 0.001), and toothache (4.7% versus 2.9%; Chi2 = 11.2; p < 0.001). Asking adolescents concerning scarlet fever and pain, a positive association between these two aspects was found only concerning abdominal pain (23.0% versus 20.5%; Chi2 = 4.2; p = 0.041) and sore throat (13.9% versus 11.9%; Chi2 = 4.1; p = 0.042).
HR-QoL after scarlet fever
In accordance with the Salmonella comparison, parents of children with a history of scarlet fever (aged 3 to 17 years) rated total HR-QoL lower than did parents of uninfected children and adolescents (76.5 versus 77.3; t = 4.2, p < 0.001). This is also reflected in the subdomains physical and mental well-being, self-worth, and family (data not shown).
Discussion
The role of GI (Salmonella) infection in the development of IBS in adults is well established.3,5 Incidence of PI-IBS varies between 4%8 and over 30%9,20 of people with a GI infection. A systematic meta-analysis4 comes to a pooled IBS incidence of 10%. In our own investigation21 we found about 30% of patients suffering from PI-IBS after a confirmed Salmonella or Campylobacter infection, but also found that the incidence of PI-IBS depends on the type of infectious event: It seems to be higher with epidemic events and lower with sporadic and individual infections.22
To the best of our knowledge, only four studies have investigated PI-IBS in children. Thabane et al.11 examined children and adolescents who were affected by a gastroenteritis outbreak due to an E.coli contamination of the water supply in Walkerton, Ontario, Canada, in 2000.23 Children under the age of 16 years at the time of the outbreak were asked about IBS symptoms in 2008: The cumulative incidence of IBS in the exposed group was reported to be 10.5% in comparison to 2.5% in a healthy control sample. Risk factors in children were shown to be similar to those in adults.11 A second study by Saps et al.10 focuses on functional GI disorders (FGID) in 44 children after stool culture-proven Salmonella, Campylobacter, or Shigella infection. A control group included 44 healthy children or children from the same hospital with a non-GI problem. Up to one-third (36%) of infected children and 11% of controls had abdominal pain after at least six months and were classified as having FGID.
Similarly to the results of Saps et al., we found 31.7% of children after Salmonella infection affected by abdominal pain during the last three months. In the non-infected group, the prevalence was significantly lower (21.9%).
Zhu et al.13 found an odds ratio of 1.29 for gastroenteritis in childhood as a risk factor for IBS in children. An even higher odds ratio of 1.92 is reported by Cremon et al.,12 who conducted a prospective controlled study with more than 200 cases of Salmonella infections in childhood and matched controls. Twenty years later they found a significantly higher IBS prevalence in the exposed than in the non-exposed group. Interestingly, individuals who were exposed as adults had no higher IBS prevalence in comparison with controls, which underlines childhood as an especially formative time period.
Children who grew up in the western part of Germany were under-represented in the Salmonella group, as were children with a migration background. We assume a reporting bias in both groups to be responsible for both differences. Different health care and reporting systems were established in the Eastern and the Western parts prior to the reunification of Germany in 1989, and different reporting behavior in countries other than Germany might also be the reason for the under-representation.
In our KiGGS sample not only abdominal pain but also headache, back pain, limb pain (arms and/or legs), and chest pain were significantly more common in children with a Salmonella infection history as compared to their counterparts. While the KiGGS data do not allow concluding on an underlying common cause of all pains, two possible explanations come into consideration: First, it is possible that the multiple pain localizations in children with a history of Salmonellosis point toward a tendency for higher somatization in the Salmonella cohort. Somatization is the tendency to report somatic symptoms in situations with psychological and environmental stress.24 In our sample of adult PI-IBS patients,21 we also found higher somatization scores in patients with moderate or severe symptoms, compared to individuals with no symptom persistence after an infection. Second, it is conceivable that Salmonella infection triggers not only post-infectious abdominal pain, but functional complaints in other bodily parts too. Infectious causes for functional syndromes such as fibromyalgia have been widely debated in the past,25 but the extent of the influence of infections in childhood on functional diseases remains unclear.
In analogy to pain reports, parents of children with a Salmonellosis rated the HR-QoL of their children lower than did parents of children in the control group. Youssef et al.26 and many others16,27 found lower QoL in children with functional abdominal pain than in respective controls. But it has to be kept in mind that the differences that we found in HR-QoL were small and most likely not clinically relevant (e.g. 76.5 versus 77.3 on a scale between 0 and 100).
Specificity
To check our findings from the Salmonella/non-Salmonella comparison concerning specificity, we compared children and adolescents with a history of scarlet fever with a scarlet fever-free sample. Surprisingly, we found similar differences to the Salmonella/non-Salmonella comparison: Children and adolescents with a scarlet fever infection reported not only more abdominal pain but also other pain localizations. This fact points toward unspecific influences of infections to functional complaints later on or to underlying causes for a higher susceptibility to infections on the one hand and pain on the other hand (confounding). Interestingly, both types of infections are associated with a higher prevalence of abdominal pain, which raises the question whether different strains may lead to FGID as discussed in the past.28
It is important to note that in adolescents, both a history of Salmonella and scarlet fever infections appear to not affect HR-QoL.
Limitations
Several limitations of our evaluation of the KiGGS data need to be acknowledged. First, KiGGS does not provide information on bowel habits and their association with abdominal pain that are needed to confirm the diagnosis of IBS or other functional bowel disorders according to the Rome criteria.29 Furthermore, owing to the design of the KiGGS study, it was not possible to exclude children with abdominal complaints prior to the reported Salmonella infection. However, since we know that the majority of all registered Salmonella cases in Germany occur between ages 1 and 4, and our analysis of the KiGGS starts with children age 3, a sequential interpretation of the data—first the infection, then increased pain reports—appears likely. Finally, higher pain prevalence and lower QoL in children with a history of a GI infection do not necessarily imply that both infection and symptoms are directly linked and are causal in nature; they may be attributable to unknown and independent factors not assessed by the KiGGS. These limitations may be overcome only with a prospective cohort study of children experiencing acute bacterial or viral enteritis.
In summary, we found evidence for increased symptoms, especially pain in the abdomen and other body parts, in children with a history of a Salmonella infection. Comparisons with scarlet fever-infected children reveal poor specificity of our data. Further prospective studies are needed to confirm GI infection as a causal factor in the development of FGID in children and adolescents.
Funding
This work was supported by a grant from the Medical Faculty, University of Tuebingen (grant number F1292013).
Conflict of interest
None declared.
References
- 1.Robert Koch-Institute. SurvStat@RKI, http://www3.rki.de/SurvStat (accessed 23 July 2014).
- 2.Borgaonkar MR, Ford DC, Marshall JK, et al. The incidence of irritable bowel syndrome among community subjects with previous acute enteric infection. Dig Dis Sci 2006; 51: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 3.Halvorson HA, Schlett CD, Riddle MS. Postinfectious irritable bowel syndrome—a meta-analysis. Am J Gastroenterol 2006; 101: 1894–1899. [DOI] [PubMed] [Google Scholar]
- 4.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007; 26: 535–544. [DOI] [PubMed] [Google Scholar]
- 5.Barbara G, Cremon C, Pallotti F, et al. Postinfectious irritable bowel syndrome. J Pediatr Gastroenterol Nutr 2009; 48(Suppl 2): S95–S97. [DOI] [PubMed] [Google Scholar]
- 6.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009; 136: 1979–1988. [DOI] [PubMed] [Google Scholar]
- 7.Chaudhary NA, Truelove SC. The irritable colon syndrome. A study of the clinical features, predisposing causes, and prognosis in 130 cases. Q J Med 1962; 31: 307–322. [PubMed] [Google Scholar]
- 8.Ilnyckyj A, Balachandra B, Elliott L, et al. Post-traveler’s diarrhea irritable bowel syndrome: A prospective study. Am J Gastroenterol 2003; 98: 596–599. [DOI] [PubMed] [Google Scholar]
- 9.McKendrick MW, Read NW. Irritable bowel syndrome—post salmonella infection. J Infect 1994; 29: 1–3. [DOI] [PubMed] [Google Scholar]
- 10.Saps M, Pensabene L, Di Martino L, et al. Post-infectious functional gastrointestinal disorders in children. J Pediatr 2008; 152: 812–816, 816.e1. [DOI] [PubMed] [Google Scholar]
- 11.Thabane M, Simunovic M, Akhtar-Danesh N, et al. An outbreak of acute bacterial gastroenteritis is associated with an increased incidence of irritable bowel syndrome in children. Am J Gastroenterol 2010; 105: 933–939. [DOI] [PubMed] [Google Scholar]
- 12.Cremon C, Stanghellini V, Pallotti F, et al. Salmonella gastroenteritis during childhood is a risk factor for irritable bowel syndrome in adulthood. Gastroenterol Nurs 2014; 147: 69–77. [DOI] [PubMed] [Google Scholar]
- 13.Zhu X, Chen W, Zhu X, et al. A cross-sectional study of risk factors for irritable bowel syndrome in children 8–13 years of age in Suzhou, China. Gastroenterol Res Pract 2014; 2014: 198461–198461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurth BM, Kamtsiuris P, Hölling H, et al. The challenge of comprehensively mapping children’s health in a nation-wide health survey: Design of the German KiGGS-Study. BMC Public Health 2008; 8: 196–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellert U, Neuhauser H, Roth-Isigkeit A. Pain in children and adolescents in Germany: The prevalence and usage of medical services. Results of the German Health Interview and Examination Survey for Children and Adolescents (KiGGS) [article in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50: 711–717. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal N, Spiegel BM. The effect of irritable bowel syndrome on health-related quality of life and health care expenditures. Gastroenterol Clin North Am 2011; 40: 11–19. [DOI] [PubMed] [Google Scholar]
- 17.Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: First psychometric and content analytical results. Qual Life Res 1998; 7: 399–407. [DOI] [PubMed] [Google Scholar]
- 18.Lange M, Kamtsiuris P, Lange C, et al. Sociodemographic characteristics in the German Health Interview and Examination Survey for Children and Adolescents (KiGGS)—operationalisation and public health significance, taking as an example the assessment of general state of health [article in German]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2007; 50: 578–589. [DOI] [PubMed] [Google Scholar]
- 19.Faensen D, Claus H, Benzler J, et al. SurvNet@RKI—a multistate electronic reporting system for communicable diseases. Euro Surveill 2006; 11: 100–103. [PubMed] [Google Scholar]
- 20.Marshall JK, Thabane M, Garg AX, et al. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 2010; 59: 605–611. [DOI] [PubMed] [Google Scholar]
- 21.Schwille-Kiuntke J, Enck P, Zendler C, et al. Postinfectious irritable bowel syndrome: Follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil 2011; 23: e479–e488. [DOI] [PubMed] [Google Scholar]
- 22.Schwille-Kiuntke J, Frick JS, Zanger P, et al. Post-infectious irritable bowel syndrome—a review of the literature. Z Gastroenterol 2011; 49: 997–1003. [DOI] [PubMed] [Google Scholar]
- 23.Hrudey SE, Payment P, Huck PM, et al. A fatal waterborne disease epidemic in Walkerton, Ontario: Comparison with other waterborne outbreaks in the developed world. Water Sci Technol 2003; 47: 7–14. [PubMed] [Google Scholar]
- 24.Lipowski ZJ. Somatization: The concept and its clinical application. Am J Psychiatry 1988; 145: 1358–1368. [DOI] [PubMed] [Google Scholar]
- 25.Buskila D, Atzeni F, Sarzi-Puttini P. Etiology of fibromyalgia: The possible role of infection and vaccination. Autoimmun Rev 2008; 8: 41–43. [DOI] [PubMed] [Google Scholar]
- 26.Youssef NN, Murphy TG, Langseder AL, et al. Quality of life for children with functional abdominal pain: A comparison study of patients’ and parents’ perceptions. Pediatrics 2006; 117: 54–59. [DOI] [PubMed] [Google Scholar]
- 27.Simrén M. Quality of life in irritable bowel syndrome: Measurement techniques and relevance of current knowledge. Expert Rev Pharmacoecon Outcomes Res 2003; 3: 75–88. [DOI] [PubMed] [Google Scholar]
- 28.McKeown ES, Parry SD, Stansfield R, et al. Postinfectious irritable bowel syndrome may occur after non-gastrointestinal and intestinal infection. Neurogastroenterol Motil 2006; 18: 839–843. [DOI] [PubMed] [Google Scholar]
- 29.Rasquin A, Di Lorenzo C, Forbes D, et al. Childhood functional gastrointestinal disorders: Child/adolescent. Gastroenterology 2006; 130: 1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]


