Abstract
Objectives
Malignant vascular tumors of the liver are rare. The aim of this study was to investigate the applicability of gray scale and contrast-enhanced ultrasonography in patients with epithelioid hemangioendothelioma (EHE) of the liver and hepatic angiosarcoma (HA) and to describe the clinical presentation.
Methods
We retrospectively analyzed all patients with epithelioid hemangioendothelioma or hemangiosarcoma of the liver from 1998 to 2011, who underwent ultrasound investigation. We describe the findings in gray scale and contrast-enhanced ultrasound and the clinical course of the disease of seven patients with EHE and five patients with HA.
Results
Ultrasound investigation in EHE showed mostly multiple hypoechoic irregular lesions close to the liver capsule and with a halo in some cases. Contrast enhancement revealed inhomogeneously and through all contrast phases vascularized tumors with a rim enhancement in 50%, with or without early wash out. All tumors had avascular parts. HA presented as multiple and irregular hypo-, iso- or hyperechoic lesions. After contrast enhancement, hypervascularization with individual patterns was evident in all patients. Of five, three had liquid parts. Patients with HA were significantly older (58 vs. 37 years, p = 0.014) and presented with lower thrombocyte counts (84 vs. 264, p = 0.0025) and with higher CEA levels (4.6 vs. 1.5, p = 0.03).
Conclusion
EHE and HA are inhomogeneous tumors, explaining the high inter-individual variability and heterogeneity in ultrasound examination. The presence of multifocal lesions, heterogeneity and undefined margins may differentiate EHE or HA from hemangioma. A biopsy is essential in the diagnosis of vascular tumors.
Keywords: Contrast-enhanced ultrasound, epithelioid hemangioendothelioma, hepatic angiosarcoma, liver tumors, vascular tumors
Introduction
Vascular tumors of the liver include hemangioma, epithelioid hemangioendothelioma (EHE) and hepatic angiosarcoma (HA).1 While hemangioma is always benign and HA is a highly malignant tumor, the clinical behavior of EHE is settled in between. Due to a relatively slow growth, 65% of the patients with EHE survive 10 years.2 On the other hand, the course of the disease can be fatal shortly after diagnosis as a consequence of infiltrative growth or bleeding complications. At diagnosis, the majority of patients have a multifocal tumor growth in the liver and more than 35% have extrahepatic manifestations.3 One major differential diagnosis of EHE is HA, accounting for only 0.4–2% of all primary liver tumors4 with an incidence of 0.002–0.013%. The prognosis of HA is poor, with a mean overall survival of 5.5 months. Diagnosis is often delayed, because EHE is rare and the clinical presentation is insidious. Uncertainty concerning the clinical outcome, diagnostic difficulties and the lack of a standard therapy are points of concern.
For both, EHE and HA, no imaging technique has sufficient sensitivity and specificity to be established as a gold standard. While the features of vascular tumors in computed tomography (CT) and magnetic resonance imaging (MRI) images and to a lesser extent in gray scale ultrasound are published in several articles,5–9 much less is known about malignant vascular tumors in contrast-enhanced ultrasound (CEUS).10
Here we present a single-center retrospective study, describing diagnostic signs of gray scale and CEUS for EHE and HA and the clinical course of the disease in seven patients with EHE and five patients with HA.
Patients and methods
We included all patients who fulfilled the following criteria: age of 18 years or more, cytologically or histologically proven EHE of the liver or HA, first diagnosis between January 1998 and December 2011, well-documented ultrasonography investigation. We analyzed clinical, laboratory and histopathological data as well as imaging reports of all patients. Comparisons of characteristics were performed using the t-test. A p-value of <0.05 was considered to be significant. The study was performed according to local laws and regulations and was approved by the local ethics committee.
Ultrasound examination
All B-mode examinations were performed by gastroenterologists highly experienced in ultrasound (DEGUM level II+III [www.degum.de]). Aplio 80 (Toshiba, Japan), Elegra Sonoline Advanced, and Sonoline Antares (Siemens, Germany) using convex arrays C 3–6 MHz and 3,5C40H were applied for ultrasonography in this study. Systematic B-mode, color Doppler and duplex examinations of all abdominal organs were performed. Examination mode and measurements were performed according to the recommendations of the German Association of Ultrasound in Medicine (DEGUM, www.degum.de). The echogenicity of a tumor was defined in comparison with the surrounding liver parenchyma (hypoechoic, isoechoic, hyperechoic and inhomogeneous). Furthermore, the diameter, the shape of the margins (regular/irregular), the presence or absence of a halo, of calcifications or necrotic zones, the localization and the number of tumors were described. After standard scanning, seven patients received low-MI contrast agent (SonoVue®, Bracco, Italy). SonoVue® was applied intravenously through the cubital vein as 2.4 ml bolus injection within 5 s followed by a 10 ml saline flush. In case of large-multinodular or bilobular spread of the tumor, up to a maximum of 2 × 2.4 ml were applied. Each liver lobe was examined separately. The tumors were observed continuously for 5 min after contrast injection. Definition of the vascular phases was based on the guidelines for the use of contrast agents in ultrasound.11
Biopsy procedure and histological examination
Ultrasound-guided percutaneous fine needle aspiration (FNA) biopsy with a 20–22 Gauges (G) needle for cytology and histology was used for puncture to minimize bleeding complications. Smears were prepared, air dried without using spray fixation and stained with Giemsa stain. Histology material was prepared using the cellblock method after formalin fixation as previously described.12 Staining was done with haematoxylin and eosin (HE), PAS, iron, Papanicolaou and silver. Additional samples were prepared for immunohistochemical studies.
Minilaparoscopy was preferred for puncture when FNA was not possible due to ascites. It was performed with a 17 G needle in the endoscopy unit of the Hannover Medical School as described elsewhere.13 CT-guided biopsy was performed with a 16 G needle when FNA was not feasible because of difficult location or overlaying anatomical structures.
Results
Clinical and laboratory data
Seven patients with EHE were included in our study (Table 1) with a mean age of 37 years. Symptoms leading to first presentation were abdominal pain (3/7), jaundice (1/7), weight loss (1/7), malaise (2/7) or elevated liver enzymes (3/7). Three patients were without symptoms at first presentation. Preexisting diagnoses were alcoholic liver cirrhosis in two patients and focal nodular hyperplasia in one patient. One patient was a painter and had had contact with solvents over many years. All patients had intrahepatic (7/7) or extrahepatic (4/7) metastases at diagnosis or during the course of the disease.
Table 1.
Patients’ characteristics
| Pat no. | Sex/ age | Liver disease | Metastases | Symptoms | Biopsies |
|---|---|---|---|---|---|
| Epithelioid hemangioendothelioma | |||||
| 1 | f/40 | no | liver | pain | 2 ×FNA full organ |
| 2 | m/58 | alcoholic liver cirrhosis | liver | no | 1 ×FNA minilap. biopsy |
| 3 | f/36 | no | liver, lungs | no | 2 ×FNA |
| vertebral column | punch biopsy | ||||
| 4 | m/27 | no | liver lung | pain, weight loss, malaise | punch biopsy |
| 5 | m/19 | no | liver | no | punch biopsy full organ |
| 6 | m/42 | alcoholic liver cirrhosis | liver, lung mesenterial | jaundice, malaise | first biopsy: n.a. full organ |
| 7 | f/43 | no | liver, vertebral column | pain | punch biopsy resection |
| Hepatic angiosarcoma | |||||
| 1 | m/73 | no | no | no | 1 ×FNA: EHE, resection: HA |
| 2 | f/47 | no | liver lung retroperitoneal | pain, weight loss, jaundice, malaise | 2 x FNA |
| 3 | m/60 | no | no | weight loss, malaise | 1 x FNA, laparoscopic biopsy |
| 4 | m/49 | no | liver, lung, vertebral column spleen | pain | laparoscopic biopsy |
| 5 | f/60 | alcoholic liver cirrhosis | bone marrow | jaundice, malaise | 1 ×FNA, bone marrow biopsy |
FNA: fine needle aspiration.
In comparison with patients with EHE, patients with HA were significantly older (mean age 57.8, p = 0.014) and more often symptomatic. Patients suffered from pain (2/5), weight loss (2/5), malaise (3/5) or jaundice (1/5). One patient was free of complaints (Table 1). One patient had a positive family history for malignancies; one had worked in a factory handling with PVC. Interestingly, three out of five patients with HA had bleeding complications in the course of the disease, one after laparoscopically performed liver biopsy. One patient had affection of the spleen and developed Kasabach–Merritt syndrome, a serious coagulopathy associated with vascular tumors. All patients with HA had a multifocal intrahepatic tumor growth at diagnosis. In addition, three of the patients had extrahepatic metastases.
In patients with EHE, the laboratory results did not contribute to the diagnosis (Table 2). In patients with HA, biochemical analysis at first presentation revealed anemia, thrombocytopenia, and an elevation of bilirubin, aminotransferases, gamma glutamyl transferase (γ-GT), alkaline phosphatase (AP) and lactate dehydrogenase. The tumor marker CA 19-9 was elevated in two out of three available samples and carcinoembryonic antigen (CEA) was elevated in all available samples (Table 2). Compared with patients with EHE, patients with HA had significantly lower thrombocyte counts (84 vs. 264, p = 0.025) and a significantly higher CEA (4.6 vs. 1.5, p = 0.03).
Table 2.
Laboratory data
| Hemangioendothelioma |
Hepatic angiosarcoma |
||||
|---|---|---|---|---|---|
| Laboratory value. normal value in brackets | Value, (SD) | N | Value, (SD) | N | p (t-test) < 0.05 |
| Hemoglobin (12–16 g/dl) | 13.49 (1.6) | 7 | 10.7 (2.4) | 4 | |
| Leukocytes (4.4–11.3 g/dl) | 7.1 (0.8) | 7 | 6.7 (1.4) | 4 | |
| Thrombocytes (150–450 × 103 per µl) | 264 (97.8) | 7 | 84 (44.5) | 4 | p = 0.0025 |
| AST (<35 U/l) | 43 (36.8) | 7 | 60.4 (264.7) | 5 | |
| ALT (<45 U/l) | 67.6 (66.4) | 7 | 44 (9.9) | 5 | |
| AP (40–129 U/l) | 385.7 (456) | 7 | 183.3 (61.2) | 3 | |
| Bilirubin (<17 mmol/l) | 39 (70.6) | 7 | 117.6 (180.1) | 5 | |
| gGT (<55 U/l) | 249 (301.4) | 7 | 342 (264.7) | 4 | |
| CRP (<8 mg/l) | 50.6 (105.3) | 5 | 29 (22.2) | 4 | |
| LDH (<248 U/l) | 262 (139.7) | 5 | 425 (243.6) | 4 | |
| CA 19-9 (<27 kU/l) | 9 (9.8) | 6 | 111.3 (114.7) | 3 | |
| AFP (<7 µg/l) | 3.3 (1.9) | 7 | 2.6 (2.1) | 3 | |
| CEA (<3 µg/l) | 1.5 (0.5) | 6 | 4.6 (1.2) | 3 | p = 0.03 |
SD: standard deviation; AST: aspartate aminotransferase; ALT: alanine aminotransferase; AP: alkaline phosphatase; gGT: gamma-glutamyltransferase; CRP: C reactive protein; LDH: lactate dehydrogenase; CA 19-9; CA 19-9: carbohydrate antigen 19-9; AFP: alphafetoprotein; CEA: carcinoembryonic antigen
All values are presented as mean.
Radiographic findings
In all patients, CT was performed (Table 3). Three patients with EHE and two patients with HA received MRI. In CT, EHE were hypodense without contrast in all cases, and in one patient a rim enhancement was evident after contrast application. In MRI studies, EHE appeared hypointense on T1 weighted images (T1WI) and hyperintense on T2WI both evaluable cases. The application of contrast medium resulted in a peripheral enhancement.
Table 3.
Ultrasonographic and contrast-enhanced ultrasonographic findings in patients with epithelioid hemangioendothelioma and hepatic hemangiosarcoma
| Ultrasonography |
CEUS |
CT |
MRI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat no | Growth pattern | Largest lesion (mm) | Echogenicity | Halo | Calcification | Ascites | PVT | Enhancement | Wash out | Non- perfused parts | Density | Intensity |
| Epithelioid hemangioendothelioma | ||||||||||||
| 1 | multifocal | 89 | hypoechoic Inhomogeneous | yes | no | no | no | hypoenhanced in all phases, non-perfused areas and peripheral enhancement, | no | yes | hypo | n.a. |
| 2 | multifocal | 23 | hypoechoic | no | no | yes | yes | no enhancement in the arterial phase, slow centripetal fill and isoenhancement in the portal phase | no | yes | hypo | T1:hypo T2:hyper |
| 3 | multifocal | 40 | hypoechoic inhomogeneous | no | no | n.a. | no | n.a. | n.a. | n.a. | hypo | n.a. |
| 4 | multifocal | 60 | hypoechoic/ isoechoic/ inhomogeneous | yes | no | no | no | in the arterial phase inhomogeneous uptake of contrast agent, isoenhanced center of the lesion and avascular periphery, inhomogeneous wash out in the late phase, pooling of contrast agent in some regions. other lesions: avascular in all phases | yes | yes | hypo | n.a. |
| 5 | multifocal | 76 | hypoechoic | yes | no | no | no | n.a. | n.a. | n.a. | hypo | T1:hypo T2:hyper |
| 6 | one | 76 | isoechoic | no | yes | no | no | n.a. | n.a. | n.a. | hypo | n.a. |
| 7 | multifocal | 1a 80 2a 16 | 1a isoechoic/ hyperechoic 2ahypoechoic | 1a yes 2a no | no | no | no | 1a in all phasesisoenhanced lesion with hypoenhanced parts. Two hypoenhanced lesions without fill up 2a peripheral vascularization with single vessels going into the lesion | 1a yes 2a no | 1a yes 2a no | 1ahypo | |
| Hepatic hemangiosarcoma | ||||||||||||
| 1 | multifocal | 110 | hyperechoic/ isoechoic Partly liquid | yes | no | no | no | 1. cotton wool-like hyperenhancement in the arterial phase 2. nodular rim enhancement and centripetal filling | late wash out | yes | hypo | T1:hypo T2:hyper |
| 2 | multifocal | 38 | hyperechoic/ isoechoic, partly liquid | yes | no | yes | no | circular hyperenhancement in the arterial phase | no | yes | n.a. | T1:hypo T2:hyper |
| 3 | multifocal | 11 | hypoechoic | no | no | ? | no | homogenous hyperenhancement in the arterial phase | no | yes | hyper | n.a. |
| 4 | multifocal | 33 | hypoechoic, | no | no | no | no | n.a. | n.a. | n.a. | hypo | n.a. |
| 5 | multifocal | n.a. | hypoechoic partly liquid | no | no | yes | no | n.a. | n.a. | n.a. | n.a. | n.a. |
: 1, before resection and 2, after recurrence of EHE; n.a.: not available; PVT: portal vein thrombosis.
In patients with HA, the findings in CT were natively heterogeneous. Exclusively hyperdense lesions were reported in two patients and hypodense lesions in two patients. In the remaining patient, hyper- as well as hypodense lesions were detected. MRI showed hypointense lesions in T1 and hyperintense lesions in T2 in both patients.
Ultrasonography
Ultrasonography was performed in all patients. CEUS was performed in four patients with EHE and three patients with HA (Table 3, Figures 1 and 2). In gray scale ultrasonography of patients with EHE, hypoechoic lesions were observed in 5/7 and hypoechoic to isoechoic lesions in 2/7. Hypoechoic tumors with a halo were described in 3/7 and calcifications were seen in one. In 3/7, the echotexture of the lesions was inhomogeneous, which was confirmed by CT scan in one patient. Tumors appeared with irregular and not clearly defined margins in 5/7, and only in one patient were the margins well defined and round. Only one patient had a solitary tumor; the other six had disseminated tumors. The diameters ranged from 23 to 89 mm. In 4/7, the tumors were close to the liver capsule. Ascites was found only in one patient with underlying liver cirrhosis and non-malignant portal vein thrombosis. Taken together, EHE presented mostly as multiple hypoechoic or inhomogeneous lesions near the liver capsule, with non-defined margins and eventually with a halo.
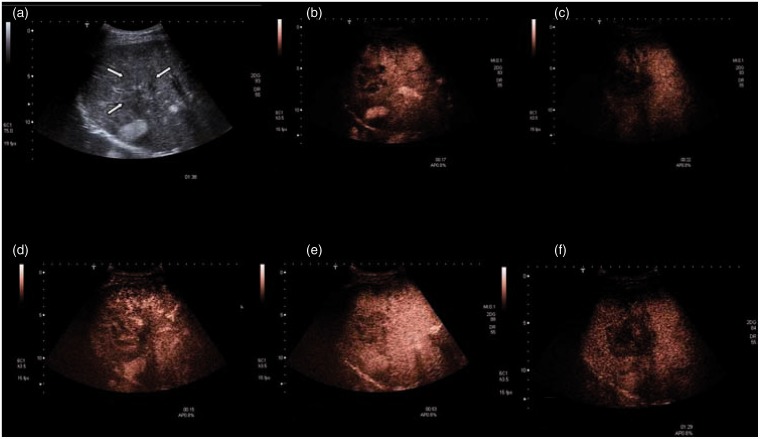
Figure 1.
Gray scale (a) and contrast-enhanced ultrasound (b–f) in a patient with epithelioid hemangioendothelioma (Table 1 + 3, patient no.4). Inhomogenous tumor with multifocal pattern. Margins are hard to define (a). The lesion shows an inhomogenous uptake of contrast agent. A central uptake is detected after 12 s being more evident after 17 s (c). d–e: The same tumor after a second injection (the surrounding tissue still contains contrast agent). Uptake of contrast agent begins in the arterial phase. The contrast agent is kept until end of portal phase (53 s). Only in late phase, we detect a wash out (f, 1:29).
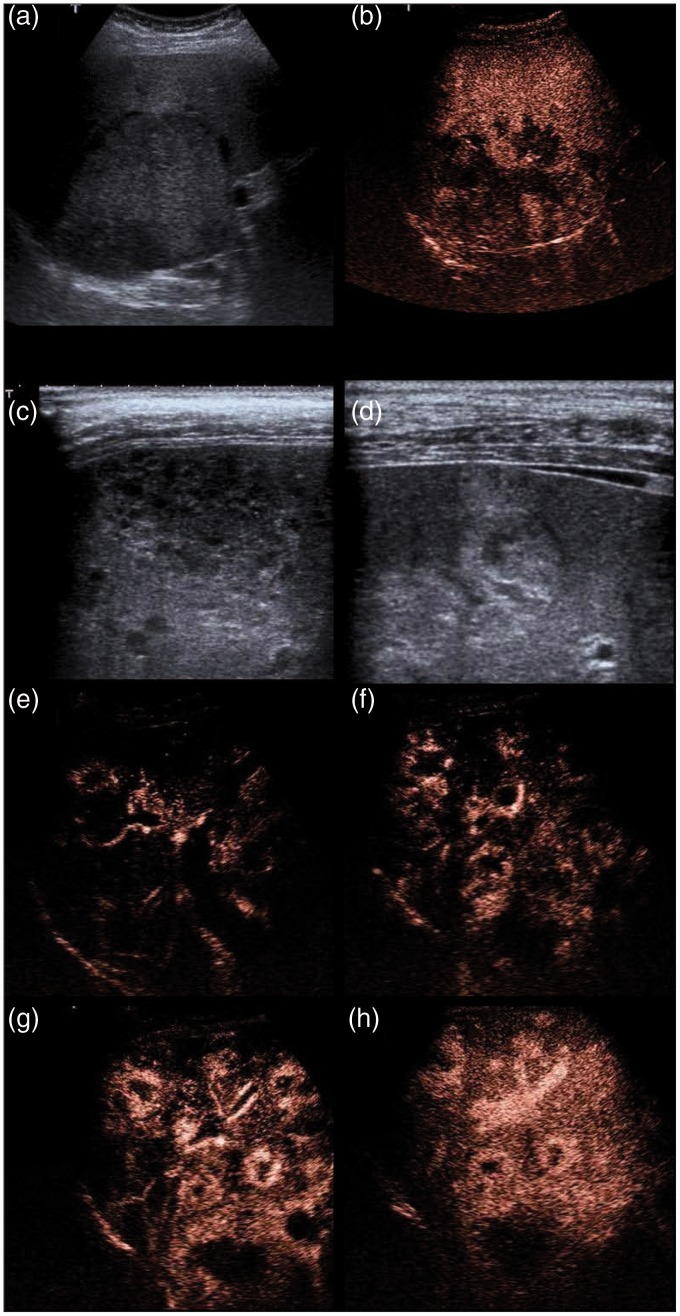
Figure 2.
Gray scale and contrast-enhanced ultrasound in patients with hepatic angiosarcoma.
a–b: (Table 1 + 3 patient 1) A large and inhomogeneous tumor in gray scale (a). In CEUS, a cotton wool-like enhancement pattern in the arterial phase (39 s) is observed.
c–h: (Table 1 + 3 patient no. 2) Multiple nodules presenting hyper- hypo and isoechoic in gray scale (c,d) and with an arterial peripheral enhancement after contrast enhancement (e–h). The central parts remain avascular after 22, 25, 30, 55 s.
CEUS revealed a contrast agent uptake of the tumors from the early arterial phase until the late phase, which reflects the vascular nature of the lesions. In two of the patients, a peripheral enhancement was described. In one patient (Table 3, no.7), the primary lesions were isoenhanced with hypoenhanced parts or hypoenhanced without filling up in later phases. An early washout was seen in two patients. All patients had tumors with avascular parts.
In patients with HA, multiple lesions could be detected ultrasonographically in all patients. The diameters ranged from 11 to 110 mm. Compared with EHE, the diameter was not significantly different (mean diameters 48 mm vs. 64 mm, p = 0.54). The lesions were hypoechoic in three and hyperechoic to isoechoic in two of five patients, respectively. The margins were irregular and not well defined. In 3/5, the tumors contained liquid parts. Two lesions had a halo. Calcifications could not be detected. Two patients had ascites, one with underlying liver cirrhosis. CEUS was performed in three patients with HA and showed an arterial hypervascularization in all patients. The pattern of arterial hypervascularization was cotton wool like, circular or homogenous. We observed a late washout in one patient.
Biopsy accuracy
Diagnosis was established by a histopathologic analysis of a tumor specimen (Figure 3, Table 1). Three patients with EHE and four patients with HA were punctured via FNA. Minilaparoscopy and CT-guided biopsy were performed in one patient with EHE. Four patients with EHE were biopsied via punch biopsy in other institutions. Histological specimens were provided in these patients. Two patients with HA were biopsied laparoscopically. One patient with HA had bleeding complications after laparoscopy. Most patients needed multiple biopsies to validate the diagnosis (Table 1). FNA was inconclusive in all patients. Also, minilaparoscopical and two punch biopsy were not diagnostic. In one patient, diagnosis was confirmed by proof of bone marrow infiltration. Interestingly, in all patients finally diagnosed with HA, the first diagnosis by cytological assessment of the fine needle biopsy was EHE.
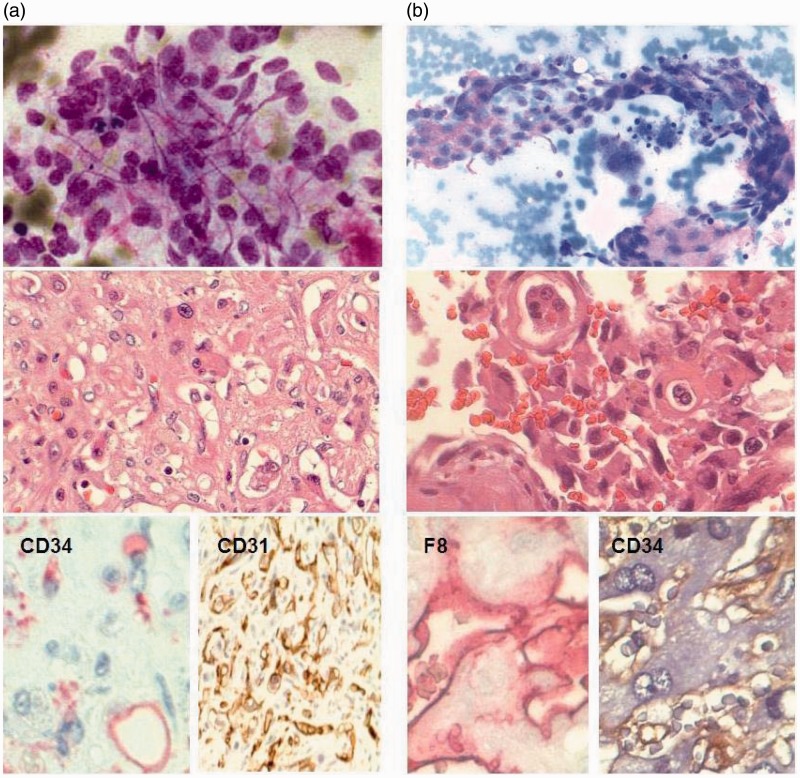
Figure 3.
(a) Cytological and histological staining of epithelioid hemangioendothelioma (Table 1 + 3, patient no.7) and (b) hepatic angiosarcoma (Table 1 + 3, patient no. 1). Papanicolaou staining shows a vascular neoplasm with atypical endothelial cells. H&E staining reveals vacuoles containing erythrocytes and with atypical endothelial cells. Immunohistochemical (IHC) positivity for endothelial markers CD34, CD31 and factor 8-related antigen and negativity for KL1 (not shown) support the diagnosis of a vascular tumor.
Discussion
EHE and HA are rare tumors of the liver. Due to a low incidence of both tumors as well as nonspecific and late onset of symptoms, diagnosis is often late. Optimization of the diagnostic path is of importance to accelerate the diagnostic process, avoid misdiagnoses and apply an appropriate therapy. CEUS is a useful tool to characterize vascularization patterns of liver tumors and allows the differentiation of benign from malignant tumors.11,14,15 CEUS has several advantages over MRI and CT, including the possibility of repeated real-time dynamic imaging and visualizing of the individual enhancement characteristics during the different vascular phases independent from a preset timing as in MRI and CT. Furthermore, echo contrast agent is a sole blood pool agent and, in contrast to MRI and CT, no diffusion is observed. In addition, CEUS has an excellent risk profile and can be applied safely in patients with renal insufficiency.
In the investigated cohort, EHE presented mostly as multiple hypoechoic or inhomogeneous lesions close to the liver capsule with irregular margins and occasionally with a halo (B-mode). These results reflect the findings of other groups.3,5,8–10 CEUS showed a peripheral enhancement in half of the patients. The detected avascular parts reflect necrotic, hemorrhagic or scarred zones within the tumor. A washout as characteristic sign for malignant tumor11 was only seen in two patients with EHE. Thus, after contrast enhancing, EHE may present in various ways sonographically. Ultrasonographic investigation of EHE shows common features of a malignant tumor in gray scale (irregular, not well-defined margins, heterogeneity) without necessarily showing typical features of a malignant tumor after contrast enhancement. Therefore, differentiation between EHE and hemangioma may be challenging. While a peripheral enhancement with a centripetal filling may be observed in both, avascular parts and heterogeneity are one hallmark of EHE but are not typically seen in hemangioma, except in large or giant hemangioma. A hypoenhanced center of the lesion in the late phase has to be interpreted with caution, as it can be observed in ∼20% of hemangiomas.14 Even a wash out is occasionally observed in hemangiomas and can result in misdiagnosing a malignant tumor.16 To differentiate EHE from hemangioma, these findings in gray scale ultrasound need to be considered: hemangioma mostly present as hyperechoic lesions with clear margins, while EHE are mostly hypoechoic or heterogeneous lesions with undefined margins. Furthermore, EHE typically presents as multiple nodules. However, hemangiomas also may appear hypoechoic and multiple, making the differentiation difficult.
HA were multifocal hypo- or hyperechoic lesions with or without a halo and contained liquid parts in three patients. In CEUS, all HA were hyperenhanced but with individual patterns. CEUS revealed a chaotic pattern of vascularization which is very suspicious. A washout was evident in only one patient. These data partly confirm the findings of Trojan et al.10 They found that HA can appear hypo-, iso- or hypoechoic or inhomogeneous with cystic areas. Consistent with our findings, HA showed a peripheral nodular or rim enhancement without centripetal filling and a reticular or chaotic pattern of arterial enhancement in this study. In contrast to our study, the tumors were hypoenhanced in the late phase.10
The inhomogeneity of malignant vascular tumors and the presence of necrotic zones might be responsible for the high rate of inconclusive histological/cytological results. FNA was used to assess these vascular tumors because of the low risk for bleeding complications and inducing metastases in comparison with punch biopsy. However, in contrast to punch biopsies, the first puncture was inconclusive in all patients and re-punctures were necessary. In all patients with HA punctured with fine needle, EHE was suspected cytologically, emphasizing the similarity of both entities. These data confirm the findings of others, who report the difficulties in diagnosing EHE via FNA.17 Minilaparoscopic biopsy is another low-risk and low-invasive method to puncture suspicious lesions, for example in patients with ascites, but this method also failed to diagnose EHE in our patient. Independently of the method used, it is very important that the cytopathologist is aware of the suspicion of a vascular tumor. Otherwise, diagnosis may be missed very easily.
For daily practice, our results indicate that clinical findings such as age, laboratory findings and presence or absence of B symptoms can help to discriminate between benign or malignant tumors and between EHE and HA. In line with that, patients with HA were significantly older than patients with EHE and had more laboratory changes, including thrombocytopenia and elevated CEA.
Taken together, our study summarizes the clinical and ultrasound findings in patients with EHE and HA. However, the heterogeneity of HA and EHE does not allow a definite diagnosis either by CEUS or by CT or MRI, which makes a biopsy obligatory for correct diagnosis. In particular, it can be challenging to differentiate EHE or HA from hemangioma.6 Our findings suggest that the presence of multifocal lesions, heterogeneity and undefined margins may be indicative of a malignant tumor. To our best knowledge, our case series is the largest cohort of CEUS in EHE and HA, and it may contribute to establish the ultrasonographic characteristics of malignant vascular tumors.
Acknowledgement
Nora Schweitzer was a Gerok stipend of the German Research Foundation (SFB/TRR77).
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Fletcher CUKMF. World Health Organisation classification of tumours. Pathology and genetics of tumours of soft tissue and bone, Lyon: IARC Press, 2002. [Google Scholar]
- 2.Kanel GC, Korula J. Atlas of Liver Pathology, 2nd ed Philadelphia: Elsevier Saunders, 2005. [Google Scholar]
- 3.Mehrabi A, Kashfi A, Fonouni H, et al. Primary malignant hepatic epithelioid hemangioendothelioma: A comprehensive review of the literature with emphasis on the surgical therapy. Cancer 2006; 107: 2108–2121. [DOI] [PubMed] [Google Scholar]
- 4.Bioulac-Sage P, Laumonier H, Laurent C, et al. Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis 2008; 28: 302–314. [DOI] [PubMed] [Google Scholar]
- 5.Lyburn ID, Torreggiani WC, Harris AC, et al. Hepatic epithelioid hemangioendothelioma: Sonographic, CT, and MR imaging appearances. Am J Roentgenol 2003; 180: 1359–1364. [DOI] [PubMed] [Google Scholar]
- 6.Buetow PC, Buck JL, Ros PR, et al. Malignant vascular tumors of the liver: radiologic-pathologic correlation. Radiographics 1994; 14: 153–166. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Yu RS, Qiu LL, et al. Contrast-enhanced multiple-phase imaging features in hepatic epithelioid hemangioendothelioma. World J Gastroenterol 2011; 17: 3544–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruegel M, Muenzel D, Waldt S, et al. Hepatic epithelioid hemangioendothelioma: Findings at CT and MRI including preliminary observations at diffusion-weighted echo-planar imaging. Abdom Imaging 2011; 36: 415–424. [DOI] [PubMed] [Google Scholar]
- 9.Lin J, Ji Y. CT and MRI diagnosis of hepatic epithelioid hemangioendothelioma. Hepatobiliary Pancreat Dis Int 2010; 9: 154–158. [PubMed] [Google Scholar]
- 10.Trojan J, Hammerstingl R, Engels K, et al. Contrast-enhanced ultrasound in the diagnosis of malignant mesenchymal liver tumors. J Clin Ultrasound 2010; 38: 227–231. [DOI] [PubMed] [Google Scholar]
- 11.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for Contrast Enhanced Ultrasound (CEUS) in the liver – update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultrasound Med Biol 2013; 39: 187–210. [DOI] [PubMed] [Google Scholar]
- 12.Boozari B, Soudah B, Rifai K, et al. Grading of hypervascular hepatocellular carcinoma using late phase of contrast enhanced sonography – a prospective study. Dig Liver Dis 2011; 43: 484–490. [DOI] [PubMed] [Google Scholar]
- 13.Beckmann MG, Bahr MJ, Hadem J, et al. Clinical relevance of transjugular liver biopsy in comparison with percutaneous and laparoscopic liver biopsy. Gastroenterol Res Pract 2009; 2009: 947014–947014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strobel D, Seitz K, Blank W, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions – diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med 2008; 29: 499–505. [DOI] [PubMed] [Google Scholar]
- 15.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver – update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013; 34: 11–29. [DOI] [PubMed] [Google Scholar]
- 16.Bernatik T, Seitz K, Blank W, et al. Unclear focal liver lesions in contrast-enhanced ultrasonography – lessons to be learned from the DEGUM multicenter study for the characterization of liver tumors. Ultraschall Med 2010; 31: 577–581. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee S, Mallick J, Pal PC, et al. Hemangioendothelioma of soft tissue: Cytological dilemma in two cases at unusual sites. J Cytol 2012; 29: 89–91. [DOI] [PMC free article] [PubMed] [Google Scholar]