Abstract
Background
Ghrelin is a peptide hormone that is involved in gastrointestinal motility and secretion; and therefore, may play a role in functional dyspepsia.
Objectives
To compare the change of serum ghrelin level in relation to meal-time, between patients with functional dyspepsia and a control group.
Materials and methods
In a cross-sectional study, 18 subjects with functional dyspepsia according to the Rome III criteria were enrolled in our study. Blood samples were collected five times: 30 minutes (min) before a standard breakfast; at the time as serving breakfast; and 30, 60 and 90 min after breakfast. Serum ghrelin concentration was measured in these patients and compared with eight normal individuals, as controls.
Results
The serum ghrelin level 30 minutes after breakfast was significantly higher in dyspepsia patients, compared to controls (751 ± 171.84 pg/ml versus 576.9 ± 195.62 pg/ml, p = 0.033). Although patients had a higher mean serum ghrelin level 30 minutes before, exactly at the time of serving breakfast and 60 min after breakfast there was no statistically significant difference between patients and controls. The shape of the curve was also different between the two groups, from 30 min until 90 min after breakfast, which is the time that most dyspeptic symptoms usually occur, although this difference was not significant (p > 0.05).
Conclusion
The significantly different ghrelin levels between dyspeptic patients and the normal population showed that ghrelin may have an important role in inducing symptoms, in functional dyspeptic patients.
Keywords: Dyspepsia, ghrelin, functional dyspepsia, mealtime, pre-prandial period, post-prandial period
Introduction
Dyspepsia is defined as a heterogenous group of symptoms with several underlying causes.1 It is divided into three subgroups: ulcer-like dyspepsia, dysmotility-like dyspepsia and the non-ulcerative ‘functional dyspepsia’ (FD).1 Dyspepsia affects 25–40% of the population in western countries, in a given year.2
The most common type of dyspepsia is FD. The diagnosis of FD implies that a complete diagnostic evaluation has been performed and any obvious structural gastrointestinal disease has been excluded.2 The Rome III criteria describe FD as having one or more of the following symptoms: postprandial fullness, early satiety and epigastric pain or burning.3 These patients have impaired accommodation, delayed gastric emptying and are hypersensitive. There are also two subcategories, named epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS). EPS is mainly characterized by intermittent epigatsric burning or pain, while PDS is characterized by bothersome post-prandial fullness and early satiation.4 Although the pathogenesis of FD has not been completely clarified, neuro-hormonal factors might play a role in its pathogenesis.5
Ghrelin, a 28-amino acid peptide that was discovered by Kojima et al.6 in 1999 in the rat stomach, is identified as the long-awaited endogenous ligand of the growth hormone secretagogue receptor.7 It is synthesized primarily by the X-A-like cells of oxynthic glands of the gastric mucosa, mostly in the corpus and fundus and in a smaller amount in the hypothalamus, and several other organs.8 Ghrelin is activated after the linking of octanoic acid to serine by the enzyme ghrelin o-acyltransferase (GOAT).8
Ghrelin is a multifunctional hormone and its role is beyond just stimulation of growth hormone in the pituitary. On the one hand, it is a hunger hormone that can increase appetite, food intake and produce weight gain9; and therefore, the level of ghrelin is higher during periods of fasting, starvation and the pre-prandial period and it is lower after eating or the postprandial period, during hyperglycemia and in obesity.10 On the other hand, ghrelin affects gastric motility by the vagal cholinergic pathway modulators and gastric acid secretion. Nitric oxide may play a role as a mediator for ghrelin secretion.11,12 Therefore, the contradictions of stimulating gastric motility, gastric emptying and stimulation to increase appetite need to be clarified.
As ghrelin affects gastric motility, emptying and secretion; this peptide may play a pathophysiological role in FD.13,14 The purpose of the present study was to compare serum ghrelin levels between patients with FD and a control group and to investigate the possible role of ghrelin as a peptide hormone in the induction of FD.
Methods and materials
Patients
Subjects with FD (diagnosed according to the Rome III criteria) were included in this study. All patients were clinically stable at the time of evaluation. A set of routine investigations, including a complete history and physical examination, the checking of fasting blood sugar (FBS), thyroid stimulating hormone (TSH) level, a complete blood count (CBC), liver function test (LFT) and a stool exam was done for every patient. An upper gastrointestinal endoscopy was performed for each patient, within 6 months prior to the inclusion day. Only subjects with normal results on these laboratory evaluations and with normal endoscopy results were enrolled in this study. If any subject had a positive Helicobacter pylori (H. pylori) infection in the endoscopic biopsy specimen, eradication therapy and confirmation of eradication with the urea breathing test was done more than 4 weeks before their enrollment in our study.
Exclusion criteria were:
Presence of organic gastrointestinal disorders, including peptic ulcer diseases, erosive gastritis, erosive reflux esophagitis;
Presence of H. pylori infection at the time of the study;
Presence of pre-malignant and malignant lesions, atrophic changes or eosinophilic gastro-duodenitis in the biopsy;
Surgical operation on gastrointestinal tract;
Atypical habitual vomiting, as with anorexia nervosa and bulimia nervosa;
Pregnancy and lactation;
Orthostatic hypotension;
Any drug consumption within 3 weeks of enrollment;
Present or past history of endocrine diseases;
Cardiac diseases;
Psychiatric diseases;
Systemic autoimmune diseases;
Body mass index (BMI) over 30 kg/m2 and below 16.5 kg/m2;
Use of tobacco products and alcohol consumption;
Age over 50 years or below 18 years old;
Symptoms compatible with irritable bowel syndrome.
Ethics and consent
Prior approval for the experimental procedure was obtained from the ethical committee of Shiraz University of Medical Sciences, Shiraz, Iran. A written informed consent, including all steps of the study, plus its possible risks and benefits were signed by all the volunteer patients, after careful discussion. The study protocol was carried out in accordance with the Helsinki Declaration, as revised in Edinbrough, Scotland in 2000.
Study design
Subjects were admitted to the endoscopy ward affiliated with Shiraz University of Medical Sciences, Shiraz, Iran. The volunteers were asked to fast from the night before the study. Then, they were served a 375 Kcal meal containing 58 gr carbohydrate, 15 gr protein and 9 gr of fat (Table 1) as a breakfast. Vital signs (blood pressure (BP), pulse rate and temperature) were measured during the study period, every 2 hours.
Table 1.
Standard 375 Kcal breakfast: 58 gr carbohydrate, 15 gr protein and 9 gr fat in total
| Food material | Fat (gr) | Protein (gr) | Carbohydrate (gr) | Energy (Kcal) |
|---|---|---|---|---|
| Bread (90 gr) | 3.600 | 8.280 | 42.48 | 243 |
| Boiled egg (50 gr) | 5.295 | 6.285 | 0.559 | 77.550 |
| Tomato (90 gr) | 0.330 | 0.851 | 4.615 | 21.000 |
| Sugar (10 gr) | 0.000 | 0.000 | 10.000 | 38.650 |
| Lemon juice (10 gr) | 0.010 | 0.043 | 0.902 | 2.699 |
| Total | 9.235 | 15.459 | 58.556 | 373.899 |
gr: grams; Kcal: kilocalories.
An intravenous cannula was placed in an ante-cubital vein for blood withdrawal and peripheral blood samples (4 ml) were collected five times: 30 min before breakfast, at the time of serving breakfast and at 30, 60 and 90 min after breakfast. All blood samples were transferred to the Endocrine and Metabolism Research Center of Shiraz University of Medical Sciences in a chilled glass tube and then centrifuged immediately at 4℃. The serum samples were frozen and stored at −20℃. We added 1 mM of p-hydroxymercuribenzoic acid in the final sample volume of the tubes, to prevent the degradation of ghrelin by protease.
Quantitative determination of total ghrelin in serum was measured by a radioimmunoassay (RIA) method with a commercially available kit (DRG Instruments GmbH, Germany). This DRG ghrelin RIA lab kit utilizes an antibody that is specific for total ghrelin and does not require the presence of the octonyl group on serine 3. A sensitivity of 93 pg/ml can easily be achieved when using a sample of 100 µl serum or plasma in a 2-day disequilibrium assay (400 µl total volume). This kit contains rabbit anti-ghrelin serum in assay buffer, iodine-125 labeled ghrelin that is high performance liquid chromatography (HPLC)-purified, and a goat anti-rabbit IgG serum as the precipitating reagent.
Statistical analysis
The statistical analysis was performed with SPSS version 18. All data were presented as the mean ± SD. The value of p < 0.05 was considered statistically significant. Data were analyzed using the Chi-square test, independent t-test, non-parametric Mann-Whitney test and the Kendall tau test, when appropriate. The power of this study was 95%.
Results
We enrolled 18 patients (15 women and 3 men) with FD according to the inclusion and exclusion criteria into our study. The mean patient age was 33.39 ± 9.84 years. Eight healthy volunteer subjects (five men and three women) with a mean age of 24.75 ± 1.282 years also enrolled in this study, as a control population. There were no significant difference between vital signs in the controls and patients during study. Mean BMI was 22.9 ± 3.4 kg/m2 in the case group and 21.3 ± 4 kg/m2 in the control group. There were no statistically significant differences between patients and controls regarding gender (p = 0.06) nor BMI (p = 0.549).
Serum ghrelin level in our study patients and controls were checked five times: 30 min before breakfast; at the time of serving breakfast; and 30, 60 and 90 min after breakfast. Patients had a higher level of mean serum ghrelin at all five sampling times compared to the controls, but the difference was statistically significant only at the period 30 min after breakfast (751 ± 171.84 pg/ml versus 576.9 ± 195.62 pg/ml, p = 0.033) (Table 2).
Table 2.
Mean serum ghrelin level (pg/ml) in patients and control group 30 min before, at the time of serving breakfast, 30 min, 60 min and 90 min after breakfast.
| Time relative to meal | Serum ghrelin (pg/ml) in patients (n = 18) | Serum ghrelin (pg/ml) in controls (n = 8) | p-value |
|---|---|---|---|
| 90 min after breakfast | 793.81 ± 159.21 | 757.71 ± 100.55 | 0.947 |
| 60 min after breakfast | 810.50 ± 202.12 | 654.71 ± 173.97 | 0.078 |
| 30 min after breakfast | 751.42 ± 171.84 | 576.9 ± 195.62 | 0.033 |
| At the time of serving breakfast | 790 ± 222.69 | 674.71 ± 143.39 | 0.241 |
| 30 min before breakfast | 887.75 ± 295.26 | 770.43 ± 130.14 | 0.293 |
min: minutes; ml: milliliter; pg: picograms.
The shape of the ‘serum ghrelin level’ curve over time was quite different in the patients with FD, compared to the control group (Figure 1). While in both groups the initial phase (−30 min till 0) was characterized by a sharp decrease, the control group continued to have the same slope till 30 min after the meal, from which point a sharp and steady rise in the ghrelin level occurred. Meanwhile, in the patient group the slope decreased after the meal time and the levels never reached the nadir for the control group. The serum ghrelin levels continued to be higher in the FD case group for the rest of the measurements, although the differences were not statistically significant (p > 0.05).
Figure 1.
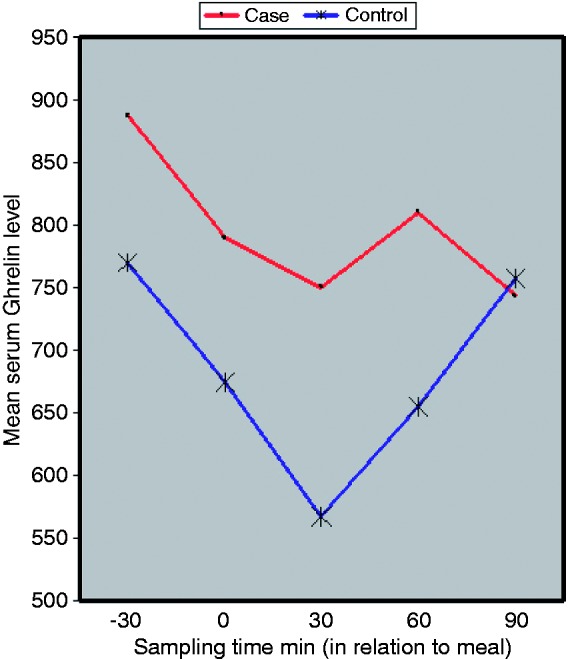
Serum ghrelin level in the FD case group and the study control group.
FD: functional dyspepsia; min: minutes.
Discussion
The changing serum ghrelin level in patients with FD is still a controversial topic. While total serum ghrelin is reported to be higher in patients with FD in some studies,15 other studies show lower levels of serum ghrelin, as compared to healthy volunteers.16 Furthermore, the pathogenic role of these alterations is still unclear. On the other hand, some studies fail to demonstrate any association in terms of fasting and post-prandial serum ghrelin between patients with FD and healthy subjects.17
As outlined in our results, the main finding of the present study was the different pattern of changing serum ghrelin levels between patients with FD and normal subjects, from 30 min before till 90 min after meal time. Although mean serum ghrelin levels between the two groups showed statistically significant differences only at 30 min after the meal, the shape of the curve is also different between the two groups, from 30 till 90 min after breakfast. This is exactly the time that most of the dyspeptic symptoms usually occur in patients affected with FD, as reported in previous studies.18 This observation supports a possible role for ghrelin in the etiology of the dyspeptic symptoms in these patients. The possible effect of ghrelin on dyspepsia could be due to the structural resemblance of ghrelin to motilin, and co-secretion of these two hormones, which may lead to concerted action and interference with gastric motility.19,20Another possibility is the stimulation of gastric acid secretion by ghrelin21 via a vagus-dependent cholinergic pathway, which may potentially induce ‘ulcer-like’ symptoms.
The highest level of serum ghrelin in both groups was at 30 min before meal time. Afterwards, the serum level of ghrelin started to decrease from meal time, and continue to fall, until it reached the through level at 30 min after breakfast. This finding is in contrast with other studies, in which the through level happened 1 hour after food was consumed.22,23 This may be due to presence of other physical or chemical stimuli other than direct contact of food materials with the gastric mucosa.24 Furthermore, ghrelin secretion is also dependent upon other hormones, like insulin, somatostatin and leptin.25,26 Measuring the serum levels of these hormones in relation to changes in serum ghrelin may better clarify our findings and should be a subject for future studies.
The serum ghrelin level then continuously increased in the normal subjects, to reach its pot-meal peak at 90 min after breakfast; compared to the dyspeptic patients, in whom the serum ghrelin level rose until 60 min after meal time, and then fell sharply to its through level. As can be seen in Figure 1, the serum ghrelin level in dyspeptic patients was always greater than in normal subjects, although it failed to reach statistical significance, except for the point 30 min after the meal.
In terms of gender, Krystyna Stec-Michalska et al.27 clearly demonstrated that in the case of gastric ghrelin, the hormone level was much higher in premenopausal women than in men,27 but some other authors claim that it is similar for both genders. Similar to previous studies,27 the difference seen in serum ghrelin level between female and male subjects was not statistically significant (p = 0.058).
Although the age difference between patients and controls was significant in this study (p = 0.002), it is unlikely that this difference explains the differences in ghrelin level between these two groups, as previous studies show that the ghrelin level does not vary from childhood to adulthood, nor undergo an age-related decrease.27
H. pylori infection is also suggested as one of the contributing factors in patients with FD. A recent review article suggests that H. pylori eradication is accompanied with significant improvement in symptoms of patients with FD.28 On the other hand, H. pylori infection is reported to be associated with changes in serum ghrelin levels, both in humans and in animals.29,30 Therefore, a pathogenic role for H. pylori in FD may be secondary to alterations in serum ghrelin levels.
Although the validity of this observation is limited because of our small sample size, it was the first study to claim a potential association between serum ghrelin level and dyspepsia symptoms. It should be noted that if ghrelin proved to be a dyspeptic modulator, the medical implications would be considerable.
In conclusion, our small study showed considerable, if not always significant, differences in the serum ghrelin levels between normal control subjects and dyspeptic patients. This potential relationship should be explored more, in larger studies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare there is no conflict of interest.
References
- 1.Jo S, Zanten VV, Flook N, et al. An evidence-based approach to the management of uninvestigated dyspepsia in the era of Helicobacter pylori. CMAJ 2000; 162: S3–S22. [PMC free article] [PubMed] [Google Scholar]
- 2.Talley NJ. What the physician needs to know for correct management of gastro-oesophageal reflux disease and dyspepsia. Aliment Pharmacol Ther 2004; 20: S23–S30. [DOI] [PubMed] [Google Scholar]
- 3.Vakil N, Halling K, Ohlsson L, et al. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol 2013; 108: 767–774. [DOI] [PubMed] [Google Scholar]
- 4.Tack J, Talley NJ. Functional dyspepsia: Symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol 2013; 10: 134–141. [DOI] [PubMed] [Google Scholar]
- 5.Montalto M, Santoro L, Vastola M, et al. Functional dyspepsia: Definition, classification, clinical and therapeutic management. Ann Ital Med Int 2004; 19: 84–89. [PubMed] [Google Scholar]
- 6.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth hormone releasing acylated peptide from the stomach. Nature 1999; 402: 656–660. [DOI] [PubMed] [Google Scholar]
- 7.Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev 2005; 85: 495–522. [DOI] [PubMed] [Google Scholar]
- 8.Van der Lely AJ, Tschop M, Heiman ML, et al. Biological, physiological, pathophysiological and pharmacological aspects of ghrelin. Endocr Rev 2004; 25: 426–457. [DOI] [PubMed] [Google Scholar]
- 9.Asakawa A, Inui T, Kaga H, et al. Ghrelin is an appetite-stimulatory signal from the stomach with structural resemblance to motilin. Gastroenterology 2001; 120: 337e45–337e45. [DOI] [PubMed] [Google Scholar]
- 10.Ukkola O, Ravussin E, Jacobson P, et al. Role of ghrelin polymorphisms in obesity based on three different studies. Obes Res 2002; 10: 782–791. [DOI] [PubMed] [Google Scholar]
- 11.Ohno T, Mochiki E, Kuwano H. The roles of motilin and ghrelin in gastrointestinal motility. Int J Pept 2010; 2010: e820794–e820794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bilgin HM, Tumer C, Diken H, et al. Role of ghrelin in the regulation of gastric acid secretion involving nitregic mechanisms in rats. Physiol Res 2008; 57: 563–568. [DOI] [PubMed] [Google Scholar]
- 13.Akamizu T, Iwakura H, Ariyasu H, et al. Ghrelin and functional dyspepsia. Int J Pept 2010; 2010: e548457–e548457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takamori K, Mizuta Y, Takeshima F, et al. Relation among plasma ghrelin level, gastric emptying and psychologic condition in patients with functional dyspepsia. J Clin Gastroenterol 2007; 41: 477–483. [DOI] [PubMed] [Google Scholar]
- 15.Lanzini A, Magni P, Petroni ML, et al. Circulating ghrelin level is increased in coeliac disease as in functional dyspepsia and reverts to normal during gluten-free diet. Aliment Pharmacol Ther 2006; 23: 907–913. [DOI] [PubMed] [Google Scholar]
- 16.Lee KJ, Cha DY, Cheon SJ, et al. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion 2009; 80: 58–63. [DOI] [PubMed] [Google Scholar]
- 17.Kim YS, Lee JS, Lee TH, et al. Plasma levels of acylated ghrelin in patients with functional dyspepsia. World J Gastroenterol 2012; 18: 2231–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology 2006; 130: 1466–1479. [DOI] [PubMed] [Google Scholar]
- 19.Ogiso K, Asakawa A, Amitani H, et al. Ghrelin: A gut hormonal basis of motility regulation and functional dyspepsia. J Gastroenterol Hepatol 2011; 26: S67–S72. [DOI] [PubMed] [Google Scholar]
- 20.Wierup N, Björkqvist M, Weström B, et al. Ghrelin and motilin are co-secreted from a prominent endocrine cell population in the small intestine. J Clin Endocrinol Metab 2007; 92: 3573–3581. [DOI] [PubMed] [Google Scholar]
- 21.Yakabi K, Kawashima J, Kato S. Ghrelin and gastric acid secretion. World J Gastroenterol 2008; 14: 6334–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natalucci G, Riedl S, Gleiss A, et al. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: Maintenance of a meal-related pattern. Eur J Endocrinol 2005; 152: 845–850. [DOI] [PubMed] [Google Scholar]
- 23.De Smet B, Mitselos A, Depoortere I. Motilin and ghrelin as prokinetic drug targets. Pharmacol Ther 2009; 123: 207–223. [DOI] [PubMed] [Google Scholar]
- 24.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000; 141: 4255–4261. [DOI] [PubMed] [Google Scholar]
- 25.Flanagan DE, Evans ML, Monsod TP, et al. The influence of insulin on circulating ghrelin. Am J Physiol Endocrinol Metab 2003; 284: E313–E316. [DOI] [PubMed] [Google Scholar]
- 26.Shimada M, Date Y, Mondal MS, et al. Somatostatin suppresses ghrelin secretion from the rat stomach. Biochem Biophys Res Comm 2003; 302: 520–525. [DOI] [PubMed] [Google Scholar]
- 27.Stec-Michalska K, Malicki S, Michalski B, et al. Gastric ghrelin in relation to gender, stomach topography and Helicobacter pylori in dyspeptic patients. World J Gastroenterol 2009; 15: 5409–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol 2013; 10: 168–174. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki H, Masaoka T, Hosoda H, et al. Helicobacter pylori infection modifies gastric and plasma ghrelin dynamics in Mongolian gerbils. Gut 2004; 53: 187–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isomoto H, Ueno H, Saenko VA, et al. Impact of Helicobacter pylori infection on gastric and plasma ghrelin dynamics in humans. Am J Gastroenterol 2005; 100: 1711–1720. [DOI] [PubMed] [Google Scholar]


