Abstract
Isoaspartate formation is a common type of protein damage normally kept in check by the repair enzyme protein-L-isoaspartyl methyltransferase (PIMT). Mice with a knockout of the gene (Pcmt1) for this enzyme (KO −/−) exhibit a pronounced neuropathology with fatal epileptic seizures at 30-60 days. HZ (+/−) mice have 50% of the PIMT activity found in WT (+/+) mice, but appear normal. To see if HZ mice exhibit accelerated aging at the molecular level, we compared brain extracts from HZ and WT mice at 8 months and 2 years with regard to (a) PIMT activity, (b) isoaspartate levels, and (c) activity of an endogenous PIMT substrate, creatine kinase B. PIMT activity declined modestly with age in both genotypes. Isoaspartate was significantly higher in HZ than WT mice at eight months and more so at two years, rising 5X faster in HZ males, and 3X faster in females. Creatine kinase activity decreased with age and was always lower in the HZ mice. These findings suggest the individual variation of human PIMT levels may significantly influence the course of age-related CNS dysfunction.
Keywords: Creatine Kinase, Deamidation, Isoaspartate, Methyltransferase, Protein Damage, Protein Repair
1. Introduction
A primary manifestation of cellular aging is the structural and functional degradation of macromolecules, especially DNA and proteins. Among the various damage reactions that affect proteins, the formation of abnormal isoaspartyl (isoAsp) peptide bonds stands out as being extremely common but relatively under explored. IsoAsp formation, through deamidation of asparaginyl residues or isomerization of aspartyl residues, constitutes a large proportion of spontaneous protein damage observed both in vitro and in vivo (Aswad, et al., 2000; Clarke, 2003; Desrosiers and Fanelus, 2011; Shimizu, et al., 2005). Generation of isoAsp sites is initiated by nucleophilic attack on the side-chain carbonyl of aspartate (Asp) or asparagine (Asn) by the C-flanking amide bond nitrogen, resulting in an intermediate succinimide that subsequently hydrolyzes to form a mixture of ca. 15-30% Asp and 70-85% isoAsp peptides (Fig. 1). Protein L-isoaspartate O-methyltransferase (PIMT; EC 2.1.1.77) selectively methylates the α-carboxyl group of L-isoaspartyl residues, and the isoAsp methyl ester formed spontaneously demethylates to reform a succinimide that can restore a normal α-linked Asp-Xaa bond. Continuing cycles of PIMT action efficiently repair L-isoAsp sites, as has been demonstrated in vitro with a number of peptides and proteins (Brennan, et al., 1994; Dimitrijevic, et al., 2014; Galletti, et al., 1988; Johnson, et al., 1987a; Johnson, et al., 1987b; McFadden and Clarke, 1987). A repair function for PIMT in vivo is supported by the observation that reduction of PIMT activity in cultured cells or PIMT knockout (KO) mice dramatically increases the level of isoAsp-containing proteins (Johnson, et al., 1993; Kim, et al., 1997; Kosugi, et al., 2008; Yamamoto, et al., 1998). A critical need for PIMT action in the brain is evident by its high specific activity in this tissue, as well as the overt neurological phenotype of PIMT-KO mice: increased brain size, abnormal neuroanatomical and electrophysiological properties of hippocampal cells, atypical open-field behavior, and fatal epileptic seizures typically at 30-60 days after birth (Ikegaya, et al., 2001; Kim, et al., 1999; Kim, et al., 1997; Vitali and Clarke, 2004; Yamamoto, et al., 1998).
Fig. 1.
Mechanism of isoaspartate formation and its PIMT-catalyzed repair. Under physiological conditions, deamidation of asparagine residues or dehydration of aspartic acid residues results in the formation of a metastable intermediate succinimide that spontaneously hydrolyzes to form a mixture of normal L-aspartyl and atypical L-isoaspartyl linkages. PIMT, using AdoMet as a methyl donor, selectively methylates the isoaspartyl α-carboxyl group to form a highly labile methyl ester. Spontaneous demethylation occurs within minutes to reform the original succinimide, with release of methanol as a by-product. This succinimide is now the starting point for further cycles of repair, resulting in near complete conversion of the isoaspartyl β-linkages to normal aspartyl α-linkages. Dotted lines indicate degradative reactions and solid lines indicate the repair pathway.
PIMT is the product of single “housekeeping” gene (Pcmt1) that is expressed in all mammalian tissues and cell types studied to date, with highest levels reported for brain, retina, and testis (Boivin, et al., 1995; DeVry, et al., 1996; Diliberto and Axelrod, 1976; Mizobuchi, et al., 1994; Qin, et al., 2014). Mice that are heterozygous (HZ, −/+) for Pcmt1 exhibit 50-52% of the enzyme activity found in wild-type (WT, +/+) mice, regardless of the tissue (Yamamoto, et al., 1998). Given the phenotype of the PIMT-KO mouse, extreme deficits in PIMT in humans would likely lead to neurodevelopmental disorders of the young, while low-normal levels might lead to an acceleration of age-related decline in CNS function. The present communication deals with this latter possibility.
Several studies indicate that PIMT activity varies significantly among individual humans. In one study, David et al. (David, et al., 1997) measured PIMT activity in red blood cell (RBC) cytosol from 299 presumed healthy residents of Minnesota, including men, women and children. PIMT activity in these samples ranged from 420 to 687 units/ml of RBC; i.e. the lowest sample had only 61% the activity of the highest sample. PIMT variation was not related to age or sex. In another study, Johnson et al. (Johnson, et al., 1991) measured PIMT activity in postmortem brain samples from 30 individuals who ranged in age from 56 to 91. Of these 30 individuals, 8 had no known neuropathology (controls), 3 were diagnosed with multiple-infarct dementia, and 19 were diagnosed with Alzheimer’s disease. PIMT activity among all 30 subjects ranged from a low of 9.0 units/mg (in a 66 year old male with multiple infarct dementia) to a high of 33.2 units/mg in a 79 year old control female. PIMT activity among the 8 controls ranged from a low of 13.2 units/mg (in a 61 year old male) to 33.2 units/mg as mentioned above. These results suggested to us that the PIMT HZ mouse might serve as a useful model to test the idea that PIMT levels might influence the rate at which CNS function declines with age in humans.
A 1999 paper by DeVry and Clarke (DeVry and Clarke, 1999) suggests that even subtle variations in PIMT activity can influence the quality of life in human aging. These workers genotyped populations of younger and older (“successfully aged”) Ashkenazi Jews with regard to a common biallelic polymorphism (Ile or Val) at position 119 in the PIMT sequence. Compared to the younger population, the aged population had a significantly higher representation of heterozygotes (Ile/Val) than would be expected from Hardy-Weinberg Equilibrium calculations. It was suggested that this “hybrid vigor” stems from a combination of greater thermostability of the Ile isoform with a higher substrate affinity of the Val isoform for many of it endogenous substrates.
With over 30 major substrates in the brain, PIMT is highly pleiotropic; thus, even a moderate reduction in its protein repair function could have a widespread effect on CNS function. This, plus the studies described above, prompted us to look for subtle but significant biochemical changes in the brains of the PIMT HZ mouse, which, until now, has been considered to have phenotype indistinguishable from the WT. We demonstrate here that, compared with WT mice, the PIMT HZ mice show significantly greater protein damage as they age from 8 months to 2 years, along with a greater loss in function of creatine kinase B (CKB), a major target of PIMT in neurons.
2. Methods
2.1 Mice
To start our colony, male PIMT +/− (HZ; heterozygous) mice were kindly provided by Dr. Mark Mamula (Yale University School of Medicine) and mated with female C57BL/6 mice purchased from The Jackson Laboratory (Bar Harbor, Me). Development of the founder mice is reported in detail elsewhere (Kim, et al., 1997). Genotyping was done by Transnetyx, Inc. (Cordova, TN) by real-time PCR analysis of tail DNA with probes for both the neo cassette and the Pcmt1 gene. For all experiments, a cohort of 4 wild type (WT; PIMT +/+) and 4 PIMT HZ mice were tested at 8 months or 22-24 months of age. Mice were housed in groups of mixed genotype and had access to food and water ad libitum in a room maintained at 19-22°C, 40-60% humidity, under a 12:12 hours light/dark cycle. Procedures were performed in compliance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by University Laboratory Animal Resources of the University of California, Irvine.
2.2 Preparation of tissue extracts
Mice were anesthetized with Euthasol® (Virbac Corporation) and sacrificed by decapitation. Brains were rapidly removed and homogenized in a buffer comprised of 5 mM K-Hepes pH 7.6, 0.5 mM EDTA, 10% sucrose, 50 mM NaF, 1 mM Na3VO4, 0.1 mM dithiothreitol, and 1% mammalian protease inhibitor cocktail (Sigma-Aldrich, product # P8340). Homogenates were centrifuged at 800 × g for 30 min at 4 °C. The supernatants (hereafter "extracts") were collected, and protein concentration was determined in triplicate using the microplate version of the Pierce BCA Protein Assay Kit and bovine serum albumin as a standard. Extracts were stored in aliquots at −80 °C.
2.3 Western blot analysis of PIMT expression
Mouse brain extracts (30 μg protein per lane) were subjected to SDS-PAGE on NuPAGE 10% Bis-Tris gels (Life Technologies). After semi-dry transfer to PVDF (Millipore), the membranes were blocked in 5% nonfat milk and probed simultaneously with primary antibodies against PIMT (1:3,000; custom polyclonal made against bovine brain PIMT), and β-actin (1:10,000; Cell Signaling Technologies, cat. no. 4970S). After incubation with HRP-conjugated donkey anti-rabbit secondary antibody (GE Healthcare), detection was performed with ECL Plus reagents (Thermo Scientific) using a Nikon D700 camera with sub-saturating exposure times (Khoury, et al., 2010). Band densities were determined with NIH ImageJ (version 1.41 for Mac OSX) and corrected for background.
2.4 Assays of PIMT activity and isoAsp levels
Assays for PIMT activity and isoAsp content in mouse brain extracts were carried out in triplicate as previously described for human brain extracts with minor modifications (Johnson, et al., 1991). The PIMT assays measure the initial rate at which 3H-methyl groups are transferred from S-adenosyl-{methyl-3H}-L-methionine (3H-AdoMet) to γ-globulin, a known methyl-acceptor for PIMT. Each 50 μl reaction contained 50 μg of mouse brain extract protein and was carried out in triplicate. For isoAsp assays, mouse brain extracts were incubated with 3H-AdoMet and sufficient recombinant PIMT to completely methylate all the available endogenous isoAsp sites . Each 18 μl reaction contained 30 μg of mouse brain extract and 5 μM PIMT, and was carried out in triplicate.
2.5 Creatine kinase B activity assays
For determination of CKB activity, brain extracts (see above) were subjected to further centrifugation at 20,000 × g for 1 h to remove interference by mitochondrial creatine kinase. Supernatants were assayed in triplicate as recently described (Dimitrijevic et al., 2014) using a coupled enzyme kit from Pointe Scientific, Inc. (catalog # C5722) that measures creatine kinase activity in the direction of ATP synthesis. Protein assays on these 20,000 × g extracts were carried out as described above for the 800 × g extracts.
3. Results
3.1 PIMT activity and expression
To study how animal age affects isoaspartyl protein damage levels in brain, we employed four groups of mice with n=4 in each group; WT males, HZ males, WT females, and HZ females. One set of four groups (mid-age) employed mice that were 8 months old, and another (aged) employed mice that were 22-24 months old.
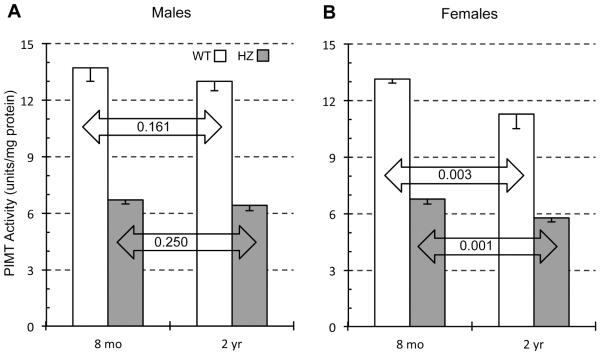
Effects of genotype and age on PIMT enzyme activity are explored in Fig. 2. The effect of heterozygosity was constant across age and sex with an HZ/WT ratio of 0.49-0.51. This is in close agreement with previous studies on PIMT activity in young mice where HZ/WT ratios of 0.50-0.55 were reported (Farrar and Clarke, 2002; Yamamoto, et al., 1998). Although the HZ/WT activity ratios do not change with age, we found that the absolute levels of PIMT activity in both genotypes drop modestly between 8 months and 2 years. For males, decreases of 5.3% and 3.8% were found for WT and HZ respectively, although the statistical significance was low. Females exhibited a greater drop that was highly significant; down 14.5% and 14.7% in WT and HZ respectively (Table 1). These data suggest that female mice lose PIMT activity in the brain faster than males.
Fig. 2.
Effect of animal age on PIMT enzyme activity in brain extracts of WT and HZ mice. Mouse brain extracts were assayed for methyltransferase activity using bovine γ-globulin as a substrate. Results are expressed as enzyme units (pmol of methyl esters formed per min) per mg of lysate protein. Data are expressed as means ± SD (n=4 mice for each group). P-values are based on a two-tailed t-test (unpaired). The quantitative data shown in Figures 2-5 is also presented in numerical form in Table 1.
Table 1.
Summary of effects of genotype and age on parameters related to isoAsp accumulation.
| Group comparisonsa | Percent difference | P-valueb |
|---|---|---|
| PIMT Protein - Males | ||
| HZ vs WT: 8 mo | − 58.6 | 0.001 |
| HZ vs WT: 2 yr | − 67.8 | 0.002 |
| WT: 2 yr vs 8 mo | − 17.3 | 0.065 |
| HZ: 2 yr vs 8 mo | − 35.6 | 0.017 |
| PIMT Protein - Females | ||
| HZ vs WT: 8 mo | − 65.1 | 0.004 |
| HZ vs WT: 2 yr | − 65.4 | <0.001 |
| WT: 2 yr vs 8 mo | − 7.5 | 0.301 |
| HZ: 2 yr vs 8 mo | − 8.3 | 0.397 |
| PIMT Activity - Males | ||
| HZ vs WT: 8 mo | − 51.3 | <0.001 |
| HZ vs WT: 2 yr | − 50.5 | <0.001 |
| WT: 2 yr vs 8 mo | − 5.3 | 0.161 |
| HZ: 2 yr vs 8 mo | − 3.8 | 0.250 |
| PIMT Activity - Females | ||
| HZ vs WT: 8 mo | − 48.7 | <0.001 |
| HZ vs WT: 2 yr | − 48.9 | <0.001 |
| WT: 2 yr vs 8 mo | − 14.5 | 0.003 |
| HZ: 2 yr vs 8 mo | − 14.7 | 0.001 |
| IsoAsp levels - Males | ||
| HZ vs WT: 8 mo | + 27.3 | 0.032 |
| HZ vs WT: 2 yr | + 55.8 | 0.014 |
| WT: 2 yr vs 8 mo | + 5.3 | 0.600 |
| HZ: 2 yr vs 8 mo | + 28.8 | 0.078 |
| IsoAsp levels - Females | ||
| HZ vs WT: 8 mo | + 19.5 | 0.038 |
| HZ vs WT: 2 yr | + 29.6 | <0.001 |
| WT: 2 yr vs 8 mo | + 4.0 | 0.627 |
| HZ: 2 yr vs 8 mo | + 12.8 | 0.005 |
| CKB Activity - Males | ||
| HZ vs WT: 8 mo | − 21.1 | 0.001 |
| HZ vs WT: 2 yr | − 17.2 | <0.001 |
| WT: 2 yr vs 8 mo | − 12.8 | 0.009 |
| HZ: 8 mo vs 2 yr | − 8.6 | 0.017 |
| CKB Activity - Females | ||
| HZ vs WT: 8 mo | − 17.3 | <0.001 |
| HZ vs WT: 2 yr | − 17.4 | <0.001 |
| WT: 2 yr vs 8 mo | − 13.5 | 0.001 |
| HZ: 2 yr vs 8 mo | − 13.6 | <0.001 |
N = 4 mice per group in every comparison.
Students T-test, unpaired, except for HZ vs WT tests comparing PIMT expression. The latter T-test used pairing of adjacent lanes of the Western blots in Figs. 2A, 2B.
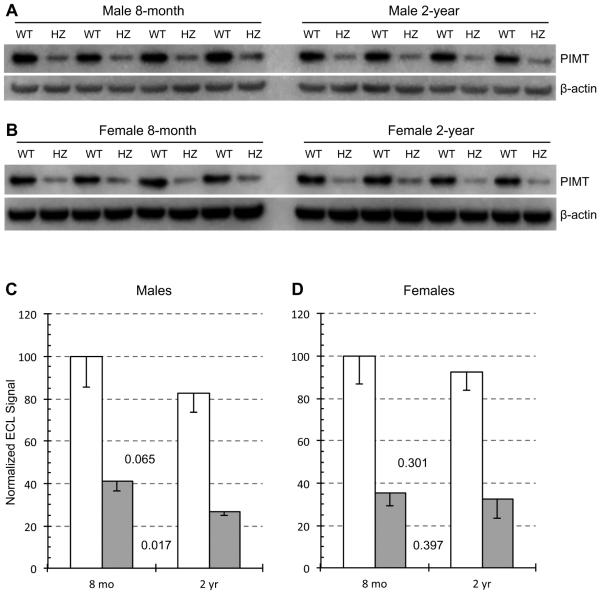
PIMT expression in mouse brain extracts was examined by Western blotting with a polyclonal antibody against full-length PIMT (Fig. 3). As expected, HZ mice exhibited a profound drop in PIMT expression relative to WT in both sexes and both age groups. HZ/WT ratios ranged from 0.32-0.41, similar to the HZ/WT expression ratios already reported for 5-week mice (Qin, et al., 2014; Yamamoto, et al., 1998). These data confirmed our genotype assignments and emphasize that PIMT expression is subject to a simple gene dosage effect.
Fig. 3.
Effect of animal age on PIMT expression in brain extracts of WT and HZ mice. Western blots used a mixture of primary antibodies to PIMT and β-actin for male mouse brain extracts (A) and female mouse brain extracts (B). Panels C and D show quantitative measurements of band intensities after normalization to β-actin. Data are expressed as means ± SD (n=4 mice for each group). P-values are based on a two-tailed t-test (paired).
Within a given genotype, PIMT expression appears to decline with age. In males, reductions of 17% and 36% were observed for WT and HZ respectively (Fig. 3C). Reductions in females were more modest at 7.5% and 8.3%, with a very low level of statistical significance (Fig. 3D). These results suggest that PIMT expression declines slightly with age and that this effect is more pronounced in males than in females.
The expression data yielded lower HZ/WT ratios (0.32-0.41) than did the activity data (0.49-0.51), which as mentioned above, has also been observed by others. This discrepancy is likely due to the non-linearity of integrated ECL signals in Western blotting, in which lower loadings of a given protein are sometimes underestimated (Charette, et al., 2010). Using the same antibody employed in the present study, we have previously found a similar non-linearity when loading different amounts of pure recombinant PIMT for Western blotting
Another notable difference between the data of Figs. 2 and 3 concerns the differential effect of aging on males vs females. Fig. 3 indicates that age-dependent loss of PIMT expression is greater in males (regardless of genotype), while Fig. 2 indicates just the opposite for PIMT activity. Because this trend is seen in both genotypes, it may not be a statistical anomaly.
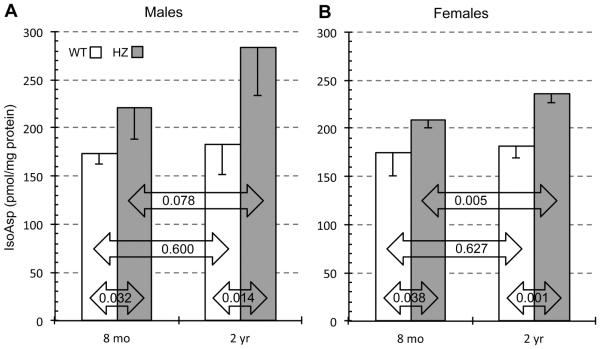
3.2 Isoaspartate levels
Using brain extracts from 5 week-old-mice, Yamamoto et al. (Yamamoto, et al., 1998) reported isoAsp levels from PIMT WT, HZ, and KO mice of 75 ± 18, 79 ± 2, and 703 ± 145 pmol/mg protein, respectively. Using brain extracts from mice at 4-5 weeks, Farrar and Clarke (2002) reported isoAsp levels of approximately 16.5, 27.0 and 534 pmol/mg (estimated from their Figure 1c) for the same genotypes. The latter data, which used mice from the same founders as ours, indicate that PIMT-HZ mice have somewhat higher isoAsp levels than WT mice even at a very young age. The guiding hypothesis of our study, as explained in our Introduction, maintains that differences in isoAsp accumulation in HZ vs WT mice will increase significantly with advanced age. The data presented in Fig, 4 indicate that this is indeed the case. In both males and females, isoAsp levels were significantly higher in HZ vs WT at both 8 months and 2 years. Of particular interest is the observation that changes with age are much greater in the HZ mice than in the WT mice. In WT males, we observed a modest aging increase of 5.3% with a very low level of statistical significance (p=0.600). In contrast, we observed a 5.4-times greater increase of 28.8% (p = 0.078) in the male HZ mice. In females, age-related increases in isoAsp with age were 4.0% (p = 0.627) for WT and 12.8% (p = 0.005) for HZ, making the HZ change 3.2-times that in the WT. These results strongly support the idea that the structural integrity of brain proteins in mice is increasingly sensitive to variations in PIMT activity as the mice age.
Fig. 4.
Effect of animal age on isoAsp levels in brain extracts of WT and HZ mice. Isoaspartyl protein content was determined for male (A) and female (B) mice using a methanol diffusion assay as described in Materials and Methods. Results are expressed as pmol isoAsp of per milligram of lysate. Data are expressed as means ± SD (n=4 mice for each group). P-values are based on a two-tailed t-test (unpaired).
We note that females lost more PIMT activity with age than did the males (Fig. 2 and Table 2). This was true for both WT and HZ mice. From this, one would expect the females to accumulate more isoAsp with age than males, but this did not happen. In fact it appears that males accumulated due in part to the greater variability of isoAsp accumulation in the older males. IsoAsp accumulation is controlled in vivo by both the rate of protein damage (isoAsp formation) and the rate of repair (isoAsp removal via PIMT). One possible explanation for the apparent discrepancy above is that females undergo a lower overall rate of protein damage as they age than do males.
Table 2.
Summary of female vs. male differences in parameters related to isoAsp accumulation.
| Group comparisonsa | Percent difference | P-valueb |
|---|---|---|
| PIMT Activity - WT | ||
| Female vs Male: 8 mo | − 4.1 | 0.202 |
| Female vs Male: 2 yr | − 13.4 | 0.009 |
| PIMT Activity - HZ | ||
| Female vs Male 8 mo | + 0.9 | 0.727 |
| Female vs Male: 2 yr | − 10.5 | 0.013 |
| isoAsp - WT | ||
| Female vs Male: 8 mo | + 0.9 | 0.912 |
| Female vs Male: 2 yr | − 0.4 | 0.967 |
| isoAsp - HZ | ||
| Female vs Male: 8 mo | − 5.3 | 0.508 |
| Female vs Male: 2 yr | − 17.1 | 0.105 |
| CKB Activity - WT | ||
| Female vs Male: 8 mo | + 3.7 | 0.353 |
| Female vs Male: 2 yr | + 2.9 | 0.292 |
| CKB Activity - HZ | ||
| Female vs Male: 8 mo | + 8.6 | 0.018 |
| Female vs Male: 2 yr | + 2.6 | 0.277 |
N = 4 mice per group in every comparison.
Students T-test, unpaired.
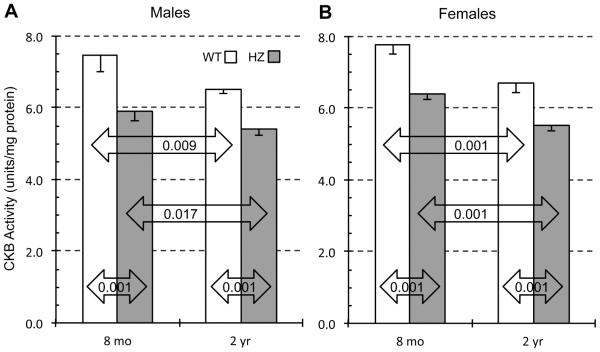
3.3 Creatine kinase activity
To see how PIMT activity and age might affect the biological function of endogenous substrates for PIMT, we measured the activity of creatine kinase B (CKB), a key enzyme in the regulation of neuronal ATP levels. CKB was found to be one of 22 major targets for PIMT in a proteomic analysis of isoAsp-rich proteins in the PIMT KO mouse brain (Zhu 2006), and has the advantage that its activity is relatively easy to measure. Recent work in our lab using 5-week mice revealed that CKB activity in PIMT HZ and KO mouse brain is reduced by 9% and 30% respectively relative to WT in both males and females (Dimitrijevic, et al., 2014). Fig. 5 shows measurements of CKB activity in 20,000 × g supernatant sub-fractions of the same brain extracts used in Figs. 2-4. Two key findings emerge: (1) CKB activity is significantly lower in HZ than WT mice at both ages, and (2) CKB activity declines significantly with age in both genotypes. The effects of genotype and age were additive and nearly identical in males and females. For both sexes, CKB was approximately 28% lower in 2-year HZ mice than in 8-month WT mice. These findings demonstrate that the isoAsp accumulation associated with decreased PIMT activity and increasing age translates into functional damage to proteins that can, in turn, lead to adverse effects on brain function.
Fig. 5.
Effect of animal age on CKB activity in brain extracts of WT and HZ mice. CKB activity was determined for male (A) and female (B) mice using a coupled enzyme assay to measure initial rates of conversion of creatine to ATP as described in Materials and Methods. Data are expressed as means ± SD (n=4 mice for each group). P-values are based on a two-tailed t-test (unpaired).
4.0 Discussion
4.1 IsoAsp levels increase with age in the PIMT HZ mouse brain
The current study was designed to test the hypothesis that a moderate (50%) reduction in PIMT activity would accelerate the rate of isoaspartyl protein damage in the aging mouse brain. The key findings presented in Fig. 4 proved this to be the case. In WT males, isoAsp levels increased only 5% between eight months and two years, whereas in the HZ males, isoAsp levels increased by 29%, a nearly 6-fold increase in rate. For females, isoAsp increases were 4% and 13% respectively. Consistent with these changes, we found that both PIMT specific activity (Fig. 2) and protein levels (Fig. 3) decreased with age in both genotypes and both sexes.
A functional consequence of increased protein damage is reflected in the activity of creatine kinase CKB (a regulator of ATP levels), known to rank high in its susceptibility to isoaspartyl damage both in vivo and in vitro (Dimitrijevic, et al., 2014; Zhu, et al., 2006). CKB KO mice have a normal life span but exhibit a phenotype that overlaps significantly with that of the PIMT KO mouse; learning deficits, decreased habituation in open field behavior, and increased susceptibility to epileptic seizures (Jost, et al., 2002). Other major targets for PIMT-dependent repair in the brain include (but are not limited to) proteins involved in cytoskeletal and membrane structure (tubulin and collapsin response mediator protein 2), synaptic vesicle release and recovery (synapsins I/II and dynamin 1), protein folding (Hsc70), and regulation of gene expression (histone H2B and 4E-BP2) (Bidinosti, et al., 2010; Zhu, et al., 2006). The plurality of substrates makes PIMT a highly leveraged enzyme that could well have a strong influence on the integrity of brain function. Insofar as PIMT KO mice show early and severe cognitive deficits associated with large increases in isoAsp levels, we anticipate from the results presented here, that a portion of cognitive decline seen during aging might be explained by variation of PIMT levels that don’t result in any obvious pathology or phenotype. As a further test of this idea, we are currently aging additional PIMT WT and HZ mice to see if, and to what extent, the +/− genotype affects the age-dependent decline in learning, memory, and retinal function.
4.2 Relevance to age related decline in CNS function in humans
Two previous studies with human subjects support the idea that PIMT activity levels may influence the age-dependent decline of CNS function. As mentioned in the introduction, DeVry and Clarke (DeVry and Clarke, 1999b) found that successful aging in an Ashkenazi Jewish population is associated with a higher than expected frequency of heterozygosity for a common Ile/Val polymorphism at position 119 in the PIMT amino acid sequence. The difference in activity of the two variants is rather subtle, indicating that even small differences in overall PIMT activity could have a profound influence on CNS of over the long term. This idea is further supported by a study on PIMT in postmortem samples of human brain (Johnson, et al., 1991) that found a strong positive correlation between PIMT activity and age at death (r = 0.51, p=0.002, n=29), and a study on human red cells demonstrating considerable variation of PIMT activity among 299 healthy subjects (David, et al., 1997).
Considerable evidence links oxidative damage to proteins with normal aging and neurodegenerative diseases. PIMT can protect cells against apoptosis induced by oxidative stress, as observed in cultured human endothelial cells and the nematode C. elegans (Cimmino, et al., 2008; Khare, et al., 2009), while oxidative conditions can promote isoAsp formation (Ingrosso, et al., 2000; O'Connor and Yutzey, 1988). IsoAsp formation has also been linked to autoimmunity, a known factor in several diseases that affect neuronal and retinal function. Mamula and colleagues showed that the presence of isoAsp can greatly increase a protein’s ability to break tolerance in the innate immune system (Doyle, et al., 2007; Mamula, et al., 1999). Oxidative damage and autoimmunity to many endogenous substrate of PIMT, including CKB, has been associated with aging and neurodegenerative diseases (Zhu, et al., 2006). Thus, it appears that isoAsp formation can have multiple deleterious effects on proteins: loss of catalytic activity, increased susceptibility to oxidation, and conversion to an autoantigen.
4.3 Conclusions
It was predicted that PIMT HZ mice might show enhanced rates of age-related protein damage in the brain compared with WT mice, even though the HZ mice live a normal life span and exhibit no overt pathology. We found that this is indeed the case, with damage manifested both as overall isoAsp accumulation and CKB activity loss. It is concluded that PIMT variation in healthy humans probably contributes to the diversity with which CNS function declines in advanced age.
Highlights.
Accelerated protein damage in brains of PIMT +/− mice; a possible model for the variability of cognitive decline in human aging.
Zhenxia Qina, Aleksandra Dimitrijevic, and Dana W. Aswad*
PIMT +/− mice have a 50% reduction in the enzyme needed to repair isoaspartyl protein damage. This reduction mimics the known range of PIMT activity in humans.
We measured levels of isoaspartyl protein damage in PIMT +/+ and +/− mouse brains at 8 mo and 2 yr and found that, between these ages, isoaspartyl protein damage increased 3-5 times faster in the +/− mice than in the +/+ mice.
We also found that the activity of creatine kinase B (a known target of PIMT-dependent repair) decreased with age in both genotypes, and was significantly lower in brains of the +/− mice than in the +/+ mice at both ages.
These results suggest that PIMT activity levels in the brain may contribute significantly to the time-course of cognitive decline that inevitable occurs in advanced age.
Acknowledgements
We thank Mark Mamula of Yale University School of Medicine for providing us with PIMT-HZ mice used to start our mouse colony. The work was initiated with funding from NIH grant NS17269, and completed with a bridge-funding award from the University of California, Irvine, Office of Research Administration. None of the authors have any conflicts of interest to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aswad DW, Paranandi MV, Schurter BT. Isoaspartate in peptides and proteins: formation, significance, and analysis. J Pharm Biomed Anal. 2000;21(6):1129–36. doi: 10.1016/s0731-7085(99)00230-7. [DOI] [PubMed] [Google Scholar]
- Bidinosti M, Martineau Y, Frank F, Sonenberg N. Repair of isoaspartate formation modulates the interaction of deamidated 4E-BP2 with mTORC1 in brain. J Biol Chem. 2010;285(25):19402–8. doi: 10.1074/jbc.M110.120774. doi:10.1074/jbc.M110.120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin D, Bilodeau D, Beliveau R. Immunochemical characterization of L-isoaspartyl-protein carboxyl methyltransferase from mammalian tissues. Biochem J. 1995;309:993–8. doi: 10.1042/bj3090993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan TV, Anderson JW, Jia Z, Waygood EB, Clarke S. Repair of spontaneously deamidated HPr phosphocarrier protein catalyzed by the L-isoaspartate-(D-aspartate) O-methyltransferase. J Biol Chem. 1994;269(40):24586–95. [PubMed] [Google Scholar]
- Charette SJ, Lambert H, Nadeau PJ, Landry J. Protein quantification by chemiluminescent Western blotting: elimination of the antibody factor by dilution series and calibration curve. J Immunol Methods. 2010;353(1-2):148–50. doi: 10.1016/j.jim.2009.12.007. doi:10.1016/j.jim.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Cimmino A, Capasso R, Muller F, Sambri I, Masella L, Raimo M, De Bonis ML, D'Angelo S, Zappia V, Galletti P, Ingrosso D. Protein isoaspartate methyltransferase prevents apoptosis induced by oxidative stress in endothelial cells: role of Bcl-Xl deamidation and methylation. PLoS One. 2008;3(9):e3258. doi: 10.1371/journal.pone.0003258. doi:10.1371/journal.pone.0003258. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Clarke S. Aging as war between chemical and biochemical processes: protein methylation and the recognition of age-damaged proteins for repair. Ageing Res Rev. 2003;2(3):263–85. doi: 10.1016/s1568-1637(03)00011-4. [DOI] [PubMed] [Google Scholar]
- David CL, Szumlanski CL, DeVry CG, Park-Hah JO, Clarke S, Weinshilboum RM, Aswad DW. Human erythrocyte protein L-isoaspartyl methyltransferase: heritability of basal activity and genetic polymorphism for thermal stability. Arch Biochem Biophys. 1997;346:277–86. doi: 10.1006/abbi.1997.0303. [DOI] [PubMed] [Google Scholar]
- Desrosiers RR, Fanelus I. Damaged proteins bearing L-isoaspartyl residues and aging: a dynamic equilibrium between generation of isomerized forms and repair by PIMT. Curr Aging Sci. 2011;4(1):8–18. [PubMed] [Google Scholar]
- DeVry CG, Clarke S. Polymorphic forms of the protein L-isoaspartate (D-aspartate) O-methyltransferase involved in the repair of age-damaged proteins. J Hum Genet. 1999;44(5):275–88. doi: 10.1007/s100380050161. doi:10.1007/s100380050161. [DOI] [PubMed] [Google Scholar]
- DeVry CG, Tsai W, Clarke S. Structure of the human gene encoding the protein repair L-isoaspartyl (D-aspartyl) O-methyltransferase. Arch Biochem Biophys. 1996;335(2):321–32. doi: 10.1006/abbi.1996.0513. doi:10.1006/abbi.1996.0513. [DOI] [PubMed] [Google Scholar]
- Diliberto EJ, Jr., Axelrod J. Regional and subcellular distribution of protein carboxymethylase in brain and other tissues. J Neurochem. 1976;26:1159–65. doi: 10.1111/j.1471-4159.1976.tb07001.x. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Qin Z, Aswad DW. Isoaspartyl formation in creatine kinase B Is associated with loss of enzymatic activity; implications for the linkage of isoaspartate accumulation and neurological dysfunction in the PIMT knockout mouse. PLoS One. 2014;9(6):e100622. doi: 10.1371/journal.pone.0100622. doi:10.1371/journal.pone.0100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle HA, Gee RJ, Mamula MJ. Altered immunogenicity of isoaspartate containing proteins. Autoimmunity. 2007;40(2):131–7. doi: 10.1080/08916930601165180. doi:10.1080/08916930601165180. [DOI] [PubMed] [Google Scholar]
- Farrar C, Clarke S. Altered levels of S-adenosylmethionine and S-adenosylhomocysteine in the brains of L-isoaspartyl (D-Aspartyl) O-methyltransferase-deficient mice. J Biol Chem. 2002;277(31):27856–63. doi: 10.1074/jbc.M203911200. [DOI] [PubMed] [Google Scholar]
- Galletti P, Ciardiello A, Ingrosso D, Di Donato A. Repair of isopeptide bonds by protein carboxyl O-methyltransferase: seminal ribonuclease as a model system. Biochemistry. 1988;27:1752–7. doi: 10.1021/bi00405a055. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Yamada M, Fukuda T, Kuroyanagi H, Shirasawa T, Nishiyama N. Aberrant synaptic transmission in the hippocampal CA3 region and cognitive deterioration in protein-repair enzyme-deficient mice. Hippocampus. 2001;11:287–98. doi: 10.1002/hipo.1043. [DOI] [PubMed] [Google Scholar]
- Ingrosso D, D'Angelo S, di Carlo E, Perna AF, Zappia V, Galletti P. Increased methyl esterification of altered aspartyl residues in erythrocyte membrane proteins in response to oxidative stress. Eur J Biochem. 2000;267(14):4397–405. doi: 10.1046/j.1432-1327.2000.01485.x. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Langmack EL, Aswad DW. Partial repair of deamidation-damaged calmodulin by protein carboxyl methyltransferase. J Biol Chem. 1987;262:12283–7. [PubMed] [Google Scholar]
- Johnson BA, Murray ED, Jr., Clarke S, Glass DB, Aswad DW. Protein carboxyl methyltransferase facilitates conversion of atypical L-isoaspartyl peptides to normal L-aspartyl peptides. J Biol Chem. 1987;262:5622–9. [PubMed] [Google Scholar]
- Johnson BA, Najbauer J, Aswad DW. Accumulation of substrates for protein L-isoaspartyl methyltransferase in adenosine dialdehyde-treated PC12 cells. J Biol Chem. 1993;268(9):6174–81. [PubMed] [Google Scholar]
- Johnson BA, Shirokawa JM, Geddes JW, Choi BH, Kim RC, Aswad DW. Protein L-isoaspartyl methyltransferase in postmortem brains of aged humans. Neurobiol Aging. 1991;12(1):19–24. doi: 10.1016/0197-4580(91)90034-h. [DOI] [PubMed] [Google Scholar]
- Jost CR, Van Der Zee CE, In 't Zandt HJ, Oerlemans F, Verheij M, Streijger F, Fransen J, Heerschap A, Cools AR, Wieringa B. Creatine kinase B-driven energy transfer in the brain is important for habituation and spatial learning behaviour, mossy fibre field size and determination of seizure susceptibility. Eur J Neurosci. 2002;15(10):1692–706. doi: 10.1046/j.1460-9568.2002.02001.x. [DOI] [PubMed] [Google Scholar]
- Khare S, Gomez T, Linster CL, Clarke SG. Defective responses to oxidative stress in protein l-isoaspartyl repair-deficient Caenorhabditis elegans. Mech Ageing Dev. 2009;130(10):670–80. doi: 10.1016/j.mad.2009.08.002. doi:10.1016/j.mad.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury MK, Parker I, Aswad DW. Acquisition of chemiluminescent signals from immunoblots with a digital single-lens reflex camera. Anal Biochem. 2010;397(1):129–31. doi: 10.1016/j.ab.2009.09.041. doi:10.1016/j.ab.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Lowenson JD, Clarke S, Young SG. Phenotypic analysis of seizure-prone mice lacking L-isoaspartate (D-aspartate) O-methyltransferase. J Biol Chem. 1999;274(29):20671–8. doi: 10.1074/jbc.274.29.20671. [DOI] [PubMed] [Google Scholar]
- Kim E, Lowenson JD, MacLaren DC, Clarke S, Young SG. Deficiency of a protein-repair enzyme results in the accumulation of altered proteins, retardation of growth, and fatal seizures in mice. Proc Natl Acad Sci U S A. 1997;94(12):6132–7. doi: 10.1073/pnas.94.12.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Furuchi T, Katane M, Sekine M, Shirasawa T, Homma H. Suppression of protein l-isoaspartyl (d-aspartyl) methyltransferase results in hyperactivation of EGF-stimulated MEK-ERK signaling in cultured mammalian cells. Biochem Biophys Res Commun. 2008;371(1):22–7. doi: 10.1016/j.bbrc.2008.03.109. doi:10.1016/j.bbrc.2008.03.109. [DOI] [PubMed] [Google Scholar]
- Mamula MJ, Gee RJ, Elliott JI, Sette A, Southwood S, Jones PJ, Blier PR. Isoaspartyl post-translational modification triggers autoimmune responses to self-proteins. J Biol Chem. 1999;274(32):22321–7. doi: 10.1074/jbc.274.32.22321. [DOI] [PubMed] [Google Scholar]
- McFadden PN, Clarke S. Conversion of isoaspartyl peptides to normal peptides: Implications for the cellular repair of damaged proteins. Proc Natl Acad Sci U S A. 1987;84:2595–9. doi: 10.1073/pnas.84.9.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi M, Murao K, Takeda R, Kakimoto Y. Tissue-specific expression of isoaspartyl protein carboxyl methyltransferase gene in rat brain and testis. J Neurochem. 1994;62:322–8. doi: 10.1046/j.1471-4159.1994.62010322.x. [DOI] [PubMed] [Google Scholar]
- O'Connor CM, Yutzey KE. Enhanced carboxyl methylation of membrane-associated hemoglobin in human erythrocytes. J Biol Chem. 1988;263:1386–90. [PubMed] [Google Scholar]
- Qin Z, Yang J, Klassen H, Aswad DW. Isoaspartyl Protein Damage and Repair in Mouse Retina. Invest Ophthalmol Vis Sci. 2014 doi: 10.1167/iovs.13-13668. doi:10.1167/iovs.13-13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Matsuoka Y, Shirasawa T. Biological significance of isoaspartate and its repair system. Biol Pharm Bull. 2005;28(9):1590–6. doi: 10.1248/bpb.28.1590. [DOI] [PubMed] [Google Scholar]
- Vitali R, Clarke S. Improved rotorod performance and hyperactivity in mice deficient in a protein repair methyltransferase. Behav Brain Res. 2004;153(1):129–41. doi: 10.1016/j.bbr.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Yamamoto A, Takagi H, Kitamura D, Tatsuoka H, Nakano H, Kawano H, Kuroyanagi H, Yahagi Y, Kobayashi S, Koizumi K, Sakai T, Saito K, Chiba T, Kawamura K, Suzuki K, Watanabe T, Mori H, Shirasawa T. Deficiency in protein L-isoaspartyl methyltransferase results in a fatal progressive epilepsy. J Neurosci. 1998;18(6):2063–74. doi: 10.1523/JNEUROSCI.18-06-02063.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JX, Doyle HA, Mamula MJ, Aswad DW. Protein repair in the brain, proteomic analysis of endogenous substrates for protein L-isoaspartyl methyltransferase in mouse brain. J Biol Chem. 2006;281(44):33802–13. doi: 10.1074/jbc.M606958200. doi:10.1074/jbc.M606958200. [DOI] [PubMed] [Google Scholar]