Abstract
Measures from diffusion magnetic resonance imaging reflect changes in the substantia nigra of Parkinson’s disease. It is the case, however, that partial volume effects from free-water can bias diffusion measurements. The bi-tensor diffusion model was introduced to quantify the contribution of free-water and eliminates its bias on estimations of tissue microstructure. Here, we test the hypothesis that free-water is elevated in the substantia nigra for Parkinson’s disease compared with controls. This hypothesis was tested between large cohorts of Parkinson’s disease and control participants in a single-site study, and validated against a multi-site study using multiple scanners. The fractional volume of free-water was increased in the posterior region of the substantia nigra in Parkinson’s disease compared with controls in both the single-site and multi-site studies. We did not observe changes in either cohort for free-water corrected fractional anisotropy or free-water corrected mean diffusivity. Our findings provide new evidence that the free-water index reflects alteration of the substantia nigra in Parkinson’s disease, and this was evidenced across both single-site and multi-site cohorts.
Keywords: Substantia nigra, Parkinson’s disease, Diffusion MRI, Free-water mapping
Introduction
In Parkinson’s disease (PD), there is a selective loss of dopaminergic neurons in the substantia nigra (SN) (Braak et al., 2003b; Hodaie et al., 2007b). Multiple mechanisms may contribute to SN neuronal loss such as mitochondrial dysfunction, protein aggregation, neurotoxins, oxidative stress, and/or alteration in innate immune activation states. Pro-inflammatory cytokines have been observed at higher levels in the cerebrospinal fluid of PD (Mogi et al., 1994a; Mogi et al., 1996; Mogi et al., 1994b), and activated microglia have been observed in animal models of PD (Barcia et al., 2004). In addition, increased oxidative damage and loss of antioxidants have been observed in the SN of individuals with PD, ultimately leading to decreased dopaminergic neuron production (Sutachan et al., 2012; Venkateshappa et al., 2012). Developing imaging markers of the substantia nigra are needed to provide direct measurement of the degenerative process related to PD (Lehéricy et al., 2012).
Using diffusion magnetic resonance imaging (MRI), several studies have reported that fractional anisotropy (FA) is reduced within the SN of PD compared with healthy controls (Lehéricy et al., 2012; Péran et al., 2010; Rolheiser et al., 2011a; Vaillancourt et al., 2009; Zhan et al., 2012), and this may reflect the loss of dopaminergic neurons within the SN. FA has been limited as a measure of tissue microstructure, since atrophy-based partial volume with free-water can bias the diffusion index (Metzler-Baddeley et al., 2012). If the fractional volume of free-water in a voxel increases then diffusion indices such as mean diffusivity can be elevated and the FA measure can be reduced (Alexander et al., 2001; Pfefferbaum and Sullivan, 2003), and these are two of the findings reported in the literature for PD (Péran et al., 2010; Scherfler et al., 2013; Vaillancourt et al., 2009). Recently, free-water diffusion MRI analysis using a bi-tensor model was developed to explicitly estimate the fractional volume of freely diffusing water molecules within the voxel (Metzler-Baddeley et al., 2012; Pasternak et al., 2009; Pierpaoli, 2004), and this measure is expected to increase with atrophy-based neurodegeneration (Wang et al., 2011). When the free-water component is eliminated, the remaining signal provides a corrected FA value (FAT) and corrected MD value (MDT) within the tissue of interest. Prior work in schizophrenia (Pasternak et al., 2012), Alzheimer’s disease (Berlot et al., 2014), and brain injuries (Pasternak et al., 2014) has shown that free-water is a key index of change for identifying microstructural changes within major white matter regions. Since SN degeneration occurs mostly in the posterior portion of the SN in PD (ie. ventrolateral tier) (Fearnley and Lees, 1991; Hodaie et al., 2007b; Kordower et al., 2013), here we test the hypothesis that free-water is elevated in the posterior SN of PD. We pursued this hypothesis as the discovery of free-water could have implications for future targeted therapies that affect mechanisms contributing to neuronal cell loss of the SN in PD (Gyoneva et al., 2014). In this study, we used the bi-tensor model in an automated pipeline to provide a novel evaluation of free-water changes within the SN of PD. The hypothesis for increased free-water in the SN of PD was evaluated using a single-site study, and subsequently validated against a multi-site study from the Parkinson’s Progressive Marker Initiative (PPMI).
Methods
2.1 Subjects
Two populations were examined, one from a single site and one from multiple sites. In the single-site study, as part of the National Institutes of Health Parkinson’s Disease Biomarker Program (PDBP), 48 subjects participated between 2011 and 2013 (control, 20; PD, 28). The PD patients were referred from the University of Florida (UF) Center for Movement Disorders and Neurorestoration, and controls were recruited from the local and surrounding communities in North Central Florida. PD patients were diagnosed by movement disorders specialists at the Center for Movement Disorders and Neurorestoration at UF based on the UK PD Society Brain Bank criteria (Hughes et al., 2001). None of the control subjects reported a history of neurological or psychiatric disease. PD patients were tested after an overnight withdrawal from PD medications. All subjects gave written informed consent, as approved by the local Institutional Review Board.
In the multi-site study, we included the baseline diffusion MRI scans of 134 subjects (control, 56; PD, 78) from the PPMI database that were available as of March 21, 2014. The study is described in detail on the PPMI website (http://www.ppmi-info.org/study-design/). All PPMI subjects were assessed comprehensively at screening and baseline visits for motor, neuropsychological, and cognitive characteristics by the site investigators. Subjects had regularly scheduled assessments to collect clinical data and to participate in biomarker studies, including the acquisition of diffusion MRI. The PPMI diffusion tensor imaging study was performed at 8 imaging sites in the US. The acquired data underwent a basic quality control check by the Center for Imaging of Neurodegenerative Diseases (VA Medical Center, San Francisco, CA), and processed images were uploaded to the PPMI website (http://www.ppmi-info.org) where they are available for download by authorized users. Demographic and clinical characteristics, including disease severity based on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III and global cognitive function as measured by the Montreal Cognitive Assessment (MoCA) were collected for both the UF and PPMI cohorts.
2.2 MRI Acquisition
For the UF cohort, diffusion-weighted images were acquired on a 3T Philips Medical Systems MRI scanner (Achieva, Best, The Netherlands) using a 32-channel head coil at the McKnight Brain Institute. The whole-brain diffusion MRI acquisition sequence consisted of the following parameters: diffusion gradient directions = 64, repetition time = 7,748 ms, echo time = 86 ms, b-values: 0, 1000 s/mm2, field of view = 224 × 224 mm , in-plane resolution = 2 mm isotropic, number of contiguous slices = 60, slice thickness = 2 mm, and SENSE factor p = 2. Participants wore protective earplugs and headphones to minimize discomfort due to instrument noise. For the UF cohort, structural images were acquired consisting of a 3D T1-weighted sequence: repetition time = 8.2 ms, echo time = 3.7 ms, flip angle = 8o, field of view = 240 mm2, acquisition matrix = 240 × 240, voxel size = 1 mm isotropic with no gap between slices (n = 170).
For the PPMI cohort, diffusion imaging was performed at various sites on a Siemens 3T TIM Trio system (Siemens AG, Munich, Germany) using a 12-channel Matrix head coil. The data acquisition parameters are publicly available on the PPMI website in the Study Design Section (http://www.ppmi-info.org/study-design/research-documents-and-sops/). The whole-brain diffusion MRI acquisition sequence consisted of the following parameters: diffusion gradient directions = 64, repetition time = 900 ms, echo time = 88 ms, b-values: 0, 1000 s/mm2, field of view = 230 × 230 mm , in-plane resolution = 2 mm isotropic, number of contiguous slices = 72, slice thickness = 2 mm, and acceleration factor = 2.
2.3 Diffusion Imaging Analysis
Data pre-processing was performed with the FMRIB Software Library (FSL; Oxford, UK) and custom UNIX shell scripts. Each brain was corrected for distortions due to eddy currents and head motion using the FSL function eddy_correct with the reference volume set as the default 0 (http://fsl.fmrib.ox.ac.uk/fsl/fsl-4.1.9/fdt/fdt_eddy.html). The gradient directions were then rotated in response to the eddy current corrections, and non-brain tissue was removed from the diffusion volumes using the FSL Brain extraction tool BET.
Free-water maps and free-water corrected diffusion tensor maps were calculated from the motion and eddy current corrected volumes using a custom written MATLAB R2013a (The Mathworks, Natick, MA) code (Pasternak et al., 2009; Pasternak et al., 2012). This code implemented a minimization procedure that fits a bi-tensor model, which quantifies the fractional volume of free-water in each voxel (free-water maps). The bi-tensor model predicts the signal attenuation in the presence of free-water contamination. It is the sum of attenuations contributed by two compartments: one that models free water, Cwater, and a tissue compartment, Ctissue, that models either gray matter or a single bundle of white matter (Alexander et al., 2001; Behrens et al., 2003):
In Equation 1, the voxel-wise modeled attenuation vector, Abi-tensor, has an entry for each diffusion orientation (or applied gradient direction). The compartments are represented by the modeled attenuation vectors Atissue and Awater. The scalar f is the fractional volume of the tissue compartment (0<f<1), and similarly (1-f) is the fractional volume of free-water. The tissue compartment follows DTI’s formalism (Basser and Pierpaoli, 1996), where the attenuation is parameterized by a diffusion tensor:
In Equation 2, b as the diffusion weightings, and qk is the k’th applied gradient orientation. The free water compartment is modeled by a degenerate case of the diffusion tensor model, where an isotropic diffusion tensor, i.e., a scalar, d, represents the bulk diffusivity. As such, all entries of the vector Awater are equal:
In Equation 3, the value d is fixed to the apparent diffusion coefficient of free water (Pasternak et al., 2009). The bi-tensor model is the simplest model accounting for both diffusion anisotropy and partial volume, and is a special case of the multiple tensors model (See Appendix 1 and 2 from Pasternak et al., 2009). Thus, it is assumed that there is no exchange of water molecules between the two compartments. Finding the parameters f and D that best fit Equation 1 is non-trivial and is described in detail in prior work (Pasternak et al., 2009).
Free-water-corrected fractional anisotropy (FAT) and mean diffusivity (MDT) maps were calculated from the free-water corrected tensor maps. To obtain standardized space representation of free-water, FAT, and MDT maps, the free-water maps were registered to a standardized T2-weighted image in MNI space (2 × 2 × 2 mm) by an affine transformation with 12 degrees of freedom and trilinear interpolation using FLIRT (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT). The transformations were then applied to the FAT and MDT maps.
2.4 Region of Interest in the Substantia Nigra
Regions of interest (ROIs) were hand-drawn on the T2-weighted atlas in MNI space (Fonov et al., 2011). Figure 1 shows a standardized free-water and FAT image at z = −12 from a PD subject. The left and right anterior and posterior SN ROIs were drawn on 3 axial slices (z = −10,−12,−14). Each ROI was comprised of one voxel on the superior (z = −10) and inferior (z = −14) axial slices. For z = −10, the coordinates were x = 8, y = 14 and x = −8, y = 14 for the left and right anterior SN ROIs and the coordinates for the left and right posterior SN ROI were x = 10, y = 20 and x = −10, y = 20, respectively. For z = −12, the coordinates for the left anterior SN ROI were x = 6 to 10, y = 12 to 16 and for the right anterior SN ROI x = 6 to −10, y = 12 to 16. The coordinates for the left posterior SN ROI at z = −12 were x = 8 to 12, y = 18 to 22 and for the right posterior SN ROI x = −8 to −12, y = 18 to 22. For z = −14, the coordinates were x = 8, y = 14 and x = −8, y = 14 for the left and right anterior SN ROIs and the coordinates for the left and right posterior SN ROIs were x = 10, y = 20 and x = −10, y = 20, respectively. The SN was identified based on prior work (Vaillancourt et al., 2012).
Figure 1.
Anterior and Posterior Substantia Nigra Regions of Interest. A free-water map (A) and free-water corrected fractional anisotropy map (B) of a PD subject in standardized space at Z = − 12 that shows the posterior and anterior regions of interest on a zoomed-in version of the top panel on the bottom panel.
2.5 Voxel-based morphometry
All structural T1-weighted MRI images from the UF cohort were co-registered to the white matter template supplied with SPM8. The resulting images were bias-corrected for field inhomogeneities and segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF). Tissues of interest (i.e., GM and WM) were normalized to the DARTEL template using linear (12-parameter affine) and non-linear transformations (Shigemoto et al., 2013). In order to correct for the local expansion or contraction inherent to the normalization process, GM and WM images were non-linearly modulated using the Jacobian of the warp field. This step was used to account for potential differences in head size. Before statistical analysis, the modulated normalized GM and WM images were smoothed with an isotropic Gaussian kernel of 8 mm FWHM. The GM and WM were compared between groups using an independent t-test corrected for multiple comparisons using the family-wise error rate. We also computed the total GM volume relative to total intracranial volume, total WM volume relative to total intracranial volume, and total cerebrospinal fluid (CSF) volume relative to total intracranial volume. These volume estimates were examined in relation to SN free-water levels.
2.6 Dopamine Active Transporter SPECT Processing
Since the SN relays dopamine to the putamen, we examined if the changes in the SN relate to dopamine transporter imaging values. DatScan single photon emission computed tomography (SPECT) imaging was acquired at PPMI imaging centers. SPECT raw projection data was imported to a HERMES (Hermes Medical Solutions, Stockholm, Sweden) system for iterative reconstructions. This was done for all imaging centers to ensure consistency of the reconstructed files. Iterative reconstruction was done without any filters applied. Attenuation correction ellipses were drawn on the images and a Change 0 attenuation correction was applied to images utilizing a site specific value that was derived from phantom data acquired during site initiation for the trial. Once attenuation correction was completed, a standard Gaussian 3D 6.0 mm filter was applied. These files were then normalized to standard MNI space so that all scans were in the same anatomical alignment. A SPECT template (non-PPMI healthy subjects) was normalized into the Montreal Neurological Institute (MNI) space using 12 degrees of freedom affine transform using the imaging software PMOD (PMOD Technologies, Zurich, Switzerland) and the SPECT image template available in the package. Subsequently, individual SPECT images were fit onto the template using 9 degrees of freedom (translation, rotation and scaling in three orthogonal directions) and the matching visually inspected (Radau et al., 2000). Next, the transaxial slice with the highest striatal uptake was identified and the 8 hottest striatal slices around it were averaged to generate a single slice image. ROIs were then placed on the left and right putamen and the occipital cortex (reference tissue). Count densities for each region were extracted and used to calculated striatal binding ratios for each striatal region. SBR was calculated as [(target region/reference region)-1].
2.7 Statistical Analysis
The mean value of the bilateral ROIs for the anterior and posterior SN was calculated for each dependent measure (FAT, MDT, and free-water) and subject from the UF and PPMI cohorts. An independent-samples t-test between PD and control was conducted on each dependent measure for each cohort. Alpha was set at p < 0.05. All statistics were performed using IBM SPSS Statistics 22 (SPSS, Inc, Chicago, IL).
Spearman’s correlations between free-water values and other demographic and clinical variables (age, MDS-UPDRS scores, and MoCA scores) were calculated for each cohort. The GM volume, WM volume, and CSF volume from T1 images were examined in relation to the posterior SN free-water measurements in the UF cohort using Pearson correlation coefficient. In addition, free-water values were correlated with SPECT striatal binding ratio (SBR) values in the PPMI cohort. To control for multiple correlation analyses, multiple comparison correction at a false discovery rate (FDR) of 0.05 will be performed using the Benjamini-Hochberg-Yekutieli method in MATLAB (http://www.mathworks.com/).
Results
Table 1 shows the demographic and clinical characteristics of the PD and control groups from both cohorts. In the UF cohort, the PD group had higher MDS-UPDRS (29.8 vs. 2.0, p < 0.001) and lower MoCA (26.0 vs. 27.4, p = 0.01) scores compared with the control group. There were no significant differences in age between the PD and control groups from UF (p = 0.55). For the PPMI cohort, the PD group had higher MDS-UPDRS (22.9 vs. 0.6, p < 0.001) and lower MoCA scores compared with the control group (27.6 vs. 28.4, p = 0.006). There was no significant difference in age between the PPMI groups (p = 0.226).
Table 1.
Subject Characteristics
| University of Florida | PPMI | |||||
|---|---|---|---|---|---|---|
| Control | PD | Test Statistic | Control | PD | Test Statistic | |
| Number of Participant (n) | 20 | 28 | na | 56 | 78 | na |
| Age (yr), mn (SD) | 62.7 (8.9) | 64.7 (8.2) | t(46)=−0.80, p=0.430 | 59.5 (11.5) | 61.6 (9.2) | t(132)=−1.22, p=0.222 |
| MDS-UPDRS III, mn (SD) | 2.0 (1.9) | 29.8 (9.2) | t(30)=−15.61, p<0.001 | 0.63 (1.2) | 22.9 (8.7) | t(80.7)=−22.2,p<.001 |
| Dis. Duration, mo (SD) | na | 40.9 (21.5) | na | na | 7.9 (7.8) | na |
| Female sex, total (%) | 11 (55%) | 9 (32.1%) | χ2(1)=2.51, p=0.113 | 21 (37.5 %) | 23 (29.5%) | χ2(1)=0.95, p=0.330 |
| MOCA, mn (SD) | 27.4 (1.5) | 26.0 (1.9) | t(46)=2.64, p=0.011 | 28.4 (1.2) | 27.6 (2) | t(128)=2.80,p=0.009 |
| Putamen SBR, mn (SD) | na | na | 1.96 (0.4) | 0.78 (0.3) | t(80.9)=17.6,p<0.001 | |
na = not available; mn = mean; SD = standard deviation
In the UF cohort, PD had a significant increase in free-water values in the posterior SN compared with controls. Figure 2 shows that, in the UF cohort, the mean free-water value for the posterior SN was higher in PD (0.18 ± 0.04) compared with controls (0.14 ± 0.03) (p < 0.001). The difference in FAT between PD (0.49 ± 0.06) and control (0.52 ± 0.04) for the posterior SN was not statistically significant in the UF cohort (p = 0.68). Figure 2B shows the mean free-water values for the anterior SN was 0.22 ± 0.09 in PD, whereas it was 0.18 ± 0.07 in controls, and this difference approached significance (p = 0.09). The difference in FAT between PD and controls for the anterior SN was not statistically significant in the UF cohort (p = 0.42). The difference in MDT between PD and controls for the anterior SN (p = 0.43) and posterior SN (p = 0.52) was also not statistically significant in the UF cohort.
Figure 2.
Mean group free-water values (A) and free-water corrected fractional anisotropy (B) from the anterior and posterior SN in the University of Florida cohort. Error bars are +1 Standard error.
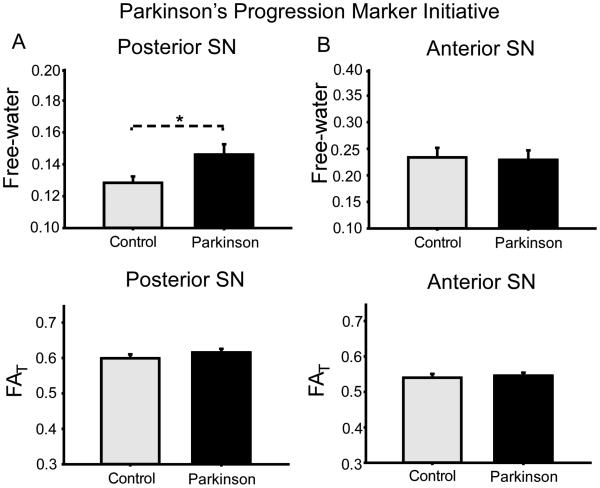
In the PPMI cohort, PD had a significant increase in free-water values in the posterior SN compared with controls. Specifically, Figure 3 shows the mean free-water value for the posterior SN was higher in PD (0.15 ± 0.05) than controls (0.13 ± 0.03) (p = 0.028). The difference in FAT between PD and controls for the posterior SN was not significant in the PPMI cohort. There was not a statistically significant difference between PD and controls in free-water (p = 0.92) or FAT (p = 0.29) for the anterior SN in the PPMI cohort. The difference in MDT between PD and controls was not statistically significant for the anterior SN (p = 0.95) or posterior SN (p = 0.87) in the PPMI cohort.
Figure 3.
Mean group free-water values (A) and free-water corrected fractional anisotropy (B) from the anterior and posterior SN in the PPMI cohort. Error bars are +1 Standard error.
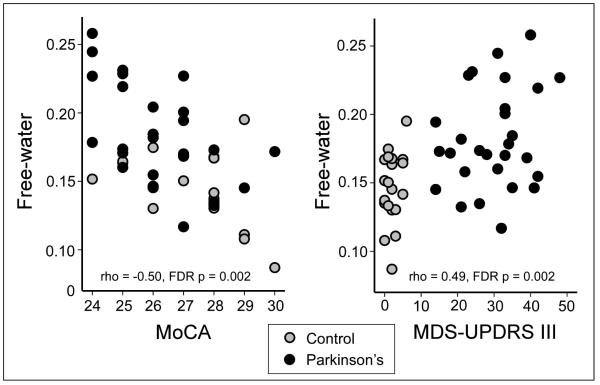
The correlation analysis was performed within each group and across all subjects from both groups included in the UF cohort. Table 2 shows the correlation Rho value, uncorrected p-value, and FDR corrected p-value. It is evident that the MoCA scores were negatively correlated with SN free-water levels within the PD and across groups. Figure 4A shows the relation between MoCA and SN free-water levels was negative across groups. It was also the case that the MDS-UPDRS-III and SN free-water levels were significantly related across groups, whereas the relation was not significant within each group (Table 2). Figure 4B shows the relation indicating that higher MDS-UPDRS-III scores were related to increased SN free-water. Table 2 also indicates that the correlations for age and free-water were not significant. In the PPMI cohort, free-water was not significantly correlated with MoCA, MDS-UPDRS-III, and age when examining within groups and across groups. Both uncorrected and FDR corrected p-values were > 0.05.
Table 2.
Correlation Analysis for University of Florida Cohort
| UF Free-water metrics vs. Demographic Information |
Rho,ρ | P-value, uncorrected |
FDR corrected p-value |
|---|---|---|---|
| SN Free-water vs. MoCA | |||
| Control | −0.37 | 0.11 | 0.20 |
| Parkinson’s | −0.48 | 0.01 | 0.03 |
| All Groups | −0.50 | <.001 | 0.002 |
| SN Free-water vs. UPDRS | |||
| Control | .151 | 0.53 | 0.60 |
| Parkinson’s | .151 | 0.44 | 0.57 |
| All Groups | 0.49 | <.001 | 0.002 |
| SN Free-water vs. Age | |||
| Control | 0.39 | 0.09 | 0.20 |
| Parkinson’s | −0.01 | 0.96 | 0.96 |
| All Groups | 0.17 | 0.25 | 0.38 |
Figure 4.
Correlation analysis between the MoCA and free-water (A) and MDS-UPDRS-III and free-water (B) for the UF Cohort. The data across groups is shown and each point is one subject.
In addition, within the PPMI cohort we observed a significant negative correlation between the contralateral posterior SN free-water values and contralateral putamen striatal binding ratio (SBR) across groups (Table 3). Contralateral refers to the side of the basal ganglia opposite to the most affected body side. We also found that contralateral SN FAT was significantly correlated with the contralateral putamen SBR within the PD group (Table 3). All other correlations for free-water and the putamen SBR were non-significant (Table 3).
Table 3.
Correlation Analysis for PPMI Cohort
| PPMI Free-water metrics vs. Striatal Binding Ratios |
Rho,ρ | P-value, uncorrected |
FDR corrected p-value |
|---|---|---|---|
| CL SN Free-water vs. Putamen SBR | |||
| Control | 0.19 | 0.17 | 0.53 |
| Parkinson’s | −0.11 | 0.34 | 0.53 |
| Both Groups | −0.23 | 0.01 | 0.05 |
| IP SN Free-water vs. Putamen SBR | |||
| Control | 0.15 | 0.25 | 0.53 |
| Parkinson’s | −0.11 | 0.33 | 0.53 |
| Both Groups | −0.05 | 0.59 | 0.64 |
| CL SN FAT vs. Putamen SBR | |||
| Control | 0.01 | 0.92 | 0.92 |
| Parkinson’s | −0.33 | 0.003 | 0.04 |
| Both Groups | −0.07 | 0.40 | 0.53 |
| IP SNFAT vs. Putamen SBR | |||
| Control | −0.13 | 0.35 | 0.53 |
| Parkinson’s | −0.09 | 0.45 | 0.54 |
| Both Groups | −0.15 | 0.18 | 0.53 |
The voxel-based morphometry analysis comparing GM and WM between PD and controls resulted in no significant between group differences. We also examined the GM, WM, and CSF measurements relative to the total intracranial volume. Correlation analysis including subjects from both groups was then examined between each volume measure and the posterior SN free-water value for the UF cohort. While the WM and CSF volumes were not significant (WM rho = 0.20; p = 0.17; FDR corrected p = 0.21; CSF rho = 0.26; p = 0.07; FDR corrected p = 0.21), the gray matter volume relative to intracranial volume was negatively correlated with posterior SN free-water values (rho = −0.46; p = 0.001; FDR corrected p < 0.05).
Discussion
The current investigation examined free-water values in the SN of PD patients and controls from a single-site and from a multi-site cohort. Free-water values were increased in the posterior region of the SN of PD participants compared with controls in both the single-site and multi-site studies. We did not observe changes in either cohort for free-water corrected FAT or free-water corrected MDT. In the PPMI PD cohort, we observed a significant correlation between the posterior SN free-water values and the putamen striatal binding ratio. In the UF cohort, we observed a significant correlation between the posterior SN free-water values and the MoCA scores. Our findings demonstrate that free-water mapping detected changes in the posterior SN in a large cohort of PD patients at a single-site and across multiple-sites, and that these changes in free-water related to dopamine transport and cognitive function.
Diffusion imaging can provide information about tissue microstructure that can be related to processes within the tissue. Several studies have reported changes in diffusion tensor indices in the SN of PD patients (Du et al., 2012; Vaillancourt et al., 2009). For example, Vaillancourt and colleagues (2009) found reduced FA values in the posterior SN of de novo PD patients using hand-drawn ROIs. Furthermore, Du et al. (2012) used a semi-automated protocol to define SN subregions and found that FA changes were evident at the time of disease diagnosis but that other imaging measures (e.g., transverse relaxation rate) were possibly more useful to capture disease progression. In the Du et al. study, the authors also found that the posterior portion of the SN was the region where PD patients had reduced FA values (these authors used the term caudal to describe the SN). In a study of moderate PD, progressive supranuclear palsy, multiple system atrophy, and essential tremor, it was found that the posterior SN was the region of interest most reliable for differentiating forms of parkinsonism from control subjects (Prodoehl et al., 2013a). In a study using a voxel-based approach evidence was found that the medial-posterior portion of the SN had different FA values between PD and controls (Peran et al. 2010). Thus, some studies in the literature have identified a similar region of the SN where degeneration occurs.
In the current study, the anterior SN was not different between groups for controls and PD in either the single-site and multi-site cohort. Both controls and PD groups in the UF cohort had elevated free-water in the anterior SN relative to the posterior SN (Figure 2), and this could be due to excessive partial volume effects near the cerebrospinal fluid. The UF PD group had elevated free-water in the anterior SN compared with controls that did not reach significance. It is possible that the close proximity of the anterior SN to the cerebrospinal fluid increases the variability of the free-water values in this region.
At the point of clinical expression of PD, pathology studies estimated that approximately half of the dopaminergic cells in the SN pars compacta are lost (Braak et al., 2003a; Hodaie et al., 2007a). In addition, the classic work of Fearnley and Lees (1991) has shown that PD-related cell loss occurs mainly in the ventrolateral tier of the SN pars compacta, and other studies have found a consistent spatial pattern in the SN (Damier et al., 1999; Ma et al., 1996). More recent work using sophisticated methods across a large range of disease duration also indicates that the ventrolateral tier of the SN is where dopaminergic cell loss is greatest in PD (Kordower et al., 2013). The ventrolateral tier is consistent with the location of the posterior SN identified in the current analysis of the single-site and multi-site cohorts. The current study is the largest cohort published to date on PD using diffusion MRI methods.
There has been wide variability in the methodology used to analyze diffusion MRI data for PD and other movement disorders (Hess et al., 2013). Some studies have used an ROI approach on individual subject data. In this approach, studies have focused on subregions of the SN (Du et al., 2012; Du et al., 2011; Prodoehl et al., 2013a; Vaillancourt et al., 2009), whereas other studies have examined one ROI in the SN (Chan et al., 2007; Menke et al., 2009; Péran et al., 2010; Rolheiser et al., 2011b; Zhan et al., 2012). There are also a series of studies that have transformed the diffusion MRI data to standardized space and used a voxel-based statistical approach (Péran et al., 2010; Zhan et al., 2012). Across these studies using different methods, the posterior portion of the SN has not always been found as the location of significant difference between PD and controls. A recent meta-analysis on DTI indices that combined data across different field strengths and analysis methods for PD indicated that disease effect size was not promising for identifying changes in the SN for PD relative to a control group (Schwarz et al., 2013). Our findings in the largest cohort to date challenge this conclusion, since the free-water metric identified changes in the same subregion of the SN in both a single-site and multi-site cohort of subjects. In addition to the analysis methods, the different findings in the literature may be related to the state of the disease of the patients, field strength of the scanner, number of diffusion directions, signal-to-noise ratio of the images, and inter-rater reliability.
Another possible explanation for differences in the literature is that the reported FA values likely included free-water contamination within the voxel, which would decrease the FA values (Pasternak et al., 2009). The current study is the first to have used the bi-tensor model for PD, and the use of the bi-tensor model helps probe the two compartments within the voxel. The use of the bi-tensor model can influence the data in two ways. First, the current study directly shows that it is the free-water within the voxel that is altered in PD within the SN, and this could be due to atrophy or cells loss related to neurodegeneration (Metzler-Baddeley et al., 2012). It is also possible that increased free-water is related to neuroinflammation (Pasternak et al., 2012), but future work is needed to determine if this is the case. Second, as shown in Figure 1 the midbrain of the free-water map tends to be more uniform in gray and white matter, whereas the FA map has a very sharp gradient from white matter (e.g. cerebral peduncle) to gray matter (e.g. SN) in the midbrain. This issue is fundamental from a methodological standpoint because any mis-registration or misplacement of the ROI can cause substantial effects for the FA value and this can increase between subject variability. In contrast, since the free-water metric is more uniform across the midbrain any slight error made in registration or placement of the ROI will not affect the outcome of the free-water value to the same extent. This has the outcome of reducing the between subject variability of the free-water values. Thus, the current findings using the bi-tensor model help to reconcile some of the differences in the PD literature that used diffusion imaging in the SN. Here, we used an automated procedure in standardized space and found that free-water values were increased at the group level in the posterior SN of PD.
Pasternak and colleagues have demonstrated that free-water mapping can provide additional information about tissue microstructure and free-water (Pasternak et al., 2009; Pasternak et al., 2012). In prior studies using free-water analysis of diffusion-weighted MRI data, it was shown that both free-water and FAT were altered in schizophrenia, but it was free-water accumulation that was the major factor across white matter tracts (Berlot et al., 2014; Pasternak et al., 2012). It has been suggested that the free-water measure is mostly related to the extracellular space because the diffusion time of most diffusion MRI sequences (e.g. as in the current UF sequence Δ = 42.4 ms) dictates that free-water mostly likely comes from spaces larger than a few tens of microns. Since cells in the brain are typically smaller, free-water is likely related to the signal from large enough water pockets consistent with the extra-cellular space (Pasternak et al., 2012; Wang et al., 2011). Further it was suggested that changes in the extracellular space can be attributed to atrophy (Maier-Hein et al., 2014), neuroinflammation (Pasternak et al., 2012) or changes in cellular density (Pasternak et al., 2014).
We used VBM analysis of the T1-weighted images and did not find macrostructural changes in the whole brain and substantia nigra consistent with most other VBM studies in non-demented PD (Price et al., 2004; Prodoehl et al., 2013b; Tessitore et al., 2012). This does not rule out the possibility that atrophy based neurodegeneration was occurring within the SN voxels because the free-water measurement could be more sensitive to neurodegeneration than VBM of the T1 image. Interestingly, the GM volume was negatively correlated with the posterior SN free-water value, suggesting that increased free-water in the SN was related to reduced GM volume in the whole brain. A similar finding was also recently reported in Alzheimer’s disease (Maier-Hein et al., 2014), strengthening the link between local microstructural changes measured with free-water and global volumetric changes, such as those measured using VBM.
The findings from the current study that observed increases in free-water of the SN extend other reports observing increased iron content in the SN of PD patients (Du et al., 2012; Péran et al., 2010). It is suggested that iron is involved in myelination, neurotransmission, and plays a role in the reduction of dopaminergic activity. Iron may play a prominent causative role in the death of neurons by inducing oxidative stress and lipid peroxidation (Mounsey and Teismann, 2012). Several reports have shown that markers of increased free radicals associated with cell membrane degradation of the SN are increased in PD (Farooqui and Farooqui, 2011; Uttara et al., 2009). As such, the increase in free-water in the SN in PD may be a consequence of mechanisms that contribute to increased oxidative stress. Further work is needed to understand the relation between various MRI measurements and free-water and to determine how free-water may reflect different aspects of PD-related changes in the SN.
In the current study, there was a negative correlation between the DAT-SPECT binding in the putamen and free-water values in the posterior SN of the PPMI cohort. In prior work using a different technique, a significant correlation between mean diffusivity and putaminal [18F] DOPA uptake was found (Scherfler et al., 2013). In this prior study, the authors examined the upper, middle, and lower parts of the substantia nigra on three separate slices, and they found effects when averaging across all three slices and when examining the middle and lower slices. The current study focused on a similar set of three slices, which were all below the subthalamic nucleus and consistent with ventral portion of the substantia nigra. We averaged across the three slices for assessing the anterior and posterior SN. Also, another difference between the study by Scherfler and colleagues is that they measured [18F] DOPA uptake whereas the current study related free-water values in the SN with DAT-SPECT binding in the putamen. Thus, our findings provide new evidence that alteration in the SN relates to dopamine transporter imaging values in the putamen.
In conclusion, the present study showed that free-water is elevated in the posterior SN of PD patients compared with controls across a single-site and multi-site cohort. This is the first study to have used the bi-tensor model in the SN of PD and control subjects. Future studies examining both animal models and human brains are needed to better understand the relationship between free-water values and metrics of neurodegenerative processes. Nevertheless, our findings suggest that free-water may be a useful measure to monitor in future therapeutic trials focused on modifying the SN in PD.
Highlights.
Bi-tensor diffusion analysis model quantifies fractional volume of free-water
We examined free-water in the SN of healthy controls and PD patients
We report increased free-water in the posterior SN for PD from a single-site and multi-site study
We propose that changes in free-water may indicate SN degeneration in PD
Acknowledgements
Data used in the preparation of this article were obtained from the PPMI database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org, Funding for PPMI was provided by the Michael J. Fox Foundation.
The authors would like to thank the participants and their families for their time and commitment to this research. PPMI, a public-private partnership, is funded by The Michael J. Fox Foundation for Parkinson’s Research and funding partners, including Abbott, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, Elan, GE Healthcare, Genentech, GlaxoSmithKline, Eli Lilly and Company, Merck, Meso Scale Discovery, Pfizer Inc., Roche Inc., and UCB Inc. The authors would also like to acknowledge the support of the Bachmann-Strauss Center of Excellence and the NPF Center of Excellence.
Study funding:
This work was supported by the National Institutes of Health (R01 NS052318, R01 NS075012) and the Bachmann-Strauss Dystonia & Parkinson Foundation. Funding for PPMI was provided by the Michael J. Fox Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander AL, Hasan KM, Lazar M, Tsuruda JS, Parker DL. Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med. 2001;45:770–780. doi: 10.1002/mrm.1105. [DOI] [PubMed] [Google Scholar]
- Barcia C, Bahillo AS, Fernández-Villalba E, Bautista V, Poza Y Poza M, Fernández-Barreiro A, Hirsch EC, Herrero M-T. Evidence of active microglia in substantia nigra pars compacta of parkinsonian monkeys 1 year after MPTP exposure. Glia. 2004;46:402–409. doi: 10.1002/glia.20015. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. doi: 10.1006/jmrb.1996.0086. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Berlot R, Metzler-Baddeley C, Jones DK, O'Sullivan MJ. CSF contamination contributes to apparent microstructural alterations in mild cognitive impairment. Neuroimage. 2014;92:27–35. doi: 10.1016/j.neuroimage.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003a;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Tredici KD, Rüb U, de Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003b;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Chan LL, Rumpel H, Yap K, Lee E, Loo HV, Ho GL, Fook-Chong S, Yuen Y, Tan EK. Case-control study of diffusion tensor imaging in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2007.121525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. Pt 8. [DOI] [PubMed] [Google Scholar]
- Du G, Lewis MM, Sen S, Wang J, Shaffer ML, Styner M, Yang QX, Huang X. Imaging nigral pathology and clinical progression in Parkinson's disease. Movement Disorders. 2012;27:1636–1643. doi: 10.1002/mds.25182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G, Lewis MM, Styner M, Shaffer ML, Sen S, Yang QX, Huang X. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson's disease. Mov Disord. 2011;26:1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui T, Farooqui AA. Lipid-mediated oxidative stress and inflammation in the pathogenesis of Parkinson's disease. Parkinsons Dis. 20112011:247467. doi: 10.4061/2011/247467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114:2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fonov V, Evans AC, Botteron K, Almli CR, McKinstry RC, Collins DL. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyoneva S, Shapiro L, Lazo C, Garnier-Amblard E, Smith Y, Miller GW, Traynelis SF. Adenosine A2A receptor antagonism reverses inflammation-induced impairment of microglial process extension in a model of Parkinson's disease. Neurobiology of Disease. 2014 doi: 10.1016/j.nbd.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess CW, Ofori E, Akbar U, Okun MS, Vaillancourt DE. The evolving role of diffusion magnetic resonance imaging in movement disorders. Curr Neurol Neurosci Rep. 2013;13:400. doi: 10.1007/s11910-013-0400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodaie M, Neimat JS, Lozano AM. The dopaminergic nigrostriatal system and Parkinson's disease: molecular events in development, disease, and cell death, and new therapeutic strategies. Neurosurgery. 2007a;60:17–28. doi: 10.1227/01.NEU.0000249209.11967.CB. discussion 28-30. [DOI] [PubMed] [Google Scholar]
- Hodaie M, Neimat JS, Lozano AM. The Dopaminergic Nigrostriatal Systemand Parkinson's Disease: Molecular Eventsin Development, Disease, and Cell Death, and New Therapeutic Strategies. Neurosurgery. 2007b;60:17–30. doi: 10.1227/01.NEU.0000249209.11967.CB. 10.1227/1201.NEU.0000249209.0000211967.CB. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 2001;57:S34–38. 1992. [PubMed] [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136:2419–2431. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Sharman MA, Santos CLD, Paquin R, Gallea C. Magnetic resonance imaging of the substantia nigra in Parkinson's disease. Movement Disorders. 2012;27:822–830. doi: 10.1002/mds.25015. [DOI] [PubMed] [Google Scholar]
- Ma SY, Rinne JO, Collan Y, Röyttä M, Rinne UK. A quantitative morphometrical study of neuron degeneration in the substantia nigra in Parkinson's disease. Journal of the Neurological Sciences. 1996;140:40–45. doi: 10.1016/0022-510x(96)00069-x. [DOI] [PubMed] [Google Scholar]
- Maier-Hein KH, Westin CF, Shenton ME, Weiner MW, Raj A, Thomann P, Kikinis R, Stieltjes B, Pasternak O. Widespread white matter degeneration preceding the onset of dementia. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2014.04.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke RA, Scholz J, Miller KL, Deoni S, Jbabdi S, Matthews PM, Zarei M. MRI characteristics of the substantia nigra in Parkinson's disease: a combined quantitative T1 and DTI study. Neuroimage. 2009;47:435–441. doi: 10.1016/j.neuroimage.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Metzler-Baddeley C, O'Sullivan MJ, Bells S, Pasternak O, Jones DK. How and how not to correct for CSF-contamination in diffusion MRI. Neuroimage. 2012;59:1394–1403. doi: 10.1016/j.neuroimage.2011.08.043. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Kondo T, Riederer P, Inagaki H, Minami M, Nagatsu T. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from parkinsonian patients. Neuroscience Letters. 1994a;180:147–150. doi: 10.1016/0304-3940(94)90508-8. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Narabayashi H, Inagaki H, Minami M, Nagatsu T. Interleukin (IL)-1β, IL-2, IL-4, IL-6 and transforming growth factor-α levels are elevated in ventricular cerebrospinal fluid in juvenile parkinsonism and Parkinson's disease. Neuroscience Letters. 1996;211:13–16. doi: 10.1016/0304-3940(96)12706-3. [DOI] [PubMed] [Google Scholar]
- Mogi M, Harada M, Riederer P, Narabayashi H, Fujita K, Nagatsu T. Tumor necrosis factor-α (TNF-α) increases both in the brain and in the cerebrospinal fluid from parkinsonian patients. Neuroscience Letters. 1994b;165:208–210. doi: 10.1016/0304-3940(94)90746-3. [DOI] [PubMed] [Google Scholar]
- Mounsey RB, Teismann P. Chelators in the Treatment of Iron Accumulation in Parkinson's Disease. International Journal of Cell Biology. 2012;2012:12. doi: 10.1155/2012/983245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Koerte IK, Bouix S, Fredman E, Sasaki T, Mayinger M, Helmer KG, Johnson AM, Holmes JD, Forwell LA, Skopelja EN, Shenton ME, Echlin PS. Hockey Concussion Education Project, Part 2. Microstructural white matter alterations in acutely concussed ice hockey players: a longitudinal free-water MRI study. J Neurosurg. 2014;120:873–881. doi: 10.3171/2013.12.JNS132090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O, Westin C-F, Bouix S, Seidman LJ, Goldstein JM, Woo T-UW, Petryshen TL, Mesholam-Gately RI, McCarley RW, Kikinis R, Shenton ME, Kubicki M. Excessive Extracellular Volume Reveals a Neurodegenerative Pattern in Schizophrenia Onset. The Journal of Neuroscience. 2012;32:17365–17372. doi: 10.1523/JNEUROSCI.2904-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péran P, Cherubini A, Assogna F, Piras F, Quattrocchi C, Peppe A, Celsis P, Rascol O, Démonet J-F, Stefani A, Pierantozzi M, Pontieri FE, Caltagirone C, Spalletta G, Sabatini U. Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain. 2010;133:3423–3433. doi: 10.1093/brain/awq212. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV. Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med. 2003;49:953–961. doi: 10.1002/mrm.10452. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Jones Derek K. Removing CSF Contamination in Brain DT-MRIs by Using a Two-Compartment Tensor Model. Proceedings 12th Annual Meeting ISMRM; Kyoto. 2004. [Google Scholar]
- Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, Fox N. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage. 2004;23:663–669. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Prodoehl J, Li H, Planetta PJ, Goetz CG, Shannon KM, Tangonan R, Comella CL, Simuni T, Zhou XJ, Leurgans S, Corcos DM, Vaillancourt DE. Diffusion tensor imaging of Parkinson's disease, atypical parkinsonism, and essential tremor. Mov Disord. 2013a;28:1816–1822. doi: 10.1002/mds.25491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prodoehl J, Planetta PJ, Kurani AS, Comella CL, Corcos DM, Vaillancourt DE. Differences in brain activation between tremor- and nontremor-dominant Parkinson disease. JAMA Neurol. 2013b;70:100–106. doi: 10.1001/jamaneurol.2013.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radau PE, Linke R, Slomka PJ, Tatsch K. Optimization of automated quantification of 123I-IBZM uptake in the striatum applied to parkinsonism. J Nucl Med. 2000;41:220–227. [PubMed] [Google Scholar]
- Rolheiser T, Fulton H, Good K, Fisk J, McKelvey JR, Scherfler C, Khan N, Leslie R, Robertson H. Diffusion tensor imaging and olfactory identification testing in early-stage Parkinson's disease. Journal of Neurology. 2011a;258:1254–1260. doi: 10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- Rolheiser TM, Fulton HG, Good KP, Fisk JD, McKelvey JR, Scherfler C, Khan NM, Leslie RA, Robertson HA. 2011b;258:1254–1260. doi: 10.1007/s00415-011-5915-2. [DOI] [PubMed] [Google Scholar]
- Scherfler C, Esterhammer R, Nocker M, Mahlknecht P, Stockner H, Warwitz B, Spielberger S, Pinter B, Donnemiller E, Decristoforo C, Virgolini I, Schocke M, Poewe W, Seppi K. Correlation of dopaminergic terminal dysfunction and microstructural abnormalities of the basal ganglia and the olfactory tract in Parkinson’s disease. Brain. 2013;136:3028–3037. doi: 10.1093/brain/awt234. [DOI] [PubMed] [Google Scholar]
- Schwarz ST, Abaei M, Gontu V, Morgan PS, Bajaj N, Auer DP. Diffusion tensor imaging of nigral degeneration in Parkinson's disease: A region-of-interest and voxel-based study at 3 T and systematic review with meta-analysis. NeuroImage. Clinical. 2013;3:481–488. doi: 10.1016/j.nicl.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto Y, Matsuda H, Kamiya K, Maikusa N, Nakata Y, Ito K, Ota M, Matsunaga N, Sato N. In vivo evaluation of gray and white matter volume loss in the parkinsonian variant of multiple system atrophy using SPM8 plus DARTEL for VBM. Neuroimage Clin. 2013;2:491–496. doi: 10.1016/j.nicl.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutachan JJ, Casas Z, Albarracin SL, Robert B, Ii S, Samudio I, Gonzalez J, Morales L, Barreto GE. Cellular and molecular mechanisms of antioxidants in Parkinson ’ s disease. 2012;15 doi: 10.1179/1476830511Y.0000000033. [DOI] [PubMed] [Google Scholar]
- Tessitore A, Amboni M, Cirillo G, Corbo D, Picillo M, Russo A, Vitale C, Santangelo G, Erro R, Cirillo M, Esposito F, Barone P, Tedeschi G. Regional gray matter atrophy in patients with Parkinson disease and freezing of gait. AJNR Am J Neuroradiol. 2012;33:1804–1809. doi: 10.3174/ajnr.A3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Current neuropharmacology. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Abraham I, Corcos DM, Zhou XJ, Comella CL, Little DM. High-resolution diffusion tensor imaging in the substantia nigra of de novo Parkinson disease. Neurology. 2009;72:1378–1384. doi: 10.1212/01.wnl.0000340982.01727.6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt DE, Spraker MB, Prodoehl J, Zhou XJ, Little DM. Effects of aging on the ventral and dorsal substantia nigra using diffusion tensor imaging. Neurobiol Aging. 2012;33:35–42. doi: 10.1016/j.neurobiolaging.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkateshappa C, Harish G, Mythri R, Mahadevan A, Srinivas Bharath MM, Shankar SK. Increased Oxidative Damage and Decreased Antioxidant Function in Aging Human Substantia Nigra Compared to Striatum: Implications for Parkinson’s Disease. Neurochemical Research. 2012;37:358–369. doi: 10.1007/s11064-011-0619-7. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH, Song SK. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan W, Kang GA, Glass GA, Zhang Y, Shirley C, Millin R, Possin KL, Nezamzadeh M, Weiner MW, Marks WJ, Schuff N. Regional alterations of brain microstructure in Parkinson's disease using diffusion tensor imaging. Movement Disorders. 2012;27:90–97. doi: 10.1002/mds.23917. [DOI] [PMC free article] [PubMed] [Google Scholar]