Abstract
Purpose
Adoptive transfer of autologous tumor infiltrating lymphocytes (TIL) can mediate durable cancer regression in selected patients with metastatic melanoma. However, the tumor antigens associated with these favorable responses remain unclear. We hypothesized that a clinical strategy involving the iterative adoptive transfer of selected autologous antigen specific T cell clones could help systematically define immunologic targets associated with successful cancer therapy, without the interpretative ambiguity of transferring polyclonal populations. Here, we evaluated the clinical efficacy of CD8+ T cell clones specific for the melanocyte differentiation antigens (MDA), gp100 and MART-1, respectively.
Experimental Design
We conducted two consecutive phase II clinical trials involving the adoptive transfer of highly selected autologous antigen specific CD8+ T cell clones against gp100 and MART-1, respectively. Fifteen HLA-A2+ treatment-refractory metastatic melanoma patients received highly avid MDA specific CD8+ T cell clones specific for either gp100 (n=10) or MART-1 (n=5) with or without intravenous interleukin-2 after a lymphodepleting myeloablative preparative regimen.
Results
Of the fifteen treated patients, we observed immune mediated targeting of skin melanocytes in eleven patients (73%) and clonal engraftment in eight patients (53%) after cell transfer. There were only transient minor tumor regressions observed, but no objective tumor responses based upon RECIST criteria.
Conclusions
Despite successful clonal repopulation and evidence of in vivo antigen targeting, the poor therapeutic efficacy after the adoptive transfer of autologous MDA specific T cells raises significant concerns regarding future immunotherapy efforts targeting this class of tumor antigens.
Keywords: Immunotherapy, CTL clones, melanocyte differentiation antigens, metastatic melanoma
Introduction
Cancer regression in patients with metastatic melanoma can now be achieved with three mechanistically distinct types of immunotherapies that augment naturally existing anti-tumor T cell responses: 1.) Systemic cytokine therapy (1, 2), 2.) Checkpoint inhibition (3–6), and 3.) Adoptive transfer of autologous tumor infiltrating lymphocytes (TIL) (7–9). These clinical findings have drawn attention to the significant therapeutic potential of exploiting endogenous T cell populations for cancer therapy. However, efforts to improve current immunotherapies are hindered by a limited understanding of the specific lymphocyte populations that were responsible for the observed tumor responses. Further, the tumor antigens associated with durable and complete cancer regression remain unclear, thus hindering the development of targeted immunotherapeutics. We hypothesized that a clinical strategy involving the iterative adoptive transfer of highly selected autologous antigen specific T cell clones could help systematically define immunologic targets associated with successful cancer therapy, without the interpretative ambiguity of transferring polyclonal T cell populations. In this approach, T cell clones could be selected ex vivo based upon high avidity recognition of specific tumor antigen epitopes, expanded to large numbers, and re-introduced into the autologous host after a lymphodepleting preparative regimen to eliminate regulatory cells and augment homeostatic expansion.
Here, we report two sequential phase II clinical trials for patients with refractory metastatic melanoma in which the class of melanocyte differentiation antigens (MDA) was targeted with highly avid CD8+ T cell clones specific for either gp100 or MART-1, respectively. The targeting of these MDAs, which are expressed in both normal melanocytes and melanoma tumors, was prompted by the significant natural immunogenicity of these proteins as evident by the high frequency of primed MDA specific CD8+ T cells found within the TIL of melanoma metastases (9–12). Further, there has been a long observed association between the development of vitiligo and uveitis due to melanocyte destruction and melanoma tumor regression in patients undergoing immunotherapy (13–17).
We previously reported a proof of concept experience isolating MDA specific CD8+ T cell clones from peripheral blood using high throughput in vitro sensitization that enabled rapid clone isolation for clinical therapy (18, 19). In the initial five patients, we found that MDA specific effector clones could target skin melanocytes in an autoimmune fashion, persist long term in peripheral blood, and undergo self-renewal to repopulate the memory pool after adoptive transfer. We now update this experience with the clinical results from fifteen metastatic melanoma patients treated with MDA specific CD8+ T cell clones. Our findings suggest that despite successful clonal repopulation with autologous MDA specific CD8+ T cells, the targeting of MDAs was insufficient to mediate meaningful cancer regression in metastatic melanoma patients. These findings raise significant concerns regarding future immunotherapy efforts directed against MDAs.
Materials and Methods
Patients and clinical protocol
HLA-A2+ patients with metastatic melanoma were treated with either gp100-specific CD8+ T cell clones (n=10) or MART-1-specific CD8+ T cell clones (n=5) at the Surgery Branch, National Cancer Institute (NCI), between January 2009 and January 2013 on two consecutive phase II clinical protocols (NCT00665470 and NCT01495572) approved by the Institutional Review Board and U.S. Food and Drug Administration. All patients gave informed consent for treatment in accordance with the Declaration of Helsinki. The patients were required to be 18 years of age or older and have measurable metastatic melanoma that expressed gp100 or MART-1 and Major Histocompatibility Complex Class I by immunohistochemistry. Prior to clone infusion, patients were transiently lymphoablated with a nonmyeloablative lymphodepleting regimen including intravenous administration of cyclophosphamide (60 mg/kg) for 2 days followed by fludarabine (25 mg/m2) for 5 days as previously described (9). One day after completion of their lymphodepleting regimen, patients received expanded CD8+ T cell clones intravenously, either with or without high-dose IL-2 (720,000 IU/kg) every 8 hours to tolerance. Patients received baseline computed tomography (CT) and/or magnetic resonance imaging (MRI) and/or positron emission tomography (PET) before treatment. Tumor size was evaluated monthly for three months and at regular intervals thereafter by CT, MRI, or documented with photography for cutaneous/subcutaneous lesion. Tumor measurements and patient responses were determined according to Response Evaluation Criteria in Solid Tumor (RECIST).
Media and cell culture
Human cultured cell lines including T2 cells (HLA-A2+ peptide transporter-associated protein deficient T-B hybrid) and melanoma tumor lines, 526 mel (HLA-A2+/MART-1+gp100+), 624 mel (HLA-A2+/MART-1+gp100+), 888 mel (HLA-A2-/MART-1+gp100+), 938 mel (HLA-A2-/MART-1+gp100+) were routinely cultured in complete medium (CM) as previously described (19). T2 cells and the melanoma cell lines, 526mel, 624mel, 938mel and 888mel, were obtained from the cell production facility in the Surgery Branch, NCI. The tumor cells had been characterized to confirm tumor morphology, antigen, and HLA expression by immunohistochemistry; they were obtained and used within six months of testing. Human PBMCs used in this study were obtained by leukapheresis from HLA-A2+ metastatic melanoma patients evaluated on IRB approved protocols at the Surgery Branch, National Cancer Institute (NCI, National Institutes of Health, Bethesda, MD). Human PBMC and CD8+ T cell clones were cultured in CM with 10% heat-inactivated human AB serum (Gemini Bio-Products).
Generation of MDA specific CD8+ T cell clones for adoptive transfer
PBMCs from HLA-A2+ melanoma patients underwent depletion of CD4+ lymphocytes by magnetic bead separation (Miltenyi) and were plated as individual microcultures in 96-well flat-bottomed plates at ~1 × 105 cells per well. The cells underwent in vitro sensitization for 10–14 days in the presence of 1μg/mL of either gp100154–162 (KTWGQYWQV) or the modified 10-mer MART-126–35(27L) (ELAGIGILTV) GMP grade peptide (Multiple Peptide Systems, San Diego, CA) and IL-2 (90 IU/ml) as previously described (19). Individual microcultures that exhibited specific peptide reactivity either by a high- throughput qPCR cytokine screening or a high-throughput flow cytometry tetramer screening assay were selected for further expansion. To derive antigen specific CD8+ T cell clones, limiting dilution was typically performed by plating between 1 and 3 T cells in each well of a 96-well U-bottomed plate in 0.2 ml of conditioned medium containing anti-CD3 mAb Orthoclone OKT3 (50 ng/ml) (Ortho-Biotech) and IL-2 (300 IU/ml) with 5 ×104 autologous irradiated (40 Gy) PBMCs. On day 5 and every 3 to 4 days thereafter, half of the medium in each well was replaced with fresh medium containing IL-2. Growth-positive culture rate was typically ~10 to 12%. Characterization of clone function was performed with enzyme-linked immunosorbent assay to quantify IFN-γ secretion in response to limiting concentrations of gp100154–162 or the native MART-127–35 (AAGIGILTV) peptide pulsed onto T2 cells and antigen-positive tumor lines. Selected clones were subsequently expanded with anti-CD3 mAb (50 ng/ml), IL-2 (300 IU/ml), and 3 ×107 irradiated allogeneic PBMCs in upright 25-cm2 flasks for 14 days. Final large scale expansion of the clones for patient therapy was performed by the Surgery Branch Cell Production Facility using methods previously described (9, 20). The production time for clone generation from in vitro stimulation to the final treatment product was approximately 6–7 weeks (18–20).
TCR gene sequencing
Confirmation of CD8+ T cell clonality and determination of TCR clonotype was performed by purifying RNA from each clone using Qiagen RNeasy kits. 5' RACE was performed using BD SmartRace reagents and protocol, using the universal 5' forward primer, and a 3' gene- specific reverse primer for the TCR α constant region, or C1 or C2 β constant regions. Results were run on an agarose gel and appropriately sized bands (800–900 bp) were excised, subcloned into pCR2.1 (Invitrogen Life Technologies) vector and sequenced.
Tetramers, mAbs, and flow cytometric immunofluorescence analysis
Phycoerythrin-conjugated MART-126–35(27L) (ELAGIGILTV) peptide/HLA-A*0201 tetramer complexes were obtained from Immunotech, Beckman Coulter. Phycoerythrin-conjugated gp100154–162 (KTWGQYWQV) peptide/HLA-A*0201 tetramer complexes were obtained from the National Institutes of Health Tetramer Facility. Anti-human CD8, CD3, CD45RO, CD62L and CD95 monoclonal antibodies were obtained from BD Biosciences. Immunofluorescence, analyzed as the relative log fluorescence of live cells, was measured using a FACSCanto II flow cytometer with FACSDiva software (BD Biosciences) and FlowJo software (Tree Star, Inc.)
ELISA based cytokine release assay
Responder cells (1×105 T cells) and stimulator cells (1 × 105 peptide pulsed T2 cells or tumor lines) were co-incubated in a 0.2-ml volume in individual wells of a 96-well plate. Supernatants were harvested from duplicate wells after 20–24 hours and IFN-γ secretion was measured in culture supernatants using commercially available IFN-γ ELISA kits (Endogen). All data is presented as a mean of duplicate samples. Cultures with IFN-γ production greater than 100 pg/ml and twice background were considered as having specific antigen reactivity.
Intracellular FACS
Antigen specific T cells were cocultured with T2 cells pulsed with the cognate peptide versus a control peptide for 2 hours. Brefeldin A (eBioscience) was added to the coculture for another 4 hours. The cells were then fixed in 2% paraformaldehyde, permeabilized, and stained with anti-CD3 and anti-CD8 (BD Biosciences) along with anti-IFN-γ-PE-Cy7 and anti-IL-2-APC (eBioscience). Cytokine staining was assessed on CD3+CD8+ gated cells.
Cytotoxicity assays
HLA matched and mismatched target tumor lines were loaded with 15uM calcein-AM (Invitrogen) for 30 min at 37oC, washed three times, then plated at 5000 cells per well/100μL CM in a 96 well round bottom plate. An equal volume of effectors were added at different concentrations to target cells (E:T ratios) as indicated in the experiment. After a 4 hr coculture, supernatants were harvested and free calcein was quantitated using Glomax UV detection system (Promega). The % of specific cytotoxicity was calculated as (experimental release-spontaneous release)/(maximum release-spontaneous release) × 100. Spontaneous release was determined by incubating the targets with 100 μL of CM instead of effector cells, and maximum release was determined by incubating the targets with 100 μl of 0.5% Triton-X. All data is presented as the mean + SEM of triplicate samples.
Determination of clonal persistence in patient PBMC
To evaluate in vivo persistence of T cells in the peripheral blood, PBMCs were prepared from samples drawn on day 0 (pre infusion) and at day 30 (post infusion) and cryopreserved so all samples could be analyzed simultaneously. 1–2 × 106 PBMC were stained for 30 min at 4–9°C with peptide–MHC tetramer-PE, anti-CD8-APC and the cell viability dye, propidium iodide, to exclude dead cells, and analyzed by flow cytometry. The degree of persistence of transferred clones is presented as the frequency of tetramer-positive, CD8+ lymphocytes over the total number of CD8+ cells. To determine long term engraftment, we analyzed PBMC samples by flow cytometry over an extended period of time (days 60, 90, 120 and 150) and derived the absolute number of infused clones (CD8+TET+Vβ+) per microliter of blood.
Results
Patient and Treatment Characteristics
A total of 15 HLA-A*0201+ patients with refractory metastatic melanoma, including five patients described in a previous report (19), were enrolled upon two consecutive clinical trials (NCT00665470 and NCT01495572) in which they received ex vivo-expanded MDA specific CD8+ T cell clones in conjunction with a non-myeloablative lymphodepleting conditioning regimen. The protocol was designed to evaluate the persistence, safety, and therapeutic efficacy of MDA-reactive clones. The characteristics of the patients and their cell therapy are shown in Table 1. At the time of enrollment, all patients had demonstrated progression of their metastatic disease after prior systemic therapy. The first ten patients were treated with CD8+ T cell clones specific for the gp100154–62 epitope and the next five patients received CD8+ T cells specific for the MART27–35 epitope. The TCR clonotype for each clone was defined by complete molecular sequencing of the beta chain variable region (Vβ). Each patient received a single clonotype except for patient M2 who received two unique clonotypes. The mean number of infused CD8+ T cell clones was 15.8 × 109 (range 0.1–58.3 × 109). Patients, who were medically eligible, received concomitant high dose IL-2 infusions that were administered to tolerance. All patients in the MART-1 cohort presented with significant clinical comorbidities and were therefore ineligible for IL-2 administration.
Table 1.
Patient Characteristics and Clinical Results
| Pt # | Age | Sex | Melanoma origin | Disease Sites | Stage | Clone Specificity | Clonotype | TCR VβNo. infused clones (×109) | IL-2doses | Autoimmunity | Tumor Response | 30 day clone persistence (% CD8+Tet+Vβ+) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GP1 | 56 | M | Cutaneous | Lu, LN | M1b | gp100 | 6.5 | 45.1 | 12 | Rash | NR | 4.1 |
| GP2 | 60 | M | Ocular | Li | M1c | gp100 | 7.6 | 0.4 | 9 | Rash | NR | 0.9 |
| GP3 | 56 | M | Cutaneous | LN | M1a | gp100 | 7.6 | 33.1 | 4 | Rash | NR | 3.1 |
| GP4 | 51 | M | Ocular | Li | M1c | gp100 | 7.6 | 22.2 | 7 | Rash | NR | 2.4 |
| GP5 | 55 | F | Mucosal | Lu, Li, SQ, LN | M1c | gp100 | 29.1 | 18.8 | 7 | Rash | NR | <0.1 |
| GP6 | 34 | F | Cutaneous | SQ, LN, brain | M1c | gp100 | 12.3 | 10.8 | 9 | None | NR | <0.1 |
| GP7 | 62 | M | Mucosal | Lu | M1b | gp100 | 7.6 | 1.5 | 9 | Rash | NR | <0.1 |
| GP8 | 55 | M | Ocular | Lu, Li | M1c | gp100 | 7.6 | 0.1 | 6 | Rash | NR | <0.1 |
| GP9 | 66 | M | Mucosal | Adrenal, SQ, LN, BM | M1c | gp100 | 9 | 1.5 | 6 | None | NR | <0.1 |
| GP10 | 45 | M | Cutaneous | SQ, LN | M1a | gp100 | 4.1 | 9.5 | 7 | Rash | NR | 0.7 |
| M1 | 65 | F | Cutaneous | Cut, SQ, LN | M1a | MART | 7.3 | 1.5 | 0 | Rash | NR | <0.1 |
| M2 | 45 | M | Cutaneous | Brain, Lu, Li, LN, ST | M1c | MART | 4.1/24.1 | 21.4/8.7 | 0 | Vitiligo | NR | 0.3/6.5 |
| M3 | 35 | M | Mucosal | Lu, Li, Bone | M1c | MART | 2 | 4.3 | 0 | None | NR | <0.1 |
| M4 | 52 | F | Cutaneous | Lu, LN | M1b | MART | 4.3 | 58.3 | 0 | Rash | NR | 7.8 |
| M5 | 42 | M | Cutaneous | Lu, Li, bone, LN | M1c | MART | 28 | 15.1 | 0 | None | NR | 3.8 |
M= Male, F= Female, Lu= Lung, LN= Lymph Node, Li= Liver, SQ= Subcutaneous, BM= Bone Marrow, Cut= Cutaneous, TCR= T Cell Receptor, NR= Non-response
Characteristics of infused MDA specific CD8+ T cell clones
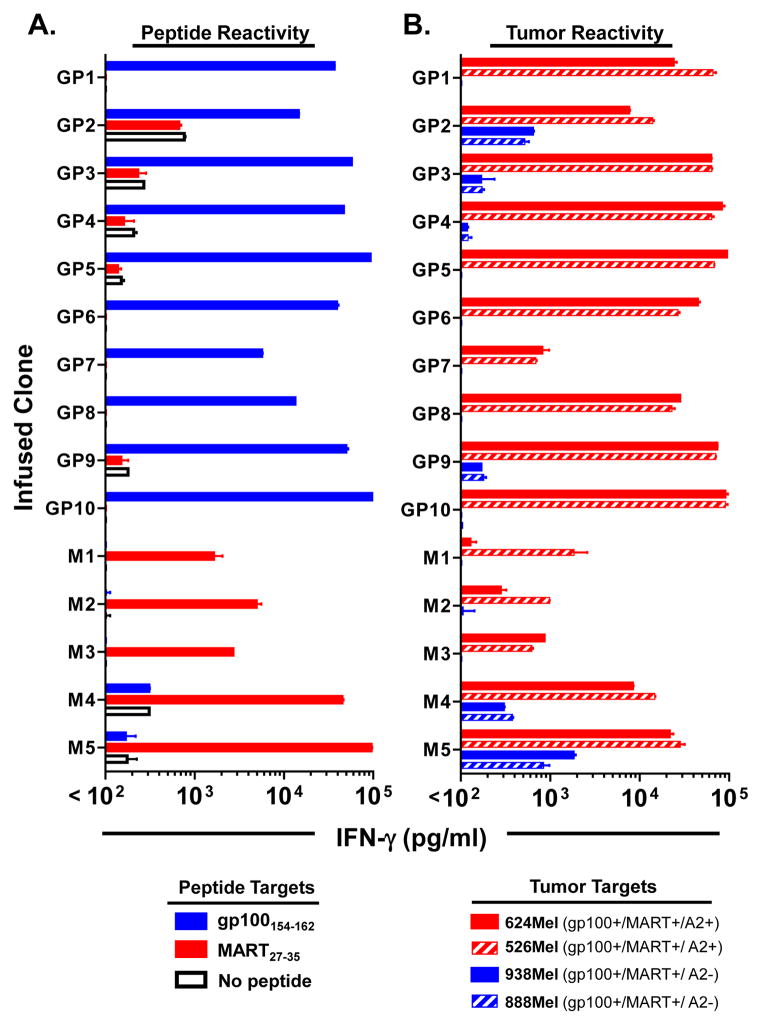
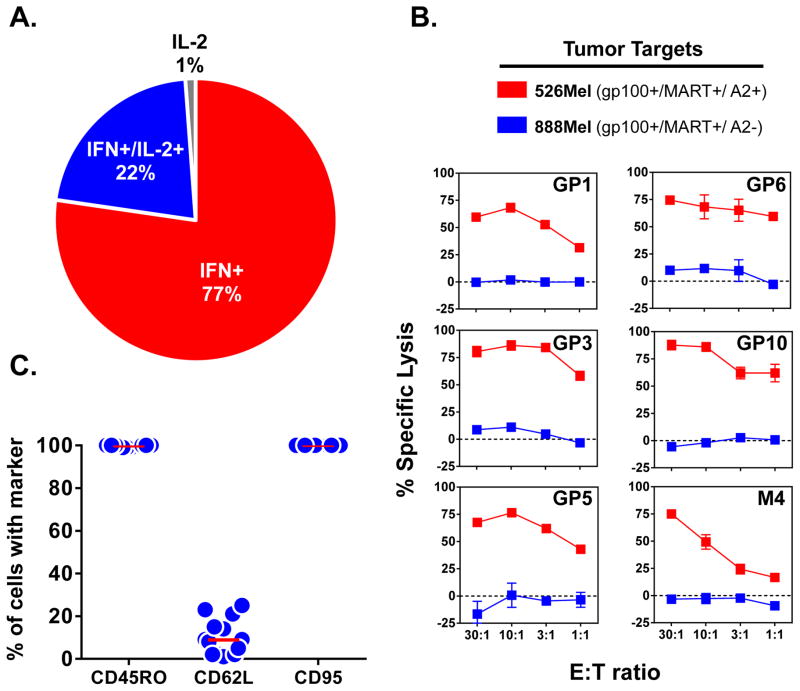
The functional and phenotypic attributes of the isolated MDA specific CD8+ T cell clones were assessed immediately prior to infusion. Each of the gp100 and MART specific CD8+ T cell clones demonstrated highly specific and avid antigen recognition by secreting significant amounts of IFN-γ in response to 100 ng/ml cognate peptide pulsed on T2 target cells (Figure 1A) and naturally presented peptide on allogeneic HLA-A2+ melanoma tumor lines (Figure 1B). Analysis of the clone reactivity by intracellular FACS for IL-2 and IFN-γ production after antigen stimulation revealed an effector cytokine profile with 77+3% of the reactive cells producing only IFN-γ, 22+4% of cells producing both IFN-γ and IL-2, and an insignificant population of cells producing only IL-2 (1+1%) (Figure 2A). Further, the transferred clones were found to be highly cytolytic and efficiently lysed tumor in an MHC-dependent manner (Figure 2B). Phenotypic profiling of the clones demonstrated high cell surface expression of CD45RO and CD95 and minimal expression of the lymph node homing molecule, CD62L, consistent with a differentiated effector status (Figure 2C). In summary, the adoptively transferred cells represented a highly selected homogenous population of lytic and differentiated effector CD8+ T cells that specifically targeted MDAs.
Figure 1. Peptide and tumor reactivity of the infused MDA specific CD8+ T cell clones.
MDA specific CD8+ T cell clones (1×105) isolated from each patient were stimulated with (A) an equal of number T2 cells pulsed with either gp100154–162, MART27–35 or no peptide (DMSO) or (B) HLA-A2+ and HLA-A2- melanoma tumor cell lines that express both gp100 and MART-1 in an overnight coculture. IFN-γ cytokine levels (pg/mL) were measured in culture supernatants by ELISA. Data shown are the mean + SEM of replicate cultures assayed. Results are representative of multiple independent assays performed on this set of clones.
Figure 2. Effector differentiation of the infused MDA specific CD8+ T cell clones.
(A) Cytokine profile of the infused effector CD8+ T cell clones. Infused MDA specific CD8+ T cell clones from 12 of the treated patients were stimulated with T2 cells (1×105) pulsed with either gp100154–162, MART27–35 or no peptide (DMSO) as a negative control. Intracellular cytokine FACS was performed for IL-2 and IFN-γ production following a 6 hour coculture. Pie chart depicts the mean frequency of peptide-reactive cells producing the denoted cytokines. (B) Cytotoxicity of the infused effector CD8+ T cell clones. CD8+ T cell clones from indicated patients were used as effector cells to perform a 4 hr calcein based cytotoxicity assay against 526 Mel (HLA-A2+) and 888 Mel (HLA-A2-) at various effector to target (E:T) ratios. Data shown are the mean + SEM of triplicate cultures assayed. (C) Phenotype of the infused effector CD8+ T cell clones. Infused CD8+ T cell clones from each patient (n=15) underwent FACS analysis to evaluate expression of the indicated differentiation markers. Shown are % of CD8+Tetramer+ cells staining with respective marker as compared to isotype. Bar on graph represents mean.
Clinical Results
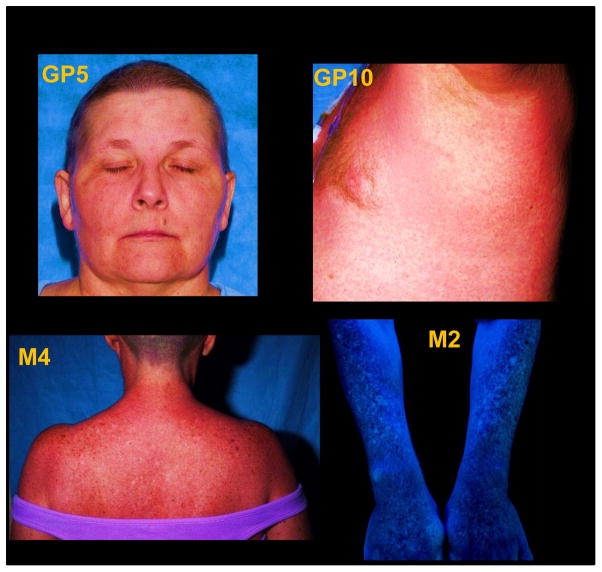
Each of the treated patients experienced transient neutropenia and thrombocytopenia induced by the lymphodepleting preparative chemotherapy regimen. The patients who received post infusion IL-2 were additionally noted to have well described self-limited toxicities associated with systemic cytokine therapy (7). All of the chemotherapy and IL-2 related adverse effects were found to be reversible with clinical symptoms and laboratory test values returning to appropriate levels within 2 weeks. With respect to the MDA specific CD8+ T cell clones, within seven days of infusion, there was evidence of immune mediated targeting of skin melanocytes. Of the fifteen treated patients, eleven patients (73%) developed a diffuse erythematous skin rash (Figure 3, Table 1) with skin biopsies revealing CD8+ T cell infiltration into the melanocytic layer consistent with autoimmune dermatitis. These histologic observations, noted at a time when the endogenous lymphocytes had been depleted, strongly suggested that the epidermal melanocytes were the targets of immune attack by the transferred clones. There were no cases of uveitis or ototoxicity detected in any of the patients, indicating that resident melanocytes found in the eyes and inner ears were not targeted. In all cases, the autoimmune dermatitis resolved without treatment after approximately 10–14 days, with the exception of patient M2, who developed progressive patchy skin vitiligo indicating ongoing melanocyte destruction after therapy.
Figure 3. Autoimmune dermatitis after MDA specific CD8+ T cell clone infusion.
Representative photographs of autoimmune dermatitis manifesting as a diffuse erythematous rash 5–7 days post clone infusion in patients GP5, GP10, and M4. Development of patchy vitiligo in patient M2 two months after clone infusion.
We next sought to evaluate the antitumor effects of the administered clones. Despite evidence of melanocyte targeting in the majority of the patients, none of the treated patients demonstrated an objective tumor response by standard oncologic RECIST criteria (Table 1). We observed mixed and minor biologic activity related to the T cell clone transfer with radiographic shrinkage of individual tumors in patients GP1, GP5, M1, and M2 (examples shown in Supplementary Figure 1), however these findings did not appear to provide meaningful clinical benefit for these patients.
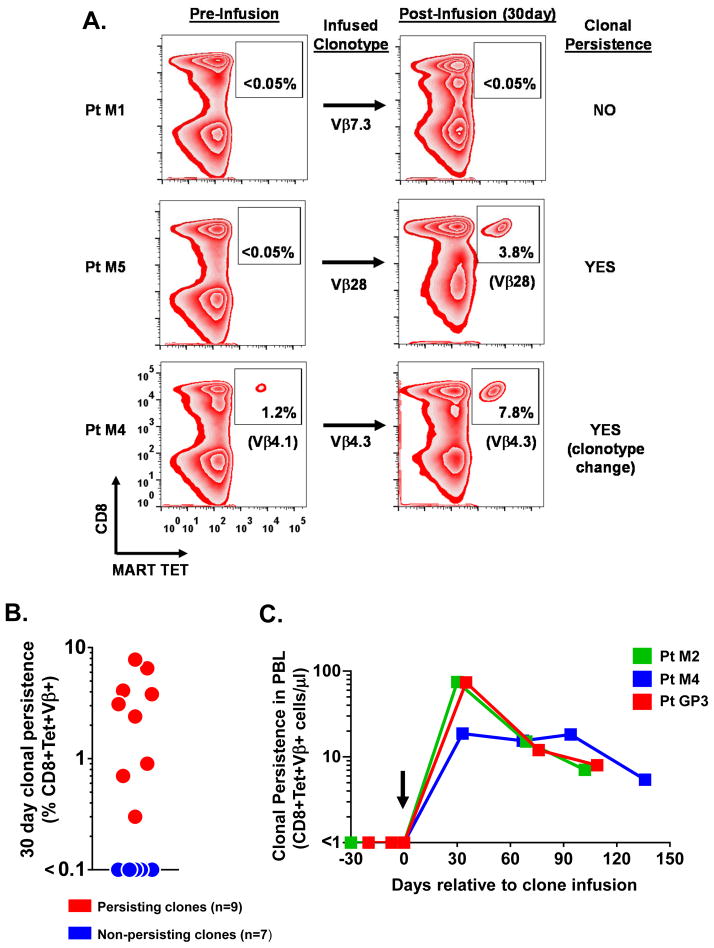
In vivo clonal persistence of MDA specific CD8+ T cell clones
In prior adoptive transfer clinical trials, the ability of the transferred cells to persist was strongly associated with tumor regression in metastatic melanoma patients (21, 22). Thus, to help understand the lack of objective tumor responses in the current trials, we sought to determine whether the MDA specific CD8+ T cell clones had successfully engrafted and repopulated the immune repertoire. To evaluate the in vivo survival of the transferred clones, peripheral blood samples, obtained prior to and one month after cell infusion, were compared by FACS for the percentage of CD8+ T cells that were tetramer positive (Figure 4A). Further, each CD8+Tetramer+ population was FACS sorted to >99% purity to allow TCR molecular sequencing to determine the antigen specific clonotypes that were present in the peripheral blood before and after clone infusion. The presence of the infused clonotype after infusion, but not before infusion, was defined as clonal persistence. The percentage of this clonotype among total CD8+ T cells was used to define the persistence frequency. Using this stringent criterion, we detected engraftment of transferred clones in eight of the fifteen patients (53%) with one month clonal persistence ranging from 0.3 to 7.8% of all circulating CD8+ T cells (Table 1, Figure 4B). To evaluate the long term fate of the persisting clones, we obtained extended peripheral blood samples from selected patients and demonstrated the sustained presence of circulating clones beyond 100 days (Figure 4C). We next assessed the ability of the persisting CD8+ T cells from all patients to re-respond to antigenic stimulation. Without culturing or addition of exogenous cytokines, peripheral blood samples obtained one month after cell infusion were assayed against T2 cells pulsed with the cognate peptide or a control peptide. Intracellular cytokine FACS for IFN-γ revealed that the persisting cells from all of the patients were highly reactive against their respective peptide targets (data not shown). Collectively, these findings demonstrated that the MDA specific CD8+ T cell clones had engrafted and persisted as a functionally active population in the immune repertoire of half of the patients after adoptive transfer.
Figure 4. In vivo persistence of transferred MDA specific CD8+ T cell clones.
(A) Defining in vivo clonal persistence. Shown are representative FACS dot plots to quantitate the percentage of CD8+ cells that were tetramer positive in patient PBMC samples obtained prior to therapy (left) and 30 days after clone infusion (right) . Numbers within the plot indicate frequency of CD8+Tetramer+ cells. CD8+Tetramer+ cells were FACS sorted to >99% purity and TCR Vβ sequencing was performed on the sorted populations to determine which antigen specific clonotypes were present in the peripheral blood before and after clone infusion (clonotype shown in brackets). (B) The magnitude of clonal persistence for all transferred MDA specific CD8+ T cell clones (n=16) in the 15 treated patients at day 30 post-infusion. (C) Long term clonal persistence. Absolute number of CD8+Tetramer+ Vβ+ cells per microliter of blood in selected patients at specified time points. Arrow denotes the day of clonal infusion.
Discussion
The adoptive transfer of autologous TIL in conjunction with lymphodepleting conditioning regimens can mediate durable complete tumor regression in selected patients with metastatic melanoma (7, 8, 23, 24). However, efforts to further improve upon these clinical findings are currently hindered by an incomplete understanding of the specific lymphocyte populations that were responsible for the tumor responses. The cellular composition of administered melanoma TIL is polyclonal, varies across individual patients, and remains largely unknown. As such, the tumor antigens that were instrumental in inducing sustained and complete immune responses are also unclear. One strategy to provide clarity to these issues involves the iterative isolation and adoptive transfer of tumor reactive T cell clones with single antigen specificity. The transfer of cloned lymphocytes would allow a precise determination of the in vivo fate and function of a genetically trackable population of antigen-specific T cells. Here, we report the results from two sequential clinical trials in which melanocyte differentiation antigens were targeted with autologous CD8+ T cell clones in patients with metastatic melanoma. The decision to target MART-1 and gp100 stemmed from a number of observations that suggested that these tumor antigens represented favorable therapeutic targets to treat melanoma. First, these antigens have been reported to be commonly and highly expressed in metastases among many melanoma patients (25, 26). Second, the MDAs are highly immunogenic. Since the original identification of HLA-A2 restricted MART-1 and gp100 epitopes recognized by naturally occurring TIL (10, 11), high frequencies of primed CD8+ T cells specific for these antigens have been routinely isolated from peripheral blood and tumor derived lymphocyte populations (11, 27, 28). Finally, there has been a long observed and intriguing association between the development of autoimmunity against normal melanocytes (for example, uveitis and vitiligo) and melanoma tumor regression in patients treated with immune therapies (13–17). Collectively, these findings prompted our prospective evaluation of the effectiveness of MART-1 and gp100 as tumor regression antigens.
We previously reported a high throughput technique that allowed the rapid isolation and expansion of high avidity MDA specific CD8+ T cell clones from peripheral blood for use in adoptive transfer clinical studies (18, 19). The current report further demonstrates that these clones could be routinely isolated and that they possess potent and specific in vitro lytic capability against MDA expressing melanoma tumor lines. In vivo evidence of clone activity after adoptive transfer was seen in the targeting of normal melanocytes residing in the skin of the majority of treated patients. Further, these clones were observed to engraft in over half of the treated patients and survive long term in the circulating immune repertoire. Despite these findings, we did not observe clinically significant tumor regression. The precise explanation for these seemingly paradoxical findings still remains unclear.
One potential deficiency of our treatment may reside in the intrinsic nature of the infused cell product. The clones generated in this study were derived from the peripheral blood and as such, these cells may lack necessary tumor trafficking and homing abilities that are present in tumor derived lymphocytes, such as TIL. Further, although our CTL clones were derived using a rapid cloning approach, the cells still underwent massive in vitro proliferation and differentiation. Murine studies have suggested that CD8+ T cells that have undergone such extensive in vitro expansion progressively lose in vivo proliferative potential and rapidly undergo apoptosis after adoptive transfer (29). However, this theory is difficult to reconcile with the observation that the clones in the current study could persist for very long periods in the host (Figure 4C), suggesting that cell survival was not a limiting factor.
Another possibility is that the degree of clonal persistence noted in our studies may not have been sufficient to mount a sustained anti-tumor response. The persistence in our treated patients was heterogeneous and ranged from 0.3% to 7.8% of the circulating CD8+ T cell population (Table 1). Previous clinical trials studying the adoptive transfer of autologous TIL have reported a significant and positive correlation between tumor response and the capability of the transferred cells to persist. However, the magnitude of persistence was not examined as a correlate of clinical response. In an effort to determine whether a threshold of persistence is critical for tumor regression, we are currently evaluating approaches to enhance in vivo persistence after cell transfer in pre-clinical murine models. Variability in the administration of high-dose IL-2 might have also influenced the level of persistence or the activation state of the transferred clones. Unfortunately, our study was not designed to address the impact of IL-2 dosing on clonal persistence and activity.
Finally, perhaps the most compelling hypothesis for the results of the current clinical trials is that MDAs are suboptimal tumor regression antigens. In fact, a comprehensive review of published adoptive transfer studies targeting the MART-1 and gp100 antigens in metastatic melanoma patients over the last decade have, similarly, found poor therapeutic efficacy using either MDA-specific clones (28, 30–35), MDA enriched polyclonal bulk infusions (36–38) or MDA TCR gene-modified PBL (17, 39, 40) (Table 2). Although these trials were conducted by various groups and had differences in their clinical design, the characteristics of the infused cell product, and the use of conditioning regimens and cytokines, they shared the exclusive targeting of either MART or gp100. Our review found few, if any, objective clinical responses by standardized oncologic criteria. The most potent in vivo targeting of MDAs was evident in the trials transferring peripheral blood lymphocytes that were genetically engineered to express high affinity TCRs against MART and gp100 (17). The transfer of these TCR transduced cells was associated with severe on target-off tumor toxicity in normal tissues in the eye, inner ear, and skin which all harbor populations of normal melanocytes. Although objective tumor responses in patients were demonstrated in these trials, the tumor shrinkage was typically partial and transient in nature. In reviewing the collective experience of published clinical trials targeting MDAs, we found a very low complete tumor response rate of 3.7%, which usually involved regression of small volume lymph node metastases. This complete response rate is significantly lower than the 22% 5-year complete response rate associated with bulk polyclonal TIL therapy in advanced melanoma patients (9). Thus, the cumulative findings of these published reports, in concert with our current study, raise significant concerns regarding future immunotherapy efforts targeting this class of tumor antigens.
Table 2.
Collective review of published adoptive transfer trials targeting MART-1 and gp100
| Year | Author | Trial phase |
Cell population |
Target antigen:epitope |
No. of pts. |
Preparative regimen |
No of cells | Concomitant therapy |
No. of Responses |
%CR | %ORR | REF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2002 | Dudley et al | I | CTL clone | MART-1: 27–35 | 2 | CY+FLU, None | 10.4 × 109 (×2) | None, LD IL-2, HD IL-2 | 0 | 0 | 0 | 34 |
| 2002 | Yee et al | I | CTL clone | MART-1: 27–35 | 5 | None | 3.3 × 109 (×4) | None, IL-2 | 0 | 0 | 0 | 30 |
| 2005 | Vignard et al | I/II | CTL clone | MART-1: 27–35 | 10 | None | 0.036–5 × 109 (×2) | IL-2, IFN-α | 1 CR | 10 | 10 | 35 |
| 2006 | Mackensen et al | I | Polyclonal CTL | MART-1: 27–35 | 11 | None | 0.11 – 13.1 × 108 (×3) | LD IL-2 | 1 CR, 1 PR | 9.1 | 18.2 | 31 |
| 2006 | Morgan et al | I | DMF4 TCR transduced | MART-1: 27–35 | 17 | CY+FLU | 0.5–34.4 × 109 | HD IL-2 | 2 PR | 0 | 11.8 | 39 |
| 2009 | Wallen et al | I | CTL clone | MART-1: 27–35 | 6 | None, FLU | 1010/m2 (×2) | None/ LD IL-2 | 0 | 0 | 0 | 32 |
| 2009 | Khammari et al | I/II | CTL clone | MART-1: 27–35 | 14 | DTIC | 1.43 – 20 × 108 | IL-2, IFN-α | 2 CR, 4PR | 14.3 | 42.9 | 36 |
| 2009 | Johnson et al | II | DMF5 TCR transduced | MART-1: 27–35 | 20 | CY+FLU | 1.5–107 × 109 | HD IL-2 | 6 PR | 0 | 30 | 17 |
| 2011 | Butler et al | I | Polyclonal CTL | MART-1: 27–35 | 9 | None | 1.84.4 × 109 | None | 1 CR | 11.1 | 11.1 | 37 |
| 2012 | Chapuis et al | I/II | CTL clone | MART-1: 27–35 | 5 | CY | 1010/m2 | LD IL-2, HD IL-2 | 1 CR | 20 | 20 | 33 |
| 2014 | Chodon et al | II | DMF5 TCR transduced | MART-1: 27–35 | 14 | CY+FLU | 0.6–4.8 × 109 | HD IL-2 + pep-pulsed DC (×3) | 0 | 0 | 0 | 40 |
| Current | Chandran et al | II | CTL clone | MART-1: 27–35 | 5 | CY+FLU | 0.4–58.3×109 | HD IL-2 | 0 | 0 | 0 | |
| 2001 | Dudley et al | I | CTL clone | gp100:209–217(210M) | 13 | None | 10.4 × 109 (×4) | None, LD IL-2, HD IL-2 | 0 | 0 | 0 | 28 |
| 2002 | Dudley et al | I | CTL clone | gp100:209–217(210M) | 12 | CY+FLU, None | 10.4 × 109 (×2) | None, LD IL-2, HD IL-2 | 0 | 0 | 0 | 34 |
| 2002 | Yee et al | I | CTL clone | gp100:154–162 | 5 | None | 3.3 × 109 (×4) | None, IL-2 | 0 | 0 | 0 | 30 |
| 2006 | Powell et al | I | Polyclonal CTL | gp100:209–217(210M) | 9 | CY+FLU | 1.3–12 × 1010 | HD IL-2, peptide/vaccine | 0 | 0 | 0 | 38 |
| 2009 | Wallen et al | I | CTL clone | gp100:154–162 | 2 | None, FLU | 1010/m2 (×2) | None, LD IL-2 | 0 | 0 | 0 | 32 |
| 2009 | Johnson et al | II | gp100 TCR transduced | gp100:154–162 | 16 | CY+FLU | 2.3–110 × 109 | HD IL-2 | 1 CR, 2 PR | 6.3 | 12.5 | 17 |
| 2012 | Chapuis et al | I/II | CTL clone | gp100:154–162 | 2 | CY | 1010/m2 | LD IL-2 | 0 | 0 | 0 | 33 |
| Current | Chandran et al | II | CTL clone | gp100:154–162 | 10 | CY+FLU | 0.4–58.3×109 | HD IL-2 | 0 | 0 | 0 | |
| MART-1 Summary | 118 | 6 CR, 13 PR | 5.1 | 16.1 | ||||||||
| gp100 Summary | 69 | 1 CR, 2 PR | 1.4 | 4.3 | ||||||||
| Overall Summary | 187 | 7 CR, 15 PR | 3.7 | 11.8 | ||||||||
CR=complete response, PR=partial response, ORR=objective response rate, CY=cyclophosphamide, FLU=fludarabine, DTIC=dacarbazine, LD= Low Dose, HD= High Dose, IL-2=interleukin-2, IFN=interferon
One major biologic limitation of targeting MDAs may be the non-essential role that these proteins play in malignant melanoma transformation and the metastatic phenotype. We recently reported the profiling of over 3000 biopsies of metastases from 1514 melanoma patients using quantitative immunohistochemistry to detect gp100, MART-1, and tyrosinase expression (41). We observed significantly low expression or complete loss of expression of each of these MDAs in approximately 30% of lesions. Further, within metastases with detectable antigen expression, there was significant heterogeneity among the tumor cells within the same metastases. This heterogeneity in MDA antigen expression may explain the partial, transient, and mixed responses observed when MDAs are immunologically targeted.
In an attempt to retrospectively define potentially more immunogenic targets responsible for the durable remissions with TIL transfer, the source tumors from these patients have recently undergone whole exomic sequencing to identify tumor-specific non-synonymous mutations. Candidate epitopes containing these mutated amino acids were screened for HLA-Class I binding motifs and those predicted by in silico algorithm to bind with high affinity to the patient’s pertinent Class I alleles were synthesized. In all cases so far, TIL were identified that immunologically recognized one or more of these mutant epitopes (42). These findings provide compelling evidence that somatic mutations in metastatic cutaneous melanoma tumors can generate neo-epitopes that can elicit a robust autologous immunologic response against mutant proteins. The search for more suitable antigenic targets has directed our current efforts toward identifying T cells that target neo-epitopes generated by cancer- specific somatic mutations and in particular, driver mutations. Classic driver mutations encode for proteins that typically contribute to tumor development and maintenance of the malignant phenotype. Unlike MDAs, the neo-proteins encoded by these mutations may serve as more effective therapeutic targets given their tumor specificity and their essential role in the survival and proliferation of metastatic cells.
Supplementary Material
Translational Relevance.
Adoptive transfer of autologous tumor infiltrating lymphocytes (TIL) can mediate durable cancer regression in selected patients with metastatic melanoma. However, the tumor antigens associated with these favorable responses remain unclear. We hypothesized that a clinical strategy involving the iterative adoptive transfer of selected autologous antigen specific T cell clones could help systematically define immunologic targets associated with successful cancer therapy, without the interpretative ambiguity of transferring polyclonal populations. Here, we report the findings from two sequential phase II clinical trials evaluating the transfer of CD8+ T cell clones specific for the melanocyte differentiation antigens (MDA), gp100 and MART-1, respectively. After clone transfer, we observed immune mediated targeting of skin melanocytes in 73% of patients and clonal engraftment in 53% of patients. Despite these findings, there were no objective tumor responses. The poor therapeutic efficacy using MDA specific T cells raises significant concerns regarding future immunotherapy efforts targeting this class of tumor antigens.
Acknowledgments
We thank the Surgery Branch cell production facility and the immunotherapy clinical and support staff for their contributions. We thank Arnold Mixon and Shawn Farid for assistance with cell sorting by flow cytometry.
Footnotes
Conflicts of Interest: None
Reference List
- 1.Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–16. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 2.Atkins MB, Kunkel L, Sznol M, Rosenberg SA. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J Sci Am. 2000;6(Suppl 1):S11–S14. [PubMed] [Google Scholar]
- 3.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prieto PA, Yang JC, Sherry RM, Hughes MS, Kammula US, White DE, et al. CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res. 2012;18:2039–47. doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134–44. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–7. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR, et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347–52. doi: 10.1084/jem.180.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakami Y, Eliyahu S, Jennings C, Sakaguchi K, Kang X, Southwood S, et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J Immunol. 1995;154:3961–8. [PubMed] [Google Scholar]
- 12.Romero P, Valmori D, Pittet MJ, Zippelius A, Rimoldi D, Levy F, et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol Rev. 2002;188:81–96. doi: 10.1034/j.1600-065x.2002.18808.x. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg SA, White DE. Vitiligo in patients with melanoma: normal tissue antigens can be targets for cancer immunotherapy. J Immunother Emphasis Tumor Immunol. 1996;19:81–4. [PubMed] [Google Scholar]
- 14.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, et al. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–44. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson MR, Chan CC, Yang JC, Rubin BI, Gracia GJ, Sen HN, et al. Cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma: a new cause of uveitis. J Immunother. 2004;27:478–9. doi: 10.1097/00002371-200411000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Jaber SH, Cowen EW, Haworth LR, Booher SL, Berman DM, Rosenberg SA, et al. Skin reactions in a subset of patients with stage IV melanoma treated with anti-cytotoxic T-lymphocyte antigen 4 monoclonal antibody as a single agent. Arch Dermatol. 2006;142:166–72. doi: 10.1001/archderm.142.2.166. [DOI] [PubMed] [Google Scholar]
- 17.Johnson LA, Morgan RA, Dudley ME, Cassard L, Yang JC, Hughes MS, et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–46. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kammula US, Serrano OK. Use of high throughput qPCR screening to rapidly clone low frequency tumour specific T-cells from peripheral blood for adoptive immunotherapy. J Transl Med. 2008;6:60. doi: 10.1186/1479-5876-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A, Chandran S, Shah SA, Chiu Y, Paria BC, Aghamolla T, et al. The stoichiometric production of IL-2 and IFN-gamma mRNA defines memory T cells that can self-renew after adoptive transfer in humans. Sci Transl Med. 2012;4:149ra120. doi: 10.1126/scitranslmed.3004306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, et al. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005;28:53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oshita C, Takikawa M, Kume A, Miyata H, Ashizawa T, Iizuka A, et al. Dendritic cell-based vaccination in metastatic melanoma patients: phase II clinical trial. Oncol Rep. 2012;28:1131–8. doi: 10.3892/or.2012.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carrio R, Rolle CE, Malek TR. Non-redundant role for IL-7R signaling for the survival of CD8+ memory T cells. Eur J Immunol. 2007;37:3078–88. doi: 10.1002/eji.200737585. [DOI] [PubMed] [Google Scholar]
- 25.Kageshita T, Kawakami Y, Hirai S, Ono T. Differential expression of MART-1 in primary and metastatic melanoma lesions. J Immunother. 1997;20:460–5. doi: 10.1097/00002371-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 26.de Vries TJ, Smeets M, de GR, Hou-Jensen K, Brocker EB, Renard N, et al. Expression of gp100, MART-1, tyrosinase, and S100 in paraffin-embedded primary melanomas and locoregional, lymph node, and visceral metastases: implications for diagnosis and immunotherapy. A study conducted by the EORTC Melanoma Cooperative Group. J Pathol. 2001;193:13–20. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH729>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 27.Benlalam H, Labarriere N, Linard B, Derre L, Diez E, Pandolfino MC, et al. Comprehensive analysis of the frequency of recognition of melanoma-associated antigen (MAA) by CD8 melanoma infiltrating lymphocytes (TIL): implications for immunotherapy. Eur J Immunol. 2001;31:2007–15. doi: 10.1002/1521-4141(200107)31:7<2007::aid-immu2007>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, et al. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 29.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, et al. Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest. 2005;115:1616–26. doi: 10.1172/JCI24480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dudley ME, Wunderlich JR, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A phase I study of nonmyeloablative chemotherapy and adoptive transfer of autologous tumor antigen-specific T lymphocytes in patients with metastatic melanoma. J Immunother. 2002;25:243–51. doi: 10.1097/01.CJI.0000016820.36510.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vignard V, Lemercier B, Lim A, Pandolfino MC, Guilloux Y, Khammari A, et al. Adoptive transfer of tumor-reactive Melan-A-specific CTL clones in melanoma patients is followed by increased frequencies of additional Melan-A-specific T cells. J Immunol. 2005;175:4797–805. doi: 10.4049/jimmunol.175.7.4797. [DOI] [PubMed] [Google Scholar]
- 33.Wallen H, Thompson JA, Reilly JZ, Rodmyre RM, Cao J, Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khammari A, Labarriere N, Vignard V, Nguyen JM, Pandolfino MC, Knol AC, et al. Treatment of metastatic melanoma with autologous Melan-A/MART-1-specific cytotoxic T lymphocyte clones. J Invest Dermatol. 2009;129:2835–42. doi: 10.1038/jid.2009.144. [DOI] [PubMed] [Google Scholar]
- 35.Chapuis AG, Thompson JA, Margolin KA, Rodmyre R, Lai IP, Dowdy K, et al. Transferred melanoma-specific CD8+ T cells persist, mediate tumor regression, and acquire central memory phenotype. Proc Natl Acad Sci U S A. 2012;109:4592–7. doi: 10.1073/pnas.1113748109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–9. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 37.Butler MO, Friedlander P, Milstein MI, Mooney MM, Metzler G, Murray AP, et al. Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Sci Transl Med. 2011;3:80ra34. doi: 10.1126/scitranslmed.3002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Powell DJ, Jr, Dudley ME, Hogan KA, Wunderlich JR, Rosenberg SA. Adoptive transfer of vaccine-induced peripheral blood mononuclear cells to patients with metastatic melanoma following lymphodepletion. J Immunol. 2006;177:6527–39. doi: 10.4049/jimmunol.177.9.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chodon T, Comin-Anduix B, Chmielowski B, Koya RC, Wu Z, Auerbach M, et al. Adoptive transfer of MART-1 T-cell receptor transgenic lymphocytes and dendritic cell vaccination in patients with metastatic melanoma. Clin Cancer Res. 2014;20:2457–65. doi: 10.1158/1078-0432.CCR-13-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartlett EK, Fetsch PA, Filie AC, Abati A, Steinberg SM, Wunderlich JR, et al. Human melanoma metastases demonstrate nonstochastic site-specific antigen heterogeneity that correlates with T-cell infiltration. Clin Cancer Res. 2014;20:2607–16. doi: 10.1158/1078-0432.CCR-13-2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–52. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.