Abstract
Sedentary lifestyle is associated with elevated cancer risk whereas regular physical activity (PA) and high cardiorespiratory fitness (CRF) have the opposite effect, with several biological mechanisms mediating such associations. There is a need for lifestyle interventions aimed at increasing the PA levels and CRF of the general population and particularly cancer survivors. Further, provocative data suggest a dose-dependent benefit of increasing levels of PA and/or CRF against cancer risk or mortality. Thus, current PA guidelines (≥150 min/week of moderate-to-vigorous PA) may not be sufficiently rigorous for preventing cancer nor for extending cancer survivorship. Research targeting this issue is urgently needed. Promoting regular PA along with monitoring indicators of CRF and adiposity may provide powerful strategies to prevent cancer in populations, help cancer patients more effectively deal with their disease and enhance secondary prevention programs in those who are affected by cancer.
1. Physical inactivity and low fitness increase cancer risk and mortality
Physical activity (PA) is defined as ‘any bodily movement produced by skeletal muscles that results in energy expenditure’ (1). In turn, physical fitness is ‘the ability to carry out daily tasks with vigor and alertness, without undue fatigue and with ample energy to enjoy [leisure] pursuits and to meet unforeseen emergencies’ (2). The PA behaviors that may be measured in health promotion studies include: frequency, duration, intensity or type of PA, and domains or settings where the activity is performed such as leisure time PA, occupational activity, active commuting, incidental energy expenditure, and sedentary behavior settings (3).
PA is the only behavioral intervention that has been proven useful to increase CRF, it should be recognized that CRF is a phenotype that has a strong genetic component in populations with heritability coefficients of the order of 50% after adjustments for age, gender, body mass and body composition (4–6). The gold standard measure of CRF is maximal oxygen uptake (VO2max), typically expressed as follows: milliliters of O2 uptake · kilograms of body mass−1 · minute−1, or metabolic equivalents (METs), where 1 MET = 3.5 ml O2 uptake · kilograms of body mass−1 · minute−1 (7). See Table 1 for explanation of the MET concept and how this translates into % of an individual’s maximum CRF.
Table 1.
Explanation of the intensity of physical activity (PA) expressed using the MET (metabolic equivalent) concept and how this translates into % of an individual’s maximal cardiorespiratory capacity (CRF). Extracted from (2, 73)
| RELATIVE INTENSITY | ABSOLUTE INTENSITY (in MET) | ||||
|---|---|---|---|---|---|
| CLASIFICATION OF EXERCISE INTENSITY | %HRmax | % of maximal CRF | Young (20–39 years) | Middle-aged (40–64 years) | Older (≥65 years) |
| Very light | <57 | <37 | <2.4 | <2.0 | <1.6 |
| Light | 57–63 | 37–45 | 2.4–4.7 | 2.0–3.9 | 1.6–3.1 |
| Moderate | 64–76 | 46–63 | 4.8–7.1 | 4.0–5.9 | 3.2–4.7 |
| Vigorous | 77–95 | 64–90 | 7.2–10.1 | 6.0–8.4 | 4.8–6.7 |
| Near-maximal to maximal | ≥96 | ≥91 | ≥10.2 | ≥8.5 | ≥6.8 |
Note: 1 MET equals an oxygen consumption of 3.5 ml/kg/min, which is the average resting energy expenditure for humans. MET-hour is an index of energy expenditure that quantifies the total amount of PA performed in a standardized manner across individuals and types of activities (US Department of Health and Human Services. 2008). It is calculated as the product of the number of mean MET associated with one PA and the number of hours the PA was performed. For example, jogging (at 7 METs) for 1 hour: 7 METs x 1 hour = 7 MET-hour.
Abbreviation: HRmax (maximum heart rate, which on average and for simplicity purposes, could be estimated as 220 minus age in years)
VO2max can be assessed with direct or indirect methods. Direct measures are obtained by ventilatory gas analysis at maximal exertion during a graded exercise ergometry test (8, 9) whereas indirect methods estimate VO2max from maximal exercise duration, the peak workload and/or heart rate (HR) responses reached during submaximal or maximal exercise ergometry, or the time required to walk or run a distance (9). While other non exercise based methods have been developed (10) those containing an exercise component (8, 9) remain the definitive standard.
According to PA guidelines issued by the US Department of Health and Human Services (11) and the World Health Organization (12), adults should undertake ≥150min/week of moderate-to-vigorous PA (MVPA). On the other hand, sedentary behaviors are defined as “any waking behavior characterized by an energy expenditure ≤1.5 METs while in a sitting or reclining posture” according to the Sedentary Behaviour Research Network (SBRN) whilst “physical inactivity” refers to those who perform insufficient amounts of MVPA (i.e., <150min/week) (13).
The link between levels of PA, CRF and cancer risk is receiving growing attention. This is a topic of paramount importance in modern medicine since ~1/3 of adults worldwide are currently inactive and the endemic inactivity trend starts in early life (14). In contrast, regular PA raises metabolic rate and increases CRF via increases in cardiovascular function, (15) muscle mitochondrial biogenesis and oxidative enzyme activity, particularly of the enzymes responsible for fat oxidation, as well as decreases in body adiposity (16–19). Thus, regular PA is recommended as an important part of a healthy lifestyle as well as for weight management by virtually all public health agencies and scientific organizations (20–24). In contrast, physical inactivity has been estimated to contribute more than 10% of the disease burden of 2 of the most prevalent cancers among westerners, i.e., breast and colon (accounting for 13.8 and 14.9% of the burden, respectively, among the Spanish population and 12.4 and 12% respectively in the US)(25). Besides the beneficial effects of exercise on energy balance, high levels of regular PA per se have also been shown to decrease the risk of cancer (22, 23, 26–32) as well as the risk of mortality among cancer survivors, particularly of breast and colorectal cancer (33).
Engaging in 8.75 or more MET-hour/week of recreational PA (equivalent to ~150min/week of brisk walking) was associated with lower colorectal cancer mortality compared with < 3.5 MET-hour/week, whereas longer leisure time spent sitting was associated with higher risk of death from colorectal cancer (34). A meta-analysis of recreational PA showed that the risk of colorectal cancer decreased by 6% per MET-hour/week, along with a 12% decreased risk per 30 min/day of recreational PA for colon cancer, by decreasing inflammation, and reducing insulin resistance resulting in lower circulating insulin levels (35). Another meta-analysis that compared highest versus lowest leisure time PA in the prevalence of colon cancer showed a 20% decreased risk in men and a 14% decreased risk in women (36). Moreover, it has been shown that breast cancer and cancers of the reproductive system are less prevalent in women who had been athletes in college compared with non-athletic controls (37). There is also evidence of a lower risk of breast cancer with higher levels of regular PA, with a dose-dependent relationship (36, 38, 39).
A recent meta-analysis from our group has shown a 40% lower standard mortality ratio due to cancer in those engaging in the highest PA levels, i.e., elite athletes of various sport disciplines (n=12,119, mostly men), including ‘Tour de France’ finishers, compared with the general population (40). While the lower mortality in elite athletes could be associated with an overall healthier lifestyle, for example reduced tobacco use, the data is compatible with the notion that regular PA confers protection against a number of cancers. Taken together the studies outlined above are suggestive of a potential dose-response relationship.
2. Potential biological underpinnings
The biological mechanisms responsible for the potential anti-tumorigenic effects of PA (independently of its influence on adiposity) remain to be elucidated, yet provocative data suggest that contracting muscle-derived molecules exerting either paracrine or endocrine effects, known as ‘myokines’, are strong candidates for mediating the PA anti-cancer effects. For instance, secreted protein acidic and rich in cysteine (SPARC) is a matricellular protein that regulates cell proliferation and migration (41) and was recently identified as a myokine (42, 43) whose expression increases with regular PA training (43). SPARC, which is in fact a potential target in cancer immunotherapy (44), might mediate the preventive effects of exercise on colon cancer by suppressing the formation of aberrant crypt foci through stimulation of apoptosis via caspase-3 and 8 (42). Circulating and muscle-transcript levels of S100A8–S100A9 complex (calprotectin) increase with acute PA bouts (45–48). One could also speculate that muscle-derived calprotectin might be cancer protective as shown by its ability to induce apoptosis in certain tumor lines (49), including colon cancer lines (50), or to inhibit matrix metalloproteinases associated with cancer invasion/metastasis (51). PA is also a powerful inducer of muscle and systemic autophagy which has been suggested to serve as a tumor suppressor pathway (52–54).
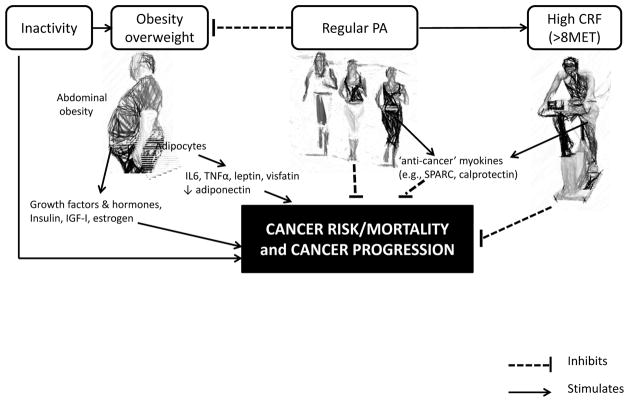
While the focus of this commentary is on PA and CRF, it is important to acknowledge that PA (particularly vigorous PA) can contribute to reduction in adipose tissue which itself can be an important contributor to decreased cancer risk, morbidity and mortality (55–59). Thus the reduction in adipose tissue associated with physical activity could potentially decrease cancer promoting potential by reducing multiple mediators including sex steroid hormones, insulin like growth factors, inflammatory cytokines and adipocytokines (60–64). Reducing adiposity could also decrease mechanical issues such as those leading to gastroesophageal reflux disease which predisposes to esophageal adenocarcinoma (65–66). In fact, the link between overweight/obesity and cancer is of significant concern, especially when taking into consideration the increasing incidence of both disorders (67–69). Thus, as illustrated in Figure 1, the combination of obesity, physical inactivity and low fitness levels can be considered a toxic triad promoting cancer incidence and mortality that should be amenable to lifestyle alterations such as PA which could potentially improve all three.
Figure 1.
Summary of the interplay between cancer and obesity, physical activity (PA), and cardiorespiratory fitness (CRF). Abbreviations: IL6, interleukin 6; SPARC, secreted protein acidic and rich in cysteine; TNFα, tumor necrosis α.
3. Cancer survivors do not take full advantage of the PA and CRF benefits
In cohorts of US cancer survivors in which PA was measured objectively (i.e., using accelerometry), mean MVPA levels were clearly below the recommended 150 min/week-threshold, i.e., ~26 (breast) and ~42 (prostate) min/week (70–71). Moreover, in a recent US National Health and Nutrition Examination Survey (NHANES) of over 7 million cancer survivors, only 4.5% met the PA recommendation while obesity prevalence was 33.9% (72).
In contrast, it was recently reported that 94% of a cohort of Spanish cancer survivors (n=204) performed more than 150min/week of MVPA (73). And yet their body weight status (mean BMI = 27.9 kg/m2, obesity prevalence = 32.7%) was similar to that of the 4 inactive US cohorts (74). Unfortunately, despite the fact that the PA levels of the abovementioned Spanish cohort was relatively high compared to current guidelines, this was not accompanied by a ‘healthy’ cardiometabolic profile. Indeed the mean CRF of this cohort (men and women with a mean age of 54 years) reached only 7.7 MET and about 1 in 2 cancer survivors did not reach a CRF of 8 MET (73). These results are consistent with those of a meta-analysis showing that CRF was substantially lower in women (mean age of the studied cohorts ~45–60 years) with a history of breast cancer compared with healthy women, especially in the post-adjuvant setting (75). The findings of such low CRF levels in cancer survivors deserve more research because any value below 8 MET is indicative of an increased risk for mortality and cardiovascular events in men and women aged 40–60 years on average (73, 76), and cardiovascular disease is the leading cause of long-term morbidity and mortality among long-term cancer survivors (77). Further, there is also epidemiological evidence supporting a protective role of CRF against bowel, colorectal and liver cancer deaths in men over a wide age range (20–88 years), with those with a CRF level below 8 MET being characterized by over a 3-fold higher risk of dying from bowel cancer compared to those with higher capacities (≥ 11 MET) (78).
Although there is convincing evidence that CRF is negatively associated with morbidity and mortality in men and women, independently of other risk factors, the clinical relevance of cardiorespiratory fitness is frequently overlooked in medical settings (25). In young and middle-aged adults, a CRF above 8 MET may be needed to provide protective benefits in cancer survivors. In this regard, a healthy body weight (BMI<25) would be a clear advantage. For instance, a 10% reduction in the BMI of the male cancer survivors of the above mentioned Spanish cohort would have translated into CRF levels of 8 and 11 MET in 66 and 28% of the subjects, respectively. Thus, regular PA and diet interventions are needed to achieve a true weight loss as well as a healthy cardiometabolic profile (79).
With regards to PA, current MVPA guidelines (>150min/week) may not be sufficient to experience all the benefits that are generated by a physically active lifestyle. We propose that future interventions aimed at primary and secondary cancer prevention should focus on vigorous PA (≥ 6 MET, e.g., very brisk walking in order to increase CRF even more and to improve the odds of meaningful weight loss (80).
4. Summary and future recommendations
In summary, regular PA and high cardiorespiratory fitness are associated with a lower incidence and better prognosis of cancer. In light of the available evidence, tantalizing but admittedly incomplete, is it appropriate at this time to call for a more proactive approach to primary and secondary prevention of cancer? We believe that a call for more proactive measures is justified on two fronts.
First, research is needed to clarify a number of issues and provide a stronger foundation where more evidence is warranted. For instance, what are differential CRF values between cancer survivors and non-survivors? What are normative CRF values for cancer survivors in each gender across the lifespan? Are demanding PA programs (perhaps focusing on vigorous PA or combining PA with other lifestyle changes, especially diet) feasible in cancer survivors and do they actually achieve ‘healthy’ levels of CRF as currently defined (> 8 MET for middle aged (40–60 years) men/women) or adiposity? Given that current MVPA guidelines emphasize >150 min/week of moderate intensity PA, what is the PA dose recommended for cancer survivors aimed at achieving a healthy cardiometabolic profile and optimizing secondary prevention?
Second, there is an urgent need for lifestyle interventions aimed at increasing PA levels and CRF not only in the general population but particularly in cancer survivors. Although current guidelines focused on >150 min/week of moderate intensity PA may be a valuable and practical public health formula, it should be investigated to determine whether higher PA intensity, duration, or frequency could achieve hidden benefits in cancer survivors. This is obviously an issue that needs to be resolved though high quality and highly targeted research to evaluate both the short and long term effects of eliminating physical inactivity and increasing CRF. More importantly, just as developmental chemotherapy research evaluates progressively higher doses of anticancer agents, it is time to evaluate benefits of vigorous compared to moderate PA. Short term effects should focus on response and tolerance to therapy, hospital utilization as well as on potential changes in CRF, BMI as well as mediators and biomarkers such as growth promoting hormones, inflammatory cytokines, antitumor and anti-inflammatory myokines. Studies on long term effects are needed to focus on effects of vigorous PA on parameters such as remission duration, time to recurrence and cancer specific and overall survival. Such interventions are difficult to support, since in the present climate, well conducted interventions are very expensive and require budgets well over caps currently imposed by most funding organizations. Nonetheless, since this research is so critically important to patient outcomes, there may be some basis for optimism in the relatively newly established Patient-Centered Outcomes Research Institute (PCORI) mission to fund comparative clinical effectiveness research authorized in the United States as part of the Patient Protection and Affordable Care Act of 2010 (81).
In the meantime, while we seek to develop more evidence based research results, oncologists and health care personnel should be made more aware of the potential downside associated with obesity, low PA levels and poor CRF. More importantly, recommending measures to improve these parameters and monitoring PA (such as recently recommended by the American Heart Association (82, 83)) along with indicators of CRF and adiposity in routine follow-up examinations would provide the information needed for healthcare professionals to consider changing their approach and favor the implementation of effective lifestyle interventions.
Acknowledgments
During the preparation of this article, the efforts of the authors were supported, in part, as follows: A. Lucia, Fondo de Investigaciones Sanitarias, FIS, # PI12/00914; C. Bouchard, John W. Barton Sr. Chair in Genetics and Nutrition and NIH HL-045670; N. A. Berger, Hanna-Payne Professor Experimental Medicine, Ellison Medical Foundation, and NIH Grants P50 CA150964 and U54 CA163060
Footnotes
Conflicts of Interests: Claude Bouchard is a consultant/advisory board member for Weight Watchers International and reports receiving fees for the above mentioned association. The other authors indicate that no other conflicts of interest exist.
References
- 1.Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100:126–31. [PMC free article] [PubMed] [Google Scholar]
- 2.Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 3.Bauman A, Phongsavan P, Schoeppe S, Owen N. Physical activity measurement--a primer for health promotion. Promot Educ. 2006;13:92–103. doi: 10.1177/10253823060130020103. [DOI] [PubMed] [Google Scholar]
- 4.Lesage R, Simoneau JA, Jobin J, Leblanc J, Bouchard C. Familial resemblance in maximal heart rate, blood lactate and aerobic power. Human heredity. 1985;35:182–9. doi: 10.1159/000153540. [DOI] [PubMed] [Google Scholar]
- 5.Bouchard C, Lesage R, Lortie G, Simoneau JA, Hamel P, Boulay MR, et al. Aerobic performance in brothers, dizygotic and monozygotic twins. Med Sci Sports Exerc. 1986;18:639–46. [PubMed] [Google Scholar]
- 6.Bouchard C, Daw EW, Rice T, Perusse L, Gagnon J, Province MA, et al. Familial resemblance for VO2max in the sedentary state: the HERITAGE family study. Med Sci Sports Exerc. 1998;30:252–8. doi: 10.1097/00005768-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Jurca R, Jackson AS, LaMonte MJ, Morrow JR, Jr, Blair SN, Wareham NJ, et al. Assessing cardiorespiratory fitness without performing exercise testing. Am J Prev Med. 2005;29:185–93. doi: 10.1016/j.amepre.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Davis JA. Direct determination of aerobic power. In: Maud PJFC, editor. Physiological assessment of human fitness. Champaign IL: 1995. pp. 9–17. [Google Scholar]
- 9.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 6. Wilkinson LW; Philadelphia: 2000. [DOI] [PubMed] [Google Scholar]
- 10.Stamatakis E, Hamer M, O’Donovan G, Batty GD, Kivimaki M. A non-exercise testing method for estimating cardiorespiratory fitness: associations with all-cause and cardiovascular mortality in a pooled analysis of eight population-based cohorts. Eur Heart J. 2012;34:750–8. doi: 10.1093/eurheartj/ehs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Physical Activity Guidelines for Americans. 2008 http://www.health.gov/paguidelines/
- 12.World-Health-Organization. Global recommendations on physical activity for health. Switzerland: 2010. http://whqlibdoc.who.int/publications/2010/9789241599979_eng.pdf. [PubMed] [Google Scholar]
- 13.Sedentary Behaviour Research N. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–2. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 14.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 15.Fiuza-Luces C, Garatachea N, Berger NA, Lucia A. Exercise is the real polypill. Physiology (Bethesda) 2013;28:330–58. doi: 10.1152/physiol.00019.2013. [DOI] [PubMed] [Google Scholar]
- 16.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–8. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 17.Hermansen L, Wachtlova M. Capillary density of skeletal muscle in well-trained and untrained men. J Appl Physiol. 1971;30:860–3. doi: 10.1152/jappl.1971.30.6.860. [DOI] [PubMed] [Google Scholar]
- 18.Ingjer F. Capillary supply and mitochondrial content of different skeletal muscle fiber types in untrained and endurance-trained men. A histochemical and ultrastructural study. Eur J Appl Physiol Occup Physiol. 1979;40:197–209. doi: 10.1007/BF00426942. [DOI] [PubMed] [Google Scholar]
- 19.Essen B, Hagenfeldt L, Kaijser L. Utilization of blood-borne and intramuscular substrates during continuous and intermittent exercise in man. J Physiol. 1977;265:489–506. doi: 10.1113/jphysiol.1977.sp011726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: executive summary. Expert Panel on the Identification, Evaluation, and Treatment of Overweight in Adults. Am J Clin Nutr. 1998;68:899–917. doi: 10.1093/ajcn/68.4.899. [DOI] [PubMed] [Google Scholar]
- 21.Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39:1423–34. doi: 10.1249/mss.0b013e3180616b27. [DOI] [PubMed] [Google Scholar]
- 22.Tsigos C, Hainer V, Basdevant A, Finer N, Fried M, Mathus-Vliegen E, et al. Management of obesity in adults: European clinical practice guidelines. Obes Facts. 2008;1:106–16. doi: 10.1159/000126822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obesity: preventing and managing the global epidemic. Report of a WHO Consultation (WHO Technical Report Series 894) 2000 http://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/ [PubMed]
- 24.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. AICR; Washington, DC: 2007. http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf. [Google Scholar]
- 25.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–47. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 27.Irwin ML. Physical activity interventions for cancer survivors. Br J Sports Med. 2009;43:32–8. doi: 10.1136/bjsm.2008.053843. [DOI] [PubMed] [Google Scholar]
- 28.Gander J, Lee DC, Sui X, Hebert JR, Hooker SP, Blair SN. Self-rated health status and cardiorespiratory fitness as predictors of mortality in men. Br J Sports Med. 2011;45:1095–100. doi: 10.1136/bjsm.2010.079855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laukkanen JA, Rauramaa R, Makikallio TH, Toriola AT, Kurl S. Intensity of leisure-time physical activity and cancer mortality in men. Br J Sports Med. 2009;45:125–9. doi: 10.1136/bjsm.2008.056713. [DOI] [PubMed] [Google Scholar]
- 30.Campbell KL, Neil SE, Winters-Stone KM. Review of exercise studies in breast cancer survivors: attention to principles of exercise training. Br J Sports Med. 2012;46:909–16. doi: 10.1136/bjsports-2010-082719. [DOI] [PubMed] [Google Scholar]
- 31.Abioye AI, Odesanya MO, Ibrahim NA. Physical activity and risk of gastric cancer: a meta-analysis of observational studies. Br J Sports Med. 2014 Jan 16; doi: 10.1136/bjsports-2013-092778. [DOI] [PubMed] [Google Scholar]
- 32.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med. 2014;48:987–95. doi: 10.1136/bjsports-2012-091732. [DOI] [PubMed] [Google Scholar]
- 33.Schmid D, Leitzmann MF. Association between physical activity and mortality among breast cancer and colorectal cancer survivors: a systematic review and meta-analysis. Ann Oncol. 2014;25:1293–311. doi: 10.1093/annonc/mdu012. [DOI] [PubMed] [Google Scholar]
- 34.Campbell PT, Patel AV, Newton CC, Jacobs EJ, Gapstur SM. Associations of recreational physical activity and leisure time spent sitting with colorectal cancer survival. J Clin Oncol. 2013;31:876–85. doi: 10.1200/JCO.2012.45.9735. [DOI] [PubMed] [Google Scholar]
- 35.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Colorectal Cancer. 2011 http://www.dietandcancerreport.org/cancer_resource_center/downloads/cu/Colorectal-Cancer-2011-Report.pdf.
- 36.World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Breast Cancer. 2010 http://www.wcrf.org/sites/default/files/Breast-Cancer-Survivors-2014-Report.pdf.
- 37.Frisch RE, Wyshak G, Albright NL, Albright TE, Schiff I, Witschi J, et al. Lower lifetime occurrence of breast cancer and cancers of the reproductive system among former college athletes. Am J Clin Nutr. 1987;45:328–35. doi: 10.1093/ajcn/45.1.328. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–82. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 39.Goncalves AK, Dantas Florencio GL, Maisonnette de Atayde Silva MJ, Cobucci RN, Giraldo PC, Cote NM. Effects of physical activity on breast cancer prevention: a systematic review. J Phys Act Health. 2014;11:445–54. doi: 10.1123/jpah.2011-0316. [DOI] [PubMed] [Google Scholar]
- 40.Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, et al. Elite athletes live longer than the general population: A meta-analysis. Mayo Clin Proc. 2014;89(9):1195–2000. doi: 10.1016/j.mayocp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–27. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- 42.Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013;62:882–9. doi: 10.1136/gutjnl-2011-300776. [DOI] [PubMed] [Google Scholar]
- 43.Norheim F, Raastad T, Thiede B, Rustan AC, Drevon CA, Haugen F. Proteomic identification of secreted proteins from human skeletal muscle cells and expression in response to strength training. Am J Physiol Endocrinol Metab. 2011;301:E1013–21. doi: 10.1152/ajpendo.00326.2011. [DOI] [PubMed] [Google Scholar]
- 44.Inoue M, Senju S, Hirata S, Ikuta Y, Hayashida Y, Irie A, et al. Identification of SPARC as a candidate target antigen for immunotherapy of various cancers. Int J Cancer. 2010;127:1393–403. doi: 10.1002/ijc.25160. [DOI] [PubMed] [Google Scholar]
- 45.Mortensen OH, Andersen K, Fischer C, Nielsen AR, Nielsen S, Akerstrom T, et al. Calprotectin is released from human skeletal muscle tissue during exercise. J Physiol. 2008;586:3551–62. doi: 10.1113/jphysiol.2008.153551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fagerhol MK, Nielsen HG, Vetlesen A, Sandvik K, Lyberg T. Increase in plasma calprotectin during long-distance running. Scand J Clin Lab Invest. 2005;65:211–20. doi: 10.1080/00365510510013587. [DOI] [PubMed] [Google Scholar]
- 47.Mooren FC, Lechtermann A, Fobker M, Brandt B, Sorg C, Volker K, et al. The response of the novel pro-inflammatory molecules S100A8/A9 to exercise. Int J Sports Med. 2006;27:751–8. doi: 10.1055/s-2005-872909. [DOI] [PubMed] [Google Scholar]
- 48.Peake J, Peiffer JJ, Abbiss CR, Nosaka K, Okutsu M, Laursen PB, et al. Body temperature and its effect on leukocyte mobilization, cytokines and markers of neutrophil activation during and after exercise. Eur J Appl Physiol. 2008;102:391–401. doi: 10.1007/s00421-007-0598-1. [DOI] [PubMed] [Google Scholar]
- 49.Yui S, Mikami M, Yamazaki M. Purification and characterization of the cytotoxic factor in rat peritoneal exudate cells: its identification as the calcium binding protein complex, calprotectin. J Leukoc Biol. 1995;58:307–16. doi: 10.1002/jlb.58.3.307. [DOI] [PubMed] [Google Scholar]
- 50.Ghavami S, Kerkhoff C, Los M, Hashemi M, Sorg C, Karami-Tehrani F. Mechanism of apoptosis induced by S100A8/A9 in colon cancer cell lines: the role of ROS and the effect of metal ions. J Leukoc Biol. 2004;76:169–75. doi: 10.1189/jlb.0903435. [DOI] [PubMed] [Google Scholar]
- 51.Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–92. doi: 10.1136/mp.54.5.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–7. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 53.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–5. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He C, Sumpter R, Jr, Levine B. Exercise induces autophagy in peripheral tissues and in the brain. Autophagy. 2012;8:1548–51. doi: 10.4161/auto.21327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 56.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–78. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 57.Azvolinsky A. Cancer risk: the fat tissue-BMI-obesity connection. J Natl Cancer Inst. 2014;106:dju100. doi: 10.1093/jnci/dju100. [DOI] [PubMed] [Google Scholar]
- 58.Bhaskaran K, Douglas I, Forbes H, Dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–65. doi: 10.1016/S0140-6736(14)60892-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kmietowicz Z. Overweight and obesity are linked to 10 common cancers and more than 12 000 UK cases. BMJ. 2014;349:g5183. doi: 10.1136/bmj.g5183. [DOI] [PubMed] [Google Scholar]
- 60.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;4:579–91. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 61.Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc. 2012;71:175–80. doi: 10.1017/S0029665111003259. [DOI] [PubMed] [Google Scholar]
- 62.Gallagher EJ, LeRoith D. Minireview: IGF, Insulin, and Cancer. Endocrinology. 2011;152:2546–51. doi: 10.1210/en.2011-0231. [DOI] [PubMed] [Google Scholar]
- 63.Hursting SD, Berger NA. Energy balance, host-related factors, and cancer progression. J Clin Oncol. 2010;28:4058–65. doi: 10.1200/JCO.2010.27.9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand G, Katz PO. Gastroesophageal reflux disease and obesity. Rev Gastroenterol Disord. 2008;8:233–39. [PubMed] [Google Scholar]
- 66.Leidner R, Chak A. Obesity and the Pathogenesis of Barrett’s Esophagus. In: Markowitz SD, Berger NA, editors. Energy Balance and Gastrointestinal Cancer. Chapter 5. Springer; 2012. pp. 77–92. [Google Scholar]
- 67.World Health Organization. Global Database on Body Mass Index. available at: http://www.who.int/topics/obesity/en/
- 68.World Health Organization, International Agency for Research on Cancer. GLOBOCAN project. available at: http://globocan.iarc.fr/Default.aspx.
- 69.De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. Journal of obesity. 2013;2013:291546. doi: 10.1155/2013/291546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lynch BM, Dunstan DW, Healy GN, Winkler E, Eakin E, Owen N. Objectively measured physical activity and sedentary time of breast cancer survivors, and associations with adiposity: findings from NHANES (2003–2006) Cancer Causes Control. 2010;21:283–8. doi: 10.1007/s10552-009-9460-6. [DOI] [PubMed] [Google Scholar]
- 71.Lynch BM, Dunstan DW, Winkler E, Healy GN, Eakin E, Owen N. Objectively assessed physical activity, sedentary time and waist circumference among prostate cancer survivors: findings from the National Health and Nutrition Examination Survey (2003–2006) Eur J Cancer Care (Engl) 2011;20:514–9. doi: 10.1111/j.1365-2354.2010.01205.x. [DOI] [PubMed] [Google Scholar]
- 72.Smith WA, Nolan VG, Robison LL, Hudson MM, Ness KK. Physical activity among cancer survivors and those with no history of cancer- a report from the National Health and Nutrition Examination Survey 2003–2006. Am J Transl Res. 2011;3:342–50. [PMC free article] [PubMed] [Google Scholar]
- 73.Ruiz-Casado A, Verdugo AS, Solano MJ, Aldazabal IP, Fiuza-Luces C, Alejo LB, et al. Objectively assessed physical activity levels in Spanish cancer survivors. Oncol Nurs Forum. 2014;41:E12–20. doi: 10.1188/14.ONF.E12-E20. [DOI] [PubMed] [Google Scholar]
- 74.Jovanovic M, Soldatovic I, Janjic A, Vuksanovic A, Dzamic Z, Acimovic M, et al. Diagnostic value of the nuclear matrix protein 22 test and urine cytology in upper tract urothelial tumors. Urol Int. 2011;87:134–7. doi: 10.1159/000330246. [DOI] [PubMed] [Google Scholar]
- 75.Peel AB, Thomas SM, Dittus K, Jones LW, Lakoski SG. Cardiorespiratory fitness in breast cancer patients: a call for normative values. J Am Heart Assoc. 2014;3:e000432. doi: 10.1161/JAHA.113.000432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301:2024–35. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 77.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: 2009. http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site. [Google Scholar]
- 78.Peel JB, Sui X, Matthews CE, Adams SA, Hebert JR, Hardin JW, et al. Cardiorespiratory fitness and digestive cancer mortality: findings from the aerobics center longitudinal study. Cancer Epidemiol Biomarkers Prev. 2009;18:1111–7. doi: 10.1158/1055-9965.EPI-08-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA. 2007;297:2081–91. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 80.Shaw K, Gennat H, O’Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database Syst Rev. 2006:CD003817. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.About Us PCORI. http://www.pcori.org/about-us.
- 82.Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the assessment of physical activity: Clinical and research applications: a scientific statement from the American Heart Association. Circulation. 2013;128:2259–79. doi: 10.1161/01.cir.0000435708.67487.da. [DOI] [PubMed] [Google Scholar]
- 83.US Department of Health and Human Services. [Accesed September 18, 2014];Physical Activity Guidelines for Americans. 2008 Available from: http://www.health.gov/paguidelines/pdf/paguide.pdf.