Abstract
Purpose
High-dose aldesleukin (HD IL-2) received FDA approval for the treatment of mRCC in 1992, producing a 14% objective response rate (ORR) and durable remissions. Retrospective studies suggested that clinical and pathologic features could predict for benefit. The Cytokine Working Group conducted this prospective trial to validate proposed predictive markers of response to HD IL-2.
Experimental Design
Standard HD IL-2 was administered to prospectively evaluate whether the ORR of mRCC patients with “good” predictive pathologic features based on an “integrated selection” model (ISM) (e.g. clear-cell histology sub-classification and carbonic anhydrase-9 (CA-9) IHC staining) was significantly higher than the ORR of a historical, unselected population. Archived tumor was collected for pathologic analysis including tumor programmed death-ligand 1 (PD-L1) expression.
Results
120 eligible patients enrolled between 11/06 and 7/09; 70% were MSKCC intermediate risk, 96% had clear cell RCC and 99% had prior nephrectomy. The independently assessed ORR was 25% (30/120, 95% CI = 17.5%–33.7%, p=0.0014) (3 CR, 27 PR) and was higher than a historical ORR. Thirteen patients (11%) remained progression-free at 3 years and the median OS was 42.8 months. ORR was not statistically different by ISM classification (“good-risk” 23% vs. “poor-risk” 30%, (p=0.39)). ORR was positively associated with tumor PD-L1 expression (p=0.01) by IHC.
Conclusions
In this prospective, biomarker validation study, HD IL-2 produced durable remissions and prolonged survival in both “good” and “poor-risk” patients. The proposed ISM was unable to improve the selection criteria. Novel markers (e.g. tumor PD-L1expression) appeared useful, but require independent validation.
Keywords: Interleukin-2, Renal, Select, Predictive, High-dose
Introduction
HD IL-2 received FDA approval in 1992 due to its ability to produce durable responses in a small percentage of patients with mRCC.(1) Data presented to the FDA showed an ORR of 14% in 255 patients with mRCC treated on 7 phase II trials.(1) Although the clinical efficacy has improved incrementally in subsequent trials largely due to clinical selection factors, investigators have attempted to develop predictive biomarkers of response that might further narrow its application to patients most likely to benefit.(2–4)
Retrospective analyses have suggested that clinical characteristics and pathologic features could predict for response (or resistance) to IL-2.(1, 3, 5–8) Leibovich et al conducted a multivariate analysis of patients who received IL-2 after nephrectomy. This study showed that survival was inversely associated with lymph node involvement, constitutional symptoms, sarcomatoid histology, metastases involving sites other than bone and lung, multiple metastatic sites, and a TSH level > 2.0 mIU/L, and led to the creation of the UCLA SANI score.(9)
Several studies have shown that responses to immunotherapy are most frequently seen in patients with clear cell (cc) RCC.(10–12) In a retrospective analysis of pathology specimens obtained from 163 patients who had received IL-2 therapy, the response rate to IL-2 was 21% for patients with clear cell tumor histology compared with 6% for patients with non–clear cell tumor histology.(12) Among the patients with ccRCC, histologic sub-classification based on the presence of “good” predictive features (e.g. more than 50% alveolar and no granular or papillary features) and the absence of “poor” predictive features (e.g., more than 50% granular or any papillary features) was associated with response to IL-2. Application of this model was reported to produce an impressive ORR (52%) in a small, prospective study.(13)
Carbonic anhydrase 9 (CA-9) has been identified as an immunohistochemical (IHC) marker that might predict the outcomes of patients with renal carcinoma. In an analysis by Bui et al, CA-9 expression in more than 85% of tumor cells (high CA-9 expression by IHC) from ccRCC was associated with improved survival and a higher objective response rate in IL-2–treated patients.(14) Building on this work, Atkins and colleagues developed a 2-component model that combined histologic sub-classification with IHC staining for CA-9.(15) In a retrospective analysis, this “integrated selection” model (ISM) was able to identify a “good” predictive features group that contained 26 (96%) of 27 responders to IL-2. Based on these findings, other investigators began to incorporate CA-9 analysis into their IL-2 based clinical trials and treatment selection decisions.(16)
In an attempt to improve the therapeutic index of IL-2, the Cytokine Working Group (CWG) designed and conducted the HD IL-2 “Select” Trial. The primary objective of this study was to evaluate prospectively whether the ISM could identify a group of patients with advanced RCC and “good’ predictive features who were significantly more likely to respond to HD IL-2–based therapy than a historical, unselected patient population.(1) During the course of this trial, retrospective analyses identified potential predictors of increased, (e.g. tumor PD-L1expression, CA-9 gene single nucleotide polymorphism (SNP)) and decreased (e.g. elevated pre-treatment levels of fibronectin and VEGF) response to immunotherapy in patients with RCC.(17–19) Additional tissue was collected and secondary study objectives were amended to determine whether other clinical and pathologic features could help to further refine the optimal population for HD IL-2-based therapy.
Materials and Methods
Patients
Patients with mRCC of any histologic type and no prior systemic therapy were enrolled. Major eligibility criteria included an Eastern Cooperative Oncology Group performance status of 0 or 1; bi-dimensionally measurable and clearly progressive disease; adequate organ function, with serum creatinine ≤1.5 mg/dl or calculated creatinine clearance >60 ml/min; forced expiratory volume in 1 sec >2.0 liter/sec or 75% of predicted value; and no evidence of ischemia on a cardiac stress test. Patients who had received prior systemic treatment and those with brain metastases, seizure disorders, organ allografts, history of another malignancy, or concurrent corticosteroid therapy were ineligible. The protocol was approved by the human investigational review board at each participating site and voluntary written informed consent was obtained from each patient.
Treatment Plan
The study was conducted by the Cytokine Working Group (CWG). Patients received IL-2, 600,000 IU/kg/dose (Prometheus Laboratories Inc. San Diego, CA) IV every 8 hours for five days (maximum of 14 doses) beginning on day 1 and again on day 15. One course generally consisted of 5 days of treatment, 9 days of rest, 5 more days of treatment, and 9 weeks of rest, followed by up to 2 additional courses of HD IL-2 for patients who benefited and tolerated most of the planned IL-2 doses. A treatment delay of up to 4 weeks was allowed for resolution of side effects between courses. Patients were eligible to receive a maximum of 3 courses of treatment.
Assessments
As HD IL-2 is a FDA-approved treatment regimen, only serious adverse events (SAEs) according to CTCAE version 3.0 were reported during the conduct of the trial. Response and progression were assessed according to standard WHO criteria and were initially determined by investigator assessment of radiographs.(20) Patients were evaluated for response during week 8 and 12 of each course. To be eligible for more than one course of treatment, patients must have had at least stable disease with evidence of minor tumor regression or objective response and had to meet baseline eligibility criteria for organ function. Progression free survival (PFS) was calculated from the date of IL-2 initiation to the date of disease progression, or death on treatment per the evaluating physician, or censored at the last documented tumor assessment for patients whose disease had not progressed. All patients who achieved a CR, PR and SD for more than 6 months had their CT scans audited by independent radiologists to confirm their response and response duration. Overall Survival (OS) was calculated from the date the first dose of IL-2 was administered to the date of death or censored at the last documented contact with the patient. Data were updated through October 31, 2013.
Correlative Laboratory Studies
Laboratory investigations were done in conjunction with the Tissue Acquisition Pathology and Clinical Data (TAPCD) Core of the Dana-Farber/Harvard Cancer Center (DF/HCC) Kidney Cancer SPORE. Examination of tumor tissue obtained prior to exposure to IL-2 was performed using a number of modalities to identify potential predictors of response to treatment. As a requirement of study enrollment, patients consented to allow access by the investigators to the original Hematoxylin and Eosin stained slides used to confirm the diagnosis of RCC for use in correlative studies. Paraffin fixed tissue, obtained from representative, satisfactory tissue blocks of primary or metastatic tumors from participating subjects, was reviewed by the TAPCD for histologic features and stained for CA-9 using the M75 antibody according to a previously published protocol.(15) Patients were then classified into the “good” or “poor” pathologic predictive factor group as proposed by the ISM.(15) Additional, exploratory laboratory investigations were performed in collaboration with co-investigators according to pre-published protocols [e.g. VEGF and fibronectin levels, CA-9 SNP and PD-L1 IHC (≥ 5% tumor membrane staining was considered “positive”)] based on preliminary data suggesting that they might predict for response to immunotherapy in RCC.(17–19, 21)
Statistical Analysis
The primary objective was to prospectively determine if the response rate to high-dose IL-2 for patients with mRCC and “good” pathologic predictive features by the ISM was significantly higher than a historical, unselected patient population. The primary endpoint, objective response rate (ORR) was defined by WHO criteria as used in previous IL-2 trials. The accrual goal was 110 patients, to enroll 66 patients in the ISM “good” pathologic predictive factor group, assuming 60% of patients would be classified as having “good” factors and a response rate between 30–40%.(12, 15) The sample size provided >80% power for a one-sample exact test with 2-sided α=0.05 to detect response proportion of 0.30 versus 0.14 and assures confidence interval precision (half-width) less than ±0.14.(1) Secondary objectives included prospectively estimating the response rate to high-dose IL-2 for patients with mRCC and “poor” pathologic predictive features by the ISM and comparing this response rate to the response rate of patients with “good” pathologic predictive features. Hypothesis-generating, univariate analyses were planned to explore whether other clinical (e.g., MSKCC and UCLA SANI score) plasma (e.g. VEGF and fibronectin levels) and tumor (e.g. immunohistochemical markers (PD-L1, B7-H3) or CA-9 gene SNP) features might be associated with HD IL-2 responsiveness in order to further refine the optimal population for HD IL 2-based therapy.(9, 11, 22) Comparisons on ORR and durable remission rate (PFS > 3 years, all evaluable patients either had progression within 3 years or were followed for more than 3 years) were assessed by the Fisher exact test. Distributions of PFS and OS were summarized with the Kaplan-Meier estimates.
Results
Study Summary
Between November 2006 and July 2009, 123 patients were enrolled at 13 participating institutions. All patients met the eligibility criteria. Three patients withdrew consent prior to receiving therapy and were not included in the analysis. Ninety-nine percent of patients had undergone prior nephrectomy, 70% were intermediate risk by MSKCC prognostic criteria and 85% intermediate risk by UCLA SANI score (Table 1). Tumor (98%) and blood (94%) samples were collected on most patients. During the first course of therapy, patients received an average of 17 doses of the planned 28 doses (61%) of HD IL-2. Doses were held due to treatment-related AEs that were typical of the prior published experience with this regimen.(1, 3) Common SAEs included: hypotension, renal failure, and hyperbilirubinemia. Treatment related AEs were reversible, except in two cases of treatment-related death. A 47-year-old male patient died during course 1, week 1 as a result of complications from a myocardial infraction. A 70-year-old male patient died following course 1, week 1 as a result of a cardiac arrhythmia.
Table 1.
Patient Clinical Characteristics of All Treated Patients
| Characteristics | n=120 |
| Median age, years (range) | 56 (28–70) |
| ECOG Performance Status* 0/1 (%) | 72/24** |
| Prior nephrectomy (%) | 99 |
| MSKCC risk factors- n (%) | |
| 0 (favorable) | 23 (19) |
| 1–2 (intermediate) | 84 (70) |
| ≥ 3 (poor) | 13 (11) |
| UCLA SANI Score n (%) | |
| Low | 10 (8) |
| Intermediate | 102 (85) |
| High | 8 (7) |
Abbreviations: n, number of patients; ECOG, Eastern Cooperative Oncology Group; MSKCC, Memorial Sloan Kettering Cancer Center; UCLA, University of California Los Angeles; SANI, Survival After Nephrectomy and Immunotherapy
Criteria as described in Oken MM, Creech RH, Tormey DC, et al: Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–655, 1982.
4% missing data
Efficacy Data
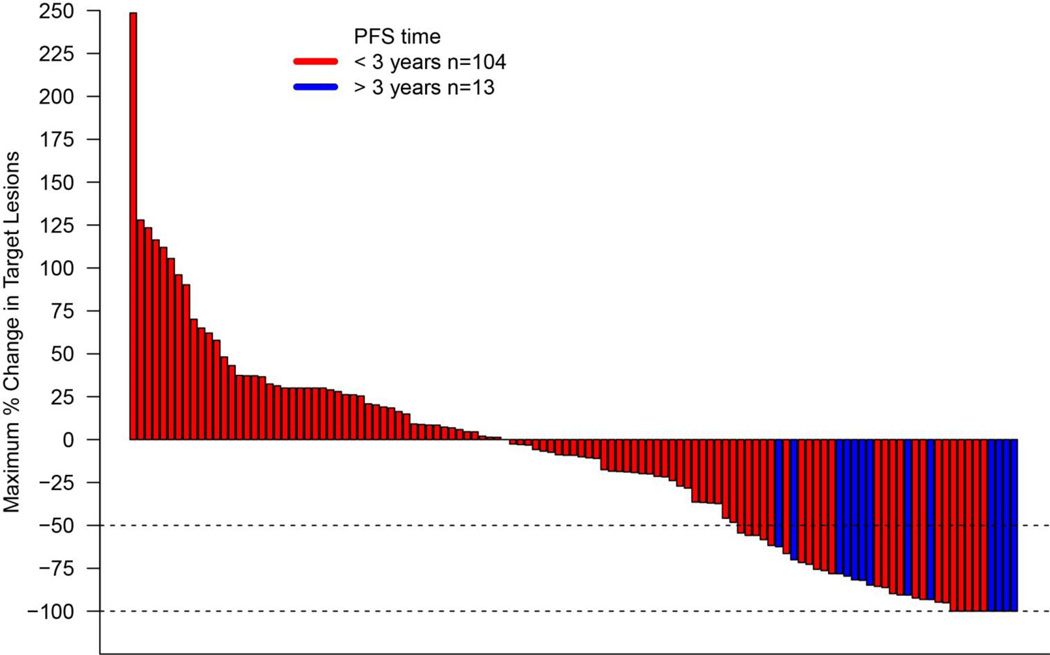
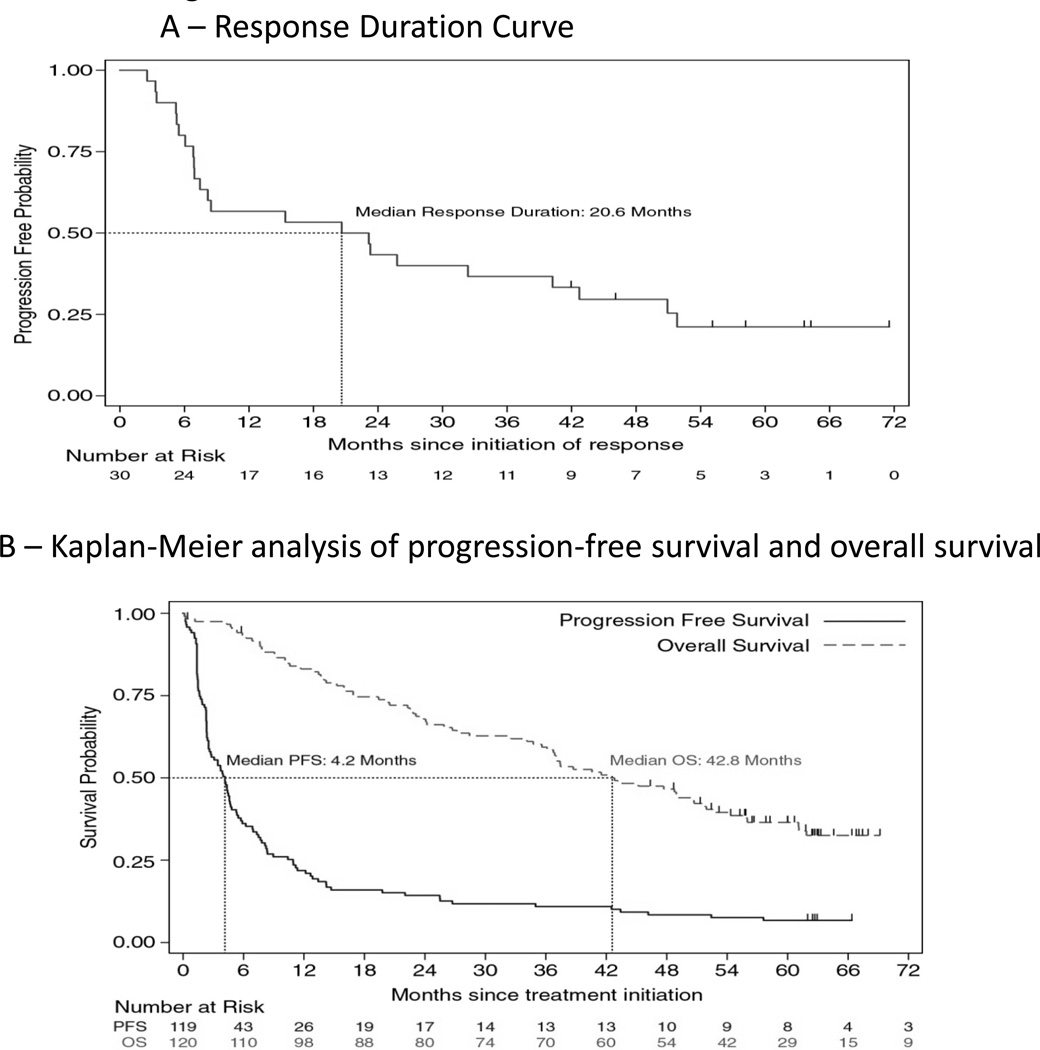
The objective response rate of all 120 patients to HD IL-2 confirmed by independent review was 25% (95% CI, 17.5 to 33.7%) (Table 2). This response was substantially greater than a historical response rate of 14% (p=0.0014).(1) Three patients had complete responses (2.5%) and 27 had partial responses (22.5%). Nine patients (7.5%) achieved stable disease that lasted more than 6 months. The overall response rate to HD IL-2 confirmed by investigator assessment was 28.3% (95% CI: 20.5 to 37.3%) (Table 2). Nine patients were classified as having complete responses (7.5%) and 25 had partial responses (20.8%). By investigator assessment, some degree of tumor regression was seen in 49 patients (42%) (Figure 1). The median response duration for the cohort was 20.6 months (95% CI, 6.9 to 42.7) (Figure 2A). The median progression-free survival was 4.2 months (95% CI, 2.5 to 4.7) (Figure 2B) and 13 patients (11%) achieved a durable remission by remaining progression-free for at least 3 years. Seven patients developed disease progression following IL-2 and were treated with local therapy (e.g. surgery or radiation) but have yet to require systemic therapy. Eighty patients received at least one cycle of VEGF targeted therapy following IL-2, which contributed to a median overall survival from time of IL-2 initiation of 42.8 months (95% CI, 35.6 to 51.9) (Figure 2B).
Table 2.
Clinical Activity of HD IL-2
| Objective Response* | N (%) | |
| Patients with measurable | 120 (100) | |
| disease (n) | ||
| Assessment | Independent | Investigator |
| Objective response rate | 30 (25.0) (95% CI: 17.5 to 33.7%) |
34 (28.3) (95% CI: 20.5 to 37.3%) |
| Complete response | 3 (2.5) | 9 (7.5) |
| Partial response | 27 (22.5) | 25 (20.8) |
| Stable disease (> 6 months) | 9 (7.5) | 9 (7.5) |
Abbreviations: n/N, number of patients, CI, confidence interval.
Objective response rates ({[CR + PR] ÷ N} × 100) have been calculated based on confirmed responses with confidence intervals calculated using the Clopper-Pearson method. Individual patient responses were adjudicated per WHO (World Health Organization) Criteria.
Fig. 1. Maximum change in summary target lesion measurements compared with baseline (WHO criteria).
Characteristics of tumor regression in patients with mRCC receiving HD IL-2 therapy by investigator assessment. Maximum reduction or minimum increase in sum of target lesion measurements compared with baseline in all treated patients with on-treatment tumor measurements. Graph shows best individual change up to first progression according to WHO criteria. Tumors were assessed after each cycle per WHO guidelines. Baseline tumor measurements were standardized to zero, tumor burden was measured as sum of the longest diameters of target lesions. Horizontal line at −50% indicates threshold for defining objective response (partial tumor regression) in the absence of new lesions or non-target disease progression according to WHO. By independent review, some degree of tumor regression was seen in 49 patients (42%). Red bars indicate patients with a PFS less than 3 years, blue bars indicate patients with a PFS greater than 3 years.
Fig. 2.
Efficacy outcomes in patients with mRCC receiving HD IL-2. Kaplan-Meier curves of response duration in 30 objective responders (A), and overall survival and progression-free survival in 120 HD IL-2 treated patients with mRCC (B). The median duration of response in 30 responding patients was 20.6 months (A). Patients with mRCC had a median overall survival of 42.8 months (B). Progression-free survival rate was 11% at 3 years, and the median 4.2 months (B). Tick marks indicate censored events, defined for overall survival as the time to last known alive date before the date of data analysis for patients without a death and for progression-free survival as the time to the last tumor assessment before the date of data analysis for patients without disease progression or death.
Predicting Responsiveness to HD IL-2
While MSKCC risk group was not associated with objective response or durable remission (PFS >3 years), patients with a high UCLA SANI score failed to respond to IL-2 (0/8 objective responses) (Table 3) and had a significantly lower PFS (median 1.4 months (CI, 0.2 to 2.5), p<0.001 by log-rank test). There were no responses seen in patients with non-clear cell histology (0/5 objective responses). Objective and durable response to IL-2 were not associated with any pathologic classification (e.g. “good” risk clear-cell histology group, high CA-9 staining or “ISM” “good” risk group) (Table 3).
Table 3.
Response by Baseline Clinical/Tumor Characteristics
| ORR (95% CI) | P- value1 |
PFS > 3 years (95% CI) |
P-value1 | |
|---|---|---|---|---|
| Clinical Characteristics | ||||
| MSKCC Risk Group | ||||
| Favorable (n=23) | 22% (7%–44%) | 0.89 | 17% (5%–39%) | 0.33 |
| Intermediate (n=84) | 25% (16%–36%) | 8% (3%–17%) | ||
| Poor (n=13) | 31% (9%–61%) | 15% (2%–45%) | ||
| UCLA SANI Score | ||||
| Low (n=10) | 20% (3%–56%) | 0.27 | 10% (0%–45%) | 0.84 |
| Intermediate (n=102) | 27% (19%–37%) | 12% (6%–20%) | ||
| High (n=8) | 0% (0%–37%) | 0% (0%–37%) | ||
| Tumor Characteristics | ||||
| Tumor Type | ||||
| Clear cell (n=114) | 26% (19%–35%) | 0.33 | 12% (6%–19%) | 0.99 |
| Non-clear cell (n=5) | 0% (0%–52%) | 0% (0%–52%) | ||
| Clear Cell Histology Risk Group | ||||
| Good (n=11) | 27% (6%–61%) | 0.89 | 18% (2%–52%) | 0.58 |
| Intermediate (n=83) | 24% (15%–35%) | 10% (4%–18%) | ||
| Poor (n=25) | 28% (12%–49%) | 12% (3%–31%) | ||
| CA-9 Score | ||||
| High (≥85% n=78) | 22% (13%–33%) | 0.19 | 9% (4%–18%) | 0.35 |
| Low (<85% n=39) | 33% (19%–50%) | 15% (6%–31%) | ||
| Integrated Selection Model Risk (ISM) Group | ||||
| Good (n=74) | 23% (14%–34%) | 0.39 | 9% (4%–19%) | 0.55 |
| Poor (n=43) | 30% (17%–46%) | 14% (5%–28%) | ||
| PD-L1+ Tumor | ||||
| Negative (n=95) | 19% (12%–28%) | 0.01 | 6% (2%–13%) | <0.01 |
| Positive (n=18) | 50% (26%–74%) | 33% (13%–59%) | ||
| B7-H3+ Tumor | ||||
| Negative (n=28) | 11% (2%–28%) | 0.08 | 7% (1%–24%) | 0.73 |
| Positive (n=86) | 29% (20%–40%) | 12% (6%–20%) | ||
| CA-9 SNP | ||||
| Homozygous (n=66) | 20% (11%–31%) | 0.28 | 12% (5%–22%) | 0.35 |
| Variant (n=12) | 33% (10%–65%) | 0% (0%–26%) |
Abbreviations: ORR, objective response rate;, PFS, progression-free survival, n, number of patients; MSKCC, Memorial Sloan Kettering Cancer Center; UCLA, University of California Los Angeles; SANI, Survival After Nephrectomy and Immunotherapy; CA-9, carbonic anhydrase-9; PD-L1, programmed death ligand-1; SNP, single nucleotide polymorphism.
Fisher exact test
The numbers (n) for each analysis do not always add up to 120 patients as data/tissue was not available in some cases.
Exploratory Correlative Analysis
ORR was not associated with CA-9 SNP status or plasma VEGF or fibronectin levels (data not shown). Response was positively associated with tumor expression of PD-L1 (p=0.01) and B7H-3 (p=0.08) (Table 3) by IHC staining.(23) Durable remission (PFS >3 years) was positively associated with tumor expression of PD-L1 (p<0.01) but not B7-H3 (p=0.73) by IHC staining.
Discussion
In this prospective biomarker study, the clinical results revealed a response rate (25%) for the entire cohort that was substantially higher than the initial experience with high-dose IL-2 in patients with mRCC.(1) Toxicity was typical of our prior published experience with this regimen.(1, 3, 4, 24) There were two treatment related deaths. Previous research by our group and others that identified potential clinical and pathologic predictors of response to immunotherapy likely contributed to enhanced pre-treatment screening (e.g. fewer patients enrolled with non-clear cell tumor histology, high UCLA SANI score or without prior nephrectomy than on previous IL-2 studies) and an improvement in antitumor activity for the entire cohort.(3, 10, 12, 15, 22, 25–27) For example, a smaller percentage of patients received HD IL-2 with their primary tumor in place on the current study than on an earlier CWG Phase III randomized trial of HD IL-2 (<1% vs. 30%).(3) In addition, the availability of other treatment options (e.g. VEGF and mTOR pathway inhibitors) for patients with mRCC likely altered the referral pattern to academic medical centers towards those patients perceived, on clinical grounds, to be most likely to benefit from IL-2. As has been reported in other studies, patients experienced durable remissions of their disease (3-year PFS rate 11%). The encouraging overall survival demonstrated in this cohort has been reported by other investigators.(3, 28–30) It is likely the result improved patient selection (as discussed above) and of the sequential application of IL-2 based immunotherapy followed, in most cases, by molecularly targeted therapy.
While the application of previously identified clinical and pathologic selection criteria improved outcomes for the entire cohort when compared to the original experience with HD IL-2, analysis of the proposed “integrated selection” model (ISM) through central histology review and immunohistochemical staining for CA-9 was unable to further improve the selection criteria. The clinical outcomes seen in this study were consistent with the more recent experience with HD IL-2.(2, 3) There are several potential explanations for this result. They include: host factors (e.g. patient immune response) and the complex biology of IL-2, which not only stimulates and expands CD8 and natural killer cells but also stimulates regulatory T cells and activation-induced T cell death which may play a larger role in determining response to IL-2 than had previously been thought;(31, 32) tumor factors are important but within a patient cohort enriched for ccRCC histology and low/intermediate UCLA SANI score, markers other than CA-9 are more predictive, or analyzed samples were not representative given the lack of standards for tumor processing at community centers and the existence of significant tumor heterogeneity.(33, 34) Given that this latter issue likely impacts many kidney cancer translational research projects, efforts to standardize tissue collection should be considered in future trials.
The complete response (CR) rate in this cohort was lower than has been reported in several prior HD IL-2 trials in RCC.(2, 3) The decline in the CR rate could be secondary to the application of independent radiology review and the enhanced resolution provided by modern CT scans. In this study, the CR rate by investigator assessment (7.5%) was greater than that reported by independent review (2.5%). Despite this discrepancy, the durable remission rate (PFS > 3 years) of 11% was consistent with the rate reported in earlier trials.(3) Responses occurred in patients in all MSKCC risk classifications, but not in the limited number of patients enrolled with non-clear cell histology or high UCLA SANI score. Based on the results of this study, while MSKCC risk classification does not identify responders, other clinical and pathologic features (e.g. UCLA SANI high score and non-clear cell histology) may identify patients unlikely to respond to HD IL-2 and probably should not receive it.
While our data did not validate the hypothesis that the ISM could predict response to HD IL-2, this trial provides further evidence for the need to confirm the value of proposed biomarkers in well-designed, prospective studies. Given that the activity of HD IL-2 was similar in both the high and low CA-9 immunohistochemical staining risk groups, this assay should not be routinely performed as a tool to identify potential patients. Attempts to develop other predictive biomarkers (e.g. PD-L1 expression, KIR (killer-cell immunoglobulin-like receptor)/KIR ligand mismatch) are ongoing to understand tumor and host factors that might predict for remissions following IL-2 therapy.(35) An improved model for IL-2 patient selection may emerge from these efforts and improve its therapeutic index. The potential correlation between tumor expression of immune inhibitory molecules (e.g. PD-L1) and response to IL-2 seen in this trial has been reported in clinical trials of PD-1/PD-L1 monoclonal antibodies.(19, 36, 37) PD-L1 expression on tumor cells is thought to be induced by infiltrating CD8+ T-cells in the tumor microenvironment.(38, 39) Given their proposed mechanism of action, it is not surprising that PD-1/PD-L1 pathway blocking antibodies would display greater clinical activity in tumors that express PD-L1. The hypothesis that IL-2 administration may be more effective in “inflamed” tumors that are infiltrated by CD8+ T-cells and express immune inhibitory molecules (e.g. PD-L1 or B7-H3) needs to be confirmed in prospective trials.(40)
Two decades of retrospective, correlative research designed to narrow the application of cytokine-based immunotherapy has culminated in the improved clinical outcomes seen in this cohort. While HD IL-2 remains a reasonable treatment option for appropriately selected patients with mRCC, this prospective trial was unable to validate proposed predictive markers of response and further improve the selection criteria for HD IL-2. However, lessons from this work may guide the development and validation of predictive biomarkers for novel immunotherapies (e.g. CTLA-4, PD-1/PD-L1 antibodies) in solid tumors.(41) As the list of effective therapies for metastatic kidney cancer grows, improvements in patient selection will be necessary to improve overall survival and the remission rate for patients with this disease.
Statement of Translational Relevance.
While this prospective trial was unable to validate proposed predictive markers of response and further improve the selection criteria for HD IL-2, this manuscript provides information on several issues that will be critical to making further advances in mRCC treatment: (1) the need to confirm the value of proposed biomarkers in well-designed, prospective studies, (2) the potential for extended overall survival through the sequential application of immunotherapy followed by targeted therapy, (3) the need for improved clinical trial endpoints that fully capture the clinical benefits of immunotherapy (e.g. 3-year PFS), and (4) the potential role of tumor PD-L1 expression as a predictor of response to immunotherapy. Finally, lessons from this work may guide the development of novel immunotherapies (e.g. CTLA-4, PD-1/PD-L1 antibodies) in RCC.
Acknowledgments
We thank the patients who participated in this study, clinical faculty, and personnel, including, Meghan French, Gretchen McGrath RN, Rachel Simonson and Alexander Carlson.
Financial Information
Research reported in this publication supported by Prometheus Laboratories Inc., Novartis International AG, the National Cancer Institute of the National Institutes of Health, and SPORE in Kidney Cancer under award number P50CA101942. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Presented in part at the 46th Annual Meeting of the American Society of Clinical Oncology, June 4–8, 2010, Chicago, IL, USA
Authors Disclosures of Potential Conflicts of Interest:
Employment: None; Leadership: None; Stock or Ownership: None; Honoraria: Janice P. Dutcher Prometheus, Pfizer, Novartis, Merck, BMS, Brendan D. Curti Prometheus, Michael K.K. Wong Merck, Ulka N. Vaishampayan Prometheus, Pfizer, Novartis; Meredith M. Regan SAIC Frederick now Leidos Biomedical Research Inc., Michael B. Atkins BMS; Consulting or Advisory Role: David F. McDermott Merck, BMS, Pfizer, Genentech, Sabina Signoretti Verastem, Joseph I. Clark Prometheus, Janice P. Dutcher Prometheus, Pfizer, Novartis, Theodore F. Logan Argos, GlaxoSmithKline, Novartis, Pfizer, Prometheus, Wyeth, Nikhil I. Khushalani Genentech, Amgen, Ulka N. Vaishampayan Prometheus, Pfizer, Novartis, Rupal S. Bhatt Eli Lilly, Eugene D. Kwon BMS, Michael B. Atkins BMS, Prometheus, Genentech; Speaker’s Bureau, Joseph I. Clark Prometheus, BMS, Pfizer, Janice P. Dutcher Prometheus, Pfizer, Novartis, Theodore F. Logan BristolMyersSquibb, GlaxoSmithKline, Novartis, Pfizer, Prometheus, Wyeth, Nikhil I. Khushalani Prometheus, Ulka N. Vaishampayan Prometheus, Pfizer, Novartis: Research Funding, David F. McDermott Prometheus Laboratories; Kim A. Margolin Prometheus, Genentech, GSK, BMS, Merck, Novartis; Joseph I. Clark Prometheus, BMS, Pfizer; Jeffrey A. Sosman Prometheus; Theodore F. Logan Abbott, Abraxis, Acceleron, Amgen, Argos, AstraZeneca, Aveo, Biovex, BristolMyersSquibb, Eisai, EliLilly, GlaxoSmithKline, Hoffman-LaRoche, Immatics, Merck, Novartis, Pfizer, Prometheus, Roche, Synta, Threshold; Brendan D. Curti Prometheus; Leonard Appleman Amgen, Bayer, Exelixis, Medivation, Astellas, PSMA Devpt, BMS, J+J; Nikhil I. Khushalani Merck,Pfizer,NCCN, Roche, Pfizer; Ulka N. Vaishampayan Prometheus, Pfizer, Novartis; Rupal S. Bhatt Sanofi; Eugene D. Kwon BMS; Meredith M. Regan Pfizer, Novartis, Ipsen, Celgene, Ferring, Merck, Veridex, OncoGenex; Patents, Royalities, Other Intellectual Property: James W. Mier Acceleron; Rupal S. Bhatt Acceleron Pharma; Eugene D. Kwon BMS, MedImmune, Amplimune, Mederex, Mayo Clinic; Expert Testimony: None; Travel Accommodations Expenses: Janice P. Dutcher Prometheus, Pfizer, Novartis, Merck, BMS; Brendan D. Curti Agonox, Prometheus, Leonard Appleman Exelixis, Bayer; Rupal S. Bhatt Eli Lilly Other Relationship: Janice P. Dutcher Data Safety & Monitoring Committee BMS, Merck
References
- 1.Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13:688–696. doi: 10.1200/JCO.1995.13.3.688. [DOI] [PubMed] [Google Scholar]
- 2.Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P, et al. Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol. 2003;21:3127–3132. doi: 10.1200/JCO.2003.02.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF, et al. Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol. 2005;23:133–141. doi: 10.1200/JCO.2005.03.206. [DOI] [PubMed] [Google Scholar]
- 4.Margolin KA, Rayner AA, Hawkins MJ, Atkins MB, Dutcher JP, Fisher RI, et al. Interleukin-2 and lymphokine-activated killer cell therapy of solid tumors: analysis of toxicity and management guidelines. J Clin Oncol. 1989;7:486–498. doi: 10.1200/JCO.1989.7.4.486. [DOI] [PubMed] [Google Scholar]
- 5.Negrier S, Perol D, Ravaud A, Chevreau C, Bay JO, Delva R, et al. Medroxyprogesterone, interferon alfa-2a, interleukin 2, or combination of both cytokines in patients with metastatic renal carcinoma of intermediate prognosis: results of a randomized controlled trial. Cancer. 2007;110:2468–2477. doi: 10.1002/cncr.23056. [DOI] [PubMed] [Google Scholar]
- 6.Negrier S, Caty A, Lesimple T, Douillard JY, Escudier B, Rossi JF, et al. Treatment of patients with metastatic renal carcinoma with a combination of subcutaneous interleukin-2 and interferon alfa with or without fluorouracil. Groupe Francais d'Immunotherapie, Federation Nationale des Centres de Lutte Contre le Cancer. J Clin Oncol. 2000;18:4009–4015. doi: 10.1200/JCO.2000.18.24.4009. [DOI] [PubMed] [Google Scholar]
- 7.Royal RE, Steinberg SM, Krouse RS, Heywood G, White DE, Hwu P, et al. Correlates of response to IL-2 therapy in patients treated for metastatic renal cancer and melanoma. Cancer J Sci. Am. 1996;2:91–98. [PubMed] [Google Scholar]
- 8.Figlin R, Gitlitz B, Franklin J, Dorey F, Moldawer N, Rausch J, et al. Interleukin-2-based immunotherapy for the treatment of metastatic renal cell carcinoma: an analysis of 203 consecutively treated patients. Cancer J Sci. Am. 1997;(3 Suppl 1):S92–S97. [PubMed] [Google Scholar]
- 9.Leibovich BC, Han KR, Bui MH, Pantuck AJ, Dorey FJ, Figlin RA, et al. Scoring algorithm to predict survival after nephrectomy and immunotherapy in patients with metastatic renal cell carcinoma: a stratification tool for prospective clinical trials. Cancer. 2003;98:2566–2575. doi: 10.1002/cncr.11851. [DOI] [PubMed] [Google Scholar]
- 10.Cangiano T, Liao J, Naitoh J, Dorey F, Figlin R, Belldegrun A. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J Clin Oncol. 1999;17:523–528. doi: 10.1200/JCO.1999.17.2.523. [DOI] [PubMed] [Google Scholar]
- 11.Motzer RJ, Bacik J, Mariani T, Russo P, Mazumdar M, Reuter V. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20:2376–2381. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 12.Upton MP, Parker RA, Youmans A, McDermott DF, Atkins MB. Histologic predictors of renal cell carcinoma response to interleukin-2-based therapy. J Immunother. 2005;28:488–495. doi: 10.1097/01.cji.0000170357.14962.9b. [DOI] [PubMed] [Google Scholar]
- 13.Shablak A, Sikand K, Shanks JH, Thistlethwaite F, Spencer-Shaw A, Hawkins RE. High-dose interleukin-2 can produce a high rate of response and durable remissions in appropriately selected patients with metastatic renal cancer. J Immunother. 2011;34:107–112. doi: 10.1097/CJI.0b013e3181fb659f. [DOI] [PubMed] [Google Scholar]
- 14.Bui MH, Seligson D, Han KR, Pantuck AJ, Dorey FJ, Huang Y, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9:802–811. [PubMed] [Google Scholar]
- 15.Atkins M, Regan M, McDermott D, Mier J, Stanbridge E, Youmans A, et al. Carbonic anhydrase IX expression predicts outcome of interleukin 2 therapy for renal cancer. Clin Cancer Res. 2005;11:3714–3721. doi: 10.1158/1078-0432.CCR-04-2019. [DOI] [PubMed] [Google Scholar]
- 16.Dudek AZ, Yee RT, Manivel JC, Isaksson R, Yee HO. Carbonic anhydrase IX expression is associated with improved outcome of high-dose interleukin-2 therapy for metastatic renal cell carcinoma. Anticancer Res. 2010;30:987–992. [PubMed] [Google Scholar]
- 17.de Martino M, Klatte T, Seligson DB, LaRochelle J, Shuch B, Caliliw R, et al. CA9 gene: single nucleotide polymorphism predicts metastatic renal cell carcinoma prognosis. J Urol. 2009;182:728–734. doi: 10.1016/j.juro.2009.03.077. [DOI] [PubMed] [Google Scholar]
- 18.Sabatino M, Kim-Schulze S, Panelli MC, Stroncek D, Wang E, Taback B, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol. 2009;27:2645–2652. doi: 10.1200/JCO.2008.19.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho DC, Sosman JA, Sznol M, Gordon MS, Hollebecque A, Hamid O, et al. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2013;31(suppl) abstr 4505. [Google Scholar]
- 20.Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer. 1981;47:207–214. doi: 10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20:289–296. doi: 10.1200/JCO.2002.20.1.289. [DOI] [PubMed] [Google Scholar]
- 23.Thompson RH, Kuntz SM, Leibovich BC, Dong H, Lohse CM, Webster WS, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006;66:3381–3385. doi: 10.1158/0008-5472.CAN-05-4303. [DOI] [PubMed] [Google Scholar]
- 24.Belldegrun A, Webb DE, Austin HA, 3rd, Steinberg SM, Linehan WM, Rosenberg SA. Renal toxicity of interleukin-2 administration in patients with metastatic renal cell cancer: effect of pre-therapy nephrectomy. J Urol. 1989;141:499–503. doi: 10.1016/s0022-5347(17)40872-x. [DOI] [PubMed] [Google Scholar]
- 25.Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 26.Fallick ML, McDermott DF, LaRock D, Long JP, Atkins MB. Nephrectomy before interleukin-2 therapy for patients with metastatic renal cell carcinoma. J Urol. 1997;158:1691–1695. doi: 10.1016/s0022-5347(01)64097-7. [DOI] [PubMed] [Google Scholar]
- 27.Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358:966–970. doi: 10.1016/s0140-6736(01)06103-7. [DOI] [PubMed] [Google Scholar]
- 28.Birkhauser FD, Pantuck AJ, Rampersaud EN, Wang X, Kroeger N, Pouliot F, et al. Salvage-targeted kidney cancer therapy in patients progressing on high-dose interleukin-2 immunotherapy: the UCLA experience. Cancer J. 2013;19:189–196. doi: 10.1097/PPO.0b013e318292e8a4. [DOI] [PubMed] [Google Scholar]
- 29.Escudier B, Michaelson MD, Motzer RJ, Hutson TE, Clark JI, Lim HY, et al. Axitinib versus sorafenib in advanced renal cell carcinoma: subanalyses by prior therapy from a randomised phase III trial. Br J Cancer. 2014;110:2821–2828. doi: 10.1038/bjc.2014.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–1939. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 31.Cesana GC, DeRaffele G, Cohen S, Moroziewicz D, Mitcham J, Stoutenburg J, et al. Characterization of CD4+CD25+ regulatory T cells in patients treated with high-dose interleukin-2 for metastatic melanoma or renal cell carcinoma. J Clin Oncol. 2006;24:1169–1177. doi: 10.1200/JCO.2005.03.6830. [DOI] [PubMed] [Google Scholar]
- 32.Lenardo M, Chan KM, Hornung F, McFarland H, Siegel R, Wang J, et al. Mature T lymphocyte apoptosis--immune regulation in a dynamic and unpredictable antigenic environment. Annu Rev Immunol. 1999;17:221–253. doi: 10.1146/annurev.immunol.17.1.221. [DOI] [PubMed] [Google Scholar]
- 33.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bahamon B, Signoretti S. Tissue Biomarkers in Renal Cell Carcinoma: Intermediate Endpoints in the Selection of Targeted Agents for RCC. In: Rathmell Kimryn, Rini Brian, Figlin Robert A., editors. Renal Cell Carcinoma: Biology, Prognostic Factors and Therapeutic Targets. 2012. Springer. pp. 69–89. [Google Scholar]
- 35.Delgado DC, Hank JA, Kolesar J, Lorentzen D, Gan J, Seo S, et al. Genotypes of NK cell KIR receptors, their ligands, and Fcgamma receptors in the response of neuroblastoma patients to Hu14.18-IL2 immunotherapy. Cancer Res. 2010;70:9554–9561. doi: 10.1158/0008-5472.CAN-10-2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grosso JHC, Inzunza D, Cardona DM, Simon JS, Gupta AK, Sankar V, et al. Association of tumor PD-L1 expression and immune biomarkers with clinical activity in patients (pts) with advanced solid tumors treated with nivolumab (anti-PD-1; BMS-936558; ONO-4538) J Clin Oncol. 2013;31(suppl) abstr 3016. [Google Scholar]
- 38.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. 2011;23:286–292. doi: 10.1016/j.coi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDermott DF, Drake CD, Sznol M, Choueiri TK, Powderly JD, Smith DC, et al. Clinical activity and safety of antiprogrammed death-1 (PD-1) (BMS-936558/MDX-1106/ONO-4538) in patients (pts) with previously treated, metastatic renal cell carcinoma (mRCC) J Clin Oncol. 2012;30(suppl) abstr 4505. [Google Scholar]