Abstract
Oxidant stress in the cardiovascular system may occur when antioxidant capacity is insufficient to reduce reactive oxygen species and other free radicals. Oxidant stress has been linked to the pathogenesis of atherosclerosis and incident coronary artery disease. As a result of this connection, early observational studies focused on dietary antioxidants, such as β-carotene, α-tocopherol, and ascorbic acid, and demonstrated an inverse relationship between intake of these antioxidants and major adverse cardiovascular events. These findings supported a number of randomized trials of selected antioxidants as primary and secondary prevention to decrease cardiac risk; however, many of these studies reported disappointing results with little or no observed risk reduction in antioxidant treated patients. Several plausible explanations for these findings have been suggested, including incorrect antioxidant choice or dose, synthetic versus dietary antioxidant as the intervention, and patient selection, all of which will be important to consider when designing future clinical trials. This review will focus on the contemporary evidence that is the basis for our current understanding of the role of antioxidants in cardiovascular disease prevention.
Keywords: antioxidants, oxidant stress, beta-carotene, ascorbic acid, alpha-tocopherol, cardiovascular disease, prevention
The concept of antioxidants as a therapeutic intervention to prevent coronary artery disease arose from the oxidized low-density lipoprotein theory of atherosclerosis. Once it was recognized that antioxidants could prevent free radical species from oxidizing lipoproteins, investigators began to explore the relationship between antioxidants and atherosclerotic cardiovascular disease in large population-based studies. Many of these early studies demonstrated an inverse relationship between antioxidant levels and adverse cardiac events. Although these observations fueled interest in randomized clinical trials, results from many trials were disappointing with little or no observed benefit of antioxidant supplementation for reducing cardiovascular risk. While several theories have attempted to explain disconnect between laboratory studies and clinical trials, to date it remains an unresolved issue. This review will examine the role of antioxidants in mediating the pathological processes involved in atherosclerosis, discuss the results from key trials of antioxidants and cardiac events, and present a rationale for further studies in this field.
Oxidant Stress, antioxidants, and atherosclerosis
In the cardiovascular system, cells constantly generate reactive oxygen species (ROS) that are utilized as signaling molecules. These ROS are an integral component of cellular homeostatic processes and operate within a redox environment that is balanced by intra- and extracellular antioxidants. The critical role of these antioxidants is realized when ROS levels exceed cellular antioxidant capacity and create a state of oxidant stress. When oxidant stress is present, ROS may oxidatively modify or damage lipids, proteins, and DNA with deleterious consequences for vascular function and structure [1,2].
The complexity of the cardiovascular antioxidant system is highlighted by the array of enzymatic and non-enzymatic factors that serve as antioxidants to reduce cellular ROS to less reactive forms (Figure 1) (reviewed in [1,2]). There are a several key cellular and circulating antioxidant systems, including the superoxide dismutases, glutathione peroxidases, and catalase that collectively reduce superoxide/hydrogen peroxide (or lipid hydroperoxides) to water (or lipid hydroxides). There are also many important small-molecule antioxidants such as α-tocopherol, ascorbic acid, β-carotene, and reduced glutathione (GSH). The enzyme glutathione reductase maintains intracellular levels of GSH by reducing glutathione disulfide in a reaction that requires NADPH, which is supplied by glucose-6-phosphate dehydrogenase. Thus, when antioxidant activity is decreased or small molecule antioxidant availability is limited, oxidant stress may occur as a result of diminished net antioxidant capacity [1,2].
Figure 1. Antioxidant signaling and mechanisms of atherosclerosis.
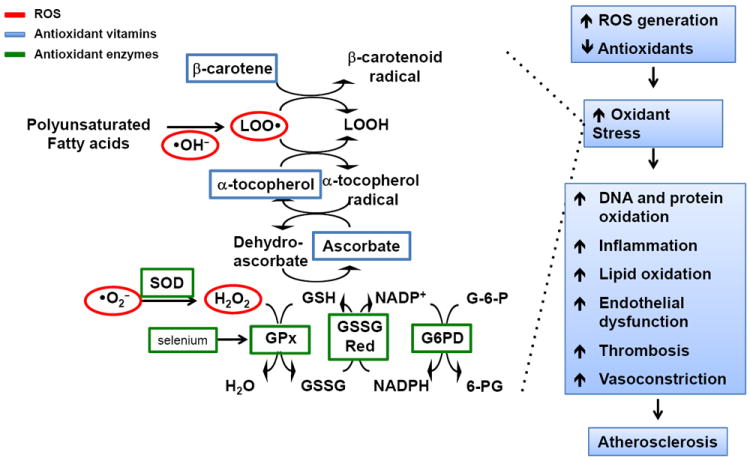
Cells generate a number of oxidizing species that can modify proteins, lipids, and DNA such as superoxide (•O2-) that is dismutated to hydrogen peroxide (H2O2), which is also an oxidant that is reduced to water by the glutathione peroxidases (GPx). This involves a series of coupled reactions that requires the small molecule antioxidant reduced glutathione (GSH) that is generated through the actions of glutathione reductase (GSSG Red) and glucose-6-phosphate dehydrogenase (G6PD). GSH is also required for the antioxidant actions of ascorbic acid and to regenerate α-tocopherol, which reduces oxidized lipids (LOO•) that are generated by hydroxyl radicals (•OH-). These oxidized lipids are reduced (LOOH) through a series of reactions that involves the antioxidant β-carotene. When there is an increase in reactive oxygen species (ROS) generation and/or a decrease in antioxidant capacity, then oxidant stress occurs. This facilitates the development of atherosclerosis by causing oxidation of proteins, lipids, and DNA as well as promoting inflammation and vascular dysfunction (reviewed in [1]).
Oxidant stress promotes atherosclerosis through a number of complementary mechanisms. When present, oxidant stress causes endothelial dysfunction; activates inflammation, immune responses, and thrombus formation; oxidizes lipids; and initiates a cascade of vascular events that is permissive for the formation of atherosclerotic plaques (reviewed in [1,2]). The link between oxidant stress and atherosclerosis has been confirmed in a number of studies that have measured elevated markers of oxidant stress in patients and shown that they are predictive for coronary artery disease [3]. For example, the F2-isoprostanes, which are considered reliable markers of oxidant stress, are an independent predictor of significant coronary artery disease (OR=9.7; 95% CI: 2.6 – 36.9, p=0.016) [4-7]. Moreover, a case-control study that found a 30.8-fold increased risk of coronary artery disease for individuals whose F2-isoprostane levels were in the highest tertile [5]. Lipid hydroperoxides are also recognized as a marker of increased oxidant stress are an independent predictor for major adverse cardiovascular events (HR = 2.23, 95% CI for relative risk; 1.44-3.44, p<0.0003) [8]. Thus, there are multiple lines of evidence to indicate that systemic oxidant stress is associated with atherosclerosis.
Another piece of confirmatory evidence relating oxidant stress to atherosclerosis was obtained from studies that examined plasma levels of antioxidants. The premise behind these studies was that lower levels of antioxidants would also increase oxidant stress. Among the circulating proteins with antioxidant function, such as uric acid, albumin, haptoglobin, transferrin, ceruloplasmin, and GSH, levels of GSH have been inversely related to atherosclerosis [9]. One study of 114 healthy individuals without known atherosclerosis found an inverse correlation between GSH levels and carotid intima-medial thickness [10]. Other studies were designed to determine the protective effects of dietary antioxidants such as β-carotene and related carotenoids, ascorbic acid, and α-tocopherol. The carotenoids are fat-soluble free radical scavengers that are typically found in in yellow-orange fruits and vegetables as well as leafy green vegetables. Of these, α- and β-carotenes are provitamin A carotenoids while lutein and lycopene cannot be converted to retinol and don’t possess vitamin A activity [9]. Ascorbic acid (vitamin C) is a water-soluble antioxidant in humans that serves as an electron donor and is capable of reducing oxidized species. Ascorbic acid content is particularly high in citrus and other fruits and is found in vegetables, including leafy greens, tomatoes, and peppers. The fat-soluble antioxidant α–tocopherol (vitamin E) exists in 8 different forms that reduce ROS by donating a hydrogen atom. In the diet, α–tocopherol is found in vegetable oils as well as whole grains, nuts and seeds, and green leafy vegetables. [9]
The relationship between levels of these antioxidants in the bloodstream in the absence of supplementation and atherosclerosis risk was examined in several cohorts of healthy individuals. In one population-based study that included 392 individuals, plasma levels of α-carotene and β-carotene were decreased significantly in tobacco users and independently and inversely associated with prevalent carotid atherosclerosis [11]. Plasma levels of ascorbic acid were also found to be inversely related to coronary calcium, a marker of atherosclerosis, in a study of 2,637 young (age 18-30 years) individuals who participated in the Coronary Artery Risk Development in Young Adults (CARDIA) study [12]. These studies indicate that in healthy individuals that are not taking supplements, lower levels of selected carotenoids and ascorbic acid compounds are associated with decreased plasma antioxidant capacity and increased risk of atherosclerosis [10].
Observational studies of antioxidants and cardiovascular risk
Owing to the fact that decreased plasma antioxidant levels were associated with an increased risk of atherosclerosis, large-scale population-based studies were investigated to confirm this association across a broad demographic. These early studies focused mainly on the antioxidants β-carotene, ascorbic acid, and α-tocopherol and how intake was related to the risk of atherosclerosis and major adverse events. This was done in part, to understand if dietary antioxidants could explain the observed differences in cardiovascular risk seen between patient populations and provide a rationale for introducing these compounds as dietary supplements to decrease cardiovascular risk.
Enthusiasm for trials of antioxidant supplementation came from studies like the Health Professionals Follow-up Study, which included 39,910 male health care professionals followed for 4 years. This study found that β-carotene decreased cardiovascular events (RR=0.71; 95% CI: 0.55-0.92, p=0.02) [13]. When the data were examined further, it turned out that current smokers had the greatest risk reduction (RR=0.30; 95% CI: 0.11-0.82) as compared to former or never smokers [13]. The Nurses Health Study that included 73,286 nurses followed for 12 years also found an inverse association between dietary carotenoids and incident coronary artery disease (RR=0.74; 95% CI:0.59-0.93) [14]. More recently, the National Health and Nutritional Examination Study (NHANES III) reported on 13,293 participants followed for up to 18 years. This study also found that higher α- and β-carotene levels were associated with lower rates of cardiovascular mortality [15].
Many of the aforementioned studies also examined the relationship between ascorbic acid or α-tocopherol and cardiovascular disease. Epidemiological studies of ascorbic acid yielded mixed results. Findings from the NHANES III and Eastern Finland Study suggested that ascorbic acid was associated with a decreased risk of cardiovascular and coronary artery disease [16,17]. The ARIC trial and Rotterdam studies both examined markers of peripheral arterial disease and also found that ascorbic acidintake was protective but only in women [18,19]. By contrast, the Health Professionals Follow-up Study, Nurses Health Study, and the Iowa Women’s Health Study all reported that ascorbic acid had no protective benefit in preventing coronary disease in either men or women.[20]
The epidemiological data supporting an association between α-tocopherolintake and cardiovascular risk reduction were much stronger. The Nurses Health Study and the Iowa Women’s Study both reported that vitamin E intake, either from food sources or supplements, had a protective effect and lowered the risk of cardiovascular disease ~34% and cardiac death by ~58% [21,22]. Findings from this study suggested further that adequate doses of α-tocopheroltaken for more than 2 years were required to achieve the antioxidant benefit. Key findings from the Health Professionals Follow-up study indicated that individuals taking 60 IU/day had an ~40% reduction of risk for cardiovascular disease compared to individuals taking <7.5 IU/day [13]. Although these epidemiological studies generated excitement regarding the protective benefit of antioxidants, when considered together it is clear that they have a number of limitations that may have influenced the outcomes. The metric by which antioxidant intake was assessed, the lack of measures of plasma antioxidant levels, and other potential confounders such as healthy lifestyle behaviors that correlate with antioxidant intake may have played a role in the positive outcomes.
Early large-scale clinical trials of antioxidants and cardiovascular disease prevention
The somewhat encouraging findings from observational studies were used as the basis to support a number of randomized primary and secondary prevention clinical trials. The primary prevention Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) study examined cardiovascular disease as a secondary outcome in 29,133 male smokers randomized to α-tocopherol, β-carotene, a combination of the two, or placebo. At 8 years follow-up, neither antioxidant was found to decrease cardiovascular disease [23-25]. Similarly, the Beta-Carotene and Retinol Efficacy Trial (CARET) was terminated early after finding that β-carotene and retinol (vitamin A) had no effect on incident cardiovascular disease [26,27]. Similarly, negative findings were also reported in the Vitamin E Atherosclerosis Prevention Study (VEAPS). This study randomized 353 participants with elevated LDL cholesterol levels (>130 mg/dL) to α-tocopherol or placebo and examined intima-media thickness every 3 months for up to 3 years. Despite evidence that plasma α-tocopherol levels were increased there was no effect of α-tocopherol on the progression of carotid atherosclerosis over the entire study period [28].
Although the early primary prevention trials reported negative findings, secondary prevention studies suggested that antioxidants reduce cardiovascular risk in patients with established disease. The Cambridge Heart Antioxidant Study (CHAOS) randomized 2,002 individuals with angiographically proven coronary artery disease to α-tocopherol (800 IU/day) or placebo. After a median follow-up of 510 days, patients randomized to α-tocopherol were found to have a reduction in the combined primary endpoint of cardiovascular death and nonfatal myocardial infarction (RR= 0.53; 95% Cl: 0.34-0.83, p=0.005) that was driven mainly by a reduction in myocardial infarction [29]. Despite this, other studies did not have such encouraging results. The Heart Outcomes Prevention Evaluation, which randomized 9,927 high-risk patients to α-tocopherol, ramipril, a combination of both, or placebo found that α-tocopherol had no effect on cardiovascular outcomes [30]. Similar negative findings were also reported by the MRC/BHF Heart Protection Study and the Women’s Angiographic Vitamin and Estrogen (WAVE) trial [9].
Contemporary clinical trials of antioxidants and cardiovascular disease prevention
Studies performed in the contemporary era have continued to explore the question of whether or not antioxidants can prevent cardiovascular disease; however, the outcomes have not changed significantly (Figure 2). In fact, several primary prevention studies yielded disappointing results. The Women’s Health Study randomized 39,876 individuals in a 2 × 2 factorial design to α-tocopherol or placebo and aspirin or placebo and followed participants for an average of 10.1 years. In this study, α-tocopherol supplementation decreased cardiovascular mortality in healthy women (RR=0.76; 95% CI:0.59-0.98, p=0.03) but had no effect on the incidence of myocardial infarction or stroke [31]. These results were confirmed by the Supplementation en Vitamine set Mineraux Antioxydants (SU.VI.MAX) study. This trial enrolled 13,017 French adults who were randomized to a capsule that contained ascorbic acid, selenium, zinc, α-tocopherol and β-carotene or placebo. There were no differences detected between the groups with respect to the incidence of ischemic cardiovascular disease after a median of 7.5 years despite the fact that the supplement did increase blood levels of the vitamins and minerals [32].
Figure 2. Outcomes from contemporary trials of antioxidants to prevent antioxidant disease.
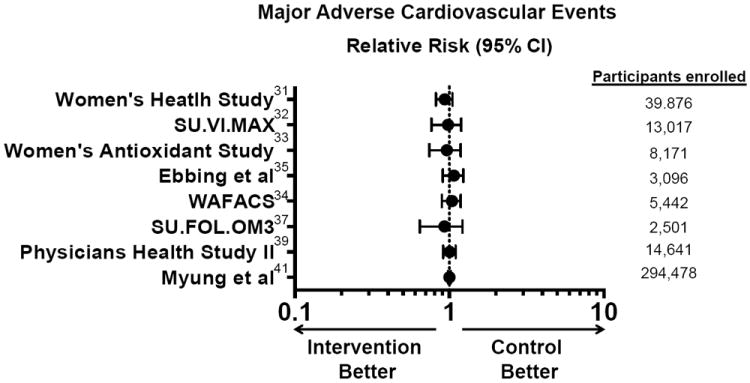
A comparison of relative risk for adverse cardiovascular events across a range of contemporary randomized clinical trials of antioxidants supplements to prevent cardiovascular disease. Adapted from [31-35, 37, 39, 41].
These disappointing findings were also seen in large-scale studies of secondary prevention. For example, the Women’s Antioxidant Cardiovascular Study examined daily ascorbic acid with every other day α-tocopherol and β-caroteneas the antioxidant cocktail. In this study, 8,171 female health care professionals with a history of cardiovascular disease or ≥ 3 risk factors were followed for a mean of 9.4 years. Antioxidant treatment had no effect on the combined endpoint of cardiovascular death, myocardial infarction, stroke, or revascularization. In a prespecified analysis, the study did report an 11% decrease in the combined endpoint in women that had established cardiovascular disease compared to those without disease [33]. In another substudy, 5,442 women were enrolled in a placebo-controlled trial to receive a combination pill that contained folic acid, vitamin B6, and vitamin B12 or placebo. After a mean follow-up of 7.3 years, there was no difference observed between the active treatment and placebo groups with respect to any cardiovascular outcome [34]. These negative findings were confirmed in a randomized trial involving Norwegian patients and in the Western Norway B Vitamin Intervention Trial [35,36]. Another study attempted to determine if the failure of B vitamins occurred as a result of missing cofactors. In this study, 2,501 French patients with a history of symptomatic cardiovascular disease were randomized to a supplement that contained 5-methytretrahydrofolate, vitamin B6, and vitamin B12 versus placebo and a supplement that contained omega-3 fatty acids or placebo in a 2 × 2 factorial design. Omega-3 fatty acids had been added as another putative antioxidant owing to their ability to prevent lipid oxidation. While homocysteine levels were decreased by 19% with the combination supplement compared with placebo, neither treatment had an effect on cardiovascular events [37]. These outcomes were also observed in the Supplementation with Folate, vitamin B6 and B12 and/or omega-3 fatty acids (SU.FOL.OM3) trial [38]. Perhaps the most significant negative report came from The Physician’s Health Study II that enrolled 14,641 male physicians, including 754 participants with a history of cardiovascular disease. This study randomized participants to a multivitamin daily or placebo and followed individuals for a median of 11.2 years. At the end of the study, multivitamin treatment was not found to decrease major cardiovascular events and had no effect on myocardial infarction, stroke, or cardiovascular mortality. These findings held up if the multivitamins were given as primary or secondary prevention [39].
In order to reconcile all the available data from clinical studies, several meta-analyses were performed. One analysis included data from 188,209 individuals that participated in 15 placebo-controlled clinical trials. Overall, this study found that antioxidant vitamins had no effect on major adverse cardiovascular events with RR=1.00, 95% CI:0.96-1.03. This meta-analysis, however, was limited by the use of pooled and not patient-level data and could not account for potential differences as a result of supplement dose and follow-up times [40]. Another meta-analysis identified 50 randomized controlled trials that included 294,478 subjects (156,663 in the treatment or intervention group and 137,815 in the control or referent group) to evaluate the effect of antioxidants on cardiovascular disease. Using a fixed effects model, the primary analysis found that vitamins or antioxidant supplements did not reduce the risk of major cardiovascular events (RR=1.00; 95% CI 0.98-1.02) leading to the conclusion that antioxidant vitamins or supplements had no beneficial effect in the primary or secondary prevention of cardiovascular disease [41].
Why have trials of antioxidants failed?
Despite the abundance of laboratory and observational study evidence indicating that antioxidants should prevent cardiovascular disease, the results from large-scale randomized clinical trials have been disappointing. A number of theories to explain the discrepancy between observational studies and clinical trials have been put forward (Table 1). First, it has been suggested that the wrong antioxidants were trialed and that the cardiovascular risk reduction seen in dietary studies was related to other compounds in the foods consumed [42]. A related theory is that synthetic versions of the antioxidants administered in clinical intervention trials may not completely mimic the natural forms of the antioxidants. Second, not all antioxidants are functionally similar and those included in clinical trials may have different threshold effects for abrogating cellular processes related to oxidant stress and the pathogenesis of atherosclerosis. Many of the studies did not include a metric to determine if the dose of the antioxidant given in the trial actually decreased oxidant stress and did not examine a dose-response [43]. This means that it is possible that individuals were either under-treated or not treated for long enough duration to demonstrate any effect. This is important to consider as different doses of α-tocopherolhave been shown to have a dose-response effect with respect to markers of oxidant stress with ~35% decrease in the oxidant stress marker F2-isoprostane after 1600 IU and an ~49% decrease after 3200 IU [43]. There is also the issue of inter-individual variability in metabolism as well as safety. As many studies were done in the era prior to standardized safety testing, it is unknown if these antioxidants are safe at the doses required to lower oxidant stress [44,45]. Finally, because patients were not screened for trials on the basis of their antioxidant capacity, it is possible that some patients were included in the studies that would derive no benefit from supplemental antioxidants. This is particularly important in an era when patients are treated with statins as studies of statins plus antioxidant vitamins have found that there was no additional benefit from the vitamin supplements [46,47].
Table 1. Reasons why antioxidants failed in clinical trials.
| Antioxidant-related |
| • Incorrect antioxidant or combination of antioxidants |
| • Insufficient cofactors or reactants |
| • Off-target effects |
| • Synthetic vs. dietary sources |
| • Reductive stress (i.e. too much antioxidant capacity) |
| Patient-related |
| • Age |
| • Baseline antioxidant capacity |
| • Diet |
| • Metabolism |
| • Comorbidities |
| • Medications |
| Trial-related |
| • Patient selection not based on oxidant stress or antioxidant levels |
| • Dose-response not tested |
| • No measure to demonstrate that the antioxidants had an effect |
| • Relatively short duration follow-up |
Other antioxidants being investigated in cardiovascular disease prevention
The suggestion that the wrong antioxidants were being studied led investigators to consider selenium as a possible newer antioxidant worthy of investigation. Selenium is a nonmetal trace element that is required for the formation of selenoproteins, such as the antioxidant enzymes glutathione peroxidase and thioredoxin reductase. Dietary sources of selenium include nuts, particularly Brazil nuts, cereals, meats, tuna, eggs, and mushrooms. Clinical trials of selenium supplementation for the primary prevention of cardiovascular disease have been conducted. Twelve randomized controlled trials involving 19,715 patients that were followed for 3 months or longer were included in a meta-analysis. This analysis found that selenium supplementation had no effect on all-cause or cardiovascular mortality and no effect on any major adverse cardiovascular event. In addition, in some trials selenium supplementation was associated with alopecia and dermatitis as adverse effects. Based on the limited trial data available, it was felt that there was not enough evidence to support the use of selenium supplementation for primary prevention of cardiovascular disease [48].
Another factor considered for its antioxidant properties is co-enzyme Q10. Co-enzyme Q10 is a lipid soluble mitochondrial electron carrier that is involved in ATP production. Co-enzyme Q10 has antioxidant functions and prevents lipid oxidation. As synthesis of co-enzyme Q10 shares the same pathway with cholesterol, statins are believed to decrease co-enzyme Q10 levels by up to 40%. There is very limited information to determine if co-enzyme Q10 would prevent cardiovascular disease; however, a meta-analysis that included 12 trials with 362 patients found that co-enzyme Q10 supplements decreased blood pressure with a drop in SBP from 11-17 mmHg and DBP 8-10 mmHg compared to placebo but owing to the unreliability of some of the included studies, no recommendations could be made [49]. Another meta-analysis that included 194 patients found that co-enzyme Q10 improves endothelial function as assessed by flow-mediated dilatation (SMD 1.70, 95% CI: 1.00–2.4, p < 0.0001) [50]. While these early studies are encouraging, this antioxidant remains to be studied in adequately powered large-scale randomized trials.
Fruit and vegetable dietary intervention
Given the findings from clinical trials of synthetic antioxidant supplements, there has been a renewed interest in studying dietary antioxidants that are consumed via fruit, vegetable, and nut intake. This has led to dietary intervention studies that have attempted to assess the effect of increasing consumption of antioxidant foods on cardiovascular disease risk. The beneficial antioxidant effects of diet have been examined extensively in the PREvención con DIeta MEDiterránea (PREDIMED) study, a multicenter, randomized, controlled, clinical trial that evaluated the effects of a Mediterranean diet on cardiovascular outcomes in individuals without cardiovascular disease. Participants were randomized to a Mediterranean diet supplemented with nuts or extra-virgin olive oil, or a control low-fat diet. After a median of 4.8 years, there was a 28-30% reduction in major adverse cardiovascular events in individuals randomized to the supplemented Mediterranean diet (Figure 3) [51]. These findings were attributed, in part, to a reduction in oxidant stress as markers of oxidant stress (i.e., oxidized low-density lipoproteins and malondialdehyde) were decreased in individuals assigned to the Mediterranean diet [52]. While it is not clear what dietary antioxidants are responsible for the cardiovascular risk reduction, it is evident that dietary intervention with the Mediterranean diet offered the best possible outcomes of all the antioxidants studied.
Figure 3. Mediterranean diet and cardiovascular outcomes.
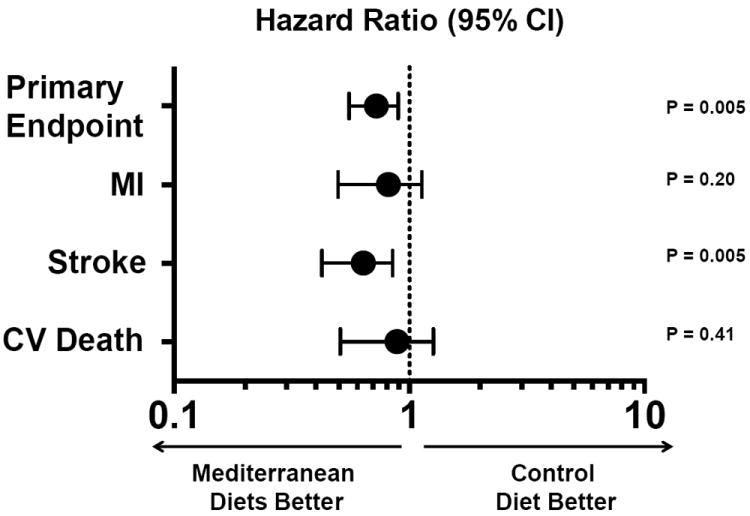
Individuals enrolled in the PREvención con DIetaMEDiterránea (PREDIMED) study were randomized to a Mediterranean diet supplemented with extra-virgin olive oil or nuts versus a low-fat diet and followed for a median of 4.8 years. The primary endpoint was a composite of myocardial infarction, stroke, or cardiovascular death. The primary endpoint was adjusted for sex, age, family history of premature coronary disease, tobacco use, body mass index, waist-to-height ratio, and the presence of hypertension, hyperlipidemia, and diabetes at baseline. Adapted from [51].
Conclusion
There is clear and convincing evidence that oxidant stress is involved in the pathogenesis of atherosclerosis and this alone provides a compelling argument that antioxidants should decrease the risk of atherothrombotic cardiovascular diseases. Yet, after 20+ years of clinical trials that studied the effect of supplemental antioxidants on incident cardiovascular disease, there is no overarching data to indicate that this intervention works. Laboratory investigations are continuing to advance our understanding of oxidant stress, the related issue of reductive stress (i.e., too much antioxidant), and to identify new antioxidants. Based on this, it is plausible that there may be some role of antioxidant therapies in the prevention of cardiovascular disease and new trials of antioxidants could start again. In order for these trials to have a chance at success, trial design will be critical. In future studies, patient selection based on distinct measures of oxidant stress and antioxidant capacity, formal dose-response and safety testing, and realistic timing of the intervention would have to be considered. Other issues to be considered include the antioxidant selected for trial and possible vascular-specific delivery using targeted small molecules or nanoparticles. Owing to the recent positive findings with the Mediterranean diet, studies should also consider dietary modification as part of the intervention. Given the intense interest in this avenue of investigation, it is likely that there will be many more trials that examine the role of antioxidants in preventing cardiovascular disease.
Acknowledgments
This work was funded by NIH/NHLBI R01 105301 and U01 125215(JAL).
References
- 1.Leopold JA, Loscalzo J. Oxidative risk for atherothrombotic cardiovascular disease. Free Rad Biol Med. 2009;47:1673–1706. doi: 10.1016/j.freeradbiomed.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lubos E, Loscalzo J, Handy D. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antiox Red Sig. 2011;15:1957–1997. doi: 10.1089/ars.2010.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsimikas S. In vivo markers of oxidative stress and therapeutic interventions. The Am J Card. 2008;101:34D–42D. doi: 10.1016/j.amjcard.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Morrow JD, Roberts LJ., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharm. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- 5.Schwedhelm E, Bartling A, Lenzen H, Tsikas D, Maas R, Brummer J, et al. Urinary 8-iso-prostaglandin f2alpha as a risk marker in patients with coronary heart disease: A matched case-control study. Circulation. 2004;109:843–848. doi: 10.1161/01.CIR.0000116761.93647.30. [DOI] [PubMed] [Google Scholar]
- 6.Wang B, Pan J, Wang L, Zhu H, Yu R, Zou Y. Associations of plasma 8-isoprostane levels with the presence and extent of coronary stenosis in patients with coronary artery disease. Atherosclerosis. 2006;184:425–430. doi: 10.1016/j.atherosclerosis.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Shishehbor MH, Zhang R, Medina H, Brennan ML, Brennan DM, Ellis SG, et al. Systemic elevations of free radical oxidation products of arachidonic acid are associated with angiographic evidence of coronary artery disease. Free Rad Biol Med. 2006;41:1678–1683. doi: 10.1016/j.freeradbiomed.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walter MF, Jacob RF, Bjork RE, Jeffers B, Buch J, Mizuno Y, et al. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: The prevent study. J Am Coll Card. 2008;51:1196–1202. doi: 10.1016/j.jacc.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 9.Willcox BJ, Curb JD, Rodriguez BL. Antioxidants in cardiovascular health and disease: Key lessons from epidemiologic studies. Am J Card. 2008;101:75D–86D. doi: 10.1016/j.amjcard.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Ashfaq S, Abramson JL, Jones DP, Rhodes SD, Weintraub WS, Hooper WC, et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Card. 2006;47:1005–1011. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 11.D’Odorico A, Martines D, Kiechl S, Egger G, Oberhollenzer F, Bonvicini P, et al. High plasma levels of alpha- and beta-carotene are associated with a lower risk of atherosclerosis: Results from the bruneck study. Atherosclerosis. 2000;153:231–239. doi: 10.1016/s0021-9150(00)00403-2. [DOI] [PubMed] [Google Scholar]
- 12.Simon JA, Murtaugh MA, Gross MD, Loria CM, Hulley SB, Jacobs DR., Jr Relation of ascorbic acid to coronary artery calcium: The coronary artery risk development in young adults study. Am J Epidemiol. 2004;159:581–588. doi: 10.1093/aje/kwh079. [DOI] [PubMed] [Google Scholar]
- 13.Rimm EB, Stampfer MJ, Ascherio A, Giovannucci E, Colditz GA, Willett WC. Vitamin E consumption and the risk of coronary heart disease in men. New Engl J Med. 1993;328:1450–1456. doi: 10.1056/NEJM199305203282004. [DOI] [PubMed] [Google Scholar]
- 14.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Manson JE, Willett WC. Dietary carotenoids and risk of coronary artery disease in women. Am J Clin Nutr. 2003;77:1390–1399. doi: 10.1093/ajcn/77.6.1390. [DOI] [PubMed] [Google Scholar]
- 15.Shardell MD, Alley DE, Hicks GE, El-Kamary SS, Miller RR, Semba RD, et al. Low-serum carotenoid concentrations and carotenoid interactions predict mortality in us adults: The third national health and nutrition examination survey. Nutrition Res. 2011;31:178–189. doi: 10.1016/j.nutres.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon JA, Hudes ES. Serum ascorbic acid and cardiovascular disease prevalence in u.S. Adults: The third national health and nutrition examination survey (nhanes iii) Ann of Epidemiol. 1999;9:358–365. doi: 10.1016/s1047-2797(99)00008-3. [DOI] [PubMed] [Google Scholar]
- 17.Nyyssonen K, Parviainen MT, Salonen R, Tuomilehto J, Salonen JT. Vitamin C deficiency and risk of myocardial infarction: Prospective population study of men from eastern finland. BMJ. 1997;314:634–638. doi: 10.1136/bmj.314.7081.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klipstein-Grobusch K, den Breeijen JH, Grobbee DE, Boeing H, Hofman A, Witteman JC. Dietary antioxidants and peripheral arterial disease : The rotterdam study. Am J Epidemiol. 2001;154:145–149. doi: 10.1093/aje/154.2.145. [DOI] [PubMed] [Google Scholar]
- 19.Kritchevsky SB, Shimakawa T, Tell GS, Dennis B, Carpenter M, Eckfeldt JH, et al. Dietary antioxidants and carotid artery wall thickness. The aric study. Atherosclerosis risk in communities study. Circulation. 1995;92:2142–2150. doi: 10.1161/01.cir.92.8.2142. [DOI] [PubMed] [Google Scholar]
- 20.Osganian SK, Stampfer MJ, Rimm E, Spiegelman D, Hu FB, Manson JE, et al. Vitamin c and risk of coronary heart disease in women. J Am Coll Card. 2003;42:246–252. doi: 10.1016/s0735-1097(03)00575-8. [DOI] [PubMed] [Google Scholar]
- 21.Stampfer MJ, Hennekens CH, Manson JE, Colditz GA, Rosner B, Willett WC. Vitamin e consumption and the risk of coronary disease in women. New Engl J Med. 1993;328:1444–1449. doi: 10.1056/NEJM199305203282003. [DOI] [PubMed] [Google Scholar]
- 22.Kushi LH, Folsom AR, Prineas RJ, Mink PJ, Wu Y, Bostick RM. Dietary antioxidant vitamins and death from coronary heart disease in postmenopausal women. New Engl J Med. 1996;334:1156–1162. doi: 10.1056/NEJM199605023341803. [DOI] [PubMed] [Google Scholar]
- 23.Group., TATBCCPS. The effect of vitamin e and beta carotene on the incidence of lung cancer and other cancers in male smokers. The alpha-tocopherol, beta carotene cancer prevention study group. New Engl J Med. 1994;330:1029–1035. doi: 10.1056/NEJM199404143301501. [DOI] [PubMed] [Google Scholar]
- 24.Rapola JM, Virtamo J, Haukka JK, Heinonen OP, Albanes D, Taylor PR, et al. Effect of vitamin e and beta carotene on the incidence of angina pectoris. A randomized, double-blind, controlled trial. JAMA. 1996;275:693–698. doi: 10.1001/jama.1996.03530330037026. [DOI] [PubMed] [Google Scholar]
- 25.Virtamo J, Rapola JM, Ripatti S, Heinonen OP, Taylor PR, Albanes D, et al. Effect of vitamin e and beta carotene on the incidence of primary nonfatal myocardial infarction and fatal coronary heart disease. Arch Int Med. 1998;158:668–675. doi: 10.1001/archinte.158.6.668. [DOI] [PubMed] [Google Scholar]
- 26.Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, et al. Effects of a combination of beta carotene and vitamin a on lung cancer and cardiovascular disease. New Engl J Med. 1996;334:1150–1155. doi: 10.1056/NEJM199605023341802. [DOI] [PubMed] [Google Scholar]
- 27.Redlich CA, Chung JS, Cullen MR, Blaner WS, Van Bennekum AM, Berglund L. Effect of long-term beta-carotene and vitamin a on serum cholesterol and triglyceride levels among participants in the carotene and retinol efficacy trial (caret) Atherosclerosis. 1999;145:425–432. doi: 10.1016/s0021-9150(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 28.Hodis HN, Mack WJ, LaBree L, Mahrer PR, Sevanian A, Liu CR, et al. Alpha-tocopherol supplementation in healthy individuals reduces low-density lipoprotein oxidation but not atherosclerosis: The vitamin e atherosclerosis prevention study (veaps) Circulation. 2002;106:1453–1459. doi: 10.1161/01.cir.0000029092.99946.08. [DOI] [PubMed] [Google Scholar]
- 29.Stephens NG, Parsons A, Schofield PM, Kelly F, Cheeseman K, Mitchinson MJ. Randomised controlled trial of vitamin e in patients with coronary disease: Cambridge heart antioxidant study (chaos) Lancet. 1996;347:781–786. doi: 10.1016/s0140-6736(96)90866-1. [DOI] [PubMed] [Google Scholar]
- 30.Yusuf S, Dagenais G, Pogue J, Bosch J, Sleight P. Vitamin e supplementation and cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. New Engl J Med. 2000;342:154–160. doi: 10.1056/NEJM200001203420302. [DOI] [PubMed] [Google Scholar]
- 31.Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Vitamin e in the primary prevention of cardiovascular disease and cancer: The women’s health study: A randomized controlled trial. JAMA. 2005;294:56–65. doi: 10.1001/jama.294.1.56. [DOI] [PubMed] [Google Scholar]
- 32.Hercberg S, Galan P, Preziosi P, Bertrais S, Mennen L, Malvy D, et al. The su.Vi.Max study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch Int Med. 2004;164:2335–2342. doi: 10.1001/archinte.164.21.2335. [DOI] [PubMed] [Google Scholar]
- 33.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: Results from the women’s antioxidant cardiovascular study. Arch Int Med. 2007;167:1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albert CM, Cook NR, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, et al. Effect of folic acid and B vitamins on risk of cardiovascular events and total mortality among women at high risk for cardiovascular disease: A randomized trial. JAMA. 2008;299:2027–2036. doi: 10.1001/jama.299.17.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebbing M, Bleie O, Ueland PM, Nordrehaug JE, Nilsen DW, Vollset SE, et al. Mortality and cardiovascular events in patients treated with homocysteine-lowering B vitamins after coronary angiography: A randomized controlled trial. JAMA. 2008;300:795–804. doi: 10.1001/jama.300.7.795. [DOI] [PubMed] [Google Scholar]
- 36.Loland KH, Bleie O, Blix AJ, Strand E, Ueland PM, Refsum H, et al. Effect of homocysteine-lowering B vitamin treatment on angiographic progression of coronary artery disease: A western norway B vitamin intervention trial (wenbit) substudy. Am J Card. 2010;105:1577–1584. doi: 10.1016/j.amjcard.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S, et al. Effects of b vitamins and omega 3 fatty acids on cardiovascular diseases: A randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blacher J, Czernichow S, Paillard F, Ducimetiere P, Hercberg S, Galan P, et al. Cardiovascular effects of b-vitamins and/or n-3 fatty acids: The su.Fol.Om3 trial. Int J Card. 2013;167:508–513. doi: 10.1016/j.ijcard.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 39.Sesso HD, Christen WG, Bubes V, Smith JP, MacFadyen J, Schvartz M, et al. Multivitamins in the prevention of cardiovascular disease in men: The physicians’ health study ii randomized controlled trial. JAMA. 2012;308:1751–1760. doi: 10.1001/jama.2012.14805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ye Y, Li J, Yuan Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PloS One. 2013;8:e56803. doi: 10.1371/journal.pone.0056803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, et al. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;346:f10. doi: 10.1136/bmj.f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Card. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Roberts LJ, 2nd, Oates JA, Linton MF, Fazio S, Meador BP, Gross MD, et al. The relationship between dose of vitamin e and suppression of oxidative stress in humans. Free Rad Biol Med. 2007;43:1388–1393. doi: 10.1016/j.freeradbiomed.2007.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jerome-Morais A, Diamond AM, Wright ME. Dietary supplements and human health: For better or for worse? Mol Nutr Food Res. 2011;55:122–135. doi: 10.1002/mnfr.201000415. [DOI] [PubMed] [Google Scholar]
- 45.Pashkow FJ. Oxidative stress and inflammation in heart disease: Do antioxidants have a role in treatment and/or prevention? Int J Inflamm. 2011;2011:514623. doi: 10.4061/2011/514623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arad Y, Spadaro LA, Roth M, Newstein D, Guerci AD. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin c, and vitamin e: The st. Francis heart study randomized clinical trial. J Am Coll Card. 2005;46:166–172. doi: 10.1016/j.jacc.2005.02.089. [DOI] [PubMed] [Google Scholar]
- 47.Brown BG, Zhao XQ, Chait A, Fisher LD, Cheung MC, Morse JS, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. New Engl J Med. 2001;345:1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]
- 48.Rees K, Hartley L, Day C, Flowers N, Clarke A, Stranges S. Selenium supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD009671.pub2. CD009671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenfeldt FL, Haas SJ, Krum H, Hadj A, Ng K, Leong JY, et al. Coenzyme q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J Hum Hyperten. 2007;21:297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 50.Gao L, Mao Q, Cao J, Wang Y, Zhou X, Fan L. Effects of coenzyme q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 51.Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D, Aros F, et al. Primary prevention of cardiovascular disease with a mediterranean diet. New Engl J Med. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. [DOI] [PubMed] [Google Scholar]
- 52.Fito M, Guxens M, Corella D, Saez G, Estruch R, de la Torre R, et al. Effect of a traditional mediterranean diet on lipoprotein oxidation: A randomized controlled trial. Arch Int Med. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]


