Abstract
Type 2 diabetes (T2D) is a metabolic disease associated with obesity-related insulin resistance (IR) and chronic inflammation. Animal studies indicate IR can be caused and/or exacerbated by systemic/tissue-specific alterations in lymphocyte differentiation and function. Human studies also indicate obesity and/or inflammation promotes IR. Nevertheless, clinical trials with anti-inflammatory therapies have yielded modest impacts on established T2D. Unlike mouse models where obesity is predominantly associated with IR, 20–25% of obese people are metabolically healthy with high insulin sensitivity. The uncoupling of obesity from IR in humans but not in animal models advocates for a more comprehensive understanding of mediators/mechanisms in human obesity-promoted IR, and better integration of knowledge from human studies into animal experiments to efficiently pursue T2D prevention and treatment.
Keywords: insulin resistance, type 2 diabetes, obesity, lymphocyte subsets
1. Overview of inflammation in the context of obesity and associated type 2 diabetes
Type 2 diabetes (T2D) is a chronic disease characterized by metabolic dysfunction including hyperglycemia, pancreatic beta cell insufficiency, and insulin resistance (IR, see glossary). IR can be driven by calorie surplus that leads to obesity, although a significant subgroup of obese individuals, the metabolic healthy obese (MHO), have healthy metabolic profiles [1, 2]. Metabolic unhealthy obese (MUHO) have metabolic profiles that mirror profiles of individuals with T2D. Up to 30% of MHO can convert to MUHO over a 5–10-year time frame [1, 3, 4], fueling the debate over the existence of a stable MHO subset. Identification of the mechanisms that promote (or prevent) the transition from MHO to MUHO to delay T2D will be crucial to counter the public health burden of metabolic disease.
Adipose tissue (AT) adipocytes undergo hyperplasia and hypertrophy to accommodate the increased demand of triglyceride storage in response to over nutrition [5]. Adipose-resident immune cells play critical roles during AT remodeling, in part through cytokine secretion that facilitates the vascular and extracellular matrix remodeling required for healthy AT expansion [6]. If AT inflammation is suppressed in this context, AT dysfunction and systemic metabolic disturbances ensue [6]. Chronic AT expansion during the development of obesity induces excessive immune cell recruitment and accumulation [7], and modulates “innate” (non-lymphocyte) and “adaptive” (lymphocyte) immune cells (Table 1) [8], with shifts in AT immune cells somewhat mirrored in blood (Table 2) and liver [9]. Chronic AT expansion also induces adipocyte transformation, including altered intracellular gene/protein expression, adipokine secretion, and apoptotic signals [10, 11, 12].
Table 1.
Lymphocyte subsets in adipose tissue.
| Signatures | Mice | Human | ||
|---|---|---|---|---|
| Obese vs Lean (T2D vs non-T2D) |
T2D vs non-T2D |
MUHO vs MHO |
Obese (non- T2D) vs Lean |
|
| CD8+ (%) | Subcutaneous and visceral – increased [29]; visceral – increased [22] |
NA | No difference [122] |
Increased [29] |
| Effector/ Memory CD8+ (%) |
Subcutaneous – increased [29] | NA | NA | NA |
| CD4+ (%) | Visceral – increased [22] | NA | No difference [122] |
Increased [29] |
| CD4+ (#) | Subcutaneous and visceral – increased [22] |
NA | NA | NA |
| Effector/ Memory CD4+ (%) |
Subcutaneous – increased [29] | NA | NA | NA |
| Th1 (%) | Subcutaneous and visceral – increased [22] |
No difference [15] |
No difference [15] |
NA |
| Th1 (#) | Subcutaneous and visceral – increased [22] |
NA | NA | NA |
| Th2 (%) | NA | No difference [15] |
No difference [15] |
NA |
| Th17 (%) | Subcutaneous – no difference [22] or increased [51]; Visceral – decreased [22] |
Increased [15] | Increased [15] | NA |
| Th17 (#) | Subcutaneous –increased [22, 51]; visceral – no difference [22] |
NA | NA | NA |
| Treg (%) | Visceral – decreased [22, 67]; | Decreased [67, 122] |
Decreased [122] |
NA |
| Treg (#) | Subcutaneous and visceral – no difference [22] |
NA | NA | NA |
| Th1:Treg | Subcutaneous and visceral – increased [22] |
NA | NA | NA |
| NKT (%) | Decreased [85, 86] | Visceral iNKT inversely correlated with BMI (females) [85] |
NA | Decreased – iNKT (BMI >40, T2D status unknown) [86, 106] |
| B cell (#) | Visceral – increased [23, 35] | NA | NA | NA |
| ILC2 (%) | Visceral – decreased [101] | NA | NA | NA |
Table 2.
Lymphocyte subsets in systemic circulation1.
| Signatures | Human | ||
|---|---|---|---|
| T2D vs non-T2D |
MUHO vs MHO | Obese (non-T2D) vs Lean | |
| CD8+ (%) | NA | Decreased [106]; No difference (women) [14] |
Decreased [106, 123]; no difference (women) [14] |
| CD8+ (#) | NA | No difference (females) [14] |
No difference (BMI >40 vs <25) [124]; no difference (females) [14] |
| CD8+ CD95+ (%) |
NA | NA | Increased (females) [123] |
| CD8+ CD25+ (%) |
NA | No difference (females) [14] |
Increased (females) [14] |
| CD8+ CD45RA+ (%) |
NA | No difference (females) [14] |
No difference (females) [14] |
| CD4+ (%) | NA | No difference (females) [14] |
Increased (females) [123] |
| CD4+ (#) | NA | Trend increase (females; p=0.06) [14] |
Increased (BMI >40 vs <25) [124]; no difference (females) [14] |
| CD4+ CD45RA+ (%) |
NA | No difference (females) [14] |
No difference (females) [14] |
| CD4+ CD25+ (%) |
NA | No difference (females) [14] |
Trend increase (females; p=0.10) [14] |
| Th1 (%) | Increased [16, 40, 43] |
Increased [43] | Increased [43], (children) [39] |
| Th1 (#) | NA | NA | No difference (BMI >40 vs <25) [124] |
| Th2 (%) | No difference [16, 40] |
NA | No difference (children) [39] |
| Th2 (#) | NA | NA | Increased (BMI >40 vs <25) [124] |
| Treg (%) | Decreased [16, 40] |
NA | NA |
| Treg (#) | NA | NA | Increased (BMI >40 vs <25) [124] |
| Th17 (%) | Increased [16, 40, 43] |
Increased [43] | NA |
| Th17 (#) | NA | NA | No difference (BMI >40 vs <25) [124] |
| Th17 cytokines |
Increased [43, 44] |
Increased [43] | Increased (females) [44] |
| iNKT (%) | NA | NA | Decreased – iNKT (BMI >40, T2D status unknown) [86] |
| B cell (%) | NA | No difference (females) [14] |
NA |
| B cell (#) | NA | No difference (females) [14] |
No difference (BMI >40 vs <25) [124]; no difference (females) [14] |
| CD19+ CD38+ (%) |
NA | No difference (females) [14] |
Increased (females) [14] |
| CD19+ CD27+ (%) |
NA | No difference (females) [14] |
No difference (females) [14] |
Lymphocyte subsets in mouse systemic circulation have not been elucidated.
Chronic “low-grade” inflammation observed in IR and T2D plays a critical role in T2D pathogenesis partially through the ability of pro-inflammatory cytokines to impair insulin signaling in insulin-sensitive tissues [13]. Adipocytes release modest amounts of pro-inflammatory cytokines during the transition from lean to obese states, but the bulk of pro-inflammatory cytokines is secreted by circulating or tissue-associated immune cells [13, 14, 15, 16, 17]. These include supra-normal concentrations of plasma interferon gamma (IFN-γ), interleukin (IL)-1β, IL-6, IL-17, IL-18, IL-22, and tumor necrosis factor (TNF)-α, many of which originate from AT macrophages (ATMs) in T2D subjects [3, 4, 14, 15, 16, 17].
ATMs are arguably the best understood immunomodulators of AT health [18]. AT from lean, healthy individuals predominantly contains non-inflammatory ATMs, including “M2”-like macrophages implicated in the proliferation and differentiation of adipocyte precursors, AT remodeling, and AT angiogenesis [18, 19]. ATMs increase in frequency and skew towards proinflammatory “M1”-like subsets in obesity, including “metabolically activated” macrophages [20, 21]. Pro-inflammatory ATM polarization in obesity is mediated by pro-inflammatory lymphocytes [22], although the ability of ATMs to differentially polarize AT lymphocytes has not been demonstrated.
Despite incontrovertible roles for ATMs in inflamed AT, the likelihood that ATMs initiate AT inflammation remains debatable. Early work showed that B [23] and T lymphocytes [24, 25] infiltrated visceral AT of high-fat diet fed mice prior to macrophage influx and polarization. However, other studies identified neutrophil infiltration into AT at day 3 [26], and elevated ATMs at day 7 [27] of high-fat diet feeding, well before B cell infiltration at 3–4 wks. Further investigation will elucidate how the timing of AT immune cell trafficking in obesity relates to chronic AT inflammation and remodeling, despite general agreement that multiple immune system subsets play pivotal roles in obesity-associated inflammation through AT infiltration, intra-AT expansion and cellular cross-talk. Herein we highlight the significance of lymphocytes as major sources of cytokines in T2D. We also use current knowledge to emphasize the importance of parallel human and animal studies of cross-talk amongst lymphocytes and other immune cell types, as well as the relevance of these studies in successfully exploiting our understanding of immunometabolism for T2D treatments.
2. Lymphocytes as sources of obesity/T2D–associated inflammation
Lymphocytes are fundamentally important to obesity-associated inflammation. The number, subset distribution, and/or function of two major lymphocyte populations, B cells and T cells, are altered in human MUHO and T2D [28, 29] to provide pro-inflammatory signals that support both AT (Table 1) and systemic (Table 2) inflammation. Cluster of differentiation 4+ (CD4+) T helper (Th), CD8+ (cytotoxic) T, and B lymphocytes are increased in AT of diet-induced obese and insulin resistant (DIO) mice, MUHO and T2D individuals [13, 29, 30, 31]. Increased AT lymphocytes are due in part to increased expression of the chemokine C-X-C motif receptor 3 (CXCR3) on T cells, which drives T cell recruitment to obese AT [32]. Furthermore, effector-memory T cells (a subset of “experienced” T cells) that produce cytokines like IFN-γ and TNFα dominate in DIO mouse AT over naïve T cells [29]. DIO mice and humans also have T cells with a less diverse T cell receptor (TCR) repertoire than T cells from lean individuals [22, 29, 33], which may reflect T cell expansion in response to an unknown antigen presented by ATMs or, in more provocative work, adipocytes themselves acting as antigen presenting cells (APCs) [34]. Limited T cell repertoires in combination with increased auto-immune antibodies from B cells of IR subjects [35] suggest an autoimmune component of T2D pathogenesis. This intriguing possibility is confounded by ageing-related auto-immunity and by the limited success of ongoing searches for putative autoantigens, likely due in part to the increase in unconventional antigens such as glycated proteins and modified lipoproteins in T2D [36].
2.1 CD4+ and CD8+ T cells
Naïve CD4+ T cells can be generally grouped into four major subsets: pro-inflammatory Th1s and Th17s, and anti-inflammatory Th2s and regulatory T cells (Tregs) [13, 31]. Subset classification is based on transcription factor expression and cytokine production [13, 31], with pro-inflammatory or anti-inflammatory subsets generally provoking or preventing obesity-associated IR, respectively. CD4+ T cell polarization is governed by the cytokine milieu following dendritic cell activation [13, 31], thus CD4+ T cell ratios are likely impacted by cytokine shifts in obesity. CD4+ T cell polarization is also determined by the strength of the signal generated by T cell receptor (TCR) engagement, which can bias naïve CD4+ T cells towards the Th1 subset [37]. Obesity-associated TCR repertoire restriction may also determine TCR signal strength [22, 33] thus CD4+ subset ratios. CD8+ cytotoxic T cells can contribute to inflammation and IR through secreting pro-inflammatory cytokines [22]. In contrast to CD4+ T cells, the pro-inflammatory responses of CD8+ T cells may be self-limiting due to the impact of perforin, an effector molecule secreted by CD8+ T cells that eliminates infected cells, and plays roles in the homeostatic regulation of all T cells by restricting their expansion in DIO mice [38]. Although both overall numbers and ratios of T cell subsets affect the final inflammatory profile in AT and in circulation, a “healthy” ratio has not been established.
2.1.1 Th1 and Th2 T cells
The frequency of circulating Th1s is increased in obese compared to lean children [39], and in obese-T2D compared to obese-non-T2D adults [16, 40]. This increase mirrors the high Th1 frequency in visceral AT of DIO mice [22]. Th1s, along with CD8+ T cells, secrete IFN-γ, which subsequently primes macrophages towards pro-inflammatory subsets [22]. Although obese-T2D patients have similar circulating Th2 cell frequencies as obese-non-T2D subjects [16, 40], visceral AT tissue from DIO mice has a paucity of Th2s [22]. Th2 differentiation in mice is inhibited by the pleiotropic cytokine IL-6 [41], suggesting that the elevated IL-6 in T2D limits the number of Th2s to decrease net production of anti-inflammatory Th2 cytokines (IL-4, IL-5, and IL-13). Th2 cytokines counter Th1s in part by priming macrophages towards less inflammatory subsets [22]. Additional effects of Th1/Th2 shifts on obesity-associated inflammation have been reviewed recently [13, 31].
2.1.2 Th17 T cells
Recent studies have focused on the roles for human Th17s, which produce inflammatory IL-17, IL-21, IL-22 and macrophage inflammatory protein-3α in obesity and associated pathologies [42]. Higher amounts of Th17 cytokines in plasma and cultured T cells concords with a higher frequency of circulating Th17s, in obese-T2D, compared to non-T2D subjects [16, 40, 43, 44]. AT from MUHO also has more IL-17- and IL-22-producing CD4+ cells, than AT from MHO or lean subjects [15]. T cell IL-17 secretion [16] or percentages of Th17s [8] positively associate with clinical parameters of T2D including hemoglobin A1c (HbA1c), a marker of long-term mean plasma glucose concentration. The overabundance of Th17s in human T2D is perhaps expected, given that classical diabetogenic cytokines like IL-1β and IL-6 support Th17 differentiation [42, 45, 46]. Leptin, an adipokine often increased in obesity-associated T2D, also stimulates IL-17 production in CD4+ T cells [46, 47]. Oxysterols, which are oxygenated cholesterol derivatives that increase in human obesity and correlate with fasting insulin [48], may also drive Th17 differentiation [49]. However, a role for Th17s in DIO mice remains controversial [50, 51, 52]. The emerging appreciation of Th17s in human T2D, together with the inconsistencies in in vivo models of T2D, rationalize a push to develop models that more accurately resemble humans relative to inflammatory profiles for in vivo assessment of roles for Th17s in T2D.
The Th17 cytokine IL-17A likely promotes obesity-associated IR through multiple mechanisms [42, 53]. IL-17A inhibits differentiation of pre-adipocytes [51, 54], and increases adipocyte IL-6 and IL-8 secretion to promote inflammation [54]. IL-17A also enhances lipolysis and impairs glucose uptake by adipocytes [54], to potentially elevate plasma free fatty acids and glucose. Importantly, the roles of Th17s and IL-17A are not necessarily identical [50, 51, 54], as IL-17A is also secreted by neutrophils, innate lymphoid and γδ T cells [51, 55, 56]. Recent work showed “pathogenic Th17s” are characterized by IL-23R and P-glycoprotein/multi-drug resistance type 1 expression [57], rather than by IL-17A secretion, calling for revised examination of Th17s in obesity [45, 58].
IL-22 is a second Th17 product downstream of IL-6 and IL-23 [42, 53]. IL-22 is also produced by the less-appreciated Th22 T cell subset [59, 60], innate lymphoid cells [61], CD8+, γδ and natural killer T cells [61]. Blood from obese-T2D subjects has an increased percentage of IL-22-producing T cells [15]. IL-22 promotes AT inflammation, based on data showing human ATMs express IL-22 receptor (IL-22R), and respond to IL-22 with more IL-1β secretion than amounts elicited by IL-17 [8, 43]. T cell IL-22 may also impact classical metabolic tissues, as evidenced by work showing that IL-22R1, a subunit of the IL-22R heterodimer [61], has highest expression in human pancreatic acinar cells [62, 63, 64]. Further roles of IL-22 in the pancreas include the ability to inhibit pancreatic cell autophagy in mice with pancreatitis [64, 65], and to increase insulin storage in diabetic, leptin receptor deficient (db/db) mice [66]. Exogenous IL-22 administration in DIO and db/db mice also reduces glucose intolerance and IR [66], with coincident preservation of intestinal mucosal barrier and endocrine function. Whether IL-22-associated metabolic effects in mice apply to humans remains unknown. Overall, work on both IL-17 and IL-22 indicate that Th17s may be alternatively “protective” and “destructive” in T2D development, but conditions that govern Th17 function remain elusive.
2.1.3 Regulatory T cells
Unlike pro-inflammatory Th1s and Th17s, CD4+ Tregs protect against obesity-associated inflammation [13, 31]. The ratio between Tregs and Th1s/Th17s in obesity and T2D is decreased in blood and AT [67], whereas adoptive transfer [68] or the induction of [69] Tregs ameliorated IR in db/db or leptin deficient (ob/ob) mice. The decline in mouse Tregs during obesity development [70], unhinges multiple mechanisms Tregs use to limit inflammation. These mechanisms include the ability of Tregs to inhibit Th1 proliferation and IFN-γ secretion through transfer of Let-7d microRNA-containing exosomes to Th1s that silence Th1-associated genes [71]. Nevertheless, the heterogeneity of Treg-microRNA exosomes is influenced by the cytokine microenvironment [72], suggesting that inflammatory status in obesity shapes Treg microRNA profiles, thus function. Hyperinsulinemia in mice also dampens anti-inflammatory Treg function by inhibiting IL-10 production [73]. In addition to Treg-extrinsic mechanisms that impact obesity-associated IR, mouse studies identified peroxisome proliferator-activated receptor gamma as a Treg-intrinsic regulator that orchestrates Treg accumulation in visceral AT of lean animals [74]. Tregs are also regulated by leptin, a hormone increased in obesity and T2D that inhibits Treg proliferation [75, 76, 77], consistent with the demonstration that circulating human Treg frequency inversely correlates with leptin and body mass index (BMI) [17]. Cell-extrinsic regulation of Tregs is further mediated by IL-2 and IL-1β, which stimulate Tregs to differentiate into Th17-like cells [78]. Although multiple physiological changes in obesity and T2D converge to disarm Tregs in DIO studies, definitive analyses of humans is critical to identify mechanistic links between Tregs and T2D.
2.2. Invariant natural killer T cells
Invariant natural killer T (iNKT) cells are a subset of “innate” T cells that express an invariant TCR, Vα24 Vβ11 in humans and Vα14 Jα18 in mice [79, 80, 81]. iNKT cells recognize glycolipid antigen presented on the major histocompatibility complex (MHC)-like molecule CD1d, and can rapidly produce a plethora of cytokines including IL-2, IL-4 and IFN-γ [80, 82]. iNKTs represent up to 10% of AT T cells in mice and humans, suggesting that omental iNKT cells are the largest population of iNKTs in vivo [83, 84, 85]. iNKT cells are under-represented in AT and blood of obese humans and mice, compared to lean controls [83, 84, 85, 86, 87]. Longitudinal studies showed iNKT frequency is increased following bariatric surgery-mediated weight loss, despite the demonstration that patient BMI in this study remained in the obese range [86]. iNKT cells are depleted in the circulation of inflamed, hyperinsulinemic obese children [87], again highlighting inverse relationships among iNKT frequency, inflammation and metabolism. DIO mice provide further evidence for roles of iNKT cells in obesity: decreased iNKTs associated with increased pro-inflammatory macrophages, adiposity and metabolic abnormalities [84, 85, 86]. Follow-up studies showed iNKT cell-deficient (Jα18−/− or CD1d−/−) DIO mice had more IR and hepatic steatosis, compared to wild-type mice [84, 85, 86], while adoptive transfer of IL-4+ and IL-10+ iNKT cells into obese Jα18−/− or CD1d−/− mice increased frequency of “M2”-like macrophages, weight loss and metabolic improvement [85, 86]. A recent study detailing a novel IL-10 producing iNKT cell subset in AT of lean mice validated the protective function of iNKTs in AT [88]. In contrast, Wu and colleagues reported that activation of iNKT cells by excess lipid promoted IR and hepatic steatosis, although iNKT frequency was still reduced in DIO mice [89]. Furthermore, CD1d but not iNKT cell deficiency resulted in metabolic disease [90]. Reasons for discordance on the roles of iNKT cells in DIO mice is unknown, but differences in experimental design, microbiota and animal age are possible explanations [86]. Overall, most studies support the concept that iNKT cells are protective in obesity and metabolic disease [84, 85, 86, 88, 91, 92], but dissention persists.
2.3. B cells
B cells are a second lymphocyte class that plays important roles in obesity-associated T2D [13, 93]. B cells accumulate in visceral AT in DIO mice [23, 35], perhaps prior to T cells [23, 30]. Importantly, B cell-depleted or B cell-null DIO mice have less obesity-associated IR and inflammation [13, 30, 35, 93], suggesting B cells promote obesity-associated metabolic dysfunction. B cells can promote IR in humans and mice through modulating T cell function [30, 35], including the ability of B cells to support human Th17 function through contact-dependent mechanisms [30]. B cells also promote IR by shifting to a pro-inflammatory cytokine profile [30, 94] and by secreting autoantibodies [35], although a relationship between putative diabetogenic autoantibodies and adult-T2D progression is not established [95]. Obesity-associated increases in serum IgG only in children [96] and younger mice [35] further question the role of antibodies in adult disease.
Both B cell-intrinsic and -extrinsic changes can facilitate B cell-mediated T2D development. B cell-activating factor (BAFF), a potent B cell survival factor produced by adipocytes, is increased in ob/ob or DIO mice [97, 98]. Adipocytes increase BAFF production following toll-like receptor (TLR) activation and oxidative stress [98, 99], two broadly impactful outcomes of obesity [97]. B cells also express TLRs, and B cells from T2D subjects secrete a pro-inflammatory cytokine profile in response to TLR ligands [30, 94]. Furthermore, the proportion of B cells expressing the ATP sensor P2-purinoceptor (P2×7) is higher in T2D compared to healthy subjects [100], and may in part underlie changes in B cell activation. Although the natural P2-purinoceptor inhibitor CD39 is required for normal glucose tolerance in animal studies [100], further work is needed to establish whether the balance between P2X7 and CD39 contribute to B cell function in T2D.
2.4. Innate lymphoid cells
Innate lymphoid cells (ILCs) are recently appreciated mediators of obesity-associated inflammation [56]. ILCs are cells with lymphoid morphology that do not recombine antigen receptor genes, and lack the phenotypic markers of myeloid and dendritic cells [56]. ILC type 2 (ILC2) is a subset of ILCs that resides in visceral AT in lean mice that declines with adiposity [101], and depletion of ILC2 results in increased weight gain and glucose intolerance [92]. ILC2s secrete the Th2 cytokines IL-5 and IL-13 to promote eosinophil accumulation and alternate macrophage activation towards the “M2”-like subsets, leading to improvements in glucose tolerance and insulin sensitivity [92, 102]. Conversely, ILC3 cells produce pro-inflammatory IL- 17A [56]. DIO mice have greater lung ILC3 accumulation than leans, and ILC3 frequency associates with obesity-promoted airway hyper-reactivity [103]. It remains unclear how ILCs modulates obesity-associated T2D in humans.
2.5. Natural Killer Cells
Natural killer (NK) cells are cytotoxic cells with a lymphoid origin, but unlike CD8+ cytotoxic T cells, NK cells can directly induce death of infected cells in the absence of specific immunization [104]. Roles for NK cells in T2D remain ambiguous, as studies observed either a lower [105, 106] or equivalent [107] frequency of circulating NK cells in obese individuals compared to leans. Obese-T2D and MUHO individuals were shown to have increased NK cells with activating markers (CD69 or NKG2A) compared to lean-healthy and MHO individuals, respectively [105, 106]. Furthermore, the frequency of spontaneous CD107a+ NK cells and their degranulation capacity post-stimulation were significantly greater in obese-T2D than lean individuals [105]. Studies in wild-type DIO mice indicated that the reduced circulating NK cells in IR individuals may not be attributable to defects in NK cell-infiltration into the AT [23]. However, lymphocyte-null DIO mice had 3-fold higher AT NK cell frequency than their lean counterpart [23], suggesting that B and/or T lymphocytes modulates murine adipose NK cell accumulation. Finally, systemic ablation of NK cells in mice decreased ATMs in intra-abdominal visceral AT and improved insulin sensitivity [108], consistent with the interpretation that NK activity may progress T2D pathogenesis.
3. Adipocytes mimic immune cell properties in obesity/T2D
AT is a recognized endocrine tissue that houses adipocytes and immune cells in close approximation, thus facilitates cross-talk amongst the various precursors and subsets [5]. Despite the many differences between adipocytes and immune cells, these cells share some functions in both lean and obese individuals. Adipocytes and immune cells both secrete cytokines including TNFα and IL-6 [10, 11, 12], which in the case of adipocytes are called “adipokines”. For both cell types, pro-inflammatory cytokine/adipokine secretion increases, while anti-inflammatory cytokines/adipokines decrease in response to obesity [10, 11, 12]. Recent reviews have highlighted how adipocyte-lymphocyte crosstalks utilize adipokines to promote IR [10, 11, 12], but cell-intrinsic changes in adipocytes play additional roles in immune responses to obesity. MHCII is the “scaffold” molecule that, in general, allows APCs to present the CD4+ T cell-activating peptide in a configuration that triggers TCR activation. Recent work indicated that, contrary to textbook paradigms that MHCII is immune cell-specific, MHCII is increased by 3–6 folds in adipocytes of obese and IR mice and humans, compared to lean counterparts [34]. Mouse adipocyte MHCII plus antigen in turn activates CD4+ T cells in some, but not all studies [34, 109]. Antigens presented by adipocytes and macrophages, a second source of MHCII activity in AT [109] have not been identified, but may be modified proteins that are uniquely generated under metabolic stress.
T cell activation requires MHCII/peptide complexes, but additional APC surface co-stimulators that respond to obesity play crucial or moderate roles in activation of naïve or effector/memory T cells, respectively. Thus, changes in APC co-stimulators may also regulate AT-associated T cell activation. The cell surface receptor CD40 is a classical T cell co-stimulator expressed in human and murine APCs, including adipocytes. CD40 is activated in response to membrane-bound or soluble CD40 ligand (CD40L) from activated T cells to further stimulate CD40-expressing cells, thus AT inflammation [110]. In turn, CD40-mediated stimulation of human pre-adipocytes promotes adipogenesis [111]. Although the soluble form of CD40L is elevated in obesity and correlates with BMI [112, 113], the role of CD40-CD40L interaction in IR remains controversial. Two studies found that CD40L–deficient DIO mice have less immune cell AT infiltration than CD40L–deficient leans [114, 115], but only one study showed improved insulin sensitivity [114]. To the contrary, CD40-deficiency exacerbated IR and AT inflammation in CD40-null DIO mice [116, 117]. The inconsistent outcomes of removing CD40 and its ligand may be attributed to the ability of CD40L to function through receptors other than CD40, including Mac-1/CD11b [118]. Similarly, CD40 activation on B cells from DIO mouse reduced release of anti-inflammatory IL-10, but had no effect on IL-6 release [30]. This observation suggests that CD40L depletion may promote B cell pro-inflammatory response, but removing CD40 receptors on B cells may not yield similar results. It is possible that CD40 expression in AT APCs is essential to initiate the AT inflammation/remodeling required for healthy AT expansion [6], such that deletion of CD40 may promote metabolic disturbance. Although the majority of data support the conclusion that the CD40-CD40L axis promotes IR, further work will be essential to clarify this idea.
CD80 and CD86 are additional T cell co-stimulators that increase in mouse and human adipocytes [34] but decrease in ATMs [119] responding to obesity. CD80/86 co-stimulate T cells through interaction with T cell surface CD28, which in turn upregulates cytotoxic T-lymphocyte-associated protein 4 (CTLA4) to dampen sustained T cell responses [119]. Surprisingly, genetic inactivation of CD80/86 in mice promoted IR and increased AT inflammation, likely due in part to reduced anti-inflammatory Treg frequency in AT [119, 120]. However, simultaneously injecting both CD80- and CD86-blocking antibodies into wild-type DIO mice improved insulin sensitivity with no obvious impact on splenic Tregs [120], suggesting that targeting a combination of CD80 and CD86 could inhibit T2D pathogenesis. Future work exploring possible differences between adipocyte and macrophage CD80/86 function, as well as expansion of studies to include the long list of known co-stimulators (iCOS, PD-1 etc.), will be needed to elucidate whether co-stimulation blockades would be expected to improve or exacerbate clinical IR.
4. Concluding remarks and future perspectives
Lymphocytes are important players in obesity-associated T2D, integrating both pro-inflammatory (Figure 1) and anti-inflammatory signals (Figure 2). Experimental animals provide versatile tools to advance our understanding of T2D, although the outcome of blocking >90% of inflammatory molecule/cells in vivo is that animals resist obesity and/or obesity-associated IR following high fat diet. Although similar outcomes from inactivation of almost any inflammatory modulator could suggest artifact, strong human data that includes comparisons of immune cells in equally obese, but metabolically distinct cohorts support the hypothesis that inflammation is important for obesity-associated T2D in humans [15, 16, 30]. Variability in diet and microbiome, among other variables of both human and animal studies, may underlie seemingly contradictory outcomes. Resource-intensive longitudinal analysis will be crucial in the future, despite challenges posed by disease latency periods of years to decades, to overcome the intrinsic “snap-shot” feature of cross-sectional human sample analyses, and the inability of such studies to differentiate cause from consequence.
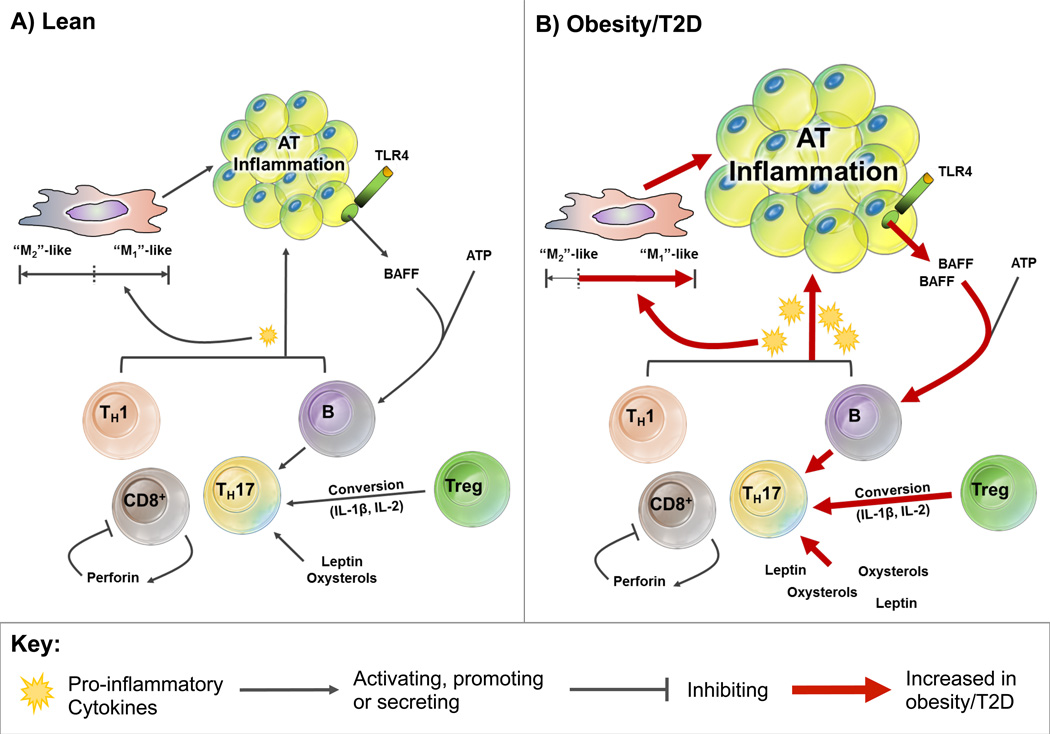
Figure 1. Lymphocyte subsets that promote adipose tissue (AT) inflammation.
(A) Lean: CD4+ Th1, Th17, CD8+ cytotoxic T cells and B cells promote AT inflammation through secreting pro-inflammatory cytokines and priming macrophages towards the pro-inflammatory “M1”-like subsets, but priming towards “M2”-like macrophages by anti-inflammatory cytokines dominates in lean AT. CD8+ cell-expansion may be self-limiting through autocrine action of perforin. Leptin and oxysterols facilitate Th17-cytokine secretion and differentiation, respectively. IL-2 and IL-1β cytokines present in AT can promote regulatory T (Treg) differentiation to Th17s, whereas B cells support Th17 function through a contact-dependent mechanism. However, the mechanisms that promote Th17 differentiation and function are minimal in lean AT. Overall, pro-inflammatory signaling within AT is limited in the lean state. (B) Obesity/T2D: In obese individuals with type 2 diabetes (T2D), pro-inflammatory cytokine secretion is increased by CD4+ Th1, Th17, CD8+ cytotoxic T cells and B cells, leading to the enhanced priming of macrophages towards the pro-inflammatory “M1”-like subsets. Both leptin and oxysterols that are increased in obesity facilitate Th17-cytokine secretion and differentiation, respectively. The increased presence of IL-2 and IL-1β in obesity also promotes increased Treg differentiation to Th17s. Obesity/T2D promotes B cell activation through toll-like receptor 4 (TLR4)-mediated secretion of B cell-activating factor (BAFF) from adipocyte, thus results in increased Th17 function through a contact-dependent mechanism.
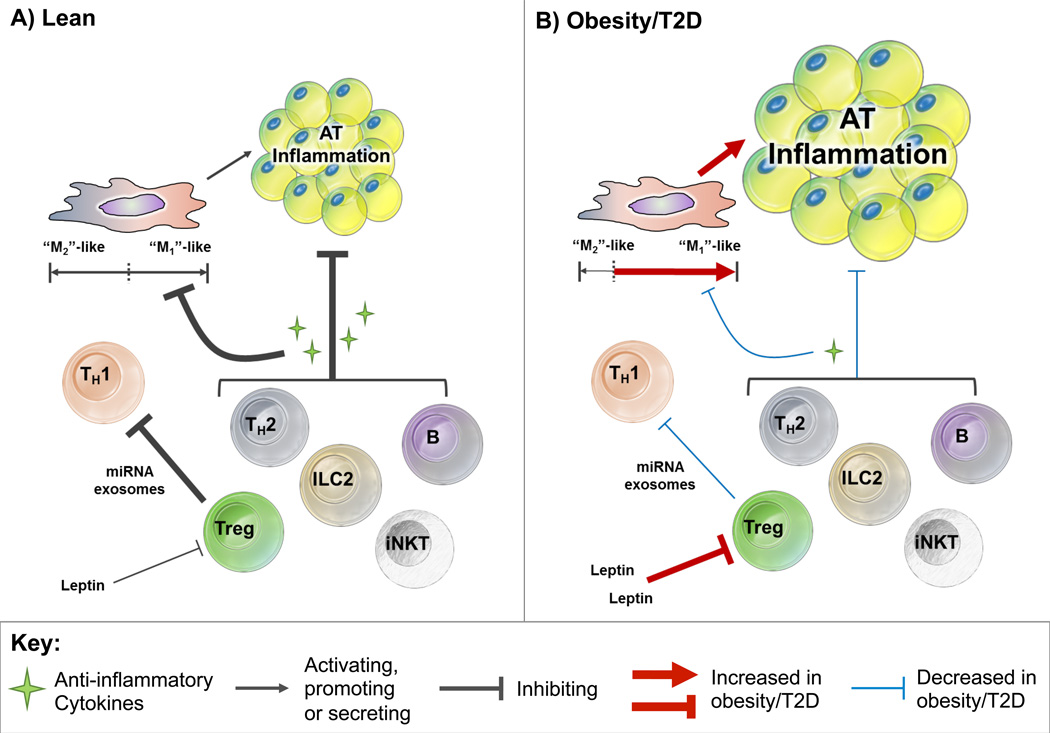
Figure 2. Lymphocyte subsets that inhibit adipose tissue (AT) inflammation.
(A) Lean: Invariant natural killer T (iNKT) cells, innate lymphoid cell type 2 (ILC2) cells, B cells, CD4+ Th2 and regulatory T (Treg) cells inhibit AT inflammation by secreting anti-inflammatory cytokines. These anti-inflammatory cytokines reduce AT inflammation by inhibiting macrophage priming towards the pro-inflammatory “M1”-like subsets and instead supporting “M2”-like macrophage generation. Treg cells can also reduce inflammation by inhibiting Th1s via transfer of microRNA-containing exosomes to Th1s and secreting anti-inflammatory cytokine IL-10. (B) Obesity/T2D: Anti-inflammatory cytokine secretion by iNKT, ILC2, B, CD4+ Th2 and Treg cells is dampened in obesity/type 2 diabetes (T2D), thus promoting macrophage priming towards the pro-inflammatory “M1”-like subsets. The anti-inflammatory effects of Treg cells are also suppressed by obesity-associated induction of leptin that inhibits Treg proliferation.
Clinical trials designed based on the logic of early immunometabolism studies show discouraging efficacy of anti-inflammatories in T2D, including drugs that neutralize TNFα, IL-1β or NF-κB [121]. Blocking one of the many inflammatory proteins implicated in T2D decreases some inflammatory indicators, but only modestly impacts glucose dysregulation in long-term T2D subjects [121]. Although these disappointments could be interpreted to show immunomodulation cannot benefit T2D subjects, we propose instead that future clinical trials should test the role of inflammation at the step often derailed in DIO animal knock-out studies: the transition from obese metabolically healthy to obese and IR. Future clinical trials may also profit from focus on validation of pathways that dominate obesity-associated inflammation in humans, rather than targeting pro-inflammatory molecules/cells in their order of appearance in the literature. We propose an approach that integrates outcomes from well-characterized human cohorts, in conjunction with outcomes from in vivo animal studies, to most effectively translate our knowledge of immunometabolism into fundamentally novel clinical approaches. This approach contrasts with the current strategy of developing concepts in animals, then retrofitting human data. Shifting effort to longitudinally collected human materials as the “gold standard” for support of new concepts will challenge both the metabolism and immunology fields, but it will be absolutely essential to streamline research towards efforts with most clinical promise, while continuing support of basic research endeavors.
Box 1: Outstanding questions.
What are the mechanisms involved in obesity-mediated modulations in lymphocyte functions?
What are the diverse functions of immunological co-stimulators (CD80/86 and similar) in obesity, insulin resistance and T2D?
How can we best relate an organ-specific effect of cytokines to the effect of cytokines on whole-body metabolism in obesity?
Numerous mechanisms converge on the development of metabolic dysfunction, but how do we identify the degree of impact for each individual player in patients?
Metabolic dysfunction is a heterogeneous disease with immense interpersonal variability. How do we best identify key player(s) involved in disease pathogenesis of an individual to design a personalized therapy?
What are the mechanisms involved in maintaining an obese individual metabolically healthy? Can we utilize this knowledge to help prevent other obese individuals from developing metabolic dysfunction?
Highlights.
Lymphocytes play complex regulatory roles in obesity-associated insulin resistance and type 2 diabetes pathogenesis.
Obesity differentially impacts the frequencies and behaviors of lymphocyte subsets.
Lymphocytes interact with other immune cells and with adipocytes to regulate adipose tissue inflammation.
Differences between type 2 diabetes in animals and humans indicate the value of a bedside-to-bench approach to maximize clinical impact of future studies.
Acknowledgments
This work was supported by the National Institutes of Health (R21DK089270, R21DE021154, NIH R56 DK096525, 5T32AI007309–25). AEH is supported by a grant from the National Children’s Research Centre.
Glossary
- Adaptive immunity
also known as acquired immunity, refers to a subset of highly specialized, systemic cells and processes that eliminate or prevent pathogen growth
- Antigen-presenting cells (APCs)
a heterologous group of immunocompetent cells that process and present antigens to TCR. APCs traditionally include macrophages, dendritic cells, langerhans cells, and B- lymphocytes
- B cells
an essential component of the humoral (antibody-mediated) immune response. B cells are produced by the bone marrow and can be distinguished from other lymphocytes by the presence of the cell surface B cell receptor (BCR)
- Chemokine C-X-C motif receptor 3 (CXCR3)
is an integral seven-transmembrane protein that specifically binds and responds to cytokines of the CXC chemokine family
- Cluster of differentiation 4 (CD4)
is a transmembrane glycoprotein on the surface of T helper cells, monocytes, macrophages, and dendritic cells. CD4 is co-receptor for T cell receptor on the surface of T helper cells that binds with major histocompatibility complex class II on APCs
- Cluster of differentiation 8 (CD8)
is a transmembrane glycoprotein and co-receptor for T cell receptor on the surface of CD8+ T cells that binds with major histocompatibility complex class I on APCs
- Innate immunity
refers to response of the immune system to molecular patterns, rather than specific antigens, provided by foreign invaders
- Innate lymphoid cells (ILCs)
a group of innate immune cells that lack B or T cell receptors. Some of their functions are analogous to helper T cells
- Insulin resistance (IR)
a physiological condition in which insulin-sensitive cells fail to respond to insulin by activating normal signal transduction cascades
- Lymphocyte
a leukocyte of fundamental importance in the immune system. Three types of lymphocytes exist: natural killer cells, T cells and B cells
- Metabolic healthy obese (MHO
Obese individuals (BMI > 30) who display healthy metabolic characteristics, including high insulin sensitivity levels, no hypertension and favorable lipid, inflammatory, hormonal, liver enzyme and immune profiles. Despite having similar total fat mass as metabolic unhealthy obese, MHO individuals present less visceral and ectopic fat including liver steatosis
- Metabolic unhealthy obese (MUHO)
Obese individuals with abnormal metabolic characteristics including abdominal obesity, insulin resistance, hypertension, dyslipidemia and increased systemic inflammation. MUHOs are at increased risk for developing metabolic syndrome, periodontal disease and some cancers
- “M1”-like macrophage
macrophages that promote inflammation, inhibit cell proliferation and cause tissue damage
- “M2”-like macrophage
macrophages that decrease inflammation, promote cell proliferation and encourage tissue repair
- Natural killer (NK) cell
a lymphoid origin cell that can directly induce death of infected cells, but is often considered as a component of the innate immunity due to the lack of antigen-specific cell surface receptors
- Natural killer T (NKT) cell
T cell that shares properties of both T cells and natural killer cells. The invariant subset of NKT cells recognizes the non-polymorphic antigen-presenting CD1d molecule that binds self and foreign lipids and glycolipids
- T helper cell (Th cell)
a type of T cell that helps the activity of other immune cells by releasing cytokines and providing co-stimulation. Mature Th cells express the surface protein CD4 and are referred to as CD4+ T cells. Uncommitted (naïve) CD4+ T cells can be induced to differentiate towards pro-inflammatory or anti-inflammatory T cells, based on the local cytokine environment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- 1.Samocha-Bonet D, et al. Metabolically healthy and unhealthy obese--the 2013 Stock Conference report. Obes. Rev. 2014;15:697–708. doi: 10.1111/obr.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Denis GV, Obin MS. ‘Metabolically healthy obesity’: origins and implications. Mol. Aspects Med. 2013;34:59–70. doi: 10.1016/j.mam.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CT, et al. White blood cell subtypes, insulin resistance and beta-cell dysfunction in high-risk individuals--the PROMISE cohort. Clin. Endocrinol. (Oxf.) 2014;81:536–541. doi: 10.1111/cen.12390. [DOI] [PubMed] [Google Scholar]
- 4.Achilike I, et al. Predicting the development of the metabolically healthy obese phenotype. Int. J. Obes. 2014 doi: 10.1038/ijo.2014.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 6.Wernstedt Asterholm I, et al. Adipocyte Inflammation Is Essential for Healthy Adipose Tissue Expansion and Remodeling. Cell Metab. 2014;20:103–118. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh DY, et al. Increased macrophage migration into adipose tissue in obese mice. Diabetes. 2012;61:346–354. doi: 10.2337/db11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalmas E, et al. T cell-derived IL-22 amplifies IL-1beta-driven inflammation in human adipose tissue: relevance to obesity and type 2 diabetes. Diabetes. 2014;63:1966–1977. doi: 10.2337/db13-1511. [DOI] [PubMed] [Google Scholar]
- 9.Gadd VL, et al. The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology. 2014;59:1393–1405. doi: 10.1002/hep.26937. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura K, et al. Adipokines: a link between obesity and cardiovascular disease. J. Cardiol. 2014;63:250–259. doi: 10.1016/j.jjcc.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Procaccini C, et al. Role of adipokines signaling in the modulation of T cells function. Front Immunol. 2013;4:332. doi: 10.3389/fimmu.2013.00332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi K, et al. Role of anti-inflammatory adipokines in obesity-related diseases. Trends Endocrinol Metab. 2014;25:348–355. doi: 10.1016/j.tem.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Nikolajczyk BS, et al. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol. Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Beek L, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63:492–501. doi: 10.1016/j.metabol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Fabbrini E, et al. Association Between Specific Adipose Tissue CD4+ T-Cell Populations and Insulin Resistance in Obese Individuals. Gastroenterology. 2013;145:366–374. doi: 10.1053/j.gastro.2013.04.010. e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jagannathan-Bogdan M, et al. Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J. Immunol. 2011;186:1162–1172. doi: 10.4049/jimmunol.1002615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner NM, et al. Circulating regulatory T cells are reduced in obesity and may identify subjects at increased metabolic and cardiovascular risk. Obesity (Silver Spring) 2013;21:461–468. doi: 10.1002/oby.20087. [DOI] [PubMed] [Google Scholar]
- 18.Sun K, et al. Adipose tissue remodeling and obesity. J. Clin. Invest. 2011;121:2094–2101. doi: 10.1172/JCI45887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaragosi LE, et al. Activin a plays a critical role in proliferation and differentiation of human adipose progenitors. Diabetes. 2010;59:2513–2521. doi: 10.2337/db10-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wentworth JM, et al. Pro-inflammatory CD11c+ CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59:1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kratz M, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winer S, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duffaut C, et al. Unexpected trafficking of immune cells within the adipose tissue during the onset of obesity. Biochem. Biophys. Res. Commun. 2009;384:482–485. doi: 10.1016/j.bbrc.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura S, et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 25.Rausch M, et al. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. 2007;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 26.Elgazar-Carmon V, et al. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008;49:1894–1903. doi: 10.1194/jlr.M800132-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen MA, et al. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J. Biol. Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 28.Wu H, et al. T-cell accumulation and regulated on activation, normal T cell expressed and secreted upregulation in adipose tissue in obesity. Circulation. 2007;115:1029–1038. doi: 10.1161/CIRCULATIONAHA.106.638379. [DOI] [PubMed] [Google Scholar]
- 29.Yang H, et al. Obesity increases the production of proinflammatory mediators from adipose tissue T cells and compromises TCR repertoire diversity: implications for systemic inflammation and insulin resistance. J. Immunol. 2010;185:1836–1845. doi: 10.4049/jimmunol.1000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DeFuria J, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proceedings of the National Academy of Sciences. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatzigeorgiou A, et al. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 32.Rocha VZ, et al. CXCR3 controls T-cell accumulation in fat inflammation. Arterioscler. Thromb. Vasc. Biol. 2014;34:1374–1381. doi: 10.1161/ATVBAHA.113.303133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H, et al. Obesity accelerates thymic aging. Blood. 2009;114:3803–3812. doi: 10.1182/blood-2009-03-213595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deng T, et al. Class II major histocompatibility complex plays an essential role in obesity-induced adipose inflammation. Cell Metab. 2013;17:411–422. doi: 10.1016/j.cmet.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winer DA, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazzone T, et al. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Panhuys N, et al. T-Cell-Receptor-Dependent Signal Intensity Dominantly Controls CD4(+) T Cell Polarization In Vivo. Immunity. 2014;41:63–74. doi: 10.1016/j.immuni.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kägi D, et al. Homeostatic regulation of CD8+ T cells by perforin. Eur. J. Immunol. 1999;29:3262–3272. doi: 10.1002/(SICI)1521-4141(199910)29:10<3262::AID-IMMU3262>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 39.Pacifico L, et al. Increased T-helper interferon-γ-secreting cells in obese children. Eur. J. Endocrinol. 2006;154:691–697. doi: 10.1530/eje.1.02138. [DOI] [PubMed] [Google Scholar]
- 40.Zeng C, et al. The imbalance of Th17/Th1/Tregs in patients with type 2 diabetes: relationship with metabolic factors and complications. J. Mol. Med. 2012;90:175–186. doi: 10.1007/s00109-011-0816-5. [DOI] [PubMed] [Google Scholar]
- 41.Mayer A, et al. Antigen presenting cell-derived IL-6 restricts Th2-cell differentiation. Eur. J. Immunol. 2014 doi: 10.1002/eji.201444646. [DOI] [PubMed] [Google Scholar]
- 42.Muranski P, Restifo NP. Essentials of Th17 cell commitment and plasticity. Blood. 2013;121:2402–2414. doi: 10.1182/blood-2012-09-378653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao R, et al. Elevated peripheral frequencies of Th22 cells: a novel potent participant in obesity and type 2 diabetes. PLoS ONE. 2014;9:e85770. doi: 10.1371/journal.pone.0085770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sumarac-Dumanovic M, et al. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 45.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chuang HC, et al. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat. Commun. 2014;5:4602. doi: 10.1038/ncomms5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu Y, et al. Cutting edge: leptin-induced RORγt expression in CD4+ T cells promotes Th17 responses in systemic lupus erythematosus. J. Immunol. 2013;190:3054–3058. doi: 10.4049/jimmunol.1203275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alkazemi D, et al. Oxysterol as a marker of atherogenic dyslipidemia in adolescence. J. Clin. Endocrinol. Metab. 2008;93:4282–4289. doi: 10.1210/jc.2008-0586. [DOI] [PubMed] [Google Scholar]
- 49.Soroosh P, et al. Oxysterols are agonist ligands of RORγt and drive Th17 cell differentiation. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1322807111. 201322807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winer S, et al. Obesity predisposes to Th17 bias. Eur. J. Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 51.Zúñiga LA, et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harley IT, et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology. 2014;59:1830–1839. doi: 10.1002/hep.26746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Korn T, et al. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 54.Shin JH, et al. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. Biochem. Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 55.Li L, et al. IL-17 produced by neutrophils regulates IFN-γ-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. The Journal of clinical investigation. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Spits H, et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 2013;13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 57.Ramesh R, et al. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J. Exp. Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee Y, et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 2012;13:991–999. doi: 10.1038/ni.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duhen T, et al. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009;10:857–863. doi: 10.1038/ni.1767. [DOI] [PubMed] [Google Scholar]
- 60.Trifari S, et al. Identification of a human helper T cell population that has abundant production of interleukin 22 and is distinct from TH-17, TH1 and TH2 cells. Nat. Immunol. 2009;10:864–871. doi: 10.1038/ni.1770. [DOI] [PubMed] [Google Scholar]
- 61.Sabat R, et al. Therapeutic opportunities of the IL-22-IL-22R1 system. Nature Reviews Drug Discovery. 2014;13:21–38. doi: 10.1038/nrd4176. [DOI] [PubMed] [Google Scholar]
- 62.Aggarwal S, et al. Acinar cells of the pancreas are a target of interleukin-22. J. Interferon Cytokine Res. 2001;21:1047–1053. doi: 10.1089/107999001317205178. [DOI] [PubMed] [Google Scholar]
- 63.Shioya M, et al. Interleukin 22 receptor 1 expression in pancreas islets. Pancreas. 2008;36:197–199. doi: 10.1097/MPA.0b013e3181594258. [DOI] [PubMed] [Google Scholar]
- 64.Xue J, et al. Aryl hydrocarbon receptor regulates pancreatic IL-22 production and protects mice from acute pancreatitis. Gastroenterology. 2012;143:1670–1680. doi: 10.1053/j.gastro.2012.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hill T, et al. The involvement of interleukin-22 in the expression of pancreatic beta cell regenerative Reg genes. Cell Regeneration. 2013;2:2. doi: 10.1186/2045-9769-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, et al. Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes. Nature. 2014;514:237–241. doi: 10.1038/nature13564. [DOI] [PubMed] [Google Scholar]
- 67.Feuerer M, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eller K, et al. Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes. 2011;60:2954–2962. doi: 10.2337/db11-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ilan Y, et al. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proceedings of the National Academy of Sciences. 2010;107:9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okeke EB, et al. Regulatory T cells restrain CD4+ T cells from causing unregulated immune activation and hypersensitivity to lipopolysaccharide challenge. J. Immunol. 2014;193:655–662. doi: 10.4049/jimmunol.1303064. [DOI] [PubMed] [Google Scholar]
- 71.Okoye IS, et al. MicroRNA-Containing T-Regulatory-Cell-Derived Exosomes Suppress Pathogenic T Helper 1 Cells. Immunity. 2014;41:89–103. doi: 10.1016/j.immuni.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kelada S, et al. miR-182 and miR-10a are key regulators of Treg specialisation and stability during Schistosome and Leishmania-associated inflammation. PLoS Pathog. 2013;9:e1003451. doi: 10.1371/journal.ppat.1003451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Han JM, et al. Insulin Inhibits IL-10-Mediated Regulatory T Cell Function: Implications for Obesity. J. Immunol. 2014;192:623–629. doi: 10.4049/jimmunol.1302181. [DOI] [PubMed] [Google Scholar]
- 74.Cipolletta D, et al. PPAR-gamma is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature. 2012;486:549–553. doi: 10.1038/nature11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Rosa V, et al. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 76.Liu Y, et al. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 2012;188:2070–2073. doi: 10.4049/jimmunol.1102835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moraes-Vieira PM, et al. Leptin deficiency impairs maturation of dendritic cells and enhances induction of regulatory T and Th17 cells. Eur. J. Immunol. 2014;44:794–806. doi: 10.1002/eji.201343592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Valmori D, et al. Human RORγt+ TH17 cells preferentially differentiate from naive FOXP3+ Treg in the presence of lineage-specific polarizing factors. Proceedings of the National Academy of Sciences. 2010;107:19402–19407. doi: 10.1073/pnas.1008247107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Porcelli S, et al. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4–8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16. doi: 10.1084/jem.178.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Exley M, et al. Requirements for CD1d recognition by human invariant Valpha24+ CD4-CD8- T cells. J Exp Med. 1997;186:109–120. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kawano T, et al. CD1d–restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 82.Matsuda JL, et al. CD1d–restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lynch L, et al. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur. J. Immunol. 2009;39:1893–1901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 84.Schipper HS, et al. Natural killer T cells in adipose tissue prevent insulin resistance. J Clin Invest. 2012;122:3343–3354. doi: 10.1172/JCI62739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ji Y, et al. Activation of natural killer T cells promotes M2 Macrophage polarization in adipose tissue and improves systemic glucose tolerance via interleukin-4 (IL-4)/STAT6 protein signaling axis in obesity. J. Biol. Chem. 2012;287:13561–13571. doi: 10.1074/jbc.M112.350066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lynch L, et al. Adipose tissue invariant NKT cells protect against diet-induced obesity and metabolic disorder through regulatory cytokine production. Immunity. 2012;37:574–587. doi: 10.1016/j.immuni.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carolan E, et al. The impact of childhood obesity on inflammation, innate immune cell frequency and metabolic microRNA expression. J Clin Endocrinol Metab. 2013 doi: 10.1210/jc.2013-3529. jc20133529. [DOI] [PubMed] [Google Scholar]
- 88.Sag D, et al. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J. Clin. Invest. 2014;124:3725–3740. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu L, et al. Activation of invariant natural killer T cells by lipid excess promotes tissue inflammation, insulin resistance, and hepatic steatosis in obese mice. Proc. Natl. Acad. Sci. U. S. A. 2012;109:E1143–E1152. doi: 10.1073/pnas.1200498109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kotas ME, et al. Impact of CD1d deficiency on metabolism. PLoS One. 2011;6:e25478. doi: 10.1371/journal.pone.0025478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huh JY, et al. A novel function of adipocytes in lipid antigen presentation to iNKT cells. Mol Cell Biol. 2013;33:328–339. doi: 10.1128/MCB.00552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hams E, et al. Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J. Immunol. 2013;191:5349–5353. doi: 10.4049/jimmunol.1301176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Winer DA, et al. B Lymphocytes in obesity-related adipose tissue inflammation and insulin resistance. Cell. Mol. Life Sci. 2014;71:1033–1043. doi: 10.1007/s00018-013-1486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jagannathan M, et al. Toll-like receptors regulate B cell cytokine production in patients with diabetes. Diabetologia. 2010;53:1461–1471. doi: 10.1007/s00125-010-1730-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dabelea D, et al. Diabetes autoantibodies do not predict progression to diabetes in adults: the Diabetes Prevention Program. Diabet. Med. 2014;31:1064–1068. doi: 10.1111/dme.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marzullo P, et al. Lymphocytes and immunoglobulin patterns across the threshold of severe obesity. Endocrine. 2014;45:392–400. doi: 10.1007/s12020-013-0006-z. [DOI] [PubMed] [Google Scholar]
- 97.Kim YH, et al. B cell activation factor (BAFF) is a novel adipokine that links obesity and inflammation. Exp. Mol. Med. 2009;41:208–216. doi: 10.3858/emm.2009.41.3.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tada F, et al. B cell activating factor in obesity is regulated by oxidative stress in adipocytes. J Clin Biochem Nutr. 2013;52:120–127. doi: 10.3164/jcbn.12-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yan H, et al. Priming of Toll-like receptor 4 pathway in mesenchymal stem cells increases expression of B cell activating factor. Biochem. Biophys. Res. Commun. 2014;448:212–217. doi: 10.1016/j.bbrc.2014.04.097. [DOI] [PubMed] [Google Scholar]
- 100.Garcia-Hernandez MH, et al. Expression and function of P2X(7) receptor and CD39/Entpd1 in patients with type 2 diabetes and their association with biochemical parameters. Cell. Immunol. 2011;269:135–143. doi: 10.1016/j.cellimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 101.Nussbaum JC, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Molofsky AB, et al. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J. Exp. Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim HY, et al. Interleukin-17-producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity-associated airway hyperreactivity. Nat. Med. 2014;20:54–61. doi: 10.1038/nm.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vivier E, et al. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guo H, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med (Maywood) 2012;237:556–562. doi: 10.1258/ebm.2012.011272. [DOI] [PubMed] [Google Scholar]
- 106.Lynch LA, et al. Are natural killer cells protecting the metabolically healthy obese patient? Obesity (Silver Spring) 2009;17:601–605. doi: 10.1038/oby.2008.565. [DOI] [PubMed] [Google Scholar]
- 107.Nieman DC, et al. Influence of obesity on immune function. J. Am. Diet. Assoc. 1999;99:294–299. doi: 10.1016/S0002-8223(99)00077-2. [DOI] [PubMed] [Google Scholar]
- 108.O'Rourke RW, et al. Systemic NK cell ablation attenuates intra-abdominal adipose tissue macrophage infiltration in murine obesity. Obesity (Silver Spring) 2014;22:2109–2114. doi: 10.1002/oby.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Morris DL, et al. Adipose tissue macrophages function as antigen-presenting cells and regulate adipose tissue CD4+ T cells in mice. Diabetes. 2013;62:2762–2772. doi: 10.2337/db12-1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Seijkens T, et al. CD40-CD40L: linking pancreatic, adipose tissue and vascular inflammation in type 2 diabetes and its complications. Diab. Vasc. Dis. Res. 2013;10:115–122. doi: 10.1177/1479164112455817. [DOI] [PubMed] [Google Scholar]
- 111.Missiou A, et al. CD40L induces inflammation and adipogenesis in adipose cells--a potential link between metabolic and cardiovascular disease. Thromb. Haemost. 2010;103:788–796. doi: 10.1160/TH09-07-0463. [DOI] [PubMed] [Google Scholar]
- 112.Poggi M, et al. The inflammatory receptor CD40 is expressed on human adipocytes: contribution to crosstalk between lymphocytes and adipocytes. Diabetologia. 2009;52:1152–1163. doi: 10.1007/s00125-009-1267-1. [DOI] [PubMed] [Google Scholar]
- 113.Unek IT, et al. The levels of soluble CD40 ligand and C-reactive protein in normal weight, overweight and obese people. Clin. Med. Res. 2010;8:89–95. doi: 10.3121/cmr.2010.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Poggi M, et al. CD40L deficiency ameliorates adipose tissue inflammation and metabolic manifestations of obesity in mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:2251–2260. doi: 10.1161/ATVBAHA.111.231357. [DOI] [PubMed] [Google Scholar]
- 115.Wolf D, et al. CD40L deficiency attenuates diet-induced adipose tissue inflammation by impairing immune cell accumulation and production of pathogenic IgG-antibodies. PLoS ONE. 2012;7:e33026. doi: 10.1371/journal.pone.0033026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guo CA, et al. CD40 deficiency in mice exacerbates obesity-induced adipose tissue inflammation, hepatic steatosis, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2013;304:E951–E963. doi: 10.1152/ajpendo.00514.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yi Z, et al. CD40-Mediated Maintenance of Immune Homeostasis in the Adipose Tissue Microenvironment. Diabetes. 2014;63:2751–2760. doi: 10.2337/db13-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zirlik A, et al. CD40 ligand mediates inflammation independently of CD40 by interaction with Mac-1. Circulation. 2007;115:1571–1580. doi: 10.1161/CIRCULATIONAHA.106.683201. [DOI] [PubMed] [Google Scholar]
- 119.Zhong J, et al. T-cell costimulation protects obesity-induced adipose inflammation and insulin resistance. Diabetes. 2014;63:1289–1302. doi: 10.2337/db13-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chatzigeorgiou A, et al. Dual role of B7 costimulation in obesity-related nonalcoholic steatohepatitis and metabolic dysregulation. Hepatology. 2014;60:1196–1210. doi: 10.1002/hep.27233. [DOI] [PubMed] [Google Scholar]
- 121.Goldfine AB, et al. Therapeutic approaches to target inflammation in type 2 diabetes. Clin. Chem. 2011;57:162–167. doi: 10.1373/clinchem.2010.148833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Esser N, et al. Obesity phenotype is related to NLRP3 inflammasome activity and immunological profile of visceral adipose tissue. Diabetologia. 2013;56:2487–2497. doi: 10.1007/s00125-013-3023-9. [DOI] [PubMed] [Google Scholar]
- 123.O'Rourke RW, et al. Alterations in T-cell subset frequency in peripheral blood in obesity. Obes. Surg. 2005;15:1463–1468. doi: 10.1381/096089205774859308. [DOI] [PubMed] [Google Scholar]
- 124.van der Weerd K, et al. Morbidly obese human subjects have increased peripheral blood CD4+ T cells with skewing toward a Treg- and Th2-dominated phenotype. Diabetes. 2012;61:401–408. doi: 10.2337/db11-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]