Abstract
Purpose
We hypothesized that platelet levels during therapy could serve as a biomarker for response to therapy and that manipulation of platelet levels could impact responsiveness to chemotherapy.
Experimental Design
The medical records of patients with recurrent or progressive ovarian cancer were retrospectively queried for changes in platelet and CA-125 levels during primary therapy. In vitro co-culture experiments and in vivo orthotopic models of human ovarian cancer in mice were used to test the effect of modulating platelet levels on tumor growth and responsiveness to docetaxel.
Results
Thrombocytosis at the diagnosis of ovarian cancer correlated with decreased interval to progression (p = 0.05) and median overall survival (p = 0.007). Mean platelet levels corrected during primary therapy and rose at recurrence. Contrary to treatment-responsive patients, in a cohort of patients refractory to primary therapy, platelet levels did not normalize during therapy. In A2780, HeyA8, and SKOV3-ip1 ovarian cancer cell lines, platelet co-culture protected against apoptosis (p < 0.05). In orthotopic models of human ovarian cancer, platelet depletion resulted in 70% reduced mean tumor weight (p < 0.05). Compared to mice treated with docetaxel, mice treated with both docetaxel and platelet-depleting antibody had a 62% decrease in mean tumor weight (p = 0.04). Platelet transfusion increased mean aggregate tumor weight 2.4-fold (p < 0.05), blocked the effect of docetaxel on tumor growth (p = 0.55) and decreased tumor cell apoptosis. Pre-transfusion aspirinization of the platelets blocked the growth-promoting effects of transfusion.
Conclusions
Platelet-driven effects of chemotherapy response may explain clinical observations.
Keywords: Platelets, docetaxel, ovarian cancer, aspirin
INTRODUCTION
Thrombocytosis, defined as >450,000 cells/μL, is found in over 30% of epithelial ovarian cancer patients and is associated with decreased progression-free and overall survival. (1) Paraneoplastic thrombocytosis, in addition to hypercalcemia, leukocytosis, and cachexia, has been shown to occur through the generation of IL-6. (1, 2) IL-6 expression correlates with ovarian cancer taxane sensitivity. (3) Platelet transfusion leads to increased tumor cell proliferation. (2) Recent clinical work supports the relationship between thrombocytosis and poor prognosis in ovarian cancer. (4, 5)
The connection between platelets and metastasis is established. (6–14) Platelets have been shown to mediate protection of micrometastases from natural-killer-cell-mediated clearance. (15) Direct signaling between platelets and tumor cells contributes to the epithelial-to-mesenchymal transition. (16) The role for platelets in metastasis has proven multifactorial, including platelet-tumor interactions involving multiple protein classes and functions. (17–21)
Exposure of human adenocarcinoma cells to platelets increases survival, proliferation, and in vitro chemoresistance through the upregulation of anti-apoptotic pathways, down-regulation of pro-apoptotic pathways, promotion of DNA synthesis, increased cyclin expression, increased DNA repair protein expression, and increased MAPK expression. (22) Induction of thrombocytopenia in a murine model of breast carcinoma results in greater taxane efficacy that correlates with and increased vascular leakage at the tumor site. (23) Platelets sequester and differentially release angiogenic and mitogenic mediators. (24–28) Release of alpha-granule contents and platelet-driven neutrophil chemotaxis are variable based on pH, suggesting a complex regulatory function. (29) Dense granules release agents known to modulate cell growth and migration. (30)
Considering the growing evidence for correlation between platelet levels and clinical outcomes, we considered whether platelet levels could serve as a biomarker of treatment response. We considered, using in vitro and pre-clinical in vivo models, whether modulation of platelet levels could influence response to chemotherapy and whether such effects could be blocked to improve sensitivity to taxane-based chemotherapy.
METHODS
Approvals
Approval for relevant studies was obtained from the University of Texas at M.D. Anderson Cancer Center Institutional Review Board (IRB). All animal experiments were approved and supervised by the MDACC Institutional Animal Care and Use Committee.
Clinical Analysis
Patients were retrospectively identified at the University of Texas at M.D. Anderson Cancer Center (MDACC), the University of Iowa, and the University of Virginia who were diagnosed with ovarian, primary peritoneal, or fallopian tube carcinoma. This database was partially overlapping with that reported by Stone, et al. (1) Patients were excluded if they did not receive primary therapy or follow-up at the institution of record. In order to explicitly focus on patterns of recurrence and progression, patients were excluded who did not recur or progress. Exclusions were made for a history of other malignancy, myeloproliferative disease, inflammatory disease, splenectomy, or other confounding cause of thrombocytosis. All patients were treated by surgical cytoreduction performed by a gynecologic oncologist in addition to adjuvant or neoadjuvant taxane- and/or platinum-based chemotherapy. Clinical data collected included patient demographics, tumor characteristics, details of treatment, and outcomes data. Platelet levels and CA-125 measurements were recorded at the time of primary evaluation, through therapy, after the completion of surgery and 6 cycles of cytotoxic chemotherapy, during the post-therapy monitoring period, and at the time of diagnosis of ovarian cancer recurrence. Thrombocytosis was defined as a platelet count greater than 450,000/μL. (31) Interval to progression was defined starting at the conclusion of six cycles of primary therapy and ending at the clinical diagnosis of recurrence by physical exam, laboratory evaluation, and/or imaging. The survival interval was also defined as starting at the conclusion of six cycles of primary chemotherapy. Patients who were known to be alive at the time of last contact were censored accordingly.
Pre-Clinical Analysis
Docetaxel
Docetaxel (Sanofi-Aventis, Paris, France) is a commonly used taxane chemotherapy shown in phase III clinic trials to be equivalent to paclitaxel in the primary therapy of ovarian cancer. (32) Docetaxel was obtained from surplus clinical samples from the clinical pharmacy associated with the University of Texas M.D. Anderson Cancer Center.
Cell lines and culture conditions
The derivation of the human ovarian cancer cell lines A2780, HeyA8, and SKOV3-IP1 are previously reported. (33) Cell lines were obtained from the institutional Cell Line Core laboratory and per institutional policy (MD Anderson policy ACA#1044) cell line authentication was performed at least once per year. In this case, authentication was performed within six months of the work described. Authentication was performed by the short tandem repeat method using the Promega Power Plex 16HS kit (Promega™, Madison, WI). Somatic mutations were detected using a Sequenom MALDI TOF MassArray system (Sequenom™, San Diego, CA). Mycoplasma detection was performed using the MycoAlert kit (Lonza™, Basel, Switzerland). The cell lines were maintained in RPMI-1640 with 15% fetal bovine serum. Cell lines were routinely genotyped to confirm identity and tested to confirm absence of Mycoplasma. Cells were maintained at 37°C in a humidified incubator infused with 20% O2 and 5% CO2.
Platelet isolation for in vitro assays
Platelets were prepared for in vitro assays in a manner that would remove plasma contents and nucleated cells. Whole blood was drawn from the inferior vena cava of anesthetized nude mice into a syringe pre-loaded with 1:9 v/v 3.8% sodium citrate and mixed 1:1 v/v with tyrodes buffer lacking Mg2+ and Ca2+. Blood was centrifuged at 1100 rpm for 3 minutes, twice, at room temperature. The platelet-rich plasma fraction was passed through a filtration column of Sepharose 2B beads (Sigma Aldrich, St Louis, MO) loaded into a siliconized glass column with a 10 μm nylon net filter (Millipore, Billerica, MA) and sepharose 2B beads previously washed in acetone 1:1 v/v, followed by 0.9% NaCl 1:1 v/v, and “Buffer 1” 1:1 v/v. Platelet-containing eluent was diluted 1:200 and platelets were counted with a hemocytometer by phase-contrast microscopy at 400x magnification.
In vitro assays
To examine potential effects of platelets on apoptosis and response to chemotherapy, we incubated cancer cells with platelets using a tissue co-culture system and observed consistent protection against apoptosis. To assess the effect of platelets on apoptosis, cells were plated in 6-well plates at 50,000 cells per plate. At 50% confluence, media was changed to serum-free for 24-hours prior to starting treatment. After serum-starvation, platelets were isolated and added to achieve a final dose of 1 × 108 platelets/mL. Docetaxel was dosed at 5 μM based on previously published IC50 levels. Controls utilized an equivalent volume of the appropriate buffer. All treatments were performed in triplicate. After 72 hours of platelet and docetaxel exposure, cell viability was assessed using Annexin V and 7-amino-actinomycin-D (7AAD) staining (BD Pharmingen™, Franklin Lakes, NJ) by flow cytometry. Indirect mediation of effect was considered by the use of an intervening cell culture insert with 0.4 μm pores (BD Falcon™, Franklin Lakes, NJ).
Proliferation was measured by flow cytometry (Click-iT EdU kit, Invitrogen, Carlsbad, CA). For platelet fixation experiments, plasma-free platelets were incubated in 1% paraformaldehyde. (2) To test the effect of aspirin in this system, a 325 mg tablet of aspirin was dissolved in deionized, distilled water and filter-sterilized. Cancer cells were plated, plasma-free platelets were isolated and co-incubated with aspirin 30 μM, and in vitro experiments were performed as described. Internal controls (n = 3) were performed for each experiment given the variability in baseline apoptosis and proliferation rates seen between experiments in order to avoid batch error.
Orthotopic model of ovarian cancer in nude mice
Female athymic nude (NCr-nu) were purchased from Taconic Farms, Inc. (Rockville, MD). The development and characterization of the orthotopic mouse model of ovarian cancer has been previously described. (34) SKOV3-IP1 (1 × 106 cells/mouse), A2780 (1 × 106 cells/mouse), or HeyA8 (0.25 × 106 cells/mouse) were lifted with trypsin/EDTA, washed with PBS, and re-suspended in 200 μL of Hank’s balanced salt solution (HBSS, Mediatech, Inc. Manassas, VA) and were injected into the peritoneal cavity of female nude mice.
Platelet-depleting antibody
To deplete platelets in mice for in vivo experiments, we used a commercially available rat anti-mouse monoclonal antibody directed against mouse GP1b-alpha (CD42b, Emfret Analytics, Eibelstadt, Germany) that causes irreversible Fc-independent platelet depletion within 60 minutes of administration without inducing platelet activation. Dose-kinetics are previously validated. (1)
Thrombocytosis, thrombocytopenia, and effect on chemotherapy in vivo
The cell lines A2780 and SKOV3-IP1 were used in the orthotopic model of nude mice. The animals were injected with tumor on Day 0 as described above. Starting on Day 7, animals were randomized and treated: twice weekly tail vein injections of Control IgG (0.5 mcg/gram); twice weekly Control IgG via tail vein injection and weekly docetaxel 35 mcg IP; platelet-depleting antibody (0.5 mcg/gram) via tail vein injection twice weekly; platelet-depleting antibody plus docetaxel; tail vein transfusion of platelet rich plasma isolated from nude mice; platelet transfusion and docetaxel. Mice were treated until they became moribund and then sacrificed.
Aspirinization of platelets
Pharmacy grade aspirin was acquired, and a single 325 mg table was dissolved in 500 mcM sodium acetate (pH 5.6). This was added 1:10 v/v to platelet rich plasma and the combination was incubated at 37°C for 15 minutes. Incubation with an equivalent sodium acetate solution without aspirin was used for control.
Effect of aspirin on thrombocytosis and malignancy in vivo
Using the A2780 orthotopic model of ovarian cancer, mice were injected with tumor on Day 0. On Day 7, the animals were randomized and treated: untreated control; intraperitoneal aspirin 20 mg/kg twice per week; 500 μL of platelet rich plasma isolated from nude mice and incubated with sodium acetate for 15 minutes (as described above) via tail vein injection weekly; tail vein transfusion of platelet rich plasma that had been incubated for 15 minutes with a 50 μM solution of aspirin in sodium acetate (as described above). Mice were treated until they became moribund and then sacrificed.
Cleaved caspase 3 immunohistochemistry
Immunohistochemistry for cleaved caspase 3 was used to measure apoptotic rates in ex vivo tumor using a rabbit polyclonal anti-human antibody to cleaved caspase 3 (BioCare Medical, Concord, CA, #CP229B). Paraffin embedded tumor sections were heated, deparaffinized, and antigen retrieval was performed by steaming, and endogenous peroxides were blocked with 3% hydrogen peroxide in methanol. Non-specific proteins were blocked with 4% fish gelatin in PBS. Slides were incubated in primary antibody (1:100), and the secondary antibody (ready-to-use) was followed by streptavidin HRP (ready-to-use). Slides were quantified by counting the number of positively-staining cells per 200x field.
Statistical considerations
A 2-sided long-rank statistic was used to compare Kaplan-Meier survival curves. Variables estimated to have a normal distribution were compared using the Student’s T-Test using Excel (Microsoft®, Redmond, Washington). The F-Test was used to compare variances where indicated. A p-value of <0.05 was considered statistically significant. For mouse experiments, sample size was estimated utilizing a two-way ANOVA model. For an effect size of 0.65, a sample size of 10 mice per group was considered sufficient to provide 80% power for α = 0.05 anticipating less than 10 groups.
RESULTS
Thrombocytosis is associated with resistance to chemotherapy
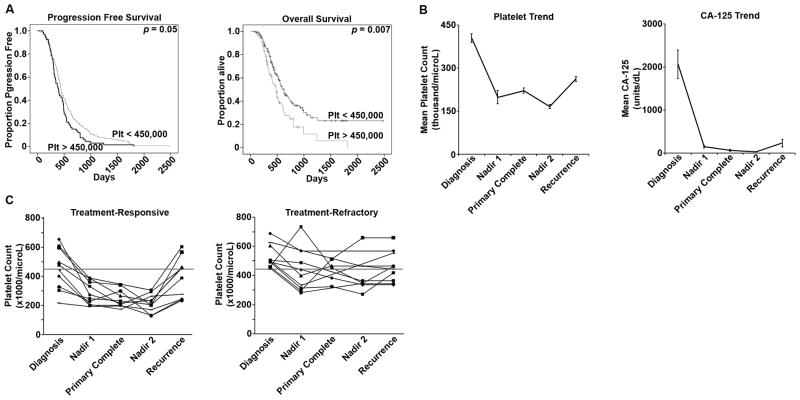
We first identified patients known to have recurrent or progressive epithelial ovarian cancer (n = 355) for whom adequate laboratory data prior to treatment, treatment data, and post-treatment follow-up data was available. Demographics (Supplemental Table 1) indicated a median age 61 years (range 31–88 years). Ninety percent had advanced stage (III or IV) and 89% had high-grade disease. For primary therapy, all patients underwent a combination of surgical cytoreduction (60% had “optimal” cytoreduction to <1cm gross residual disease) and taxane-based chemotherapy, most commonly paclitaxel and carboplatin. In this population, in which all patients developed disease recurrence, the mean platelet level was 409,000/μL (range 134,000–1,122,000 cells/μL) at diagnosis. Thirty-two percent had a mean platelet level of >450,000 cells/μL at the time of diagnosis. Even after patients without diagnosed recurrence were excluded, thrombocytosis at diagnosis was associated with worse median progression free survival (12.9 vs. 14.7 months, p = 0.05, figure 1A) and median overall survival (16 vs. 20.8 months, p = 0.007, figure 1A).
Figure 1.
A. Patients with recurrent ovarian cancer (n = 341) were identified and stratified according to their platelet counts at diagnosis into two groups: those with thrombocytosis (>450,000 cells/μL) and those with normal platelet counts (<450,000 cells/μL). The patients with thrombocytosis at the time of diagnosis had a significantly shorter median interval to progression (12.9 vs. 14.7 months, p = 0.05). The patients with thrombocytosis at the time of diagnosis had a significantly shorter median overall survival (16 vs. 20.8 months, p = 0.007).
B. Patients with recurrent ovarian cancer for whom longitudinal data was available through treatment and surveillance (n = 96). The mean platelet level at diagnosis was 403,000 cells/μL. During primary therapy, the mean platelet nadir was 198,000 cells/μL. At the conclusion of therapy, the average platelet level was 221,000 cells/μL, and this remained stable in the surveillance period, with the mean nadir 166,000 cells/μL during this time. At recurrence, mean platelet levels increased 27% to 262,000 cells/μL (p < 0.001). Of the patients with available longitudinal data, the mean level at diagnosis was 332 units/mL (normal <35 units/mL). Only 86% had a normal CA-125 level at the conclusion of primary therapy with a mean 63 units/mL, and the mean post-treatment nadir was 23 units/mL. At the clinical diagnosis of disease recurrence, CA-125 was elevated in 75% of patients, with a median 229 units/mL.
C. A subgroup of the patients with complete longitudinal data was identified who experienced progression of disease through first-line therapy (n = 10). These patients were matched to a cohort who experienced a durable response to therapy lasting more than 6 months. In the subgroup of patients with a durable response, only 50% had thrombocytosis (>450,000 cells/μL) at the time of diagnosis, and all patients in this subgroup achieved normal platelet counts during therapy. In the treatment-refractory cohort, all patients had thrombocytosis at the time of diagnosis, and platelet levels were more heterogeneous during primary therapy, with only 50% having normalized platelet counts by the completion of primary therapy.
A subgroup of 96 patients was identified whose available laboratory data were adequate to consider platelet and CA-125 trends through primary diagnosis, primary treatment, surveillance, and until the clinical diagnosis of recurrence. (Figure 1B, Supplemental Table 2). CA-125 is a standard tumor marker followed in ovarian cancer to track the efficacy of primary therapy and in surveillance for recurrence. In this group of patients, only 86% of patients had a normal CA-125 level (<35 units/mL) at the conclusion of primary therapy. In contrast, all patients had a normal platelet count <450,000 cells/μL (mean 206,000 cells/μL) after primary therapy. At the clinical diagnosis of disease recurrence or progression, CA-125 was elevated in 75% of patients. In parallel, at the diagnosis of recurrence, mean platelet counts were found to be increased 57.8% to 262,000 cells/μL compared to nadir levels found after primary therapy was completed (p<0.001, figure 1B, Table 2). Among patients with a CA-125 <35 units/mL at the time of recurrence, platelet levels were increased by 49% (mean increase 108,400 cells/μL, p<0.01) at the diagnosis of recurrence compared to the conclusion of primary therapy.
Among ovarian cancer patients, approximately 10% will not respond to primary therapy and are considered to have “refractory” disease. From the 96 patients with complete longitudinal data, 10 patients were identified who had disease refractory to primary treatment. Ten additional patients (matched for stage, grade, histology, and primary therapy) were identified for comparison who experienced a complete response to primary therapy that was durable for at least 6 months. In the patients who experienced a compete response to therapy that was durable for >6 months, 50% had thrombocytosis at diagnosis, and all of these patients consistently normalized platelet levels by the end of primary therapy (figure 1C). In the treatment-refractory cohort, all patients had thrombocytosis at the time of diagnosis, and platelet levels were far more heterogeneous during primary therapy, with only 50% having normalized platelet counts by the completion of primary therapy (figure 1C). These data suggest a correlation between the normalization of platelet counts during primary therapy and disease response to that therapy.
Platelets mediate resistance against chemotherapy-induced apoptosis in vitro
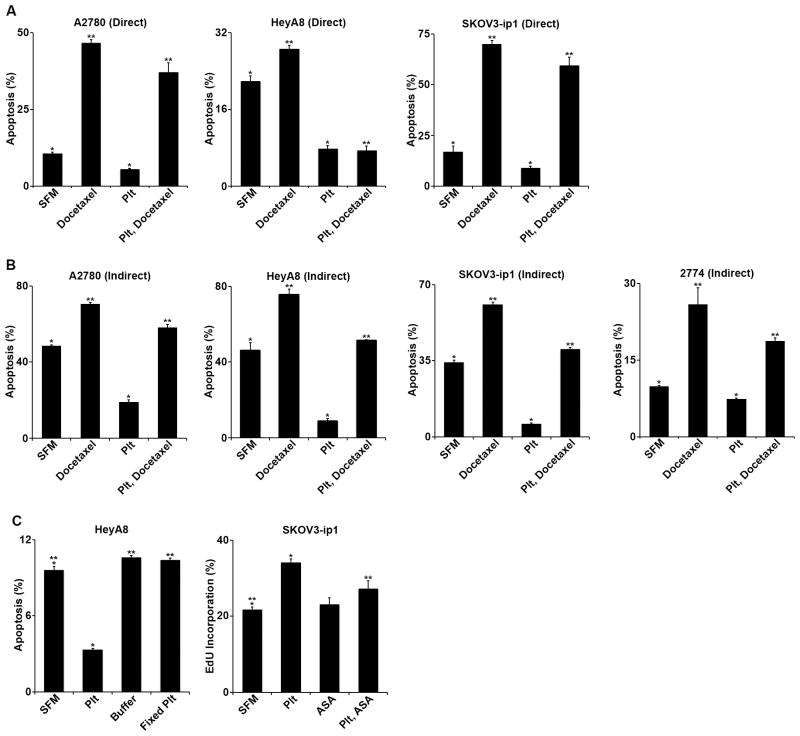
Tissue co-culture with platelets demonstrated consistent protection against apoptosis, both directly and indirectly, and with and without exposure to docetaxel. Platelet activation was evident by the aggregation of platelets within the initial hours of 37°C incubation. Direct incubation of the A2780, HeyA8, and SKOV3-ip1 cells with platelets in serum-free conditions reduced apoptosis 46.7% (p = 0.002), 64.4% (p < 0.001), and 47.3% (p = 0.004) respectively (figure 2A). After incorporating docetaxel, direct incubation of the same cell lines with platelets reduced apoptosis by 20.4% (p = 0.004), 74.0% (p < 0.001), and 15.1% (p = 0.007) respectively (figure 2A). To consider whether direct contact between platelets and tumor cells was required to observe these changes in apoptotic rates, ovarian cancer cells were indirectly incubated with platelets across a barrier with 0.4 μm pores for 72 hours in a serum free environment with and without docetaxel 5 nM. Indirect incubation of A2780, HeyA8, SKOV3-ip1, and 2774 cells with platelets in serum-free conditions reduced apoptosis by 60.8% (p < 0.001), 80.7% (p = 0.001), 82.3% (p < 0.001), and 25.3% (p = 0.002), respectively (figure 2B). After incorporating docetaxel, direct incubation of the same cell lines with platelets reduced apoptosis by 17.4% (p < 0.001), 31.9% (p < 0.001), 33.9% (p < 0.001), and 27.5% (p = 0.03), respectively (figure 2B). These data suggest that platelets have an anti-apoptotic effect on cancer cells, and they suggest that this effect does not require direct contact between platelets and tumor cells.
Figure 2.
A. In vitro, A2780 directly co-cultured with platelets (plt) 10×107/mL +/− docetaxel 5 nM, platelets decreased apoptosis from 10.7% to 5.7% (p = 0.004) compared to serum free media (SFM). With the addition of docetaxel, platelets decreased apoptosis from 46.6% to 37.1% (p = 0.007). HeyA8 directly co-cultured with platelets +/− docetaxel, platelets decreased apoptosis from 21.8% to 7.8% (p < 0.001) compared to serum free media. With the addition of docetaxel, platelets decreased apoptosis from 28.6% to 7.5% (p < 0.001). SKOV3-ip1 directly co-cultured with platelets +/- docetaxel, platelets decreased apoptosis from 17.1% to 9.0% (p = 0.008) compared to serum free media. With the addition of docetaxel, platelets decreased apoptosis from 70.1% to 59.5% (p = 0.013).
B. In vitro, A2780 indirectly co-cultured with platelets +/− docetaxel, platelets decreased apoptosis from 48.5% to 19.0% (p < 0.001) compared to serum free media (SFM). With the addition of docetaxel, platelets decreased apoptosis from 70.4% to 58.2% (p < 0.001). HeyA8 indirectly co-cultured with platelets +/− docetaxel, platelets decreased apoptosis from 46.1% to 8.9% (p < 0.001) compared to serum free media. With the addition of docetaxel, platelets decreased apoptosis from 75.8% to 51.6% (p = 0.001). SKOV3-ip1 indirectly co-cultured with platelets +/− docetaxel, platelets decreased apoptosis from 34.3% to 6.1% (p < 0.001) compared to serum free media. With the addition of docetaxel, platelets decreased apoptosis from 60.8% to 40.2% (p = 0.001). 2774 indirectly co-cultured with platelets +/− docetaxel, platelets decreased apoptosis from 9.9% to 7.4% (p = 0.003) compared to serum free media. With the addition of docetaxel, platelets decreased apoptosis from 25.9% to 18.8% (p = 0.033).
C. In vitro, HeyA8 cells were incubated in SFM, platelets, and buffer from platelet washing after paraformaldehyde fixation, and paraformaldehyde-fixed platelets. Normal platelets decreased apoptosis from 9.6% to 3.3% (p < 0.001). In contrast, platelet fixation had no significant effect on tumor cell apoptosis (10.4%, p = 0.28). SKOV3-ip1 cells were incubated with SFM, platelets, and/or aspirin (ASA) 30 μM. EdU incorporation was used to measure proliferation by flow cytometry. Platelet co-culture increased proliferation from 21.8% to 34.1% (p = 0.004) while aspirin had no effect. When ASA was added to the platelets, the degree of proliferation was decreased to 27.3% (p = 0.22 compared to SFM control).
To determine whether platelet activation was necessary for the apoptosis protection, the above experiments were repeated using platelets fixed with paraformaldehyde. Fixation of platelets abrogated the anti-apoptotic effect (p = 0.28, figure 2C), suggesting that platelet activation is necessary for the anti-apoptotic effects. Platelet co-culture has previously been shown to induce increased tumor cell proliferation, which was abrogated by platelet fixation. (2) Acknowledging that aspirin is a moderate inhibitor of platelet activation that can be overcome by adequate accumulation of adenosine di-phosphate (ADP), and given that ADP accumulates in vitro over time, aspirin pre-treatment of platelets was utilized to block the pro-proliferative effects of platelets in vitro. The cell line SKOV3-ip1 was co-cultured with platelets with and without aspirin 30 μM for 24 hours and evaluated by flow cytometry for EdU incorporation as a proxy for proliferation. As anticipated, platelet co-culture increased proliferation by 56.5% (p = 0.004). Inclusion of aspirin abrogated the effect of platelet co-culture (figure 2C)
Effects of platelets on tumor growth and response to chemotherapy in vivo
All ovarian cancer cell lines utilized here are known to cause increased platelet counts. (1) To simulate the effects of excess platelet volume, allogeneic platelet transfusions were performed. Noting that platelet activation was apparently necessary for the anti-apoptotic effects in vitro, we considered whether in vivo effects from platelet transfusion might be blocked by utilizing aspirin. Nude mice were given IP injections of A2780 cells, and 7 days later, they were randomized to the following treatment groups (n = 10 mice/group): untreated control, IP aspirin, platelet transfusion, and aspirinized platelet transfusion. Platelet transfusion resulted in a 1.9-fold increase in the aggregate mean tumor weight compared to control (p = 0.01; figure 3A). Intraperitoneal aspirin therapy did not have any significant effect on aggregate tumor weight. In contrast, pre-aspirinization of the platelets blocked the pro-growth effect of platelet transfusion (p = 0.01 compared to platelet transfusion; p = NS compared to control; figure 3A).
Figure 3.
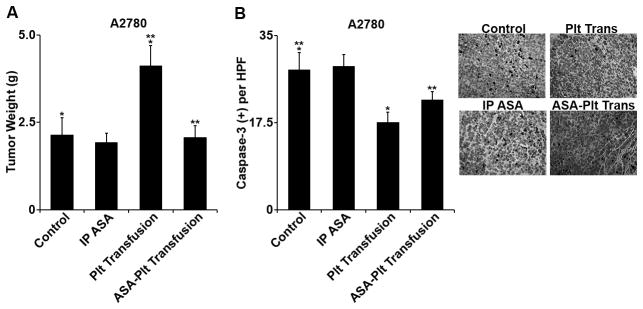
A. In vivo, A2780-bearing nude mice were allocated into the following groups (n = 10): untreated control, IP aspirin, platelet transfusion, and transfusion of aspirinized platelets. Aspirin by itself had no significant effect on mean aggregate tumor weight at necropsy. Platelet transfusion increased mean aggregate tumor weight from 2.1 g to 4.1 g (p = 0.03). Pre-transfusion aspirinization of platelets abrogated the increased tumor growth (2.1 g versus 2.1 g, p = NS).
B. Immunohistochemistry for cleaved caspase-3 demonstrated reduction in apoptosis in tumors of mice receiving platelet transfusion from 28.1/hpf to 17.6/hpf (p = 0.009). Pre-transfusion aspirinization of platelets decreased the reduction of apoptosis to 22.2% (p = 0.11 compared to control). IP ASA had no statistically significant effect on the rate of apoptosis.
In resected tumor specimens, ex vivo immunohistochemistry demonstrated that platelet transfusion resulted in a 37% lower rate of apoptosis (activated caspase-3 immunohistochemistry) compared to control (p = 0.009; figure 3B). Aspirin delivered IP did not significantly change the apoptotic rate in tumor (p = 0.86; figure 3B). In contrast, aspirinizing platelets prior to transfusion blocked the anti-apoptotic effect of platelets on tumor (p = 0.11; figure 3B).
We next studied the effect of platelets on response to taxane-based chemotherapy in vivo by reducing platelet counts using an anti-platelet antibody (APA) that causes non-activating sequestration of circulating platelets and has been previously validated in our laboratory. (1) Seven days following IP injection of A2780 cancer cells, mice were randomized to the following treatment groups: control IgG, APA, control IgG with docetaxel, or APA with docetaxel. After five weeks, mice treated with either APA had 65% decrement in mean aggregate tumor weight compared to control (p = 0.008, figure 4A) that was similar to the 70% decrease that resulted from treatment with docetaxel (p = 0.004, figure 4A). There was no statistical difference between the APA treatment and docetaxel treatment (p = 0.35, figure 4A). By comparison, mice treated with both the APA and docetaxel had an additional 62% reduction in aggregate tumor weight compared to that achieved by docetaxel alone (p = 0.04, figure 4A).
Figure 4.
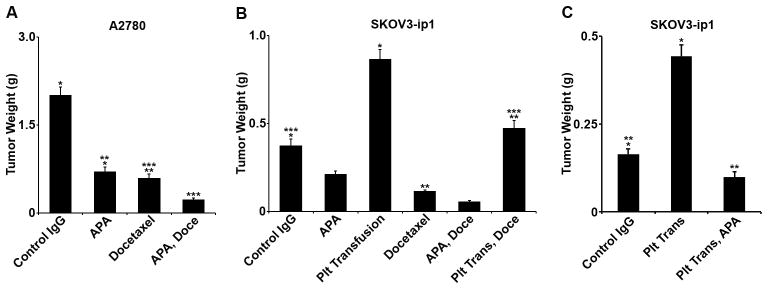
A. In vivo, A2780-bearing nude mice were treated with a control IgG, a platelet-depleting IgG anti-platelet antibody (APA), docetaxel, or a combination of the docetaxel and APA. Animals treated with APA had a 65% decrease in mean aggregate tumor weight compared to control (p = 0.008) that was similar to the 70% decrease that resulted from treatment with docetaxel (p = 0.004 compared to control). There was no statistical difference between the APA treatment and docetaxel treatment (p = 0.35). Mice treated with both the APA and docetaxel had an additional 62% reduction in aggregate tumor weight compared to that achieved by docetaxel alone (p = 0.04).
B. In vivo, SKOV3-ip1-bearing nude mice were treated with control IgG, APA, and docetaxel, and/or platelet transfusion. Platelet depletion with APA resulted in a 43% decrease in mean aggregate tumor weight of borderline significance (p = 0.07). Docetaxel resulted in a similar reduction in mean aggregate tumor weight (69%, p = 0.006). Mice given platelet transfusions had a 2.4-fold increase in mean aggregate tumor weight compared to control (p = 0.01). Compared to mice treated with docetaxel, mice treated with docetaxel and platelet transfusion had a 4-fold increase in mean aggregate tumor weight (p = 0.004). Mice given platelet transfusions and treated with docetaxel had a similar mean aggregate tumor weight to that of untreated controls (p = 0.55). Compared to mice treated with docetaxel, mice treated with APA and docetaxel had a 51% decrease in mean tumor weight (p = 0.02).
C. In vivo, SKOV3-ip1-bearming nude mice were treated with control IgG, platelet transfusion, or platelet transfusion with APA. Platelet transfusion resulted in a 70% increase in mean aggregate tumor weight (p = 0.001) whereas the combination of platelet transfusion with APA resulted in a non-significant 40% decrease in mean aggregate tumor weight compared to control (p = 0.06).
To confirm this finding and consider the effect of platelet transfusion, nude mice were given IP injections of SKOV3-ip1 cells, and after seven days were randomized to the following groups: control IgG, APA, twice weekly platelet transfusion, control IgG with docetaxel, APA with docetaxel, and platelet transfusion with docetaxel. Platelet depletion and docetaxel resulted in similar reductions in tumor size at necropsy (figure 4B). Mice given platelet transfusions had a 2.4-fold increase in mean aggregate tumor weight compared to controls (p = 0.01, figure 4B). Compared to mice treated with docetaxel, treatment with docetaxel and platelet transfusion resulted in a 4-fold increase in mean aggregate tumor weight (p = 0.004, figure 4B). Mice given platelet transfusions and treated with docetaxel had a similar mean aggregate tumor weight to that of untreated controls (p = 0.55, figure 4B). Compared to mice treated with docetaxel, mice treated with APA and docetaxel had a 51% decrease in mean tumor weight (p = 0.02, figure 4B). In a confirmatory experiment using the SKOV3-ip1 model, the animals were randomized to control IgG, twice weekly platelet transfusion, or platelet transfusion with APA. Platelet transfusion resulted in a 70% increase in mean aggregate tumor weight (p = 0.001, figure 4C) whereas the combination of platelet transfusion with APA resulted in a non-significant decrease in mean aggregate tumor weight compared to control (p = 0.06, figure 4C).
DISCUSSION
It is increasingly recognized that there are multiple biological components that participate in a cooperative relationship between the host and tumor cells. Cross-talk between various cell types, including platelets, leukocytes, and endothelial cells, has been shown to participate in the epithelial-to-mesenchymal transition, metastasis, as well as arrest of tumor emboli with the establishment of the metastatic niche. (16, 35) Platelets have been shown to sequester angiogenesis regulators in addition to other mitogens (24) and release these compounds from alpha-granules in a manner that modulates angiogenesis. (36) There is evidence that exposure to anticoagulants decreases platelet release of vascular endothelial growth factor, suggesting anticoagulants may alter the potential of platelets to facilitate angiogenesis. (37)
In a cohort of patients enriched for recurrence of disease, we found that elevated platelet levels correlated with a decreased interval to progression and decreased overall survival. Overall survival as a trial end-point is influenced by therapeutic crossover; therefore it is notable that thrombocytosis correlates with worsened overall survival, suggesting that platelet effects may be agnostic to the types of therapy used. Furthermore, we demonstrated that platelet counts might be useful as a tumor marker, in parallel to CA-125 levels, to follow treatment response and follow in surveillance for recurrence. These data were limited by provider variation in the frequency of both CA-125 and CBC checks. Standardization as well as prospective analysis could allow the development of prospective algorithms to test for the predictive value of platelet response as a biomarker for tumor response.
In breast cancer models, chemotherapy was found to be more effective in the context of thrombocytopenia, and the effect was attributed to intra-tumoral hemorrhage facilitated by leukocytes and deficiency in β-2 or β-3 integrins. (26, 38) Based on our observation in patients with ovarian cancer that elevated platelet counts are associated with higher rates of relapse and lower rates of response to chemotherapy, we hypothesized and confirmed that platelets might confer resistance to apoptosis, including that induced by taxane chemotherapy. Co-incubation resulted in obvious platelet aggregation, and blockade of activation abrogated these effects. Aspirin at least partially blocked the increased tumor cell proliferation attributed to platelet co-culture.
A series of meta-analyses of randomized and case-control studies have indicated a significantly reduced risk of malignancy in individuals treated with low-dose aspirin. (39–42) In our model, platelet transfusion resulted in accelerated tumor growth that was partially blocked by pre-treatment of the platelets with aspirin, however IP administration of aspirin did not have a clear effect. Aspirin is a moderate inhibitor of platelet activation and aggregation, and it is known that other activating stimuli (e.g. shear force, catecholamines, thrombin, and ADP) are capable of activating platelets despite aspirinization through non-thromboxane-dependent mechanisms. (43) The IP dose utilized here may not have been adequate to overcome these mechanisms. Alternatively, it may be the case that aspirin only provides an observable effect above a threshold number of platelets.
The potential impact of platelet transfusion on cancer progression or survival has not been well studied. Concern has been identified that erythropoiesis-simulating agents are associated with decreased tumor progression and survival. (44–46) In this context, some centers are exploring the effects of agents such as romiplostim (a thrombopoietin receptor agonist) to maintain platelets >100 × 109/L in patients being treated with cytotoxic chemotherapy. Limited data report a 15% DVT rate and are not adequate to consider impact on progression and/or survival. (47) Our model would suggest that care should be taken when platelet transfusions are considered and as thrombopoietin receptor agonists are developed to carefully consider the possibility of stimulating tumor growth through the intervention.
We further demonstrated that reduction of platelet counts in vivo reduced tumor growth to the same extent as chemotherapy, and platelet transfusion strongly counter-acted the antitumor effect of chemotherapy. Thrombocytopenia is a common toxicity of front-line chemotherapy, and clinical trials will decline to enroll, delay therapy, or remove patients from protocols based on persistent platelet levels less than 10×105 cell/μL. (48) The effect of relative thrombocytopenia and platelet transfusion on the response to chemotherapies needs to be investigated in a larger number of patients in a controlled setting. If our results are confirmed, the risks of platelet transfusion in a patient population may be greater than previously thought. Further, relative thrombocytopenia may be of therapeutic benefit, and within carefully defined safety parameters, the use of anti-platelet reagents may be considered as chemosensitizers.
Supplementary Material
Supplemental Table 1: Patient Demographics
Supplemental Table 2: Patterns of platelet level changes through treatment and surveillance until recurrence.
TRANSLATIONAL RELEVANCE.
Thrombocytosis is known to correlate with poor clinical outcomes in cancer, is known to be caused by tumor cells, and platelets are known to participate in metastasis. In this work, we show that in patients with recurrent ovarian cancer, elevated platelet levels at diagnosis correlated with decreased interval to progression and decreased overall survival. Changes in platelet levels during and after therapy may be a biomarker for response to that therapy and recurrence. Platelets protect ovarian cancer cells from apoptosis in a manner not requiring direct contact. Platelet transfusion results in increased tumor growth that can be at least partially blocked with aspirin. Further, platelet transfusion decreases the efficacy of taxane-based chemotherapy and platelet depletion increases the efficacy of the same therapy. These models argue for reconsideration of the dangers of platelet transfusion and thrombopoietin receptor agonists as well as the consideration of anti-platelet reagents as chemosensitizers.
Acknowledgments
GRANT SUPPORT
JBM, HJD, RLS, BZ, RP, and EK are supported by NIH T32 Training Grant CA101642. This work was also supported in part by NIH grants (CA177909, P50CA083639, CA109298, P50CA098258, U54CA151668, UH2TR000943, CA016672, U54CA96300, and U54CA96297), CPRIT RP110595 and RP120214, an Ovarian Cancer Research Fund Program Project Development Grant, Department of Defense grants (OC120547 and OC093416), the Betty Ann Asche Murray Distinguished Professorship, the RGK Foundation, the Gilder Foundation, the Judi A. Rees Ovarian Cancer Research Fund, the Chapman Foundation, the Meyer and Ida Gordon Foundation, and the Blanton-Davis Ovarian Cancer Research Program. MH is supported by a Research Fellowship of the Deutsche Forschungsgemeinschaft (DFG). STR DNA fingerprinting was done by the Cancer Center Support Grant-funded Characterized Cell Line core, NCI # CA016672.
Footnotes
CONFLICT OF INTEREST STATEMENT: The authors disclose no potential conflicts of interest.
J.B-M. designed research, performed research, analyzed data, and wrote the paper
H-J.C. designed research, performed research, and analyzed data
H.D. performed research and analyzed data
R.S. designed research, performed research, and analyzed data
M.C. designed research, performed research, and analyzed data
M.H. performed research and analyzed data
A.N. designed research, performed research, and analyzed data
S.P. designed research, performed research, and analyzed data
B.Z. performed research and analyzed data
R.P. performed research and analyzed data
C.P. performed research and analyzed data
W.H. performed research and analyzed data
S.L. performed research and analyzed data
V.A-K. designed research, performed research, analyzed data, and wrote the paper
A.S. designed research, performed research, analyzed data, and wrote the paper
References
- 1.Stone RL, Nick AM, McNeish IA, Balkwill F, Han HD, Bottsford-Miller J, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–8. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–72. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duan Z, Foster R, Bell DA, Mahoney J, Wolak K, Vaidya A, et al. Signal transducers and activators of transcription 3 pathway activation in drug-resistant ovarian cancer. Clin Cancer Res. 2006;12:5055–63. doi: 10.1158/1078-0432.CCR-06-0861. [DOI] [PubMed] [Google Scholar]
- 4.Allensworth SK, 1, Langstraat CL, Martin JR, Lemens MA, McGree ME, Weaver AL, et al. Evaluating the prognostic significance of preoperative thrombocytosis in epithelial ovarian cancer. Gynecol Oncol. 2013;130:499–504. doi: 10.1016/j.ygyno.2013.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen JG, Tran AQ, Rimel BJ, Cass I, Walsh CS, Karlan BY, et al. Thrombocytosis at secondary cytoreduction for recurrent ovarian cancer predicts suboptimal resection and poor survival. Gynecol Oncol. 2014;132:556–9. doi: 10.1016/j.ygyno.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proc Natl Acad Sci USA. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gasic GJ, Gasic TB, Galanti N, Johnson T, Murphy S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int J Cancer. 1973;11:704–18. doi: 10.1002/ijc.2910110322. [DOI] [PubMed] [Google Scholar]
- 8.Hilgard P. The role of blood platelets in experimental metastases. Br J Cancer. 1973;28:429–35. doi: 10.1038/bjc.1973.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearlstein E, Salk PL, Yogeeswaran G, Karpatkin S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proc Natl Acad Sci USA. 1980;77:4336–9. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 11.Karpatkin S, Pearlstein E, Salk PL, Yogeeswaran G. Role of platelets in tumor cell metastases. Ann N Y Acad Sci. 1981;370:101–18. doi: 10.1111/j.1749-6632.1981.tb29726.x. [DOI] [PubMed] [Google Scholar]
- 12.Radomski MW, Jenkins DC, Holmes L, Moncada S. Human colorectal adenocarcinoma cells: differential nitric oxide synthesis determines their ability to aggregate platelets. Cancer Res. 1991;51:6073–8. [PubMed] [Google Scholar]
- 13.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, et al. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci USA. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA. 1998;95:9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, et al. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–41. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–90. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klepfish A, Greco MA, Karpatkin S. Thrombin stimulates melanoma tumor-cell binding to endothelial cells and subendothelial matrix. Int J Cancer. 1993;53:978–82. doi: 10.1002/ijc.2910530620. [DOI] [PubMed] [Google Scholar]
- 18.Cheresh DA, Spiro RC. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987;262:17703–11. [PubMed] [Google Scholar]
- 19.Kramer RH, McDonald KA, Crowley E, Ramos DM, Damsky CH. Melanoma cell adhesion to basement membrane mediated by integrin-related complexes. Cancer Res. 1989;49:393–402. [PubMed] [Google Scholar]
- 20.Roberts DD, Sherwood JA, Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987;104:131–9. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Radziwon-Balicka A, Medina C, O’Driscoll L, Treumann A, Bazou D, Inkielewicz-Stepniak I, et al. Platelets increase survival of adenocarcinoma cells challenged with anticancer drugs: mechanisms and implications for chemoresistance. Br J Pharmacol. 2012;167:787–804. doi: 10.1111/j.1476-5381.2012.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demers M, Ho-Tin-Noé B, Schatzberg D, Yang JJ, Wagner DD. Increased efficacy of breast cancer chemotherapy in thrombocytopenic mice. Cancer Res. 2011;71:1540–9. doi: 10.1158/0008-5472.CAN-10-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, et al. Platelets actively sequester angiogenesis regulators. Blood. 2009;113:2835–42. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Fox L, Klement GL, et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010;85:487–93. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 26.Peterson JE, Zurakowski D, Italiano JE, Jr, Michel LV, Connors S, Oenick M, et al. VEGF, PF4 and PDGF are elevated in platelets of colorectal cancer patients. Angiogenesis. 2012;15:265–73. doi: 10.1007/s10456-012-9259-z. [DOI] [PubMed] [Google Scholar]
- 27.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–33. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etulain J, Negrotto S, Carestia A, Pozner RG, Romaniuk MA, D’Atri LP, et al. Acidosis downregulates platelet haemostatic functions and promotes neutrophil proinflammatory responses mediated by platelets. Thromb Haemost. 2012;107:99–110. doi: 10.1160/TH11-06-0443. [DOI] [PubMed] [Google Scholar]
- 30.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. doi: 10.1160/TH03-07-0440. [DOI] [PubMed] [Google Scholar]
- 31.Downing SR, Klement GL. Isolation and proteomic analysis of platelets by SELDI-TOF MS. Methods Mol Biol. 2012;818:153–70. doi: 10.1007/978-1-61779-418-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasey PA, Jayson GC, Gordon A, Gabra H, Coleman R, Atkinson R, et al. Phase III randomized trial of docetaxel-carboplatin versus paclitaxel-carboplatin as first-line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682–91. doi: 10.1093/jnci/djh323. [DOI] [PubMed] [Google Scholar]
- 33.Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–76. [PubMed] [Google Scholar]
- 34.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–44. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 35.Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2:1091–9. doi: 10.1158/2159-8290.CD-12-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–69. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Battinelli EM, Markens BA, Kulenthirarajan RA, Machlus KR, Flaumenhaft R, Italiano JE., Jr Anticoagulation inhibits tumor cell-mediated release of platelet angiogenic proteins and diminishes platelet angiogenic response. Blood. 2014;123:101–12. doi: 10.1182/blood-2013-02-485011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho-Tin-Noé B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD. Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol. 2009;175:1699–708. doi: 10.2353/ajpath.2009.090460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thun MJ, Jacobs EJ, Patrono C. The role of aspirin in cancer prevention. Nat Rev Clin Oncol. 2012;9:259–67. doi: 10.1038/nrclinonc.2011.199. [DOI] [PubMed] [Google Scholar]
- 40.Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379:1591–601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 41.Rothwell PM, Price JF, Fowkes FG, Zanchetti A, Roncaglioni MC, Tognoni G, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379:1602–12. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 42.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13:518–27. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 43.Folts JD, Schafer AI, Loscalzo J, Willerson JT, Muller JE. A perspective on the potential problems with aspirin as an antithrombotic agent: a comparison of studies in an animal model with clinical trials. J Am Coll Cardiol. 1999;33:295–303. doi: 10.1016/s0735-1097(98)00601-9. [DOI] [PubMed] [Google Scholar]
- 44.Sheikh S, Littlewood TJ. Erythropoiesis-stimulating agents for anemic patients with cancer. Expert Rev Hematol. 2010;3:697–704. doi: 10.1586/ehm.10.64. [DOI] [PubMed] [Google Scholar]
- 45.Morais C, Johnson DW, Vesey DA, Gobe GC. Functional significance of erythropoietin in renal cell carcinoma. BMC Cancer. 2013;13:14. doi: 10.1186/1471-2407-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trost N, Stepisnik T, Berne S, Pucer A, Petan T, Komel R, et al. Recombinant human erythropoietin alters gene expression and stimulates proliferation of MCF-7 breast cancer cells. Radiol Oncol. 2013;47:382–9. doi: 10.2478/raon-2013-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parameswaran R, Lunning M, Mantha S, Devlin S, Hamilton A, Schwartz G, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22:1217–22. doi: 10.1007/s00520-013-2074-2. [DOI] [PubMed] [Google Scholar]
- 48.Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Patient Demographics
Supplemental Table 2: Patterns of platelet level changes through treatment and surveillance until recurrence.