Abstract
Use of community-based participatory research (CBPR) approaches is increasing with the goal of making more meaningful and impactful advances in eliminating cancer-related health disparities. While many reports have espoused its advantages, few investigations have focused on comparing CBPR-oriented recruitment and retention. Consequently, the purpose of this analysis was to report and compare two different CBPR approaches in two cancer prevention studies. We utilized frequencies and chi-squared tests to compare and contrast subject recruitment and retention for two studies that incorporated a randomized, controlled intervention design of a dietary and physical activity intervention among African Americans. One study utilized a de-centralized approach to recruitment in which primary responsibility for recruitment was assigned to the general AA community of various church partners whereas the other incorporated a centralized approach to recruitment in which a single lay community individual was hired as research personnel to lead recruitment and intervention delivery. Both studies performed equally well for both recruitment and retention (75 and 88% recruitment rates and 71 and 66% retention rates) far exceeding those rates traditionally cited for cancer clinical trials (~5%). The de-centralized approach to retention appeared to result in statistically greater retention for the control participants compared to the centralized approach (77 vs 51%, P<0.01). Consequently, both CBPR approaches appeared to greatly enhance recruitment and retention rates of AA populations. We further note lessons learned and challenges to consider for future research opportunities.
Keywords: African American, Recruitment, Retention, Behavioral intervention, Community based participatory research, Cancer prevention
Introduction
Community-based participatory research (CBPR) strategies are a promising way to address cancer-related and other health disparities [1–8] and to find relevant answers to biomedicine’s and public health’s most vexing questions [8,9]. Through the development of authentic partnerships with the target audience and stakeholders, cultural and contextual relevance of recruitment is increased [7]. When integrated into recruitment and retention plans CBPR approaches may improve retention and recruitment as these approaches include feedback from the targeted population regarding ways to approach and inform the community about the proposed study. In the Southeastern United States, African-American (AA) communities are often geographically isolated and characterized by limited health-related resources, factors that hamper the ability of persons to engage in effective cancer prevention behaviors and to seek appropriate health care services [10–14]. Additionally, the South has a long, sordid history of racial oppression and discrimination, a significant and persistent barrier to consider when developing interventions and recruiting and retaining AA participants for intervention research [15–17].
When conducted in collaborations between high-risk communities and academic partners CBPR-based recruitment may address factors of distrust, especially when the target population has experienced negative interactions with “research” [5,6,8,9,18–35]. This is of particular relevance when racial and ethnic minorities, such as AAs, are the focus of recruitment efforts. Integrating a CBPR-based recruitment approach may help AA community members to increase familiarity with research, thus mitigating mistrust of researchers and research experiences [7,22].
In addition to increasing the participation of AA in research trials, CBPR also enhances the retention rates of AA participants [36]. Participant attrition reduces the external validity of research findings and thus it is important determine how various features of research trials impact participant retention rates [37,38]. Features of CBPR and CBPR-based recruitment strategies, such as community engagement in the development and implementation of culturally appropriate interventions and recruitment strategies as well as the involvement of members of the target population in project activities, have been shown to enhance the retention of AAs in health-related research [39,40]. Despite this evidence, no studies have examined the relationship between recruitment strategies and their effects on participant retention rates.
Another benefit of CBPR is that long-neglected health issues can be addressed. Recruitment partnerships with AA churches, a cornerstone of AA culture and heritage, and involvement of local leaders in intervention research may assist with providing trusted information and advice [22,41]. While some of the benefits of CBPR have been documented, few studies have examined the strengths and weakness of different CBPR-based recruitment strategies [42,43]. Examining CBPR-based recruitment approaches will provide insight regarding strategies to increase minority representation in intervention research
We analyzed recruitment data from two large CBPR intervention studies that focused on increasing physical activity (PA) and healthy eating among exclusively AA participants. Therefore, the purpose of this paper is to compare and contrast recruitment and retention for a centralized versus a decentralized CBPR recruitment plan.
Methods
Design
The methodology employed in this paper constitutes a post-hoc analysis of recruitment data from the SISTAS and HEALS studies. As participants were recruited and followed over time to assess attendance at subsequent study assessments, we have incorporated a cohort study design.
De-Centralized and Centralized Approaches Defined
We define “de-centralized” recruitment in which all recruitment of participants into the study and implementation of the intervention are conducted by the partnering site and not study staff. A “centralized” recruitment schema is one in which all participants were recruited by community members hired as full-time study personnel to recruit and implement the intervention. The Healthy Eating and Active Living in the Spirit (HEALS) Study incorporated a “decentralized” recruitment strategy, which relied upon church-based education teams. Church education teams (CETs) were numerous and specific to each church in the study. The other study, Sistas Inspiring Sistas Through Activity and Support (SISTAS), incorporated a “centralized” recruitment strategy. In this case, study personnel remained the same for the duration of recruitment and implementation.
HEALS: A De-centralized Approach
The HEALS Study is a group-randomized controlled trial with two arms, focused on diet, physical activity, and related factors (e.g., obesity). The control arm of the study was waitlisted for the intervention; however, subjects did not receive any education for diet, physical activity and related factors and just attended data collection clinics during the first year of study involvement. The intervention arm was designed collaboratively by the AA faith-based community and researchers to test the effectiveness of a community-designed, family-based dietary and PA intervention aimed at modifying levels of inflammatory markers associated with risk of cancer and a host of other chronic diseases [44]. The trial consisted of a 12-week intervention followed by nine months of “booster sessions” (total intervention time of 1 year). To participate in the research study, individuals had to be at least 30 years old without a cancer history or any condition that would limit participation. The primary outcome was inflammatory markers (high sensitivity C-reactive protein [hsCRP], tumor necrosis factor-alpha [TNF-α], and interleukin [IL]-6), which were assessed (along with other measures) at baseline, 3 months post baseline, and 1-year post baseline. Monetary incentives were provided to participants ($0 at baseline, $15 at 3 months, and $20 at 1-year).
HEALS had a Community Advisory Board (CAB) consisting of 12 members representing participating churches in the catchment area (Columbia, South Carolina [SC] Metropolitan Statistical Area). In some instances, a CAB member also served as a member of a CET for a church. The CAB advised the project on the development, recruitment, implementation, and evaluation of the entire project. It also directed the study team for development of the study logo and all marketing materials.
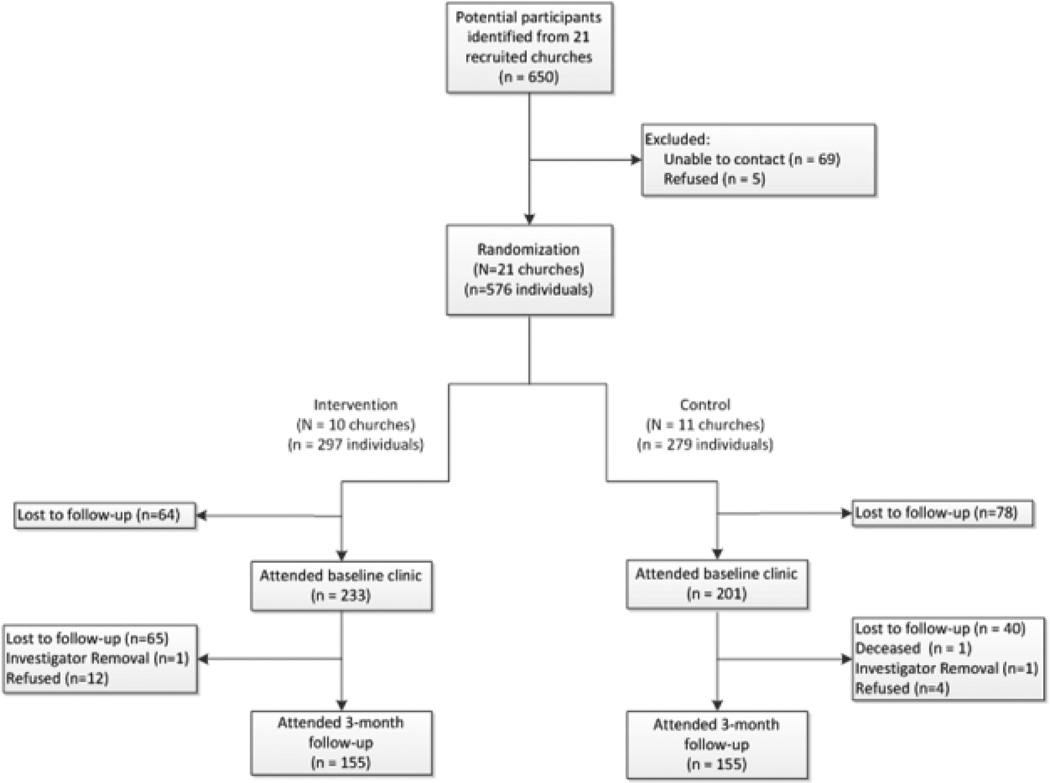
The HEALS Study identified an influential individual interested in health issues and who had a connection to the community. This individual, referred to as a project liaison, connected with the church pastor on behalf of the research project team. Brochures and flyers describing the study were mailed to all interested churches identified by the project liaison. If the pastor was interested, then the research team secured a Memorandum of Agreement (MOA) about the relationships between the research project and the church. Next, the research team made an informational presentation highlighting the specifics of the study to church members at a congregational meeting; after which, participants completed enrollment forms. To assist in further recruitment and project implementation, each pastor named up to three individuals to serve on the CET. CETs recruited additional participants. The unit of randomization for the study was the church (see Figure 1 for the flow diagram). Ultimately, the project liaison recruited churches in three waves (intervention and comparison churches).
Figure 1.
Recruitment flow diagram for HEALS study (2008–2013)
SISTAS: A Centralized Approach
The SISTAS Study was designed as a two-arm, randomized clinical trial of a one-year dietary and PA intervention for breast cancer prevention among AA women. Recruitment was conducted from the general community of AA women aged 30 years or older with no chronic inflammatory conditions or previous cancer diagnoses. Participants were randomized into either an intervention or control arm. As with HEALS, the intervention arm consisted of 12 weekly two-hour classes followed by nine monthly booster sessions for a total period of participation of one year. The control arm participants did not attend any classes but received biweekly correspondence of small participation gifts for the first three months and monthly materials for the following nine months. All participants were scheduled for data collection at three times throughout the duration of participation: baseline, 12-weeks post baseline, and one-year post baseline. Monetary incentives were provided to participants at each timepoint (baseline: $15, 12-week: $15, 1-year: $20). It should be noted that the intervention plan was identical for both SISTAS and HEALS. The primary outcome for the SISTAS trial was inflammation as measured by hsCRP, TNF-α, and IL-2 receptor.
Given that recruitment was conducted in the general community, a community-wide marketing campaign was developed by an 8-member Community Advisory Panel and a 5-member Professional Advisory Panel (composed of members from Florence, SC who had experience working with AA populations). In partnership with advisory panels, we created a study brand which included an acronym (SISTAS), study colors and logo which were used on brochures, flyers, and posters. In addition, recruitment venues were identified by the advisory panels, including churches, employee list serves of local businesses, health fairs, hair salons, support groups, the local chapter of an AA sorority, the local public library, and the mammography clinic of the largest local hospital. The research staff formed partnerships with local AA churches and conducted brief presentation during key church events such as Bible studies or church conferences. A social marketing campaign also was developed and implemented, which included Facebook™, Twitter™ and Instagram™ connected to a project-specific, centralized e-mail address.
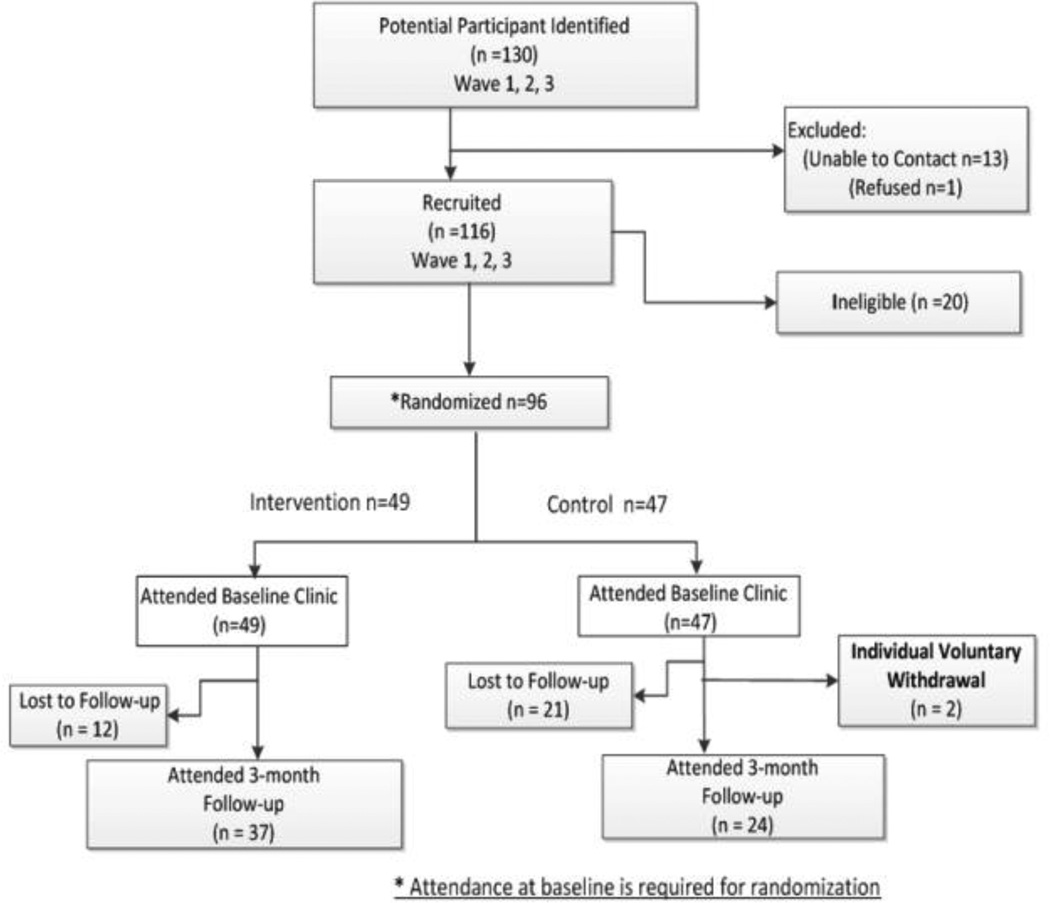
Recruitment was conducted in Florence, SC and its general outlying community approximately one hour from the study management site (Columbia, SC). Subsequently, one full-time and one part-time employee were hired from the general Florence community and maintained study offices in their home. A centralized study coordinator (based in Columbia, SC), oversaw all recruitment activities for the study. The unit of randomization for the study was the individual (see Figure 2 for flow diagram). Because the intervention involved group classes, participants were enrolled in waves consisting of an intervention group and control group. This paper reports on the first three waves of recruitment.
Figure 2.
Recruitment flow diagram for SISTAS study (2010–2015)
Data Sources
Two separate tracking databases were developed in Microsoft Access 2007® to track all subjects contacted and recruited for both studies. These databases are saved on a secure server in separate folders and only key study personnel have access to the databases to ensure security and confidentiality of participants’ information. For SISTAS, all potential participants from the community who contacted study personnel (in person, via telephone, or electronically) were entered into the subject tracking database. For HEALS, participants were recruited by CET members who participated in the study. For both studies, during initial contact, staff completed a screening form to assess pre-eligibility to collect as much information as possible including name, address, phone, date of birth, and other important information. This information was then entered in the study-specific databases. The HEALS and SISTAS databases included fields to track completion of the various aspects of the recruitment and retention process including eligibility screening, material mailings, and clinic scheduling. There also were fields to update all communication attempts and outcomes.
Statistical Methods and Evaluation
This paper reports on three waves of participants for both HEALS and SISTAS who had completed the baseline and the 12-week post baseline clinics (see Figures 1 and 2). For both SISTAS and HEALS, we calculated recruitment rates by dividing the total number of recruited participants by the number of individuals who were eligible, ineligible, and refused, as well as those we were unable to contact. We further calculated attendance rates for the 12-week post baseline visit. Descriptive analyses were performed using SAS analytical software package (version 9.3, Cary, NC)®. Chi-Square tests were used to assess differences in recruitment and retention rates for the two studies.
Results
The overall pool for the HEALS study consisted of 650 potential participants (see Figure 1 and Table 1). From this group, 11% (69/650) could not be contacted for further follow-up on enrollment. In addition, 0.8% (5/650) declined to participate after hearing additional information about the study. From the original 650 potential participants, 89% (n=576) were eligible to enroll in the study and 75% (434/576) of those eligible ultimately attended the first, baseline clinic. Of those attending the baseline clinic, 71% (310/434) returned to the clinic at time point 2 (3 month follow-up). Interestingly, 67% of the intervention arm participants (155/233) attended time point 2 clinic and 77% (155/201) attended from the control arm (see Table 2).
Table 1.
Recruitment proportion comparisons for HEALS (2008–2013) and SISTAS (2010–2013)
| Proportion | HEALS* | SISTAS | p-value |
|---|---|---|---|
| % eligible from total* | 576/650 = 89% | 96/116 = 84% | 0.08 |
| % unable to contact from total* | 69/650 = 11% | 13/130 = 10% | 0.83 |
| % refused from total* | 5/650 = 0.01% | 1/130 = .01% | 0.99 |
| % attended baseline from all eligibles | 434/576 = 75% | 96/109 = 88% | <0.01 |
| % attended TP2** | 310/434 = 71% | 63/96 = 66% | 0.26 |
Denominator numbers may be under-estimated due to de-centralization of initial recruitment which took place within the regular congregational meetings of the church outside of the study purview.
Time point 2 (Three month follow-up)
Heals, Healthy Eating and Active Living in the Spirit; SISTAS, Sistas Inspiring Sistas Through Activity and Support; TP2: time point 2
Table 2.
Retention proportion comparisons for HEALS (2008–2013) and SISTAS (2010–2013) by intervention status.
| HEALS - Intervention |
HEALS - Control |
SISTAS - Intervention |
SISTAS - Control |
p- value** |
p-value† | |
|---|---|---|---|---|---|---|
| % did not attend TP2 | 78 / 233 = 33% | 46 / 201 = 23% | 12/49=24.5% | 23/47 = 45% | 0.22 | <0.01 |
| % attended TP2 from baseline | 155 / 233 = 67% | 155 / 201 = 77% | 37/49 = 75.5% | 24/47 = 51% | ||
| Within study p-value | 0.01 | 0.01 | ||||
TP2 = Time point 2 (three month follow-up)
Comparing intervention arms between studies
Comparing control arms between studies
Heals, Healthy Eating and Active Living in the Spirit; SISTAS, Sistas Inspiring Sistas Through Activity and Support; TP2: time point 2
For the SISTAS study, a total of 130 potential participants were identified (see Figure 2 and Table 1). From this pool, we were unable to contact 10% (13/130) for further follow-up of their participation. Of the original, potential participants that could be assessed for eligibility, 84% (96/116) were enrolled into the study and only 0.8% refused participation (1/130). Of the 130 original contacts, 88% (96/109) attended the baseline clinic to be consented into the study. From this group, 66% (63/96) attended the clinic for time point 2 (3 month follow-up). Upon stratification by intervention arm, 76% (37/49) of those randomized to the intervention group and 51% (24/47) of those randomized to the control group attended time point 2 (see Table 2).
Both studies had a high percentage of eligible participants from the overall pool of interested individuals. However, SISTAS had marginally significantly fewer eligible participants when compared to HEALS participants (84% vs. 89%, p=0.08). Interestingly, SISTAS study participants had significantly higher attendance among those eligible at the baseline data collection clinic compared to the HEALS study (88% vs. 75%, p <0.01). While overall attendance at the second data collection point was not statistically different between the two studies, a significantly greater proportion of control participants in SISTAS attended the clinic compared to HEALS controls (77% vs. 51%, p< 0.01). No statistically significant differences were noted between the intervention participants attendance of the two studies (see Table 2).
Discussion
This investigation confirms that CBPR approaches are highly effective in recruitment of AAs to behavioral studies (88% and 75% in SISTAS and HEALS, respectively). In our examination of the two different CBPR approaches, interesting differences emerged when comparing a ‘de-centralized’ to a ‘centralized’ recruitment infrastructure. A centralized infrastructure (SISTAS) was significantly more likely to retain individuals from initial recruitment until the first baseline clinic (88%). While there were no overall differences in retention between baseline and time point 2, a de-centralized approach (HEALS) was significantly more likely to retain the control subjects than a centralized one.
It is worth noting that both recruitment methods were highly effective at attracting potential AA participants and minimizing refusals (0.8% for both studies). Furthermore, all noted recruitment proportions are much greater than seen in traditional oncology clinical trials (i.e. ≈5%) [45]. Even more traditional general community recruitment proportions for this population yield fairly low percentages (14–41%) [46].
When comparing retention between arms in the same study some fascinating trends emerged in that the de-centralized schema (HEALS) retained a higher proportion of the control participants (77 % vs. 51%, p<0.01). We speculate that the church environment created greater peer influence, perhaps through greater social connection and networking, for control individuals to return to subsequent data collection clinics whereas the SISTAS control participants were isolated from each other. On the other hand, intervention arm retention was equally high in both recruitment schemas (76 vs. 67, p=0.22) possibly due to influence of the class leaders. The slight increase may be explained by the fact that the SISTAS intervention leader remained the same for the duration of all waves and classes. There also was greater maturation and building of group leadership skills over multiple waves that could have occurred with a single instructor that was impossible with multiple CETs.
Lessons learned about RECRUITMENT and RETENTION
We have noted some other points that underscore “best practice” for CBPR recruitment strategies. Participant testimonials, rather than University staff initiating contact all the time, seemed particularly effective to promote recruitment of both churches and individuals for both projects. Having a representative who is a part of the African-American community and/or church provides an innate sense of comfort in their understanding of cultural traditions, shared identity and trust that may not naturally exist with university staff [47]. Updating instructions and reinforcement of program details (procedures, data collection instruments, etc) is helpful in promoting retention. Reminders and helpful guides to participants ensure that they remain engaged and compliant with the complete process. It is imperative to include both-groups-intervention and control. By keeping the participants engaged in the study it allows them to feel a sense of ownership and a feeling that they are really a part of making a difference. We found that asking the participants to assist in the recruitment process by sharing their experience was productive. This also ensures they return for the remaining clinics. Flexibility with data collection times and procedures while costly due to lack of efficiency, can result in more complete data. For both projects we began offering “make-up sessions” for follow-up data collection (3 month follow-up). This gave participants multiple opportunities to take part in follow-up measurements and still receive their incentives (i.e. measurement feedback, monetary, insulated tote). This also adds additional flexibility for participants, especially delayed participants, who may not have that specific day of the week available or may live closer to another host site (as lack of gas/budget/no vehicle are sometimes reasons for not being able to attend their clinic appointment).
CBPR approaches have been espoused as an important way to have sustainability in community initiatives for health promotion after research projects are complete. As these two projects have neared completion, we have encountered the natural outcome of two different recruitment approaches related to sustainability. Since sustainability training was built into the recruitment for HEALS, time and energy was spent up front in assuring that church members were trained in conducting the program. Very little additional investment is needed as the study concludes. Conversely, the SISTAS study has required the development of sustainability plan now that the study is concluding. The SISTAS sustainability plan has been developed by the community advisory panels and involves training of interested people so that the health program could continue as well locating “community repositories” of participant education materials for public use.
Challenges Related to RECRUITMNET and RETENTION
As often happens in this resource-scarce funding environment, understanding the differences in geographical areas and the resources needed in order to recruit properly is critical to meeting recruitment goals. Things that may have worked in past studies may not work now. For the SISTAS project, brochures, flyers and health fairs were critical tools in recruitment for the Florence, SC community, but in the Columbia, SC community social network was a great asset in the recruitment process. Our Columbia community appeared to have a larger “technology-comfortable population”, thus it was feasible to send out text messages or Facebook notes (although it’s a more informal approach it was been more productive than personal phone calls), in the Florence community participants preferred to receive calls. While we attended health fairs; our recruitment consisted of word-of-mouth, family and friends, church members and social network. All of these venues are costly, both financially and from personnel investment so that the more complete the knowledge is of which strategies work best in which environment helps to ensure that resources are used efficiently.
Data collection tools, which may be specifically chosen by a community advisory panel, do not always yield the return that was anticipated. In the HEALS project, the church education teams and staff commented that it was challenging to get participants to complete the length dietary and physical activity assessments which were self-administered. Consequently, the community advisory panel and the research team of SISTAS decided to collect this same data via interviewer-administered phone calls. Unfortunately, low completion of data collection was still problematic in the SISTAS study. In solution-seeking brainstorming sessions, it was revealed that participants did not completely understand the time commitment of the calls (e.g. were not prepared for a 45 minutes phone call) and felt disconnected from the dietary specialists who were required to administer the telephone calls. Best scientific practice, dictates that phone-administered dietary and physical activity recall interviews are conducted by blinded and highly trained registered dieticians. Many participants were expecting their intervention leader or staff that they had seen at the study clinics to do follow-up calls because of the rapport built during the multiple study-driven interactions. They felt “let down” when an unknown stranger contacted them.
Database access on teams with multiple members can often be problematic. In the past, our projects have been best served by having a single database in which all participant contacts and responses were cataloged from the initial recruitment contact to the conclusion of the study. Our teams, which consisted of 5 to 8 members at any one time, often were delayed in tracking participant information because another team member was currently utilizing the database for another participant. Thus, having a separate database would have helped to cut down on any single staff person monopolizing the database during high call volumes. While having staff revised considerable overlap in staff training and responsibilities, we also found that it could lead to some challenges. We found that it was useful to have staff members who were designated specifically to administer technical assistance calls (TAs). Having one designated person will help in the future to streamline calls: so that multiple staff members are not making multiple calls to one participant about scheduling clinic appointment time; reminder calls about intervention class; confirming/updating mailing address, email, or best contact number; and completing any missing information on assessment forms after the clinic.
Another challenge to conducting the study was the large number of “missed” appointments. In spite of multiple reminder phone calls just before participants needed to arrive, we had a high proportion of individuals who never reported for their data collection measures. In a centralized recruiting mechanism, participants might never have to see the research staff in the future. Thus, it was felt that participants were not concerned about having to “maintain face” after missing an appointment. In a de-centralized schema, it was the church teams who were making appointments for data collection, so participants might have felt that they some modest level of “accountability” for missed appointments. This is especially critical in Southern culture where “politeness” is emphasized and valued.
Conclusions
CBPR approaches hold great promise for increasing relevance to increase the likelihood of answering important public health questions and to improve the prospects for reducing cancer- related and other health disparities. The effectiveness of CBPR initiatives can be maximized by the strategic adoption of recruitment strategies. The strengths of centralized and de-centralized recruitment vary by the community context and the phase of the study. An examination of these and other CBPR recruitment strategies has the potential to enhance both CBPR and investigator-driven research trials.
Acknowledgments
Study Acknowledgments
We wish to acknowledge the contributions of Ms. Ernie Weisner and Ms. Kendrea Knight to participant or church recruitment. The authors also wish to acknowledge the community partners who made significant contributions to the conduct of the study including Maureen Byrd, McLeod Hospital, Cumberland United Methodist Church, Mt. Zion African Methodist Episcopal Church, Monumental Baptist Church, Majority Missionary Baptist Church, Trinity Baptist Church, Levi Park Community Center, and Florence County Parks and Recreation.
Funding Acknowledgments
Funding was provided by the National Cancer Institute, National Institute on Minority Health and Health Disparities (NIMHD) for the Healthy Eating and Active Living in the Spirit (HEALS) study [R24 MD002769 Hebert, JR (PI)]. The Sistas Inspiring Sistas Through Activity and Support (SISTAS) was funded by the National Cancer Institute for the South Carolina Cancer Disparities Community Network-II [U54CA153461 Hébert, JR (PI)]. Dr. Hébert also was supported by an Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute [K05 CA136975 Hébert, JR (PI)]. Dr. Wirth’s participation was supported through an ASPIRE-II Grant from the University of South Carolina, Office of Research and by the South Carolina Cancer Prevention and Control Research Network funded under Cooperative Agreement Number 3U48DP001936-01 from the Centers for Disease Control and Prevention and the National Cancer Institute. Ms. Farr’s participation was supported by a Research Supplement to Promote Diversity in Health Related Research from the Center to Reduce Cancer Health Disparities of the National Cancer Institute (3U54CA153461-04S2 PI: Hebert/PD: Farr). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest
All authors do not have conflicts of interest.
Contributor Information
Swann Arp Adams, Associate Professor, College of Nursing, Department of Epidemiology & Biostatistics, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 1601 Greene Street, Williams Brice Bldg, Rm 618, Columbia, SC 29208, USA, swann.adams@sc.edu, Ph: 803-777-7635.
Sue P. Heiney, Dunn-Shealy Professor of Nursing, College of Nursing, University of South Carolina, 1601 Greene Street, Columbia, SC 29208, USA, heineys@mailbox.sc.edu.
Heather M. Brandt, Associate Professor, Department of Health Promotion, Education, and Behavior, Core Faculty, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA, hbrandt@sc.edu.
Michael D. Wirth, Research Assistant Professor, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA, wirthm@mailbox.sc.edu.
Samira Khan, Research Associate\Data manager Cancer Prevention and Control Program Arnold School of Public Health University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA khans@mailbox.sc.edu.
Hiluv Johnson, Project Coordinator, South Carolina Cancer Disparities Community Network II, Arnold School of Public Health, Cancer Prevention and Control Program, 915 Greene Street, Columbia, SC 29208, USA, hsjohnso@mailbox.sc.edu.
Lisa Davis, HEALS Program Coordinator, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA, ldavis@mailbox.sc.edu.
Cassandra M. Wineglass, SISTAS Program Coordinator I Cancer Prevention and Control Program Arnold School of Public Health University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA cwinegla@mailbox.sc.edu.
Tatiana Y. Warren-Jones, Department of Exercise Science, Arnold School of Public Health, Public Health Research Center, University of South Carolina, 921 Assembly Street-318A, Columbia, SC 29208, USA, warrenty@mailbox.sc.edu.
Tisha M. Felder, Research Assistant Professor, College of Nursing, Cancer Prevention & Control Program, Arnold School of Public Health, University of South Carolina, 1601 Greene Street, Columbia, SC 29208, USA, feldert@mailbox.sc.edu.
Ruby F. Drayton, Field Coordinator, Community Clinical Trials Team Cancer Prevention and Control Program Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA, draytonr@mailbox.sc.edu.
Briana Davis, HEALS Intervention Coordinator, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia, SC 29208, USA, brianad@mailbox.sc.edu.
Deeonna E. Farr, Department of Health Promotion, Education, and Behavior, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Columbia SC 29208, USA, farrde@email.sc.edu.
James R. Hébert, Health Sciences Distinguished Professor, Carolina Trustees Professor, Department of Epidemiology and Biostatistics, Director, Statewide Cancer Prevention & Control Program, Cancer Prevention and Control Program, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Suite 241-2, Columbia, SC 29208, USA, jhebert@sc.edu.
References
- 1.Freeman HP. Commentary on the meaning of race in science and society. Cancer Epidemiology, Biomarkers & Prevention. 2003;12:232s–236s. [PubMed] [Google Scholar]
- 2.Freeman HP. Poverty, culture, and social injustice: determinants of cancer disparities. CA: A Cancer Journal for Clinicians. 2004;54:72–77. doi: 10.3322/canjclin.54.2.72. [DOI] [PubMed] [Google Scholar]
- 3.Freeman HP, Chu K. Determinants of cancer disparities: barriers to cancer screening, diagnosis, and treatment. Surgical Oncology Clinics of North America. 2005;14:655–669. doi: 10.1016/j.soc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Kerner J, Guirguis-Blake J, Hennessy K, et al. Translating research into improved outcomes in comprehensive cancer control. Cancer Causes & Control. 2005;16:27–40. doi: 10.1007/s10552-005-0488-y. [DOI] [PubMed] [Google Scholar]
- 5.Minkler M, Wallerstein N. MMNW . Community-based participatory research for health. San Francisco, CA: Josey-Bass; 2003. [Google Scholar]
- 6.Viswanathan M, Ammerman A, Eng E, et al. Community-based participatory research: Assessing the evidence. Rockville, MD: Agency for Healthcare Research and Quality; 2004. AHRQ Pub. No. 04-E022-1 AHRQ Pub. No. 04-E022-1. [PMC free article] [PubMed] [Google Scholar]
- 7.Wallerstein N, Duran B. Community-based participatory research contributions to intervention research: the intersection of science and practice to improve health equity. American Journal of Public Health. 2010;100:S40–S46. doi: 10.2105/AJPH.2009.184036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braun KL, Nguyen TT, Tanjasiri SP, et al. Operationalization of Community-Based Participatory Research Principles: Assessment of the National Cancer Institute's Community Network Programs. American Journal of Public Health. 2011;102(6):1195–1203. doi: 10.2105/AJPH.2011.300304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hebert JR, Brandt HM, Armstead CA, Adams SA, Steck SE. Interdisciplinary, translational, and community-based participatory research: finding a common language to improve cancer research. Cancer Epidemiology & Biomarkers Prevention. 2009;18:1213–1217. doi: 10.1158/1055-9965.EPI-08-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey MM, Call KT, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? American Journal of Preventive Medicine. 2001;21:182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 11.Moscovice I, Rosenblatt R. Quality-of-care challenges for rural health. Journal of Rural Health. 2000;16:168–176. doi: 10.1111/j.1748-0361.2000.tb00451.x. [DOI] [PubMed] [Google Scholar]
- 12.Nemet GF, Bailey AJ. Distance and health care utilization among the rural elderly. Social Science & Medicine. 2000;50:1197–1208. doi: 10.1016/s0277-9536(99)00365-2. [DOI] [PubMed] [Google Scholar]
- 13.Bronstein JM, Morrisey MA. Determinants of rural travel distance for obstetrics care. Medical Care. 1990;28:853–865. doi: 10.1097/00005650-199009000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Coughlin SS, Thompson TD, Seeff L, Richards T, Stallings F. Breast, cervical, and colorectal carcinoma screening in a demographically defined region of the southern U.S. Cancer. 2002;95:2211–2222. doi: 10.1002/cncr.10933. [DOI] [PubMed] [Google Scholar]
- 15.Gamble VN. A legacy of distrust: African Americans and medical research. American Journal of Preventive Medicine. 1993;9:35–38. [PubMed] [Google Scholar]
- 16.Gamble VN. Under the shadow of Tuskegee: African Americans and health care. American Journal of Public Health. 1997;87:1773–1778. doi: 10.2105/ajph.87.11.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scharff DP, Mathews KJ, Jackson P, et al. More than Tuskegee: understanding mistrust about research participation. Journal of Health Care for the Poor and Underserved. 2010;21:879–897. doi: 10.1353/hpu.0.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dancy BL, Wilbur J, Talashek M, Bonner G, Barnes-Boyd C. Community-based research: barriers to recruitment of African Americans. Nursing Outlook. 2004;52:234–240. doi: 10.1016/j.outlook.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 19.Wallerstein NB, Duran B. Using community-based participatory research to address health disparities. Health Promotion Practice. 2006;7:312–323. doi: 10.1177/1524839906289376. [DOI] [PubMed] [Google Scholar]
- 20.Wallerstein N. Commentary: challenges for the field in overcoming disparities through a CBPR approach. Ethnicity and Disease. 2006;16:S146–S148. [PubMed] [Google Scholar]
- 21.Cashman SB, Adeky S, Allen AJ, et al. The power and the promise: working with communities to analyze data, interpret findings, and get to outcomes. American Journal of Public Health. 2008;98:1407–1417. doi: 10.2105/AJPH.2007.113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher T, Burnet D, Huang E, Chin M, Cagney K. Cultural leverage: interventions using culture to narrow racial disparities in health care. Medical Care Research and Review. 2007;64:243S–282S. doi: 10.1177/1077558707305414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman DB, Young VM, Freedman DA, et al. Reducing cancer disparities through innovative partnerships: a collaboration of the South Carolina cancer prevention and control research network and federally qualified health centers. Journal of Cancer Education. 2012;27:59–61. doi: 10.1007/s13187-011-0272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman DA, Whiteside YO, Brandt HM, et al. Assessing readiness for establishing a farmers' market at a community health center. Journal of Community Health. 2012;37:80–88. doi: 10.1007/s10900-011-9419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scarinci I, Garcia FAR, Kobetz E, et al. Cervical Cancer Prevention: New Tools and Old Barriers. Cancer. 2010;116:2531–2542. doi: 10.1002/cncr.25065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams SA, Fleming A, Brandt HM, et al. Racial disparities in cervical cancer mortality in an African American and European American cohort in South Carolina. Journal of South Carolina Medical Association. 2009;105:237–244. [PMC free article] [PubMed] [Google Scholar]
- 27.Wright MS, Brandt HM, Corwin SJ, Friedman D, Hebert JR. Assessing Psychosocial, Cultural, and System-Level Barriers to Colorectal Cancer Screening Among African Americans. Columbia: University of South Carolina, Arnold School of Public Health; 2008. [Google Scholar]
- 28.Wilcox S, Sharpe PA, Parra-Medina D, Granner M, Hutto B. A randomized trial of a diet and exercise intervention for overweight and obese women from economically disadvantaged neighborhoods: Sisters Taking Action for Real Success (STARS) Contempory Clinical Trials. 2011;32:931–945. doi: 10.1016/j.cct.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharpe PA, Wilcox S, Rooney LJ, et al. Adherence to accelerometer protocols among women from economically disadvantaged neighborhoods. Journal of Physical Acttivity & Health. 2011;8:699–706. doi: 10.1123/jpah.8.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bynum SA, Brandt HM, Sharpe PA, Williams MS, Kerr JC. Working to close the gap: identifying predictors of HPV vaccine uptake among young African American women. Journal of Health Care for the Poor and Underserved. 2011;22:549–561. doi: 10.1353/hpu.2011.0060. [DOI] [PubMed] [Google Scholar]
- 31.Bynum SA, Brandt HM, Annang L, et al. Do health beliefs, health care system distrust, and racial pride influence HPV vaccine acceptability among African American college females? [Published online before print July 8, 2011];Journal of Health Psychology. 2011 doi: 10.1177/1359105311412833. [DOI] [PubMed] [Google Scholar]
- 32.Sharpe PA, Burroughs EL, Granner ML, et al. Impact of a community-based prevention marketing intervention to promote physical activity among middle-aged women. Health Education & Behavior. 2010;37:403–423. doi: 10.1177/1090198109341929. [DOI] [PubMed] [Google Scholar]
- 33.Peck LE, Sharpe PA, Burroughs EL, Granner ML. Recruitment strategies and costs for a community-based physical activity program. Health Promotion Practice. 2008;9:191–198. doi: 10.1177/1524839906292819. [DOI] [PubMed] [Google Scholar]
- 34.Ureda JR, Byrd TL, Calderon-Mora JA, et al. The use of illustrated story mapping to enhance focus group discussion. Health Promotion Practice. 2011;12:74–78. doi: 10.1177/1524839909341027. [DOI] [PubMed] [Google Scholar]
- 35.McFall SL, Ureda J, Byrd TL, Valdes A, Morales P, et al. What is needed for informed decisions about prostate cancer screening: perspectives of African-American and Hispanic men. Health Education Research. 2009;24:280–291. doi: 10.1093/her/cyn018. [DOI] [PubMed] [Google Scholar]
- 36.De Las Nueces D, Hacker K, DiGirolamo A, Hicks LS. A systematic review of community-based participatory research to enhance clinical trials in racial and ethnic minority groups. Health Services Research. 2012;47:1363–1386. doi: 10.1111/j.1475-6773.2012.01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shadish W. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton Mifflin; 2001. [Google Scholar]
- 38.Greiner KA, Friedman DB, Adams SA, et al. Effective recruitment strategies and community-based participatory research: community networks program centers' recruitment in cancer prevention studies. Cancer Epidemiology, Biomarkers & Prevention. 2014;23:416–423. doi: 10.1158/1055-9965.EPI-13-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yancey AK, Ortega AN, Kumanyika SK. Effective recruitment and retention of minority research participants. Annual Review of Public Health. 2006;27:1–28. doi: 10.1146/annurev.publhealth.27.021405.102113. [DOI] [PubMed] [Google Scholar]
- 40.Burns D, Soward ACM, Skelly AH, Leeman J, Carlson J. Effective recruitment and retention strategies for older members of rural minorities. Diabetes Educator. 2008;34:1045–1052. doi: 10.1177/0145721708325764. [DOI] [PubMed] [Google Scholar]
- 41.Schlueter DF, Thompson WW, Mason TA, Rayton M, Arriola KJ. A qualitative evaluation of the Avon Foundation Community Education and Outreach Initiative Patient Navigation Program. Journal of Cancer Education. 2010;25:571–576. doi: 10.1007/s13187-010-0073-2. [DOI] [PubMed] [Google Scholar]
- 42.Larkey LK, Gonzalez JA, Mar LE, Glantz N. Latina recruitment for cancer prevention education via Community Based Participatory Research strategies. Contemporary Clinical Trials. 2009;30:47–54. doi: 10.1016/j.cct.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 43.Horowitz CR, Brenner BL, Lachapelle S, Amara DA, Arniella G. Effective recruitment of minority populations through community-led strategies. American Journal of Preventive Medicine. 2009;37:S195–S200. doi: 10.1016/j.amepre.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebert JR, Wirth M, Davis L, et al. C-reactive protein levels in African Americans: a diet and lifestyle randomized community trial. American Journal of Preventive Medicine. 2013;45:430–440. doi: 10.1016/j.amepre.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Doctors, patients face different barriers to clinical trials. [Accessed 1-18-08, 2008];2001 :1–3. Available at: http://www.cancer.gov/clinicaltrials/developments/doctors-barriers0401.
- 46.Motzer SA, Moseley JR, Lewis FM. Recruitment and retention of families in clinical trials with longitudinal designs. Western Journal of Nursing Research. 1997;19:314–333. doi: 10.1177/019394599701900304. [DOI] [PubMed] [Google Scholar]
- 47.Corbie-Smith G, Goldmon M, Isler MR, et al. Partnerships in health disparities research and the roles of pastors of black churches: potential conflict, synergy, and expectations. Journal of the National Medical Association. 2010;102(9):823–831. doi: 10.1016/s0027-9684(15)30680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]