Abstract
BACKGROUND
This study investigated the dynamics of cognitive control instability in methamphetamine (MA) abuse, as well its relationship to substance-induced psychiatric symptoms and drug use patterns.
METHODS
We used an ex-Gaussian reaction time (RT) distribution to examine intraindividual variability (IIV) and excessively long RTs (tau) in an individual’s RT on a Stroop task in 30 currently drug-abstinent (3 months to 2 years) MA abusers compared with 27 nonsubstance-abusing control subjects. All subjects underwent functional magnetic resonance imaging while performing the Stroop task, which allowed us to measure the relationship between IIV and tau to functional brain activity.
RESULTS
Elevated IIV in the MA compared with the control group did not reach significance; however, when the MA group was divided into those subjects who had experienced MA-induced psychosis (MAP+) (n = 19) and those who had not (n = 11), the MAP+ group had higher average IIV compared with the other groups (p < .03). In addition, although control subjects displayed a relationship between IIV and conflict-related brain activity in bilateral prefrontal cortex such that increased IIV was associated with increased activity, the MAP+ group displayed this relationship in right prefrontal cortex only, perhaps reflecting elevated vigilance in the MAP+ group. Greater IIV did not correlate with severity of use or months MA abstinent. No group differences emerged in tau values.
CONCLUSIONS
These results suggest increased cognitive instability in those MA-dependent subjects who had experienced MA-induced psychosis.
Keywords: Cognitive Control, fMRI, Methamphetamine, Psychosis, RT Variability, Stroop
A subset of individuals who chronically abuse methamphetamine (MA) also develop severe psychotic symptoms that are associated with high levels of psychiatric hospitalization and serious social dysfunction (1-5). Premorbid risk factors for MA psychosis include a familial history of psychiatric illness and age of first MA use (6,7), as well as abnormal measures of cognitive function early in development (8). Approximately 60% of MA abusers report a history of paranoia, delusions, and hallucinations while under the influence of the drug (2,4,8-10), with MA capable of triggering psychotic episodes in individuals with no history of psychiatric illness. (11). Early symptom descriptions of MA psychosis that hold true today include the following: 1) paranoid delusions with ideas of reference; 2) persecutory delusions; and 3) auditory and visual hallucinations that occur in a state of clear consciousness (12). The highly addictive nature of MA, as well as the ability to produce psychotic symptoms, make this drug a major public health concern (13).
Impaired attention has long been considered a marker for developing psychotic illnesses, including schizophrenia (14-18) and bipolar disorder (19-23). Sustained attention and cognitive control impairments have consistently been implicated across psychiatric disorders (24-26). Cognitive control is defined as a set of cognitive and neural operations linked to goal-driven behavior and is associated with brain regions such as the dorsolateral prefrontal cortex, anterior cingulate cortex, and the inferior parietal lobes (27-30). Disrupted cognitive control has been shown across addictive disorders (31-35) with concomitant reductions in frontal, cingulate, and parietal brain function (36-39). In the context of addiction, cognitive control can be interpreted as the inhibition of a prepotent response (e.g., habitual drug use) to carry out behaviors associated with long-term rewards and positive outcomes (e.g., abstaining from drug use).
Cognitive paradigms, such as the Stroop paradigm, have long been used to evaluate the integrity of cognitive control processes in a variety of clinical disorders (24,40). Traditionally, the number of correct responses and number of differing reaction times (RT) across different conditions, such as the transition from congruent to incongruent stimuli in the Stroop, have been used as a measure of differing attention and control demands. Incongruent stimuli introduce competing information that taxes top-down attention control by increased demands on selective attention and working memory due to interference between competing task-relevant and taskirrelevant information (i.e., word reading versus color naming) and by activating two competing motor responses and generating response conflict (response to the word meaning versus the color ink). However, behavioral measures such as the number of correct responses and RT to different stimulus classes may stem from a variety of impairments from perceptual problems to problems with top-down control through to response preparation and response selection impairments. More recently, intraindividual RT variability (IIV) has been employed to tease apart different aspects of cognitive impairment in clinical groups. Highly variable and/or unusually long RTs (compared with mean RT to other items in a similar stimulus class) have been associated with lapses in attention or cognitive control [e.g., (41-43)]. High levels of RT variability have been associated with an inability to effectively engage cognitive control in situations of increased cognitive demands (44), sustained attention impairments (45,46), and problems in regulating energetic states (47-50). Cognitive control may bias attention in favor of task-related stimuli and reduce attention to nontask-related stimuli or thoughts, resulting in fairly homogenous RTs, whereas poor attention control allows taskirrelevant information to interfere with performance, resulting in highly variable RT. Increased RT variability has been noted in clinical disorders associated with cognitive impairments, including attention-deficit/hyperactivity disorder (51-54), autism (55), traumatic brain injury (56-59), Alzheimer’s dementia (60,61), frontal lobe damage (62), schizophrenia (63,64), and bipolar disorder with psychotic symptoms (19).
Traditional methods of measuring RT variability have recently been argued to be somewhat flawed. This is because RT distributions are not best represented by the normal curve but instead contain a right-sided tail that captures numbers of abnormally long RT. The values within this tail can skew the RT distribution, making the mean RT appear to be longer than its true value. Furthermore, the use of standard measures of RT variability in research with clinical groups may be problematic as the standard deviation of RT is highly correlated with the mean RT (65-67), which often differs significantly in clinical groups. The ex-Gaussian distribution can be decomposed into the summation of two independent components: a symmetric Gaussian component and an exponential component that captures the right-sided skew in RT data. The model permits the measurement of the mean (mu) and standard deviation (sigma) of the normal (Gaussian) component, as well as the mean and standard deviation (tau) of the exponential component. Tau is often considered to represent trials on which lapses in attention have occurred (46,68,69), whereas the standard deviation of the normal component is considered to capture response preparation/execution problems (44,70-72).
Increased RT variability has been associated with poor performance on cognitive control tasks, such as response inhibition tasks (44,73,74), as well as with increased number of omission errors to Go trials. Hyperactivity in brain regions associated with cognitive control has been linked to RT variability in healthy control subjects (44,74). The authors interpreted increased RT variability as reflecting a lack of consistency of topdown cognitive control (44) or an overreliance on higher order processes when more successful performance was associated with more automatic response patterns (74). In a recent study of RT variability and attention lapsing in a group of subjects with schizophrenia (41), we found evidence that long RTs in the Stroop paradigm were associated with increased engagement of the cognitive control network, including bilateral dorsolateral prefrontal cortex, anterior cingulate, and parietal cortex. We also found that the schizophrenia group engaged these cognitive control regions to a lesser degree than control subjects.
The goal of the present study was to investigate the dynamics of cognitive control instability in MA abuse using the Stroop task during functional magnetic resonance imaging. We sought to examine both IIV, as well as trials with uncharacteristically long RT, as measured by tau, via the ex-Gaussian distribution of RT. We hypothesized that MAdependent subjects would display both increased IIV and increased tau compared with control subjects, reflecting lapses in cognitive control. Guided by previous imaging studies of brain activity associated with RT variability (44,74), we predicted that across groups, trials with elevated IIV and tau would be associated with increased activity in prefrontal cortex (PFC) due to increased engagement of control during these trials. We also hypothesized that this engagement of cognitive control related activity would be attenuated in MAdependent individuals. As elevated RT variability has been associated with psychosis (19,63,64,75,76), we sought to examine if RT patterns were correlated with MA-induced psychiatric symptoms (i.e., psychosis) as well as drug use patterns.
METHODS AND MATERIALS
Subjects
Two groups were studied: 30 MA-abusing subjects and 27 nonsubstance-abusing control subjects. Neuroimaging data from this cohort have been previously published, but the analyses employed in the current study are novel and have never been previously reported (77). The MA abusers met DSM-IV criteria for lifetime MA dependence determined from the Structured Clinical Interview for DSM (SCID) (78) but were currently drug abstinent for a minimum of 3 weeks. All MA subjects were interviewed using the Methamphetamine Experience Questionnaire (MEQ), which is an interview based on the Cocaine Experience Questionnaire (79,80). The MEQ is designed to assess the lifetime frequency of psychotic episodes associated with MA use and conditions in which psychotic episodes occur, as well as the persistence of these symptoms. In this study, the MEQ was administered in conjunction with the SCID to determine the presence or absence of MA-induced paranoia. Nineteen of the MA subjects reported lifetime psychotic symptoms associated with MA abuse and 11 reported no psychosis (Table 1).
Table 1.
Demographic and Clinical Characteristics of 30 Methamphetamine Abusers and 27 Control Subjects
| Methamphetamine Abusers (n =30) | Control Subjects (n = 27) | |
|---|---|---|
| Demographic Variables | ||
| Age, years, mean (SD) | 35.5 (7.9)a | 28.5 (7.2) |
| Range | 21 to 48 years | 20 to 47 years |
| Female subjects | 15 | 11 |
| Subject’s education, years, mean (SEM) | 12.5 (1.6)a | 15.4 (1.2) |
| Parental education, years, mean (SEM) | 13.1 (2.4) | 14.7 (2.0) |
| NART | 108.2 (4.8)a | 111.9 (4.8) |
| Clinical Variables | ||
| Methamphetamine use | – | |
| Duration, years, mean (SD) | 14.0 (6.4) | – |
| Range | 4 to 28 years | |
| Months abstinent, mean (SD) | 13.7 (15.4) | – |
| Range | 2 to 60 months | |
| Age of first MA use, years, mean (SD) | 17.7 (4.4) | – |
| Mean daily MA dosage (grams) | 1.3 (.85) | – |
| History of cannabis abuse | 24 | – |
| Age of first cannabis use, years, mean | 15.0 (3.8) |
NART, National Adult Reading Test; MA, methamphetamine.
Significantly different from control group.
Procedure
Stroop Stimuli
A computerized single-trial version of the Stroop color-word task was administered to all subjects in the scanner. Three colors were employed in this experiment: red, green, and blue. The conflict stimuli (i.e., incongruent trials [I]) were created by printing each of the three color names in the two other ink colors. The nonconflict stimuli (i.e., congruent stimuli [C]) were created by printing each of the three color names in its own color. Stimuli were back-projected on a screen that could be viewed by the subjects through a mirror on the head coil.
Task Procedure
Subjects were instructed to respond to the color ink of colored words that were either congruent or incongruent by depressing a button on a response device attached to their dominant hand. Each button was mapped to a specific color (red button, green button, or blue button). Stimuli were presented for 1500 milliseconds with a fixed intertrial interval of 2500 milliseconds. Both speed and accuracy of responses were emphasized. To increase the level of conflict elicited by incongruent trials as well as maintain error rates, 70% of trials were nonconflict trials (i.e., C) and 30% of the trials were conflict trials (i.e., I). A commission error was defined as an incorrect button press. The task included a total of 6 runs of 62 trials each. All subjects completed two blocks of task practice trials outside the scanner before the functional magnetic resonance imaging session.
Behavioral Analysis
The numerical algorithm quantile maximum probability estimator (54) was used to estimate the ex-Gaussian parameters. RT variability was estimated within each word type (I and C) for all subjects. We defined intraindividual RT variability as the variability of Gaussian component (σ) and the exponential component (τ) as expressed by the following equation: IIV = σ + τ.
To ascertain whether any potential differences in IIV were explained by an increase in the number of excessively long RTs, we also examined tau separately.
Analysis of variance (ANOVA) procedures for repeated measures were used to analyze the data in a 2 × 2 mixed ANOVA with group as a between-subjects factor (MA vs. control subjects) and word type (I vs. C) as within-subject variables, as well any possible group × word type interaction on Stroop percent correct, errors, correct RT, and RT variability measures. Incorrect responses were not included within the analysis of variance for RT. As we wished to examine the effects of combined tau and IIV (I plus C) in addition to the relative effects across conditions (I and C), independent samples t tests between groups (MA, control) also compared combined IIV and combined tau separately. One-way ANOVA with psychosis status (methamphetamine-dependent subjects who had experienced MA-induced psychosis [MAP+]; methamphetamine-dependent subjects who had not experienced MA-induced psychosis [MAP−]; and control subjects) as a between-subjects factor examined combined IIV and tau separately. As group differences in trial-to-trial adjustments were observed between MA and control subjects in our previous study (81), we also examined whether MAP+ and MAP− subjects would differ in RT adjustments across trial sequences (Supplement 1).
Imaging Procedure and Analysis
Functional magnetic resonance imaging data were collected on a 3T Siemens Trio Total Imaging Matrix MRI System (Erlangen, Germany) (see Supplement 1 for scanning parameters and image preprocessing details). Analyses were performed using a general linear model as implemented in SPM5 (www.fil.ion.ucl.ac.uk/SPM5). In a first-level analysis, individual subject general linear models were fitted to each subject’s functional data. The statistical models included regressors coding for seven covariates: congruent trials preceded by other congruent OR incongruent trials (cC and iC, respectively), incongruent trials preceded by other incongruent OR congruent trials (iI and cI, respectively), errors, posterrors, and nonresponses. The coding of trial-to-trial regressors allowed us to examine both within-trial effects (e.g., I − C), as well as trial-to-trial adjustments (e.g., iI − cI). Parameter estimates obtained from this first-level analysis were used to compute maps of the contrasts of interest for effects of RT conflict (I − C).
Whole-Brain Analysis
Only correct responses were included in the reported contrasts. To examine how changes in IIV between conditions related to conflict-related brain activity (I − C) for control and MA groups separately, we employed I − C IIV as a regressor. We also conducted between-group analyses (control vs. MA) on these regression maps to investigate any potential group differences between groups in variability-associated brain activity. All between-group contrasts were thresholded at the voxel level with p < .01 and clusters were considered significant if they survived cluster-level family-wise error correction of p < .05. Due to the relatively small number of subjects in the MA group (n = 11), we did not conduct these regressions separately for the MAP+ and MAP− groups. Instead, we conducted exploratory analyses within regions of interest (ROI) plotting the betas from the I − C contrast maps generated from our previously published study (81) against combined IIV in the control, MAP+, and MAP− groups separately.
Region of Interest Analyses
As the PFC has consistently emerged as a region of interest with regard to elevated RT variability, as well as an area of between-group difference in our previous study of RT variability in schizophrenia (41), we opted to examine prefrontal ROIs derived from our previous article based on the same data set as used here (81), namely bilateral Brodmann area (BA) 10. Within these two ROIs, we examined withingroup (control, MAP+, MAP−) relationships between IIV and betas derived from the conflict contrast maps using Spearman’s rho.
Conjunction Analyses
To assess any potential shared regions of IIV-related activity between the MA and control groups, we conducted a conjunction analysis based on the global null (82) thresholded at a more conservative voxel level at p = .001 and cluster corrected as described above.
RESULTS
Behavioral Data
Reaction Time Analyses
Analyses revealed main effects of Stroop word type (F1,55 = 140.93, p < .0001) but no group by word type interaction (p = .56). No group differences were observed on within-trial Stroop conflict effects (F < 1) (81). When examining combined IIV and tau, independent samples t tests revealed no significant between-group (MA and control) differences (IIV: t55 = −1.51, p = .14; tau t55 = −1.32, p = .19), suggesting that the MA group as a whole did not differ significantly from control subjects in terms of RT variability (Table 2 and Figure 1).
Table 2.
Behavioral Results from 30 Methamphetamine Abusers and 27 Control Subjects
| Methamphetamine Abusers (n = 30) | Control Subjects (n = 27) | |
|---|---|---|
| Stroop Effects, Median (SD) | ||
| Congruent RT (msec) | 642.8 (86.9) | 617.2 (93.3) |
| Incongruent RT (msec) | 786.5 (143.0) | 742.9 (136.7) |
| Stroop conflict effect RT (msec) | 143.7 (90.5) | 125.7 (58.9) |
| Percent conflict errors | .05 (.04) | .06 (.06) |
| Percent nonconflict errors | .02 (.02) | .02 (.02) |
| Combined IIV (msec) | 179.85 (56.74) | 162.62 (39.23) |
| Combined Tau (msec) | 146.97 (60.23) | 126.10 (41.16) |
IIV, intraindividual reaction time variability; RT, reaction time.
Figure 1.
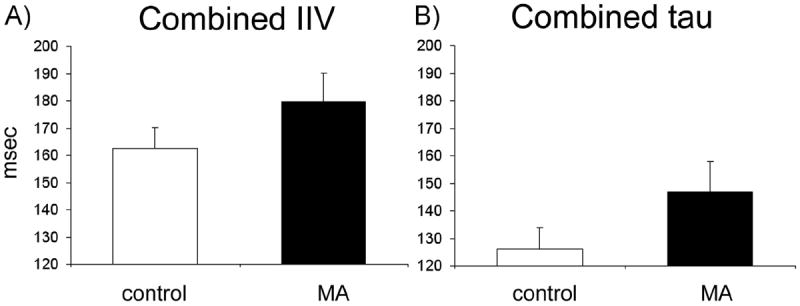
Intraindividual reaction time variability (IIV) and tau during the Stroop task in methamphetamine (MA) and control groups. (A) Combined IIV (congruent + incongruent IIV) in the control and MA groups measured in milliseconds. There was no significant group difference in combined IIV between MA and control groups. (B) Combined tau (congruent + incongruent tau) in the control and MA groups measured in milliseconds. There was no significant group difference in combined tau between MA and control groups.
We also examined differences between the MAP+ and MAP− subjects in IIV and tau (Table 3 and Figure 2). Results revealed a significant main effect of group (MAP+, MAP−, control) in IIV only (F2,54 = 3.74, p = .03; tau p = .15). Post hoc comparison (least significant difference) revealed that MAP+ subjects displayed increased IIV combined across I and C conditions compared with both MAP− subjects (p = .02) and control subjects (p = .03). Due to a lack of significant group difference in tau, the rest of our analyses focused on IIV only. An analysis of trial-to-trial RT adjustments between the MAP+ and MAP− subjects also failed to reach significance (p = .15).
Table 3.
Behavioral Results from 19 Methamphetamine Abusers with Psychosis (MAP+) and 11 Methamphetamine Abusers Without Psychosis (MAP−)
| MAP+ (n = 19) |
MAP− (n = 11) |
|
|---|---|---|
| Stroop Effects, Median (SD) | ||
| Congruent RT (msec) | 664.87 (90.5) | 604.77 (68.10) |
| Incongruent RT (msec) | 818.93 (141.3) | 730.54 (133.9) |
| Stroop conflict effect RT (msec) | 154.06 (96.0) | 119.6 (81.1) |
| Percent conflict errors | .05 (.04) | .05 (.05) |
| Percent nonconflict errors | .02 (.02) | .02 (.02) |
| Combined IIV | 195.39 (55.03) | 153.00 (51.32)a |
| Combined Tau | 156.21 (64.60) | 131.08 (50.65) |
IIV, intraindividual reaction time variability; RT, reaction time.
Significantly different from control group.
Figure 2.
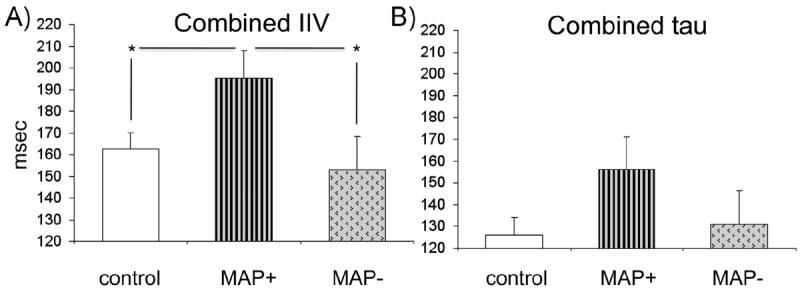
Intraindividual reaction time variability (IIV) and tau among methamphetamine subjects with psychosis (MAP+) and methamphetamine subjects without psychosis (MAP−) compared with control subjects. (A) Combined IIV significantly differed between groups; the MAP+ subjects displayed significantly greater IIV than both MAP− and control subjects. *Significance at p < .05. (B) There was no significant between-group difference in combined tau.
Error Analyses
Analyses revealed a main effect of word type (F1,55 = 34.78, p = .0001) with both groups making significantly more errors in the incongruent condition (5%) than in the congruent condition (2%). There were no group differences in incongruent (p = .54) or congruent errors (p = .43).
Correlations between IIV and Clinical Measures
Given the patterns with IIV and presence of MA-induced paranoia, we ran statistical correlations with the frequency of MA-induced paranoid episodes and IIV in the 19 MAP+ subjects. Despite the differences in IIV patterns between the MAP+ and MAP− subjects, the correlations between IIV and frequency of MA paranoia failed to reach significance. There were no significant correlation patterns that emerged between RT IIV and drug use patterns (years use, time abstinent, or age of first MA use) among the MA subjects.
Imaging Analyses
Whole-brain regression analyses within the control and MA groups separately revealed a relationship between IIV and activity in the MA group in a broad network of brain regions, including those involved in cognitive control such as bilateral prefrontal and left parietal cortices (Figure 3; Table 4). Greater variability was associated with increased activity in similar regions in control subjects, although these clusters did not reach significance. Between-group comparisons of these regression maps revealed greater activity in the MA over control group in three regions, including the left precentral gyrus (Table 4). Conjunction analysis examining any potential shared IIV-related activity between the MA and control groups revealed one significant region in the left inferior frontal gyrus (BA 45) and insula extending laterally into BA 46.
Figure 3.
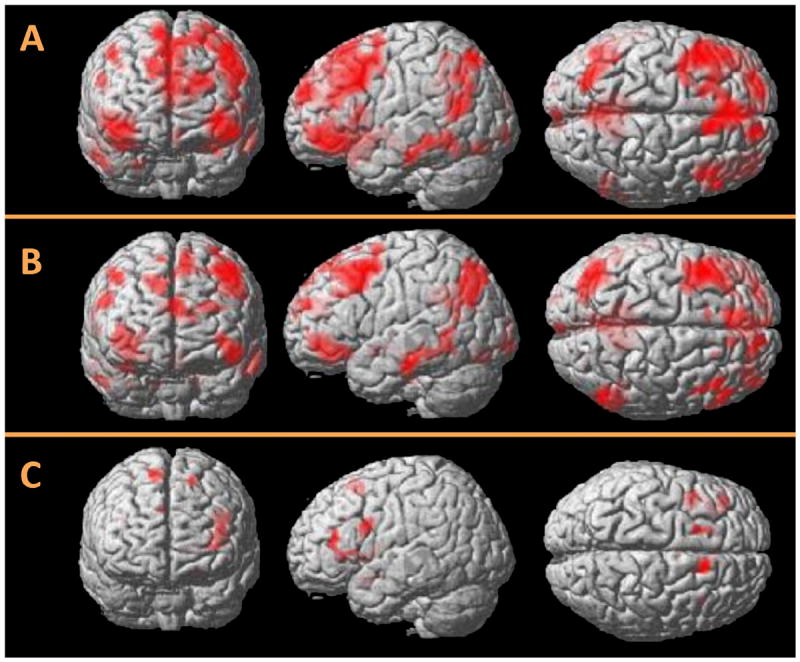
Whole-brain regression maps displaying the relationship between conflict-related brain activity and intraindividual reaction time variability (IIV) in the Stroop paradigm. (A) Activity in the methamphetamine (MA) group (n = 30). Greater IIV was associated with more activity in cognitive control regions including bilateral frontal and parietal cortex. (B) Areas of greater activity in the MA group over control group (n = 27). Greater IIV-related activity was associated with more activity in MA users in left precentral gyrus, left middle and inferior temporal gyrus, and posterior cingulate cortex. (C) An area of common IIV-related activity in participants with MA dependence and control subjects was found in the left inferior frontal gyrus extending into insula and middle frontal gyrus (Brodmann area 46).
Table 4.
Brain Regions with Significant Whole-Brain Differences Associated with Reaction Time Variability Conflict in 30 Methamphetamine Abusers in Regions Where MA Abusers Activated Significantly More Than 27 Control Subjects and in Regions Common to MA Abusers and Control Subjects
| Region | Brodmann Area | Number Voxels | MNI Coordinates | ||
|---|---|---|---|---|---|
| x | y | z | |||
| MA Abusers | |||||
| Right inferior frontal gyrus | 47 | 1884 | 44 | 26 | −10 |
| Right precuneus | 31 | 1581 | 4 | −56 | 32 |
| Right lentiform nucleus | 1308 | 10 | −4 | −2 | |
| Left inferior frontal gyrus | 47 | 1805 | −38 | 38 | −2 |
| Left middle frontal gyrus | 9 | 5026 | −44 | 14 | 40 |
| Left angular gyrus/inferior parietal cortex | 22 | 1417 | −38 | −60 | 16 |
| MA > Control Subjects | |||||
| Left precentral gyrus | 9 | 1549 | −44 | 14 | 42 |
| Left middle/inferior temporal gyrus | 21 | 1673 | −54 | −24 | −24 |
| Posterior cingulate cortex | 31 | 1640 | 0 | −56 | 28 |
| MA + Control Subject Conjunction Analysis | |||||
| Left inferior frontal gyrus/insula | 45/46 | 175 | −34 | 28 | 4 |
MA, methamphetamine; MNI, Montreal Neurological Institute.
ROI Analysis
To further probe any potential between-group differences in the relationship between brain activity and IIV, we examined bilateral prefrontal ROIs as outlined above. Conflict-related activity in right and left BA10 correlated positively with IIV in control subjects (r27 = .40, p = .04; r27 = .42, p = .03, respectively). Conflict-related activity in the right (r19 = .49, p = .03) but not left (p = .33) BA 10 correlated positively with IIV in the MAP+ group. There were no significant correlations between brain activity in either BA 10 region and IIV in the MAP− group (p > .50) for both correlations.
DISCUSSION
The current study examined the dynamics of cognitive control instability in MA abusers using a RT distributional analysis. The findings revealed an increase in RT variability in those MA subjects with psychosis compared with both MA abusers without psychosis and control subjects. Contrary to our hypothesis, we did not find a significant increase in the degree of unusually long RT (i.e., tau) in the MA abusers compared with control subjects. Our results from whole-brain activation analyses revealed that in participants with MA dependence increased IIV was associated with more activity in cognitive control regions, including bilateral PFC, as well as the right parietal lobe. Activity in the control group in these regions did not reach significance. However, conjunction analysis revealed an area of common activation in both groups in the left prefrontal cortex. Significantly greater activity associated with increased IIV in the MA group compared with control group was evident in a number of regions including the left precentral gyrus. When we examined the relationship between brain activity and IIV separately in those MA abusers who had experienced MA psychosis and those who had not, different patterns were observed. MA abusers who had experienced MA-induced psychosis, like control subjects, displayed a significant correlation between IIV and conflict-related activity in lateral PFC, whereas MA abusers who had never experienced MA-induced psychosis did not. Unlike control subjects, however, the relationship between IIV and brain activity in the MAP+ group was not distributed bilaterally but limited to the right PFC only.
Increased IIV has previously been associated with inefficiency in response preparation and selection (72,83), topdown self-regulation (84,85), and problems with arousal (50). Although our analysis revealed no behavioral differences in IIV between the MA and control groups, differences emerged when participants with MA dependence were classified as those who had experienced (MAP+) and had not experienced MA-induced psychosis (MAP−); MAP+ experienced elevated IIV compared with MAP− and control subjects. This suggests that in this subgroup of MA users there is an overarching problem with cognitive control in terms of attention control, response preparation, and/or response selection. This difference cannot be explained by a preponderance of unusually long RT, representing mind-wandering episodes, as we did not find any significant differences specifically in tau. This is in agreement with previous studies that have also found a relationship between increased RT variability and psychosis (19,41,63,64,75,76).
We then sought to examine the patterns of conflict-related functional brain activity associated with IIV in the MA and control groups. Previous research in healthy control subjects has linked activity in cognitive control regions with increased RT variability (44,74). Although we did not find any behavioral difference in IIV between the overall MA group and control subjects, the MA group displayed an increase in IIV-related brain activity compared with control subjects in regions associated with cognitive control. Conjunction analyses revealed one common region of IIV-related activity within left prefrontal cortex in both the MA and control groups. Left lateral PFC has been associated with storing task goals and rules, maintaining task set, and re-establishing top-down attention processes or preparing response rules (86-92). Previous research (74) found that in healthy control subjects, increased RT variability was associated with increased activity in cognitive control regions, whereas subjects with lower IIV displayed great activity in regions associated with motor preparation and execution. The authors posited that a more successful performance strategy, indicated by lower levels of RT variability, was reflected in a more automatic response tendency rather than overreliance on higher order cognitive control regions. In other words, poor performance was linked to overthinking one’s performance. In the current study, the MA-dependent group displayed increased IIV-related activity in cognitive control regions despite comparable behavioral measures of IIV. It may be that MA-dependent participants engaged cognitive control to a greater degree than the control group to achieve a similar behavioral result.
As the presence of a history of MA-induced psychosis resulted in significantly elevated rates of IIV, we next chose to examine whether conflict-related brain activity was differentially related to IIV in MAP+ and MAP− groups. To this end, we examined activity in bilateral PFC (BA 10) in each group separately and found that only the MAP+ group displayed a relationship between IIV and brain activity, whereby those individuals with greater IIV were also those individuals that activated PFC more. However, although control subjects showed a relationship between conflict-related activity in bilateral PFC and IIV, the MAP+ group displayed that relationship in the right hemisphere only. The different patterns of lateralized activity between the MAP+ group and control subjects may suggest the employment of different strategies. The right lateral PFC has been associated with vigilance, alerting, and arousal processes (93-101). As the MAP+ group displayed a relationship between increased activity in right PFC and IIV in this task, it is possible that a history of psychosis may be related to hypervigilant behavior, causing an increased level of concentration on cognitive tasks. Although somewhat speculative, published data from our lab are consistent with this model in that MAP+ subjects exhibit hyperalerting on tasks of attentional orienting (102,103). Collectively, these findings between drug-induced paranoia and cognitive function could suggest greater vigilance and focused attention in those MA subjects with a history of druginduced psychosis. As we would not predict to see increased vigilance in the MAP− group, this might explain the lack of PFC activation across either hemisphere. It is also possible that the relatively small number of subjects in this group (n = 11) did not allow us to detect this relationship.
Limitations
Potential limitations of this study include the use of subjects in our MA group that were recruited from treatment facilities rather than from a generalized community sample. Thus, our MA group may not be representative of the MA-dependent population at large. With regard to sample size, our MAdependent group without a history of drug-induced psychosis was relatively small (n = 11), which somewhat limited our ability to examine brain activity specifically related to this group. However, we conducted hypothesis-based regionspecific analyses to probe brain activity in MAP+ and MAP− groups. Nonetheless, it is possible that our relatively small sample size limited our ability to detect potential relationships between behavioral and clinical variables. It is also possible that the MAP+ group had preexisting psychosis before drug use; however, careful screening for preexisting psychosis was conducted using two structured interviews (i.e., SCID and MEQ) to distinguish between drug-induced psychosis and psychosis unrelated to drug use.
Conclusion
The results of the present study inform our understanding of the nature of impaired cognitive control by showing elevated IIV during Stroop task performance in a subset of MAdependent individuals with a history of MA-induced psychosis. Our findings suggest that cognitive control tasks that measure within-subject RT variability and recruit prefrontal regions can be powerful tools to detect meaningful relationships with substance-induced psychiatric symptoms. Future research in substance dependence should consider the inclusion of RT variability measures in addition to simple RT and measures of performance accuracy.
Supplementary Material
Acknowledgments
This work was funded by National Institute on Drug Abuse Grant DA021847 to RS.
We thank Jerry Sonico for his support and technical assistance with magnetic resonance data collection.
Footnotes
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.07.028.
DISCLOSURES
The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Chen CK, Lin SK, Sham PC, Ball D, Loh EW, Hsiao CC, et al. Pre-morbid characteristics and co-morbidity of methamphetamine users with and without psychosis. Psychol Med. 2003;33:1407–1414. doi: 10.1017/s0033291703008353. [DOI] [PubMed] [Google Scholar]
- 2.McKetin R, McLaren J, Lubman DI, Hides L. The prevalence of psychotic symptoms among methamphetamine users. Addiction. 2006;101:1473–1478. doi: 10.1111/j.1360-0443.2006.01496.x. [DOI] [PubMed] [Google Scholar]
- 3.Sato M. A lasting vulnerability to psychosis in patients with previous methamphetamine psychosis. Ann N Y Acad Sci. 1992;654:160–170. doi: 10.1111/j.1749-6632.1992.tb25965.x. [DOI] [PubMed] [Google Scholar]
- 4.Dore G, Sweeting M. Drug-induced psychosis associated with crystalline methamphetamine. Australas Psychiatry. 2006;14:86–89. doi: 10.1080/j.1440-1665.2006.02252.x. [DOI] [PubMed] [Google Scholar]
- 5.Pasic J, Russo JE, Ries RK, Roy-Byrne PP. Methamphetamine users in the psychiatric emergency services: A case-control study. Am J Drug Alcohol Abuse. 2007;33:675–686. doi: 10.1080/00952990701522732. [DOI] [PubMed] [Google Scholar]
- 6.Tsuang MT, Stone WS, Faraone SV. Genes, environment and schizophrenia. Br J Psychiatry. 2001;40:s18–s24. doi: 10.1192/bjp.178.40.s18. [DOI] [PubMed] [Google Scholar]
- 7.Chen CK, Lin SK, Sham PC, Ball D, Loh el W, Murray RM. Morbid risk for psychiatric disorder among the relatives of methamphetamine users with and without psychosis. Am J Med Genet B Neuropsychiatr Genet. 2005;136:87–91. doi: 10.1002/ajmg.b.30187. [DOI] [PubMed] [Google Scholar]
- 8.Salo R, Nordahl TE, Leamon MH, Natsuaki Y, Moore CD, Waters C, Carter CS. Preliminary evidence of behavioral predictors of recurrent drug-induced psychosis in methamphetamine abuse. Psychiatry Res. 2008;157:273–277. doi: 10.1016/j.psychres.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 9.Degenhardt L, Baker A, Maher L. Methamphetamine: Geographic areas and populations at risk, and emerging evidence for effective interventions. Drug Alcohol Rev. 2008;27:217–219. doi: 10.1080/09595230801956538. [DOI] [PubMed] [Google Scholar]
- 10.Schuckit MA. Comorbidity between substance use disorders and psychiatric conditions. Addiction. 2006;101(suppl 1):76–88. doi: 10.1111/j.1360-0443.2006.01592.x. [DOI] [PubMed] [Google Scholar]
- 11.LeDuc PA, Mittleman G. Schizophrenia and psychostimulant abuse: A review and re-analysis of clinical evidence. Psychopharmacology (Berl) 1995;121:407–427. doi: 10.1007/BF02246489. [DOI] [PubMed] [Google Scholar]
- 12.Connell PH. Amphetamine Psychosis. BMJ. 1957;1:582. [Google Scholar]
- 13.United Nations Office on Drugs and Crime. World Drug Report. New York: United Nations; 2009. [Google Scholar]
- 14.Brewer WJ, Francey SM, Wood SJ, Jackson HJ, Pantelis C, Phillips LJ, et al. Memory impairments identified in people at ultrahigh risk for psychosis who later develop first-episode psychosis. Am J Psychiatry. 2005;162:71–78. doi: 10.1176/appi.ajp.162.1.71. [DOI] [PubMed] [Google Scholar]
- 15.Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, et al. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790–1794. doi: 10.1176/appi.ajp.160.10.1790. [DOI] [PubMed] [Google Scholar]
- 16.Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, et al. Generalized and specific cognitive performance in clinical high-risk cohorts: A review highlighting potential vulnerability markers for psychosis. Schizophr Bull. 2006;32:538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornblatt BA, Keilp JG. Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull. 1994;20:31–46. doi: 10.1093/schbul/20.1.31. [DOI] [PubMed] [Google Scholar]
- 18.Boutros NN, Gooding D, Sundaresan K, Burroughs S, Johanson CE. Cocaine-dependence and cocaine-induced paranoia and mid-latency auditory evoked responses and sensory gating. Psychiatry Res. 2006;145:147–154. doi: 10.1016/j.psychres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Bora E, Vahip S, Akdeniz F. Sustained attention deficits in manic and euthymic patients with bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1097–1102. doi: 10.1016/j.pnpbp.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Jabben N, Arts B, Jongen EM, Smulders FT, van Os J, Krabbendam L. Cognitive processes and attitudes in bipolar disorder: A study into personality, dysfunctional attitudes and attention bias in patients with bipolar disorder and their relatives. J Affect Disord. 2012;143:265–268. doi: 10.1016/j.jad.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 21.Najt P, Bayer U, Hausmann M. Right fronto-parietal dysfunction underlying spatial attention in bipolar disorder. Psychiatry Res. 2013;210:479–484. doi: 10.1016/j.psychres.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Robinson LJ, Thompson JM, Gallagher P, Gray JM, Young AH, Ferrier IN. Performance monitoring and executive control of attention in euthymic bipolar disorder: Employing the CPT-AX paradigm. Psychiatry Res. 2013;210:457–464. doi: 10.1016/j.psychres.2013.06.039. [DOI] [PubMed] [Google Scholar]
- 23.Walshe M, Schulze KK, Stahl D, Hall MH, Chaddock C, Morris R, et al. Sustained attention in bipolar I disorder patients with familial psychosis and their first-degree relatives. Psychiatry Res. 2012;199:70–73. doi: 10.1016/j.psychres.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Lesh TA, Niendam TA, Minzenberg MJ, Carter CS. Cognitive control deficits in schizophrenia: Mechanisms and meaning. Neuropsychopharmacology. 2011;36:316–338. doi: 10.1038/npp.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latalova K, Prasko J, Diveky T, Velartova H. Cognitive impairment in bipolar disorder. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2011;155:19–26. doi: 10.5507/bp.155.2011.003. [DOI] [PubMed] [Google Scholar]
- 26.Vohringer PA, Barroilhet SA, Amerio A, Reale ML, Alvear K, Vergne D, Ghaemi SN. Cognitive impairment in bipolar disorder and schizophrenia: A systematic review. Front Psychiatry. 2013;4:87. doi: 10.3389/fpsyt.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 28.Braver TS, Paxton JL, Locke HS, Barch DM. Flexible neural mechanisms of cognitive control within human prefrontal cortex. Proc Natl Acad Sci U S A. 2009;106:7351–7356. doi: 10.1073/pnas.0808187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 30.Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- 31.Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, et al. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawton-Craddock A, Nixon SJ, Tivis R. Cognitive efficiency in stimulant abusers with and without alcohol dependence. Alcohol Clin Exp Res. 2003;27:457–464. doi: 10.1097/01.ALC.0000056620.98842.E6. [DOI] [PubMed] [Google Scholar]
- 33.Nestor LJ, Ghahremani DG, Monterosso J, London ED. Prefrontal hypoactivation during cognitive control in early abstinent methamphetamine-dependent subjects. Psychiatry Res. 2011;194:287–295. doi: 10.1016/j.pscychresns.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salo R, Nordahl TE, Buonocore MH, Natsuaki Y, Waters C, Moore CD, et al. Cognitive control and white matter callosal microstructure in methamphetamine-dependent subjects: A diffusion tensor imaging study. Biol Psychiatry. 2009;65:122–128. doi: 10.1016/j.biopsych.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salo R, Nordahl TE, Galloway GP, Moore CD, Waters C, Leamon MH. Drug abstinence and cognitive control in methamphetaminedependent individuals. J Subst Abuse Treat. 2009;37:292–297. doi: 10.1016/j.jsat.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004;61:73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 37.Nordahl TE, Salo R, Leamon M. Neuropsychological effects of chronic methamphetamine use on neurotransmitters and cognition: A review. J Neuropsychiatry Clin Neurosci. 2003;15:317–325. doi: 10.1176/jnp.15.3.317. [DOI] [PubMed] [Google Scholar]
- 38.Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- 39.Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- 40.Henik A, Salo R. Schizophrenia and the stroop effect. Behav Cogn Neurosci Rev. 2004;3:42–59. doi: 10.1177/1534582304263252. [DOI] [PubMed] [Google Scholar]
- 41.Fassbender C, Scangos K, Lesh TA, Carter CS. RT distributional analysis of cognitive-control-related brain activity in first-episode schizophrenia. Cogn Affect Behav Neurosci. 2014;14:175–188. doi: 10.3758/s13415-014-0252-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fassbender C, Zhang H, Buzy WM, Cortes CR, Mizuiri D, Beckett L, Schweitzer JB. A lack of default network suppression is linked to increased distractibility in ADHD. Brain Res. 2009;1273:114–128. doi: 10.1016/j.brainres.2009.02.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nat Neurosci. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- 44.Bellgrove MA, Hester R, Garavan H. The functional neuroanatomical correlates of response variability: Evidence from a response inhibition task. Neuropsychologia. 2004;42:1910–1916. doi: 10.1016/j.neuropsychologia.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Hervey AS, Epstein JN, Curry JF, Tonev S, Eugene Arnold L, Keith Conners C, et al. Reaction time distribution analysis of neuropsychological performance in an ADHD sample. Child Neuropsychol. 2006;12:125–140. doi: 10.1080/09297040500499081. [DOI] [PubMed] [Google Scholar]
- 46.Leth-Steensen C, Elbaz ZK, Douglas VI. Mean response times, variability, and skew in the responding of ADHD children: A response time distributional approach. Acta Psychol (Amst) 2000;104:167–190. doi: 10.1016/s0001-6918(00)00019-6. [DOI] [PubMed] [Google Scholar]
- 47.Andreou P, Neale BM, Chen W, Christiansen H, Gabriels I, Heise A, et al. Reaction time performance in ADHD: Improvement under fast-incentive condition and familial effects. Psychol Med. 2007;37:1703–1715. doi: 10.1017/S0033291707000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuntsi J, Oosterlaan J, Stevenson J. Psychological mechanisms in hyperactivity: I. Response inhibition deficit, working memory impairment, delay aversion, or something else? J Child Psychol Psychiatry. 2001;42:199–210. [PubMed] [Google Scholar]
- 49.Scheres A, Oosterlaan J, Sergeant JA. Response execution and inhibition in children with AD/HD and other disruptive disorders: The role of behavioural activation. J Child Psychol Psychiatry. 2001;42:347–357. [PubMed] [Google Scholar]
- 50.Sergeant JA, Geurts H, Huijbregts S, Scheres A, Oosterlaan J. The top and the bottom of ADHD: A neuropsychological perspective. Neurosci Biobehav Rev. 2003;27:583–592. doi: 10.1016/j.neubiorev.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 51.Loo SK, Specter E, Smolen A, Hopfer C, Teale PD, Reite ML. Functional effects of the DAT1 polymorphism on EEG measures in ADHD. J Am Acad Child Adolesc Psychiatry. 2003;42:986–993. doi: 10.1097/01.CHI.0000046890.27264.88. [DOI] [PubMed] [Google Scholar]
- 52.Buzy WM, Medoff DR, Schweitzer JB. Intra-individual variability among children with ADHD on a working memory task: An ex-Gaussian approach. Child Neuropsychol. 2009;15:441–459. doi: 10.1080/09297040802646991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: The search for endophenotypes. Nat Rev Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 54.Heathcote A, Brown S, Cousineau D. QMPE: Estimating Lognormal, Wald, and Weibull RT distributions with a parameterdependent lower bound. Behav Res Methods Instrum Comput. 2004;36:277–290. doi: 10.3758/bf03195574. [DOI] [PubMed] [Google Scholar]
- 55.Verte S, Geurts HM, Roeyers H, Oosterlaan J, Sergeant JA. Executive functioning in children with autism and Tourette syndrome. Dev Psychopathol. 2005;17:415–445. doi: 10.1017/s0954579405050200. [DOI] [PubMed] [Google Scholar]
- 56.Segalowitz SJ, Dywan J, Unsal A. Attentional factors in response time variability after traumatic brain injury: An ERP study. J Int Neuropsychol Soc. 1997;3:95–107. [PubMed] [Google Scholar]
- 57.Stuss DT, Stethem LL, Hugenholtz H, Picton T, Pivik J, Richard MT. Reaction time after head injury: Fatigue, divided and focused attention, and consistency of performance. J Neurol Neurosurg Psychiatry. 1989;52:742–748. doi: 10.1136/jnnp.52.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tinius TP. The intermediate visual and auditory continuous performance test as a neuropsychological measure. Arch Clin Neuropsychol. 2003;18:199–214. [PubMed] [Google Scholar]
- 59.Whyte J, Polansky M, Fleming M, Coslett HB, Cavallucci C. Sustained arousal and attention after traumatic brain injury. Neuropsychologia. 1995;33:797–813. doi: 10.1016/0028-3932(95)00029-3. [DOI] [PubMed] [Google Scholar]
- 60.Duchek JM, Balota DA, Tse CS, Holtzman DM, Fagan AM, Goate AM. The utility of intraindividual variability in selective attention tasks as an early marker for Alzheimer’s disease. Neuropsychology. 2009;23:746–758. doi: 10.1037/a0016583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gorus E, De Raedt R, Lambert M, Lemper JC, Mets T. Reaction times and performance variability in normal aging, mild cognitive impairment, and Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2008;21:204–218. doi: 10.1177/0891988708320973. [DOI] [PubMed] [Google Scholar]
- 62.Cismaru R, Chertkow H. Is variability a frontal lobe function? Confirmation in dementia subjects. Brain Cogn. 1999;40:84–85. [Google Scholar]
- 63.Kaiser S, Roth A, Rentrop M, Friederich HC, Bender S, Weisbrod M. Intra-individual reaction time variability in schizophrenia, depression and borderline personality disorder. Brain Cogn. 2008;66:73–82. doi: 10.1016/j.bandc.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 64.van den Bosch RJ, Rombouts RP. Coping and cognition in schizophrenia and depression. Compr Psychiatry. 1997;38:341–344. doi: 10.1016/s0010-440x(97)90930-5. [DOI] [PubMed] [Google Scholar]
- 65.Wagenmakers EJ, Brown S. On the linear relation between the mean and the standard deviation of a response time distribution. Psychol Rev. 2007;114:830–841. doi: 10.1037/0033-295X.114.3.830. [DOI] [PubMed] [Google Scholar]
- 66.Adams ZW, Roberts WM, Milich R, Fillmore MT. Does response variability predict distractibility among adults with attention- deficit/hyperactivity disorder? Psychol Assess. 2011;23:427–436. doi: 10.1037/a0022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Epstein JN, Langberg JM, Rosen PJ, Graham A, Narad ME, Antonini TN, et al. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. Neuropsychology. 2011;25:427–441. doi: 10.1037/a0022155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tamm L, Narad ME, Antonini TN, O’Brien KM, Hawk LW, Jr, Epstein JN. Reaction time variability in ADHD: A review. Neurotherapeutics. 2012;9:500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee RW, Jacobson LA, Pritchard AE, Ryan MS, Yu Q, Denckla MB, et al. Jitter reduces response-time variability in ADHD: An ex-Gaussian analysis. J Atten Disord. 2012 doi: 10.1177/1087054712464269. published online ahead of print November 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schall JD, Hanes DP. Neural mechanisms of selection and control of visually guided eye movements. Neural Netw. 1998;11:1241–1251. doi: 10.1016/s0893-6080(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 71.Schall JD. Neural correlates of decision processes: Neural and mental chronometry. Curr Opin Neurobiol. 2003;13:182–186. doi: 10.1016/s0959-4388(03)00039-4. [DOI] [PubMed] [Google Scholar]
- 72.Vaurio RG, Simmonds DJ, Mostofsky SH. Increased intraindividual reaction time variability in attention-deficit/hyperactivity disorder across response inhibition tasks with different cognitive demands. Neuropsychologia. 2009;47:2389–2396. doi: 10.1016/j.neuropsychologia.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aggarwal A, Lillystone D. A follow-up pilot study of objective measures in children with attention deficit hyperactivity disorder. J Paediatr Child Health. 2000;36:134–138. doi: 10.1046/j.1440-1754.2000.00464.x. [DOI] [PubMed] [Google Scholar]
- 74.Simmonds DJ, Fotedar SG, Suskauer SJ, Pekar JJ, Denckla MB, Mostofsky SH. Functional brain correlates of response time variability in children. Neuropsychologia. 2007;45:2147–2157. doi: 10.1016/j.neuropsychologia.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 75.Kieffaber PD, Kappenman ES, Bodkins M, Shekhar A, O’Donnell BF, Hetrick WP. Switch and maintenance of task set in schizophrenia. Schizophr Res. 2006;84:345–358. doi: 10.1016/j.schres.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 76.Rentrop M, Rodewald K, Roth A, Simon J, Walther S, Fiedler P, et al. Intra-individual variability in high-functioning patients with schizophrenia. Psychiatry Res. 2010;178:27–32. doi: 10.1016/j.psychres.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 77.Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: A functional magnetic resonance imaging study. Biol Psychiatry. 2009;65:706–709. doi: 10.1016/j.biopsych.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID). I: History, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 79.Gelernter J, Kranzler HR, Satel SL, Rao PA. Genetic association between dopamine transporter protein alleles and cocaineinduced paranoia. Neuropsychopharmacology. 1994;11:195–200. doi: 10.1038/sj.npp.1380106. [DOI] [PubMed] [Google Scholar]
- 80.Leamon MH, Flower K, Salo RE, Nordahl TE, Kranzler HR, Galloway GP. Methamphetamine and paranoia: The methamphetamine experience questionnaire. Am J Addict. 2010;19:155–168. doi: 10.1111/j.1521-0391.2009.00014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Salo R, Fassbender C, Buonocore MH, Ursu S. Behavioral regulation in methamphetamine abusers: An fMRI study. Psychiatry Res. 2013;211:234–238. doi: 10.1016/j.pscychresns.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–396. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- 83.Mostofsky SH, Simmonds DJ. Response inhibition and response selection: Two sides of the same coin. J Cogn Neurosci. 2008;20:751–761. doi: 10.1162/jocn.2008.20500. [DOI] [PubMed] [Google Scholar]
- 84.Douglas VI. Cognitive control processes in attention-deficit/hyperactivity disorder. In: Quay HC, Horgan A, editors. Handbook of Disruptive Behavior Disorders. New York: Plenum; 1999. pp. 105–138. [Google Scholar]
- 85.Stuss DT, Murphy KJ, Binns MA, Alexander MP. Staying on the job: The frontal lobes control individual performance variability. Brain. 2003;126:2363–2380. doi: 10.1093/brain/awg237. [DOI] [PubMed] [Google Scholar]
- 86.Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Prefrontal and midline interactions mediating behavioural control. Eur J Neurosci. 2009;29:181–187. doi: 10.1111/j.1460-9568.2008.06557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Frith C, Dolan R. The role of the prefrontal cortex in higher cognitive functions. Brain Res Cogn Brain Res. 1996;5:175–181. doi: 10.1016/s0926-6410(96)00054-7. [DOI] [PubMed] [Google Scholar]
- 88.Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: Inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 89.Hakun JG, Ravizza SM. Cognitive control: Preparation of task switching components. Brain Res. 2012;1451:53–64. doi: 10.1016/j.brainres.2012.02.046. [DOI] [PubMed] [Google Scholar]
- 90.Hazy TE, Frank MJ, O’Reilly RC. Towards an executive without a homunculus: Computational models of the prefrontal cortex/basal ganglia system. Philos Trans R Soc Lond B Biol Sci. 2007;362:1601–1613. doi: 10.1098/rstb.2007.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koechlin E, Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cogn Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 92.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 93.Breckel TP, Giessing C, Thiel CM. Impact of brain networks involved in vigilance on processing irrelevant visual motion. Neuroimage. 2011;55:1754–1762. doi: 10.1016/j.neuroimage.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 94.Coull JT, Frackowiak RS, Frith CD. Monitoring for target objects: Activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–1334. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- 95.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A frontoparietal network for rapid visual information processing: A PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 96.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 97.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: An ASL perfusion study of the time-on-task effect. Neuroimage. 2010;49:3426–3435. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Manly T, Owen AM, McAvinue L, Datta A, Lewis GH, Scott SK, et al. Enhancing the sensitivity of a sustained attention task to frontal damage: Convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–349. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- 99.McCarthy G, Luby M, Gore J, Goldman-Rakic P. Infrequent events transiently activate human prefrontal and parietal cortex as measured by functional MRI. J Neurophysiol. 1997;77:1630–1634. doi: 10.1152/jn.1997.77.3.1630. [DOI] [PubMed] [Google Scholar]
- 100.Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, et al. Functional anatomy of intrinsic alertness: Evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 101.Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- 102.Salo R, Gabay S, Fassbender C, Henik A. Distributed attentional deficits in chronic methamphetamine abusers: Evidence from the Attentional Network Task (ANT) Brain Cogn. 2011;77:446–452. doi: 10.1016/j.bandc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salo R, Nordahl TE, Possin K, Leamon M, Gibson DR, Galloway GP, et al. Preliminary evidence of reduced cognitive inhibition in methamphetamine-dependent individuals. Psychiatry Res. 2002;111:65–74. doi: 10.1016/s0165-1781(02)00111-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


