Abstract
Extracellular ATP has been shown to either inhibit or promote cancer growth and migration; however the mechanism underlying this discrepancy remained elusive. Here, we demonstrate the divergent roles of ATP and adenosine released by bone osteocytes in breast cancers. We showed that conditioned media (CM) collected from osteocytes treated with alendronate (AD), a bisphosphonate drug, inhibited the migration of human breast cancer MDA-MB-231 cells. Removal of the extracellular ATP by apyrase in CM abolished this effect, suggesting the involvement of ATP. ATP exerted its inhibitory effect through the activation of purinergic P2X receptor signaling in breast cancer cells evidenced by the attenuation of the inhibition by an antagonist, oxidized ATP, as well as knocking down P2X07 with siRNA, and the inhibition by an agonist, BzATP. Intriguingly, ATP had a biphasic effect on breast cancer cell behavior–lower dosage inhibited, but higher dosage promoted its migration. The stimulatory effect on migration was blocked by an adenosine receptor antagonist, MRS1754, ARL67156, an ecto-ATPase inhibitor, and A2A receptor siRNA, suggesting that in contrast to the action of ATP, adenosine, a metabolic product of ATP, promoted migration of breast cancer cells. Consistently, non-hydrolyzable ATP, ATPγS, only inhibited, but did not promote cancer cell migration. ATP also had a similar inhibitory effect on the Py8119 mouse mammary carcinoma cells; however, adenosine had no effect due to the absence of the A2A receptor. Consistent with the results of cancer cell migration, ATPγS inhibited, while adenosine promoted anchorage-independent growth of breast cancer cells. Our in vivo xenograft study showed a significant delay of tumor growth with the treatment of ATPγS. Moreover, the extent of bone metastasis in a mouse intratibial model was significantly reduced with the treatment of ATPγS. Together, our results suggest the distinct roles of ATP and adenosine released by osteocytes, and the activation of corresponding receptors P2X7 and A2A signaling on breast cancer cell growth, migration and bone metastasis.
Keywords: adenosine nucleotides, breast cancer, bone metastasis
INTRODUCTION
Bone metastases are major, potentially fatal complications associated with advanced cancers including breast cancer1,2,3,4. Bone tissues are preferred sites of cancer metastasis due to their microenvironment, which provides a fertile setting in which tumor cells can grow5. There are three major cell types in bone tissues: osteocytes, osteoblasts, and osteoclasts. Osteocytes comprise over 95% of total bone cells and play an essential role in orchestrating the bone remodeling process by coordinating activities from the osteoclasts and osteoblasts6,7. The roles of osteoblasts and osteoclasts in bone metastasis have been linked to the release of growth factors from the bone matrix, which stimulates tumor growth4. However, the role of osteocytes, the most abundant cell type in bone tissue, in bone metastases remains unexplored.
Bisphosphonate drugs are commonly used to treat skeletal complications such as bone pain, pathological fracture, osteopenia and osteoporosis. This class of drugs is also used to treat bone cancer metastasis and is associated with decreased tumor growth in addition to reduced bone destruction and pain8. It has been reported that alendronate (AD), a bisphosphonate drug, induces the opening of hemichannels, a channel permeable to small molecules (Mr < 1 kDa) in osteocytes9. In addition, osteocytes permit the release of ATP in response to mechanical loading10. However, it is unknown whether the ATP molecules which are released from osteocytes mediate the inhibitory effect of bisphosphonates on bone metastasis.
Several studies have established the anti-neoplastic activity of extracellular ATP to inhibit the growth of several cancer cell lines, including prostate cancer cells, colon adenocarcinoma cells, melanoma cells, and bladder cancer cells11,12,13. Previous studies point to the possibility that ATP, through its binding to P2 purinergic receptors, exhibits an anti-cancer effect12. The activation of purinergic signaling is also reported to inhibit proliferation and migration of human acute myeloblastic leukemia cells in immune-deficient mice14. Additionally, in vivo studies show that daily injections of ATP significantly inhibit tumor growth, prolong survival time and inhibit weight loss in mice15. However, the effect of adenosine nucleotides on cancer bone metastasis is largely unexplored. Our study demonstrates that ATP released from bone osteocytes exerts inhibitory effects on breast cancer cells. ATPγS, a nonhydrolyzable analogue of ATP, has a similar inhibitory effect on breast cancer cell migration. In contrast to the effect by ATP, adenosine, a metabolic product, promoted human breast cancer cell migration, and this stimulatory effect was attenuated with an adenosine receptor antagonist. Moreover, we showed the inhibitory effect by ATP and the stimulatory effect by adenosine were primarily mediated by the activation of P2X7 and A2A receptors, respectively. These results suggest that adenosine nucleotides released from osteocytes and their activating signaling mechanisms have significant impacts on the migration and growth of tumor cells and cancer metastasis to the bone.
RESULTS
ATP released by AD-treated osteocytes inhibits the migration of human breast cancer cells
To determine the underlying mechanism of the bisphosphonates in suppressing cancer metastasis to the bone, we treated osteocytic MLO-Y4 cells with AD and collected CM. The result from the transwell cell migration assay showed that CM collected from the MLO-Y4 osteocytes treated with AD significantly decreased the migration of MDA-MB-231 cells (127±12 cells to 38±12 cells) (Figure 1A). To eliminate the possibility of any effects from proliferation, the WST-1 cell proliferation assay was performed by incubating the MDA-MB-231 breast cancer cells in the identical CM and time duration as used in the transwell migration assay. The proliferation of the MDA-MB-231 cells incubated in CM from MLO-Y4 cells treated with 20 μM AD (CM-AD) was similar to that of the MDA-MB-231 cells incubated in untreated CM (CM) (Figure 1B). To determine whether ATP released from osteocytes would have an effect on MDA-MB-231 cell migration, we depleted ATP from the CM collected from MLO-Y4 cells using apyrase, an ATP hydrolyzing enzyme. The addition of apyrase increased MDA-MB-231 cell migration by 2.5 fold in untreated CM and 7.7 fold in CM-AD (Figure 1A). To exclude the possibility that AD might have direct effects on MDA-MB-231 cells, we performed the transwell cell migration assay with the MDA-MB-231 cells with AD added directly to the CM collected from MLO-Y4 cells. The results showed that there was no difference in migration when incubated with AD (Figure 1C). These results suggest that ATP released from osteocytes upon AD treatment can inhibit the migration of human breast cancer cells.
Figure 1.
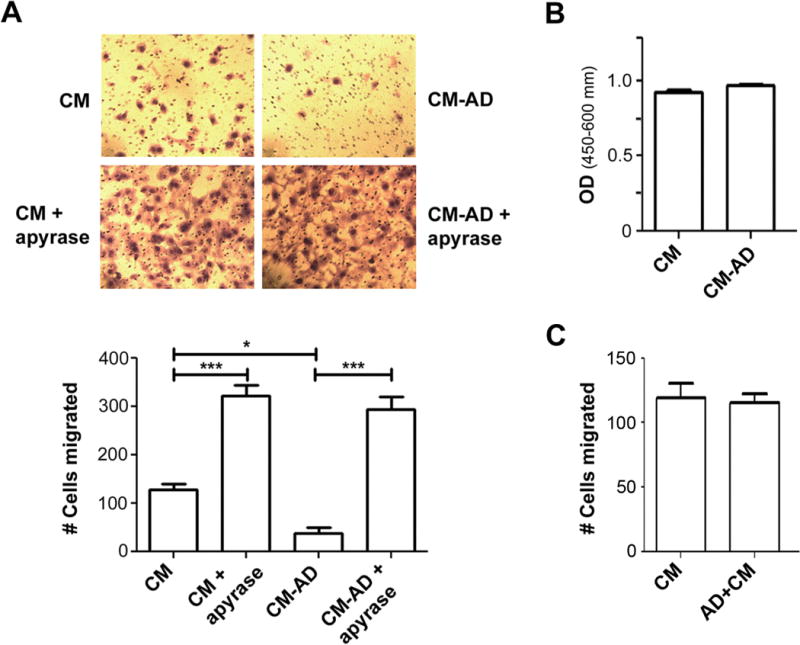
ATP released by osteocytes treated with AD has inhibitory effect on migration of human breast cancer cells. (A) Depletion of ATP by apyrase from CM collected from AD-treated osteocytes increases breast cancer cells migration. CM was collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr and was then treated with or without apyrase (5 units/ml), an ATP hydrolyzing enzyme for 4 hr prior to being used to culture MDA-MB-231 cells in transwells. The cells migrated through the transwell filter were stained with Hema 3 Stat Pack (Fisher Scientific) (upper panel). The numbers of the cells migrated were quantified. Data were presented as mean ± SEM, n = 3. *, P < 0.05; ***, P < 0.001. (B) CM collected from AD-treated MLO-Y4 cells has no effect on human breast cancer cell proliferation. MDA-MB-231 breast cancer cells were incubated for 18 hr in CM collected from MLO-Y4 cells with (CM-AD) or without (CM) 20 μM AD for 48 hr. Cell proliferation was analyzed by WST-1 assay. Data presented as mean ± SEM, n = 3. (C) AD has no effect on human breast cancer cell migration. CM was collected from MLO-Y4 cells without (CM) AD for 48 hr. Prior to the incubation with MDA-MB-231 breast cancer cells for 18 hr, 20 μM AD was directly added to the CM. Data presented as mean ± SEM, n = 3.
To test the effect of purinergic signaling activated by ATP on breast cancer cell migration, we treated the CM with oxidized ATP (oATP), a potent inhibitor of P2X purinergic receptors. The addition of oATP significantly attenuated the inhibitory effect of CM-AD on MDA-MB-231 cell migration (Figure 2A). Consistently, the addition of BzATP, a nonhydrolyzable P2X7 receptor agonist, caused a significant, dose-dependent decrease in breast cancer migration (0 μM = 110±11.6 cells, 10 μM = 99±7.8 cells, 100 μM = 64±4.4 cells, 200 μM = 39±2.6) (Figure 2B). The result from the WST-1 assay showed that the treatment with BzATP at concentrations 1–200 μM had minimal effects on cell proliferation, and a significant reduction was only observed at 400 μM (Figure S1). These data support an inhibitory role for P2X receptor activation in the migration of human breast cancer cells.
Figure 2.
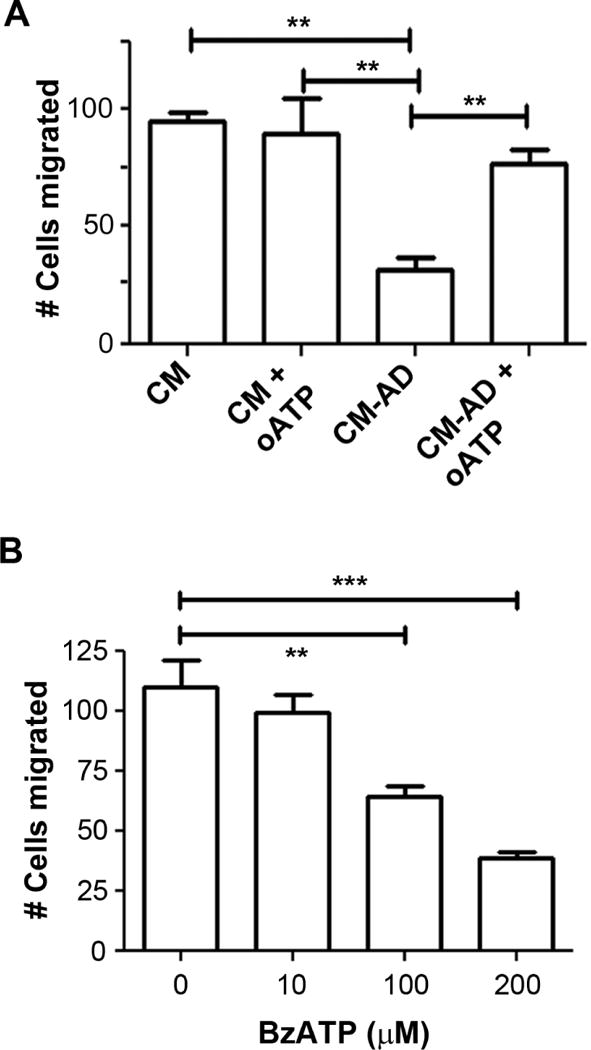
The migration of human breast cancer cells is inhibited by the activation of purinergic P2X receptor. (A) oATP, a P2X antagonist, attenuates the decrease in migration of human breast cancer cells when treated with CM collected from MLO-Y4 cells treated with 20 μM AD. MDA-MB-231 cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr and with or without 300 μM oATP and numbers of cells migrating in the transwell plates were quantified. Data presented as mean ± SEM, n = 3. **, P < 0.01. (B) BzATP, a P2X7 agonist, decreases migration of human breast cancer cells. MDA-MB-231 cells were treated with various concentrations of BzATP (0 – 200 μM) for 18 hr and numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. **, P < 0.01; ***, P < 0.001.
ATP inhibits, but adenosine promotes the migration of breast cancer cells
To determine the direct involvement of ATP, we applied ATP into the CM. Surprisingly, the treatment of 200 μM ATP did not decrease, but increased the migration of MDA-MD-231 cells in both CM collected from AD and non-AD-treated MLO-Y4 cells (153±21.1 vs. 88±10.7 and 188±33.5 vs. 127±2, respectively) (Figure 3A). To further test the effect of ATP, we treated MDA-MB-231 cells with ATP at varying concentrations (Figure 3B). We found that the inhibitory effect of ATP was only observed at lower concentration (0 μM = 150±4.8 cells vs 50 μM = 100±17.7 cells), but higher concentration instead promoted cancer cell migration (200 μM = 257±26 cells, 400 μM = 240±0.9 cells). The effect of ATP on cell migration was not caused by alterations of cell proliferation (Figure S2). This increase in migration is possibly due to higher levels of adenosine formed as a product of the increased break down of ATP at higher concentrations, since extracellular ATP is known to be readily hydrolyzed to adenosine by a group of enzymes known as ectonucleotidases16. To test for the possible effects of adenosine as a result of ATP hydrolysis, a potent adenosine receptor antagonist, MRS1754 was used. As a selective adenosine receptor antagonist, MRS1754 specifically inhibits A2B receptors at low concentrations, but at higher concentrations, MRS1754 is known to inhibit other P1 receptors (A1 and A2A). The addition of high concentration (500 nM) of MRS1754 attenuated the stimulatory effect of ATP on the migration (Figure 3A). Moreover, MRS1754 further augmented the inhibitory effect of CM-AD on cell migration, suggesting these adverse effects were mediated by adenosine. To verify the effects of MRS1754 by itself on MDA-MB-231 cell migration and to reveal the specific P1 receptor(s) involved, we treated the media directly with a low concentration (2 nM) and high concentration (500 nM) of MRS1754 (Figure S3A). There was no significant difference in migration with the low concentrations (A2B receptors blocked), but at high concentrations (A1, A2A, and A2B receptors blocked), the migration was reduced by over 2.6 fold (Figure S3A). The cell viability was not affected by the different concentrations of MRS1754 (Figure S3B). As further confirmation, we applied an ecto-ATPase inhibitor, ARL67156, which prevents the breakdown of ATP. The addition of ARL67156 attenuated the stimulatory effect of higher dosage of ATP on the migration of the breast cancer cells (Figure 3C). The addition of ARL67156 to media also triggered a decrease in migration (Figure S4A). However, the effect of ARL67156 on cell migration was not caused by changes in cell proliferation (Figure S4B). To demonstrate the effect of ATP in the absence of its break down, we used a nonhydrolyzable ATP analogue, ATPγS, which is a broad agonist of P2 receptors. The application of this reagent to control CM significantly reduced the migration of MDA-MB-231 cells (112±2.4 cells to 63±9.6 cells) (Figure 3D). The significant reduction of cancer cell migration by ATPγS was further demonstrated in a dose-dependent manner (0 μM = 69±3.8 cells, 50 μM = 46±9.3 cells, 100 μM = 33±3.3 cells, 200 μM = 11±1.7, 400 μM = 9±1.5 cells) (Figure 3E). These data confirm the inhibitory role of ATP on breast cancer cell migration and imply the opposite role of adenosine.
Figure 3.
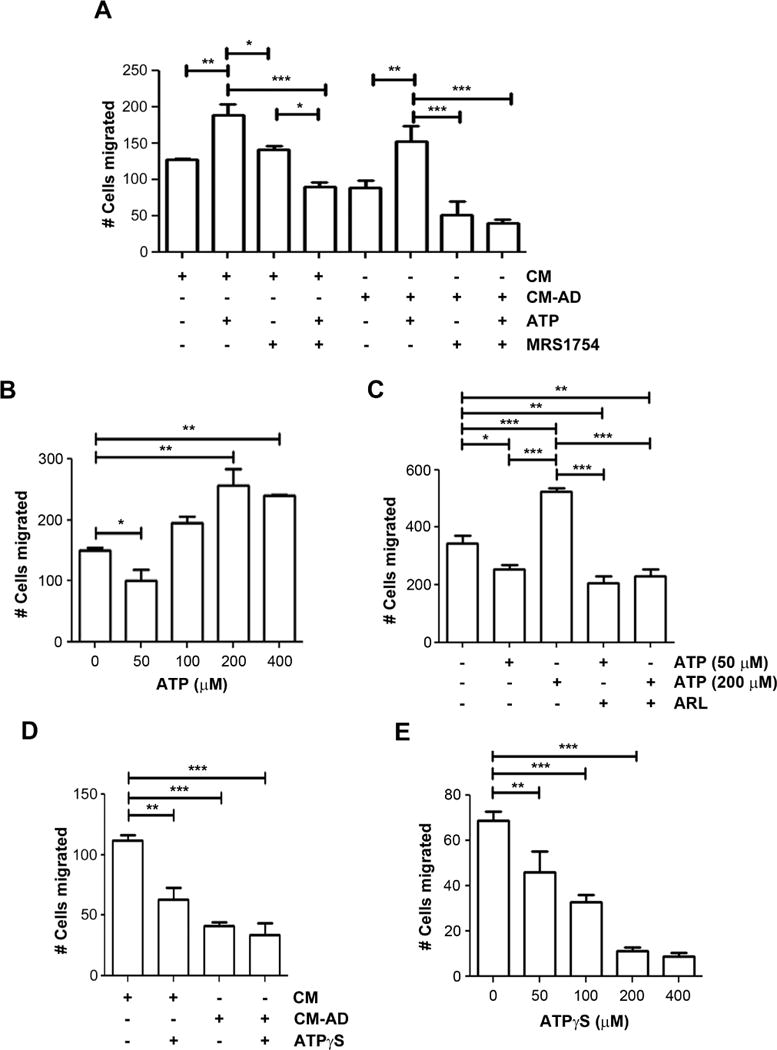
Antagonist of adenosine receptor and non-hydrolysable ATP inhibit the migration of human breast cancer cells. (A) Addition of ATP increases the migration of breast cancer cells and this increase is attenuated by adenosine receptor antagonist, MRS1754. MDA-MB-231 cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr in the absence or presence of 200 μM ATP and/or 500 nM MRS 1754, a potent P1 adenosine receptor antagonist. Numbers of the migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Lower dosage of ATP decreases, but higher dosage increases the migration of human breast cancer cells. MDA-MB-231 cells were incubated with various concentrations of ATP (0 – 400 μM) for 18 hr and the numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. *, P < 0.05; **, P < 0.01. (C) ARL 67156 attenuates the increase in breast cancer migration from higher concentrations of ATP. MDA-MB-231 breast cancer cells were incubated with 50 μM or 200 μM ATP with or without the addition of 200 μM ARL67156. Data presented as mean ± SEM, (n=3); *, P < 0.05; **, P < 0.01;, ***, P < 0.001. (D) ATPγS decreases the migration of human breast cancer cells. MDA-MB-231 breast cancer cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr and with or without 100 μM of the non-hydrolyzable ATP analogue, ATPγS. The numbers of cells by transwell assay were quantified. Data presented as mean ± SEM, n =3. **, P < 0.01; ***, P < 0.001. (E) ATPγS decreases the migration of human breast cancer cells in a dose-dependent manner. MDA-MB-231 cells were incubated with various concentrations of ATPγS (0 – 400 μM) for 18 hr and the numbers of migrating cells migrated by transwell assay were quantified. Data presented as mean ± SEM, n = 3. **, P < 0.01; ***, P < 0.001.
We further tested the effect of adenosine on MDA-MB-231 cell migration. CM collected from MLO-Y4 cells were treated with or without adenosine. MRS1754 was also added to verify the specific effect from adenosine. The treatment of adenosine increased MDA-MB-231 cell migration, whereas this increase was completely attenuated with the addition of 500 nM MRS1754 (Figure 4A). The enhanced cell migration by adenosine was not a result of increased cell proliferation since the treatment of adenosine at various concentrations had minimal effects on cell proliferation (Figure S5). These data indicated the possible involvement of A1 and A2A receptors. To further determine if a similar effect was also observed with the hydrolysis of ATP, we added apyrase to the CM. Consistently, the increase of the migration as a result of apyrase treatment was significantly attenuated by MRS1754 (Figure 4B). To ascertain the specific P1 receptor subtype which is stimulated by adenosine to enhance breast cancer cell migration, we utilized a selective A2A receptor antagonist, ANR94. The viability of MDA-MB-231 cells is unaffected by ANR94 up to concentrations of 100 μM; therefore, we used this concentration in future experiments (Figure S6). The blocking of A2A receptors by ANR94 dramatically decreased the migration of MDA-MB-231 cells (Figure 4C). Based on these data, we concluded that adenosine has a stimulatory role on breast cancer cell migration and this effect is likely mediated through the adenosine A2A receptor. These results further suggest the divergent roles of ATP and adenosine on breast cancer cell migration; the inhibitory role by ATP and the stimulatory role by adenosine.
Figure 4.
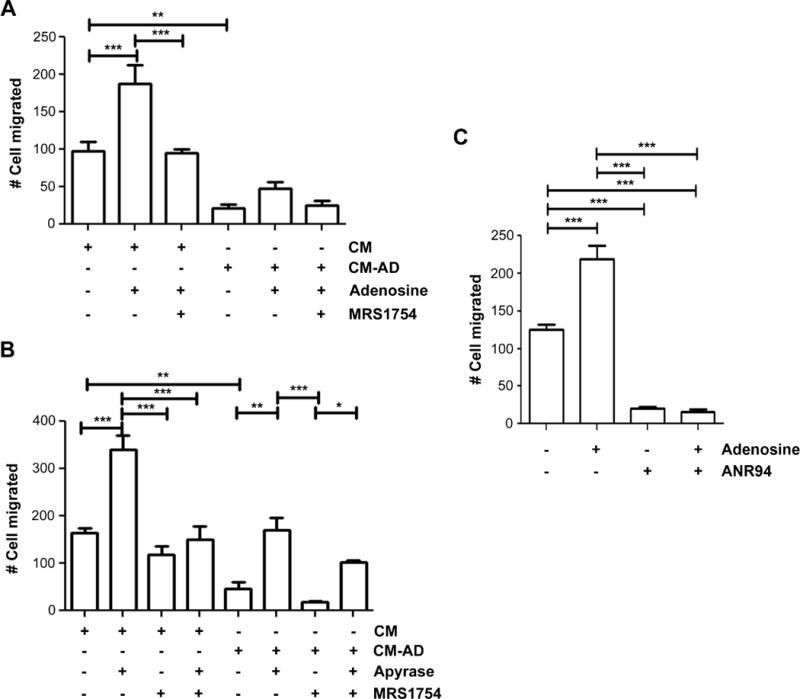
Adenosine increases the migration of human breast cancer cells and this increase is attenuated by an adenosine receptor antagonist. (A) Adenosine and a P1 adenosine receptor antagonist increase the migration of MDA-MB-231. MDA-MB-231 breast cancer cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr in the absence or presence of 200 μM adenosine and/or 500 nM of MRS 1754. The numbers of migrating cells by transwell assay were quantified. Data is presented as mean ± SEM, n = 3. **, P < 0.01; ***, P < 0.001. (B) The increased migration of breast cancer cells by apyrase is attenuated by MRS1754. MDA-MB-231 breast cancer cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr and then treated with or without apyrase (5 units/ml) and/or 500 nM MRS 1754. Data presented as mean ± SEM, n = 3. The numbers of migrating cells by transwell assay were quantified. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) The antagonist of A2A adenosine receptors inhibits breast cancer cell migration. MDA-MB-231 breast cancer cells were incubated in the presence or absence of 200 μM adenosine and/or 100 μM ANR 94. The numbers of migrating cells by transwell assay were quantified. Data is presented as mean ± SEM, n = 3. ***, P < 0.001.
The levels of ATP and adenosine in the CM (MLO-Y4 cells treated with AD for 48 hr) were measured by liquid chromatography–mass spectrometry (LC-MS). 10 μM or 20 μM AD increase the ATP by 102.1±17.85 or 221.6±18.45 fold compared to the control CM, respectively. Adenosine levels were also increased in the CM from MLO-Y4 cells treated with 20 μM AD, to a lesser degree compared with ATP levels (4.8±0.1 fold compared to control CM). This data further supports the notion that ATP released by osteocytes treated with AD is responsible for the inhibitory effect on the breast cancer cells. On the other hand, the lesser increase of adenosine possibility due to the hydrolysis of ATP in CM-AD failed to exert a predominant effect on promoting breast cancer cells.
P2X7 mediates the migration-inhibiting effects from ATPγS, while A2A mediates the migration-promoting effects from adenosine in MDA-MB-231 cells
We investigated the specific involvement of P2X7 in the ATP-induced inhibition of migration as well as A2A receptors in adenosine-induced increase of migration by silencing the expression of P2X7 and A2A using siRNA. We confirmed that mRNA expression of P2X7 and A2A receptors were decreased by transfection with a P2X7 and A2A siRNA, respectively (Figure 5A). In P2X7 knocked down cells, ATPγS failed to induce a decrease in migration, whereas a dramatic decrease is seen with control siRNA transfected cells (Figure 5B). Additionally, adenosine failed to promote migration in A2A knocked down cells, while control cells transfected with control siRNA exhibited significant increase in migration (Figure 5C). These results indicate the specific involvement of P2X7 and A2A receptors in ATP and adenosine-mediated migration effects, respectively.
Figure 5.
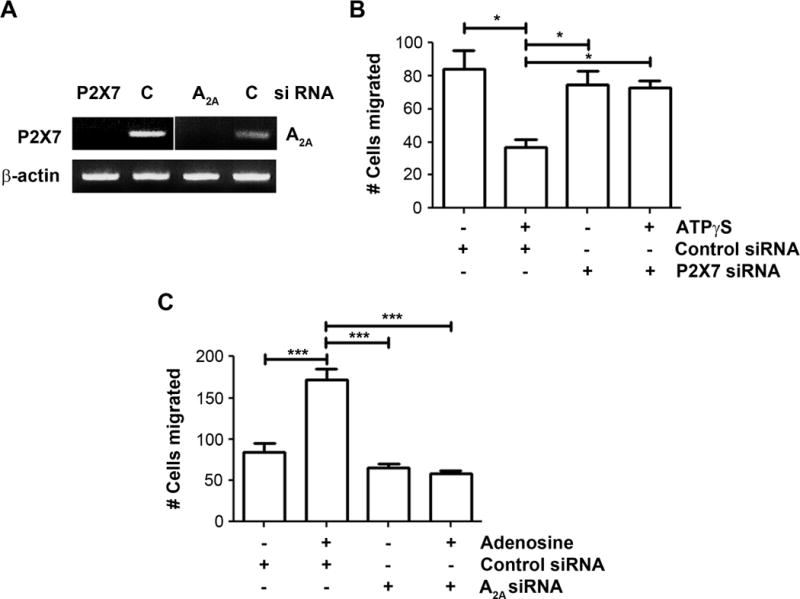
P2X7 and A2A receptors are involved in the ATP and adenosine-induced migration effects, respectively. (A) MDA-MB-231 cells were transfected with 90 nM of P2X7 siRNA, 60 nM of A2A siRNA, or 90 nM silencer negative control (C) siRNA and incubated for 48 hr. The expression of P2X7 and A2A receptors were detected by RT-PCR as described. (B) P2X7 and control siRNA-transfected MDA-MB-231 cells were incubated with or without 100 μM ATPγS for 18 hr and numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. *, P < 0.05. (C) A2A and control siRNA-transfected MDA-MB-231 cells were incubated with or without 200 μM adenosine for 18 hr and numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. ***, P < 0.001.
ATPγS inhibits, but adenosine promotes anchorage-independent growth of human breast cancer cells
To determine if ATP and adenosine have similar effects on the anchorage-independent growth of human cancer cells, we cultured MDA-MB-231 breast cancer cells in soft agar (Figure 6). Similar to their effects on the cell migration, ATPγS significantly inhibited colony formation of MDA-MB-231 cells (82±4.5 colonies to 47 ± 6.2 colonies), while adenosine had an opposite effect by significantly promoting colony formation (132 ± 13.7 colonies). These results suggest that ATP and adenosine not only affect cell migration, but also have a major impact on human cancer cell growth.
Figure 6.
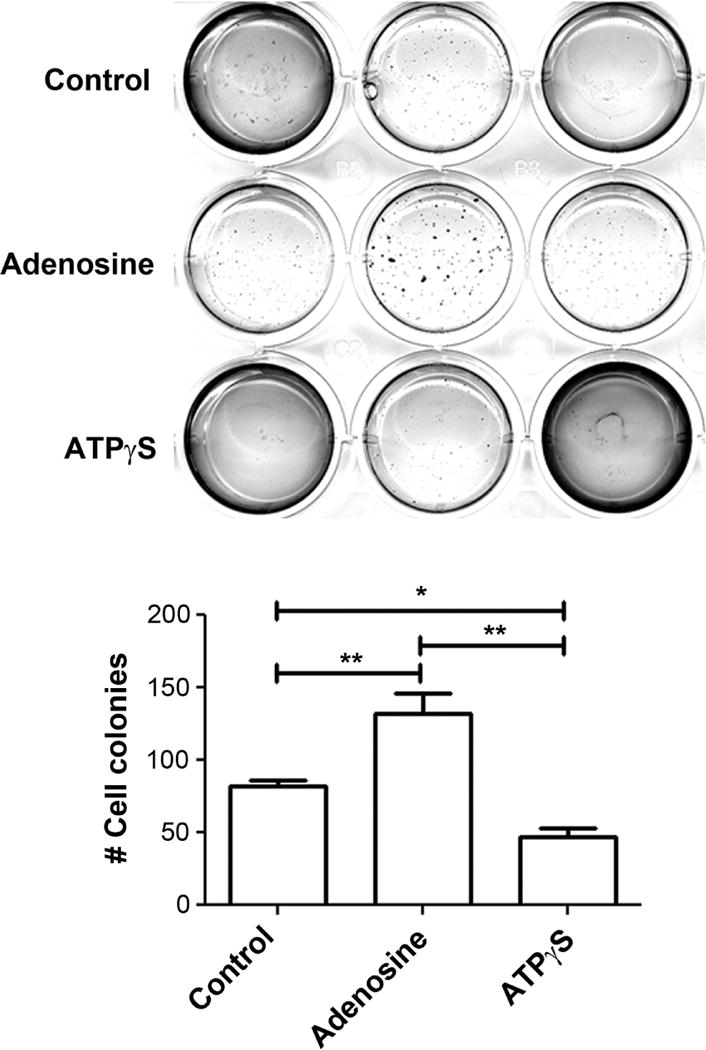
The anchorage-independent growth of human breast cancer cells is inhibited by ATPγS, but stimulated by adenosine. MDA-MB-231 breast cancer cells were plated on soft agar and were treated with 100 μM ATPγS, 200 μM adenosine, or without for about 2 weeks. Cells growing on soft agar plates were imaged (upper panel) and quantified (lower panel). Data presented as mean ± SEM, n = 3. *, P < 0.05; **, P < 0.01.
ATP and ATPγS inhibited the migration of mouse mammary carcinoma cells
We tested adenosine nucleotides on Py8119, a mouse mammary carcinoma cell line, since this cell is capable of metastasizing to other tissues in immunocompetent wild-type mice and has been used as an in vivo metastatic model17. ATP at varying concentrations was added to the CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD. The transwell cell migration assay was conducted with Py8119 cells incubated in these CM. With the increase of dosage, the migration of the Py8119 cancer cells decreased, with the most significant effect at 400 μM (254±25.9 cells to 159±7.8 cells with CM and 127±.3 cells to 88±10.3 cells for CM-AD) (Figure 7A). The migration of Py8119 cells was also decreased with the treatment of ATPγS (Figure 7B). These results suggest that similar to MDA-MB-231 breast cancer cells, ATP has an inhibitory role on Py8119 mouse mammary carcinoma cells and further implies a broad role of ATP on breast cancer bone metastasis. We then tested whether adenosine has a similar stimulatory effect on the Py8119 cells as it had on the MDA-MB-231 cells. The transwell migration assay was conducted with Py8119 cells incubated in media containing various concentrations of adenosine (Figure 7C). We found that the migration of Py8119 cells was not changed, regardless of the concentration of adenosine added. Furthermore, Py8119 cell migration was unaffected by either low or high concentrations of MRS1754 (Figure S3C). Blocking the specific A2A receptors with ANR94 treatment did not affect migration patterns either (Figure 7D). This indicates that unlike the human breast cancer cell line MDA-MB-231, the migration of the mouse mammary carcinoma cell line Py8119 is not sensitive to adenosine.
Figure 7.
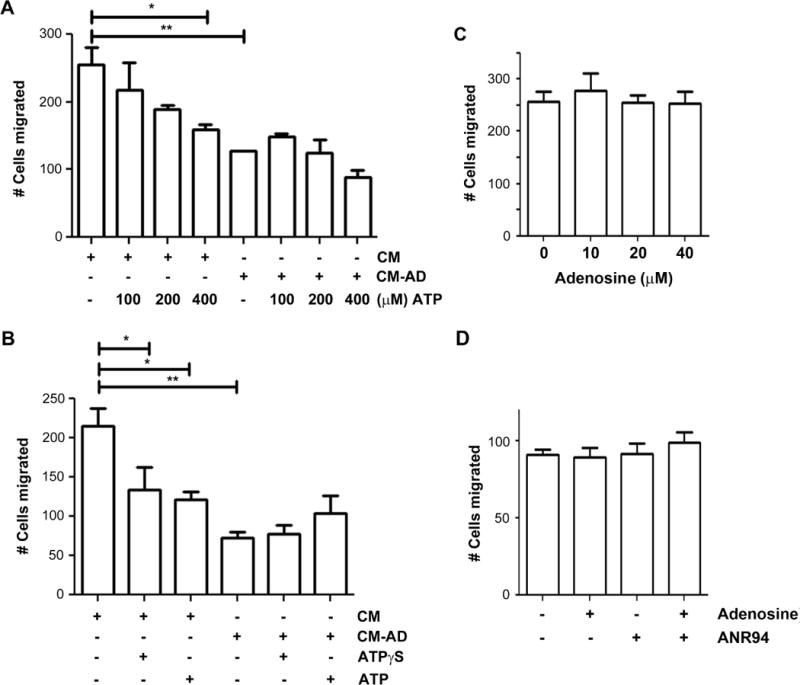
The reduction of mouse mammary cancer cells by ATP and ATPγS. (A) ATP reduces the migration of murine mammary cancer cells in a dose-dependent manner. Py8119 mouse mammary cancer cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr and ATP in concentrations ranging from 0–400 μM. The numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n=3. *, P < 0.05; **, P < 0.01. (B) ATPγS decreases the migration of murine mammary cancer cells. Py8119 mouse mammary cancer cells were incubated in CM collected from MLO-Y4 cells treated with (CM-AD) or without (CM) 20 μM AD for 48 hr in the absence or present of 100 μM ATPγS or 200 μM ATP. Data presented as mean ± SEM, n = 3. *, P < 0.05; **, P < 0.01. (C) Adenosine has no effect on murine mammary cancer cell migration. Py8119 cells were incubated with various concentrations of adenosine (0 – 40 μM) for 18 hr and numbers of migrating cells by transwell assay were quantified. Data presented as mean ± SEM, n = 3. (D) The antagonist of A2A adenosine receptors had no effect on mouse breast cancer cell migration. Py8119 mouse mammary carcinoma cells were incubated in the presence or absence of 200 μM adenosine and/or 100 μM ANR 94. The numbers of migrating cells by transwell assay were quantified. Data is presented as mean ± SEM, n = 3.
The insensitivity of Py8119 cells to adenosine could be caused by the absence of certain adenosine receptors. To investigate the specific purinergic receptor subtypes which are expressed on MDA-MB-231 and Py8119 cells, we examined the mRNA expression of all P1 and several P2 receptors. We chose P2X5, P2X7, P2Y1, P2Y2, and P2Y11, as these are the P2 receptor subtypes most implicated in cancer11,12. MDA-MB-231 cells express all P1 receptors but A3, as well as all 5 P2 receptors assessed (Figure S7). Py8119 cells express all P1 receptors except A2A, as well as P2X5, P2X7, and P2Y2. Receptor P2Y11 was not evaluated in Py8119 cells as there has not been a rodent ortholog of P2Y11 reported18. These results suggest that the different response of Py8119 cells to adenosine stimulation could be due to its lack of the A2A receptor since this receptor subtype as shown above is responsible for the effect of adenosine on MDA-MB-231 cells.
ATPγS inhibited the tumor growth of human mammary carcinoma cells in nude mouse xenografts
Our in vitro data demonstrated the inhibitory effect of ATP on breast cancer cell growth and migration. To test if ATP has a similar, inhibitory effect on tumor growth in vivo, we used an orthotopic mouse model. MDA-MB-231 cells were orthotopically implanted into the mammary fat pads of athymic female nude mice. After the mice were randomly assigned into 3 different treatment groups, the mice were treated with or without ATPγS or adenosine. Dosages were determined by a previous study showing no toxicity from IP injections of up to 50 mM of adenine nucleotides into mice for 10 days15. At the end of the study, the tumors were excised and weighed. We found that the mice treated with ATPγS exhibited significantly reduced tumor growth rate in comparison to the control group, while the adenosine treated mice had an increase in tumor growth rate (Figure 8A). The reduced mean tumor volume of the treatment group was statistically significant after 17 days of ATPγS treatment. In post mortem analysis, the tumors excised from the mammary fat pads showed significantly (over 4 fold) decreased sizes in the ATPγS-treated group as compared to the control group. Additionally, the adenosine-treated group had 50% increased tumor sizes compared to the control group tumors (Figure 8B). These results reveal that systemic administration of ATPγS had an inhibitory effect on the growth of human breast cancer cells in vivo.
Figure 8.
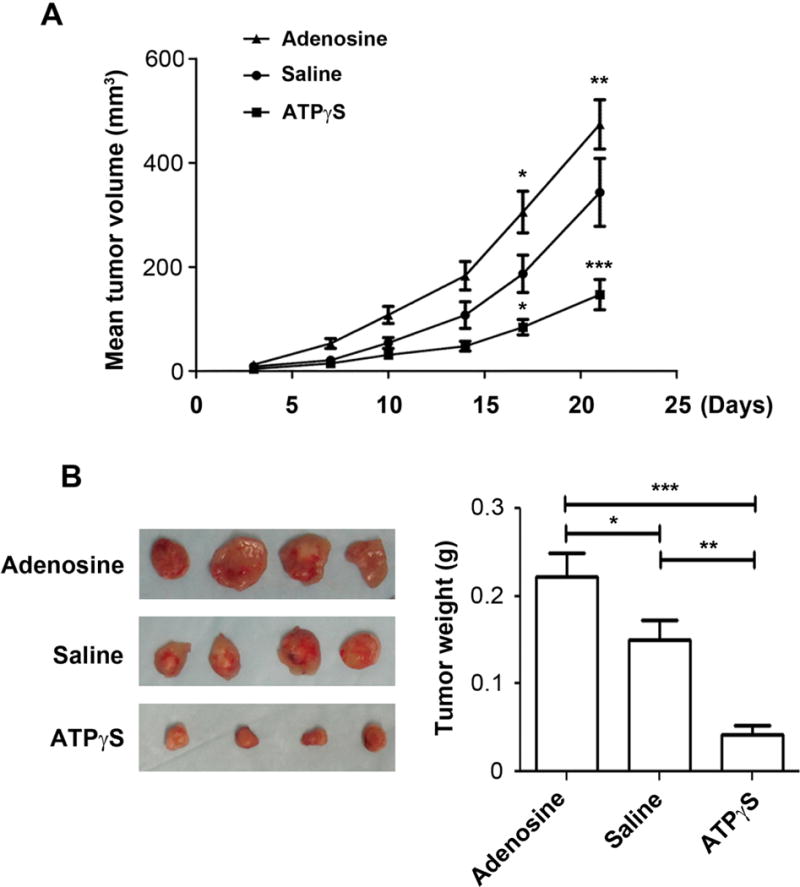
Systemic administration of ATPγS reduces the growth of MDA-MB-231 mammary cells in vivo. MDA-MB-231 cells were injected into the mammary fat pads of nude female mice at 1×106 cells per mouse. The mice were treated three times a week IP with 500 μl of saline or saline containing 400 μmol of ATPγS or adenosine. (A) Tumor volumes were calculated with the equation V= (L×W2) ×0.5 (mm3), where L is length and W is width of a tumor (n = 14 measurements per group). Data presented as mean ± SEM. *, P < 0.05; **, P < 0.01; ***, P < 0.001. (B) Left: Photomicrographs of orthotopic tumors excised from mice. Right: Tumor weight of the orthotopic tumor tissues from saline or ATPγS treated mice. Data presented as mean ± SEM (n=14 per group); *, P < 0.05; **, P < 0.01; ***, P < 0.001.
ATPγS inhibited the tumor growth and metastasis of mouse mammary carcinoma cells in vivo
To assess how systemic treatment with ATPγS may affect the growth of breast cancer cells in the bone microenvironment of a syngeneic host, we performed intratibial injections in wild-type C57bl/6 female mice using the mouse mammary carcinoma cell line Py8119. The mammary tumor cells were injected into the right tibias of the female mice, and the tumor growth was monitored with whole animal imaging once a week for 4 weeks. Bioluminescence analysis of the animals revealed that treatment with ATPγS significantly inhibited tumor growth in the tibias (Figure 9). Our results indicate that mice injected with ATPγS had a dramatic reduction in tumor burden after 4 weeks of treatment as reflected by bioluminescence signals from the images taken in both the dorsal (right panels) and ventral (left panels) positions. Quantification data (lower panels) further confirmed the significant decrease of tumor growth in bone with the treatment of ATPγS.
Figure 9.
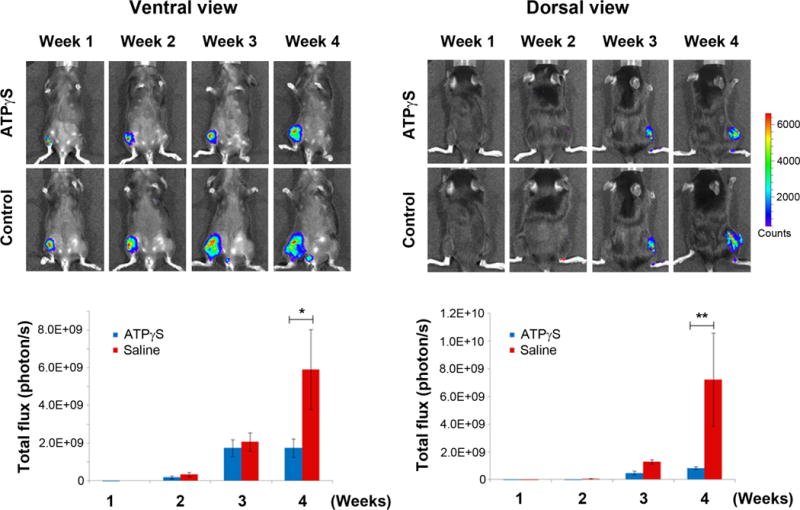
Systemic administration of ATPγS reduces the growth of Py8119 mammary carcinoma cells in bone. Py8119/Luc-GFP cells were injected into the right tibias of WT female mice at 1×105 cells per mouse. The mice were treated three times a week IP with 500 μl of saline or saline containing 400 μmol of ATPγS. Whole body imaging analysis of mice (n = 5 per group). Both ventral (left panel) and dorsal (right panel) views are shown. Total photon flux was taken once a week after tumor cell injection. Luciferase signals were quantified by using Living Image 3.2. Data presented as mean ± SEM (n = 5 per group). *, P < 0.05; **, P < 0.01.
DISCUSSION
The growth and migration of tumor cells are largely influenced by their microenvironment, and bone is one of the most preferred sites for cancer metastasis4. Osteocytes, the most abundant bone cell type, are known to release several factors, including prostaglandin, nitric oxide and ATP by mechanical stimulation19. Although osteocytes comprise over 95% of total cells in the bone, their involvement in cancer bone metastasis is unknown. We observed that CM collected from AD-treated osteocytes decreased numbers of breast cancer cells migrating to the other side of the transwell membrane. This decrease is caused by a reduction of cell migration but not a reduction of cell viability, as the WST-1 assay failed to detect any alteration in cell number. Furthermore, direct treatment with AD did not result in any change, indicating that the decrease in cell migration was not due to direct effects from AD. The inhibitory effect is likely to be mediated by ATP since depletion of ATP by apyrase or application of an antagonist of P2X receptors completely attenuated such effects. Additionally, the use of a P2X7 agonist and P2X7 siRNA inhibited the migration. We would expect that direct treatment with ATP could inhibit the migration. However, we observed a biphasic effect of ATP on the migration of MDA-MB-231 breast cancer cells; low concentration inhibited, but high concentrations promoted. Extracellular ATP can be hydrolyzed by ectonucleotidases released from the cell16. We suspected that products of ATP hydrolysis, such as adenosine, might exert an inverse effect from ATP on cancer cell migration. Indeed, prevention of ATP hydrolysis by an ecto-ATPase inhibitor, ARL67156, averted the stimulation of higher dosage of ATP on breast cancer cell migration. Moreover, treatment with non-hydrolyzable ATP analogues, ATPγS or BzATP, significantly attenuated this adverse effect. Additionally, ATPγS reduced MDA-MB-231 cell growth assessed by the soft agar colony formation assay. Consistently, we showed that adenosine has an opposite effect from ATP in promoting the growth and migration of human breast cancer cells. The treatment of an adenosine receptor antagonist or A2A siRNA attenuated the stimulatory effect of adenosine on cancer cells. Therefore, we have to be cautious when investigating the involvement of ATP on tumorigenesis or other diseases since it could largely depend on the stability of this molecule in the body.
Extracellular nucleotides and nucleosides have been shown to participate in signal transduction through purinergic receptors and affect a variety of cellular functions and processes such as inflammation, development and regeneration, and cancer20. Several previous studies have shown the increased levels of adenosine nucleotides in the tumor microenvironment. Adenosine concentrations increase under metabolically unfavorable conditions, such as cancer. Studies have demonstrated higher levels of extracellular adenosine in a solid tumor microenvironment compared to normal tissue (about two-fold increase)21. The physiological ATP concentration in human blood is normally submicromolar (20 – 100 nM), and it is estimated that the pericellular ATP concentration is in the low nanomolar range. Based on one study, tumor cells generate a microenvironment in which the ATP concentration is in the hundred micromolar range22. We quantified the ATP and adenosine in our CM and discovered over a hundred fold increase in ATP levels into the media from osteocyte release after AD treatment. The increased adenosine level was much less dramatic, although it was several fold difference compared to untreated osteocytes. The levels of ATP and adenosine released from the osteocytes into the CM are comparable to what is found in the aforementioned studies. This suggests that adenosine nucleotides may have a role in bone metastasis.
The main pathway leading to high adenosine levels is the hydrolysis of ATP by ectonucleotidases, such as CD39 and CD73. CD73 catalyzes the conversion of AMP to adenosine and has been associated with a pro-metastatic phenotype in breast cancer. CD73 is overexpressed in ER negative breast cancer cell lines and clinical samples23. Its expression is upregulated in both primary tumors and lymph node metastases. Additionally, knocking down CD73 in MDA-MB-231 breast cancer cells leads to growth suppression and inhibition of invasion and migration both in vivo and in vitro24. Furthermore, CD73 promotes the migration and invasion of both T47D and MDA-MB-231 breast cancer cells as confirmed with the use of overexpression and inhibition, revealing that adenosine increases the mobility of T47D and MDA-MB-231 cells25. As an enzyme that produces adenosine, CD73 is able to tightly regulate adenosine receptor interaction in many tissues. Therefore, it is important to not exclude the possibility that the stimulatory effects that we observe in breast cancer cell migration and invasion may be partly due to the ectonucleotidase control of adenosine signaling.
Previous literature reported conflicting observations with regard to the roles of ATP and purinergic receptors in tumorigenesis. In accordance with our study, published studies have indicated biphasic effects of ATP on cancer cells. Multiple studies indicated that the action of ATP on P2 purinergic receptors leads to an anticancer effect12. On the other hand, other studies have shown that activation of P2 receptors in some breast cancer cell lines could cause an increase in cell migration and invasion26. This discrepancy could possibly be due to varying expression levels of P2 ATP receptors reported among different breast cancer cells types or activation of P1 adenosine receptors due to the ATP hydrolyzation by endonucleases. Additionally, there is increased expression of certain P2X receptors in breast tissue undergoing malignant change compared to normal breast tissue12.
Consistent with our observation of human breast cancer cells, a similar stimulatory effect of adenosine on cancer cell chemotaxis has been observed previously for A2058 melanoma cells and this response was inhibited by adenosine receptor antagonists27. Bladder and prostate carcinomas seem to be inhibited by the activation of the P1 adenosine receptors, and anti-proliferative, pro-apoptotic and pro-necrotic effects have been reported in several other different cell types11,13. It has also been reported that human primary breast tumor tissues express higher levels of P1 adenosine receptors than in matched normal breast tissues28. However, none of the published reports uncovered the biphasic effects of ATP as a result of its degradation and the adverse effect of adenosine in the same cancer cells. We found that the treatment with adenosine promoted human breast cancer cell migration. To further determine the specific roles of P2X7 and A2A receptors in influencing breast cancer cell migration, we used an RNAi method to knock down the expression of P2X7 or A2A with siRNA transfection. We found that the downregulation of P2X7 in MDA-MB-231 cells resulted in no effect when a P2 receptor agonist, ATPγS, was added. Furthermore, downregulation of A2A lead to no effect when a P1 receptor agonist, adenosine, was added. These data indicate the specific roles of P2X7 and A2A receptors in the inhibition and promotion of MDA-MB-231 cell migration, respectively.
We sought further confirmation of the role of ATP/adenosine in breast cancer cells by using an additional mammary carcinoma cell line from mouse, Py8119. Intriguingly, in contrast to the observation in MDA-MB-231 cells, Py8119 cell migration was inhibited by ATP, even at high concentrations. Through further investigation, we found that this discrepancy is due to the insensitivity of Py8119 cell migration to adenosine. MDA-MB-231 migration was inhibited by P1 receptor antagonists, such as MRS1754 (blocks A1, A2A, and A2B) and ANR94 (blocks A2A), whereas Py8119 cell migration was unaffected by treatment with either pharmacologic antagonist. Consistent with this, assessment of the purinergic receptor mRNA expression in both MDA-MB-231 and Py8119 cells revealed that Py8119 cells lack A2A expression, suggesting a possible reason for the lack of response to adenosine. These results suggest that A2A receptors could be the major purinergic receptor subtype involved in mediating the effect of adenosine in promoting breast cancer migration. Further investigation is warranted to determine the involvement of this receptor in other breast cancer cells as well as during cancer progression and metastasis.
We used two in vivo models to demonstrate the differential effects of adenosine nucleotides on breast cancer growth and bone metastasis. Firstly, we applied a breast cancer xenograft model where MDA-MB-231 human breast cancer cells were orthotopically injected into the mammary fat pad of nude female mice. This method is often used to examine primary tumor growth29. We chose this model to study the influence of adenosine nucleotides on breast cancer cell growth. Consistent with our in vitro observation of the anchorage-independent cell growth assay, human breast cancer cell growth was significantly attenuated with the treatment of ATPγS, while adenosine inversely enhanced the tumor growth in situ. The intracardiac metastasis model is often used to study the metastatic behavior of cancer cells into different organs30. However, due to the difficulty of producing bone metastases using the intracardiac injection metastasis model with breast cancer cells, we opted for the intratibial injection method. Intratibial injections are often performed for the purpose of studying the relationship of cancer cells and bone after a tumor has already metastasized to bone29. We directly injected tumor cells into the bone marrow cavity of the tibia to study the effects of ATPγS on the growth of mammary tumor cells in a bone microenvironment. Our in vivo studies demonstrate that systemic administration of the nonhydrolyzable ATPγS had an inhibitory effect on breast cancer growth and migration in both the human MDA-MB-231 breast cancer cells as well as the mouse Py8119 mammary carcinoma cells.
Clinically, intravenous ATP has been safely trialed in patients with advanced lung cancer31. In another randomized controlled trial, ATP infusions in patients with advanced non-small cell lung cancer had significantly increase overall survival at 9.3 months for ATP-treated vs. 3.5 months for control-treated patients32. There was a particularly significant survival benefit in the subgroup of patients with stage IIIB non-small cell lung adenocarcinoma, as opposed to stage IV, suggesting that the treatment is more effective at earlier stages. In another study, ATP treatment in 99 preterminal cancer patients (life expectancy <6 months) increased the short-term (0–8 weeks) and long-term (0–6 months) survival33. Although the exact mechanism of the patient response remains unclear, it is evident that ATP has considerable effect on survival. Together, our results point to the differential roles of adenosine nucleotides and purinergic receptors in breast cancer invasion and metastasis, and imply the possibility of utilizing these purinergic receptors as potential targets in cancer metastasis therapeutics.
MATERIALS & METHODS
Materials
MLO-Y4 osteocytic cells derived from mouse long bones were kindly provided by Lynda Bonewald (University of Missouri at Kansas City). Alendronate (4-amino-1-hydroxybutylidene-1, 1-bisphosphonic acid), ATP, ATPγS (adenosine 5′-[γ-thio] triphosphate tetralithium salt), BzATP (2′(3′)-O-(4-Benzoylbenzoyl)adenosine–5′-triphosphate tri(triethylammonium) salt), oxidized ATP (oATP), adenosine, apyrase, and MRS1754 were purchased from Sigma. ARL67156 was purchased from R&D systems.
Cell lines and cell cultures
The human breast carcinoma cell line MDA-MB-231 cells were grown in McCoy’s 5A Modified Media (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS) (Hyclone, Logan, UT, USA). The murine mammary carcinoma cell line Py8119 cells were grown in F12K nutrient media (Gibco) supplemented with 5% Fetal Clone II (Fisher Scientific). MLO-Y4 cells were cultured on rat tail collagen type I (BD Biosciences, San Jose, CA, USA) coated cell culture plates. Cells were cultured in α-modified essential medium (α-MEM) (Gibco) supplemented with 2.5% FBS and 2.5% bovine calf serum (BCS) (Hyclone). All cell lines were incubated in a 5% CO2 incubator at 37°C.
Conditioned Media (CM) preparation
MLO-Y4 cells were seeded onto 150 mm dishes (Corning, Corning, NY, USA) and incubated for 24 hr to allow attachment, after which media was removed and changed with α-modified essential medium (α-MEM) without phenol red (Gibco) supplemented with 2.5% FBS and 2.5% bovine calf serum (BCS) (Hyclone). MLO-Y4 cells were incubated in the absence or present of 20 μM AD in a 5% CO2 incubator at 37°C for 48 hr and the CM was collected.
Cell proliferation assay
Cell proliferation was assessed using the WST-1 (Water Soluble Tetrazolium salts) assay (Roche, Basel, Switzerland). A single cell suspension was plated in 96-well plates at 2.0 × 104 cells/well and allowed to attach to the plates at 37°C for 2 hr. The cells were then treated with CM collected from MLO-Y4 cells treated with or without 20 μM AD for 18 hr. After the treatment, cell viability was measured by adding 10 μl of Cell Proliferation Reagent WST-1 to each well and incubated for 1 hr at 37°C in a 5% CO2 incubator. The cell proliferation was measured at an emission wavelength of 450 nm with a Synergy HT Multi-Mode Microplate Reader (Biotek, Winooski, VT, USA).
Cell migration assay
Migration assays were performed in transwell membrane filter inserts in 24-well tissue culture plates (BD Biosciences, San Jose, CA, USA). The transwell membrane filter inserts contained 6.5-mm diameter, 8-μm pore size, 10-nm thick polycarbonate membranes. The breast cancer cell lines were harvested and resuspended in CM from MLO-Y4 cells with or without other compounds. Five-hundred microliter breast cancer cell suspensions were added to the upper side of the inserts at a density of 10 × 104 cells/insert and 750 μl CM with or without other compounds was added to the lower wells. Cells were incubated at 37°C for 18–20 hr. Cells that did not migrate through the filters were removed using cotton swabs, and cells that migrated through the inserts were fixed and stained with Hema 3 Stat Pack (Fisher Scientific). The number of migrated cells in 5 fields of view per insert was counted under a light microscope at magnification 10X.
Small interfering RNA (siRNA) transfection
MDA-MB-231 cells were transfected with 90 nM of P2X7 siRNA, 60 nM of A2A siRNA, or 90 nM Silencer Negative Control siRNA (Ambion, Austin, TX) using DharmaFECT 4 Transfection Reagent (Thermo Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Decreased mRNA expression of P2X7 or A2a was confirmed at 48 hr after transfection.
RT-PCR
Total RNA was extracted from MDA-MB-231 and Py8119 cells using the GeneJet RNA Purification kit (Thermo Scientific, Waltham, MA, USA). cDNA was synthesized by incubating 11 μl RNA (50 ng/μl) with 1μl Random Primers (Promega, Fitchburg, WI, USA) at 70°C for 10 min, transferred to ice for 3 min, and then was incubated with 4 μl of 5X First Buffer (Invitrogen, Carlsbad, CA, USA), 1 μl of 10 mM dNTP mix, and 2μl of 0.1 M DTT (Promega, Fitchburg, WI, USA). The reaction mix was incubated at 42°C for 5 min and then was incubated with 1 μl Superscript Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) at 42°C for 1 hr. The reaction was terminated by incubating at 70°C for 15 min and then with 1 μl RNase H (Thermo Scientific, Waltham, MA, USA) at 37°C for 20 min. Primer pairs for PCR were designed and verified by basic local alignment search tool (BLAST) to detect specific P1 and P2 receptors (Table 1). Optimal annealing temperatures for each primer pair was determined by testing PCR with genomic DNA using annealing temperatures ranging from 55 to 64°C. PCR was performed using JumpStart Taq DNA polymerase (Sigma-Aldrich, St. Louis, MO) and PCR products were separated on 2% agarose gels. Negative controls comprised of no template (water) to monitor for PCR product contamination. Genomic DNA was used as the positive control.
Table 1.
| Receptor | Species | Primer Sequence | Expected size | |
|---|---|---|---|---|
| A1 | Hum | Fwd | 5′-TGGCAATTGCTGTGGACCGCTACC-3′ | 122 |
| Hum | Rev | 5′-GCCCGCTCCACCGCACTCAGATT-3′ | ||
| A2A | Hum | Fwd | 5′-CCATCTTCAGTCTCCTGGCC-3′ | 94 |
| Hum | Rev | 5′-CGATGGCAAACGACAGCACCC-3′ | ||
| A2B | Hum | Fwd | 5′-TGGCCGTGGCAGTCGACAGATACC-3′ | 67 |
| Hum | Rev | 5′-CCAAAGGCAAGGACCCAGAGGACA-3′ | ||
| A3 | Hum | Fwd | 5′-CGCTGTGGACCGATACTTG-3′ | 78 |
| Hum | Rev | 5′-ATCCCACCAGGAATGACAC-3′ | ||
| P2X5 | Hum | Fwd | 5′-AGCACGTGAATTGCCTCTGCTTAC-3′ | 183 |
| Hum | Rev | 5′-ATCAGACGTGGAGGTCACTTTGCTC-3′ | ||
| P2X7 | Hum | Fwd | 5′-CTGCTCTCTTGAACAGTGCCGAAA-3′ | 270 |
| Hum | Rev | 5′-AGTGATGGAACCAACGGTCTAGGT-3′ | ||
| P2Y1 | Hum | Fwd | 5′-ACCTCAGACGAGTACCTGCGAAGT-3′ | 353 |
| Hum | Rev | 5′-AGAATGGGGTCCACACAACTGTTGAG-3′ | ||
| P2Y2 | Hum | Fwd | 5′-GTGTCTGGGCGTCTTACGACCTCT-3′ | 215 |
| Hum | Rev | 5′-GCATGACTGAGCTGTAGGCCACGAA-3′ | ||
| P2Y11 | Hum | Fwd | 5′-AGAAGCTGCGTGTGGCAGCGTTGGT-3′ | 369 |
| Hum | Rev | 5′-ACGGTTTAGGGGCGGCTGTGGCATT-3′ | ||
| A1 | Mus | Fwd | 5′-TCGTCTGGGTGCTGCCGCCATTG-3′ | 93 |
| Mus | Rev | 5′-GATCTTGAGCTCCTTCCCGTAGTA-3′ | ||
| A2A | Mus | Fwd | 5′-CCATCTTCAGTCTCCTGGCC-3′ | 94 |
| Mus | Rev | 5′-CGATGGCAAACGACAGCACCC-3′ | ||
| A2B | Mus | Fwd | 5′-GCCTCTTCCTCGCCTGCTTCGTG-3′ | 101 |
| Mus | Rev | 5′-GCAATGATCCCTCTCGCTCGTGTC-3′ | ||
| A3 | Mus | Fwd | 5′-ACGGTTACCACTCAAAGAAGAATA-3′ | 69 |
| Mus | Rev | 5′-TTTTGAGAGCTCGCTAAGGTTGC-3′ | ||
| P2X5 | Mus | Fwd | 5′-TCCCGGATGGCGAGTGTTCAGAGG-3′ | 58 |
| Mus | Rev | 5′-GTAGAGTTCCCCACCCGTAGACAG-3′ | ||
| P2X7 | Mus | Fwd | 5′-ACAGAGGGTGGGGTGACGA-3′ | 76 |
| Mus | Rev | 5′-ACTTGGCCTTCTGACTTGACATAG-3′ | ||
| P2Y1 | Mus | Fwd | 5′-GATAATTGTCCTGACGGTGTTTGCTGTG-3′ | 376 |
| Mus | Rev | 5′-TGTTGCTTCTTCTTGACCTGTGTA-3′ | ||
| P2Y2 | Mus | Fwd | 5′-GCTGCCGGTGCGCTGATGAACT-3′ | 92 |
| Mus | Rev | 5′-TGAGGCAGGAAACAGGAAGAACAG-3′ |
Soft agar colony formation assay
For anchorage-independent cell growth, MDA-MB-231 cells were plated in 0.4% agarose with complete medium supplemented with either 100 μM ATPγS or 200 μM adenosine on top of a 0.8% agarose base supplemented with complete medium. Cells were maintained for about 2 weeks before staining with p-iodonitrotetrazolium violet (Sigma-Aldrich, St. Louis, MO). The pictures were taken by scanner and the numbers of colonies were counted.
Quantification of ATP and adenosine using liquid chromatography-mass spectrometry (LC-MS)
ATP and adenosine were measured from the CM directly after filtering through a_5,000 NMWL Ultrafree-MC filter unit (Millipore, Bedford, MA) containing adenosine-13C10, 15N5-triplosphate (ATP13C, 15N) as internal standard, modified from Zhang et al (2011)34. LC-MS analysis was conducted on a Thermo Fisher Q Exactive mass spectrometer with on-line separation using a Thermo Fisher/Dionex Ultimate 3000 HPLC. HPLC conditions were: Waters XTerra-MS C18 column (3.5 μm, 150 mm × 2.1 mm i.d.); mobile phase A, 5 mM Hexylamine and 0.5% diethylamine in water, pH10; mobile phase B, 50% acetonitrile in water; flow rate, 400 μL/min; gradient, 1% B to 20% B over 10 min and followed by 20% B to 30% B over 10 min. Full scan mass spectra were acquired in the orbitrap using negative ion detection over a range of m/z 300 – 800 at 70,000 resolution (m/z 300). Identification was based on the metabolite accurate mass (± 5 ppm) and agreement with the HPLC retention time of authentic standards. Quantification was made by integration of extracted ion chromatograms of ATP and adenosine followed by comparison with the corresponding standard curves.
Animals
Four-week-old female athymic nude mice (Harlan Sprague–Dawley, Indianapolis, IN, USA) were used for the mammary fat pad injections. Four- to five-week old female C57bl/6 mice were used for the intratibial injections. Animals were maintained under the care and supervision of the Laboratory Animal Research facility at the University of Texas Health Science Center, San Antonio, Texas. The animal protocol was approved and monitored by the Institutional Animal Care and Use Committee.
In vivo xenograft experiment
MDA-MB-231 cells were injected subcutaneously in the mammary fat pad of 4-week-old female nu/nu athymic nude mice. Each mouse received bilateral subcutaneous inoculation in both the left and right inguinal mammary fat pad areas with 100 μl of cell suspension containing ~1 × 107 cells/ml in serum-free media. Animals were randomly assigned to 3 different groups, and solid tumors were allowed to form up to about 5 mm3 volume before treatments began. ATPγS, at 400 μmol/500 μl saline, adenosine, at 400 μmol/500 μl saline, or 500 μl of saline, were administered intraperitoneally (IP) three times a week for 3 weeks. The growth of xenograft tumors was monitored twice a week and tumor size was measured with a caliper in two dimensions. Tumor volumes were calculated with the equation V = (L×W2) × 0.5 (mm3), where L is length and W is width of a tumor.
Intratibial injections
Mice were anesthetized by isoflurane and were also given buprenorpine-HCl (0.3 mg/ml) as an analgesic. Intratibial injections were performed as described previously35. Py8119 cells expressing Luc-GFP (1 × 105 in 20 μl of PBS) were inoculated into the bone marrow area of right tibias through the pre-made hole by a Hamilton syringe fitted with a 30-gauge needle. PBS was injected into the left tibias as control. ATPγS, at 400 μmol/500 μl saline or 500 μl of saline, was administered IP twice a week for 5 weeks, beginning from day 1. Intratibial tumor growth was monitored with bioluminescence imaging with a Xenogen IVIS-Spectrum imaging system (Xenogen, Alameda, CA, USA) every week starting from 3 days after tumor cell inoculation.
Bioluminescence imaging analysis
Mice were anesthetized and D-luciferin (Caliper Life Sciences, Alameda, CA) was injected IP at 75 mg/kg in PBS. Xenogen IVIS Spectrum Imaging system was used to acquire bioluminescence images at 10 min after injection. Acquisition time was set at 60 sec at the beginning and reduced later on in accordance with signal strength to avoid saturation. Analysis was performed using LivingImage software (Xenogen) by measurement of photon flux (measured in photons/sec/cm2/steradian) with a region of interest (ROI) drawn around the bioluminescence signal to be measured. Tumor burden was taken by drawing an ROI around the major bioluminescence signal from the hind limb.
Statistical analysis
Unless otherwise specified in the Figure Legends, the data are presented as the mean ± S.E.M. of at least three determinations. Asterisks indicate the degree of significant differences compared with the controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Oneway analysis of variance (ANOVA) and Student Newman-Keuls test were used to compare groups using GraphPad Prism 5.04 software (GraphPad, La Jolla, CA, USA).
Supplementary Material
Acknowledgments
The authors would like to thank Drs. Sumin Gu, Sweta Mishra and Tanuka Biswas for technical assistance. The work was supported by Welch Foundation grant AQ-1507 and National Institutes of Health grant EY012085 to JXJ, ES022057 to LZS, CA118182 to LGE, and the NCI Cancer Center Support Grant 2 P30 CA054174-17 to Cancer Therapy and Research Center.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Kohno N. Treatment of breast cancer with bone metastasis: bisphosphonate treatment – current and future. Int J Clin Oncol. 2008;13:18–23. doi: 10.1007/s10147-007-0726-2. [DOI] [PubMed] [Google Scholar]
- 2.Jin JK, Dayyani FGallick GE. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer. 2011;128:2545–2561. doi: 10.1002/ijc.26024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welch DR, Harms JF, Mastro AM, Gay CVDonahue HJ. Breast cancer metastasis to bone: evolving models and research challenges. J Musculoskelet Neuronal Interact. 2003;3:30–38. [PubMed] [Google Scholar]
- 4.Roodman GD. Mechanism of bone metastasis. N Engl J Med. 2004;350:1655–1664. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 5.van der Pluijm G, Sijmons B, Vloedgraven H, Deckers M, Papapoulos SLowik C. Monitoring metastatic behavior of human tumor cells in mice with species-specific polymerase chain reaction: elevated expression of angiogenesis and bone resporption stimulators by breast cancer in bone metastases. J Bone Miner Res. 2001;16:1077–1091. doi: 10.1359/jbmr.2001.16.6.1077. [DOI] [PubMed] [Google Scholar]
- 6.Bonewald LF. Osteocytes as dynamic multifucntional cells. Ann N Y Acad Sci. 2007;1116:281–290. doi: 10.1196/annals.1402.018. [DOI] [PubMed] [Google Scholar]
- 7.Matsuo K. Cross-talk among bone cells. Curr Opin Nephrol Hypertens. 2009;18:292–297. doi: 10.1097/MNH.0b013e32832b75f1. [DOI] [PubMed] [Google Scholar]
- 8.Brown SAGuise TA. Cancer-associated bone disease. Cur Osteopor Rep. 2007;5:120–127. doi: 10.1007/s11914-007-0027-8. [DOI] [PubMed] [Google Scholar]
- 9.Plotkin LI, Manolagas SCBellido T. Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem. 2002;277:8648–8657. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- 10.Genetos DC, Kephart CJ, Zhang Y, Yellowley CEDonahue HJ. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol. 2007;212:207–214. doi: 10.1002/jcp.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shabbir MBurnstock G. Purinergic receptor-mediated effects of adenosine 5′-triphosphate in urological malignant diseases. Int J Urol. 2009;16:143–150. doi: 10.1111/j.1442-2042.2008.02207.x. [DOI] [PubMed] [Google Scholar]
- 12.White NBurnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27:211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Rapaport E, Fishman RFGercel C. Growth inhibition of human tumor cells in soft-agar cultures by treatment with low levels of adenosine 5′-triphosphate. Cancer Res. 1983;43:4402–4406. [PubMed] [Google Scholar]
- 14.Salvestrini V, Zini R, Rossi L, Gulinelli S, Manfredini R, Bianchi E, Piacibello W, Caione L, Miqliardi G, Ricciardi MR, Tafuri A, Romano M, Salati S, Di Vigilio F, Ferrari S, Baccarani M, Ferrari DLemoli RM. Purinergic signaling inhibits human acute myeloblastic leukemia cell proliferation, migration, and engraftment in immunodeficient mice. Blood. 2012;119:217–226. doi: 10.1182/blood-2011-07-370775. [DOI] [PubMed] [Google Scholar]
- 15.Rapaport E. Experimental cancer therapy in mice by adenine nucleotides. Eur J Cancer Clin Oncol. 1988;24:1491–1497. doi: 10.1016/0277-5379(88)90340-9. [DOI] [PubMed] [Google Scholar]
- 16.Deli TCsernoch L. Extracellular ATP and cancer: an overview with special reference to P2 purinergic receptors. Pathol Oncol Res. 2008;14:219–231. doi: 10.1007/s12253-008-9071-7. [DOI] [PubMed] [Google Scholar]
- 17.Savariar EN, Felsen CN, Nashi N, Jiang T, Ellies LG, Steinbach P, Tsien RYNguyen QT. Real-time in vivo molecular detection of primary tumors and metastases with ratiometric activatable cell-penetrating peptides. Cancer Res. 2013;73:855–864. doi: 10.1158/0008-5472.CAN-12-2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KAWeisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Batra N, Kar RJiang JX. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim Biophys Acta. 2012;1818:1909–1918. doi: 10.1016/j.bbamem.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burnstock G. Unresolved issues and controversites in purinergic signalling. J Physiol. 2008;586:3307–3312. doi: 10.1113/jphysiol.2008.155903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams SSitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103:13132–13137. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Falzoni S, Donvito GDi Virgilio F. Detecting adenosine triphosphate in the pericellular space. Interface Focus. 2013;3:20120101. doi: 10.1098/rsfs.2012.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leth-Larsen R, Lund R, Hansen HV, Laenkholm AV, Tarin D, Jensen ONDitzel HJ. Metastasis-related plasma membrane proteins of human breast cancer cells identified by comparative quantitative mass spectrometry. Mol Cell Proteomics. 2009;8:1436–1449. doi: 10.1074/mcp.M800061-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhi X, Chen S, Zhou P, Shao Z, Wang L, Ou ZYin L. RNA interference of ecto-5′-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Zhou X, Zhou T, Ma D, Chen S, Zhi X, Yin L, Shao Z, Ou ZZhou P. Ecto-5′-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PubMed] [Google Scholar]
- 26.Jelassi B, Chantôme A, Alcaraz-Pérez F, Baroja-Mazo A, Cayuela ML, Pelegrin P, Surprenant ARoger S. P2X(7) receptor activation enhances SK3 channels- and cystein cathepsin-dependent cancer cells invasiveness. Oncogene. 2011;30:2108–2122. doi: 10.1038/onc.2010.593. [DOI] [PubMed] [Google Scholar]
- 27.Woodhouse EC, Amanatullah DF, Schetz JA, Liotta LA, Stracke MLClair T. Adenosine receptor mediates motility in human melanoma cells. Biochem Biophys Res Commun. 1998;246:888–894. doi: 10.1006/bbrc.1998.8714. [DOI] [PubMed] [Google Scholar]
- 28.Gessi S, Merighi S, Sacchetto V, Simioni CBorea PA. Adenosine receptors and cancer. Biochim Biophys Acta. 2011;1808:1400–1412. doi: 10.1016/j.bbamem.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 29.Campbell JP, Merkel AR, Masood-Campbell SK, Elefteriou F, Sterling JA. Models of bone metastasis. J Vis Exp. 2012:e4260. doi: 10.3791/4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kretschmann KLWelm AL. Mouse models of breast cancer metastasis to bone. Cancer Metastasis Rev. 2012;31:579–583. doi: 10.1007/s10555-012-9378-4. [DOI] [PubMed] [Google Scholar]
- 31.Agteresch HJ, Dagnelie PC, Rietveld T, van den Berg JW, Danser AHWilson JH. Pharmacokinetics of intravenous ATP in cancer patients. Eur J Clin Pharmacol. 2000;56:49–55. doi: 10.1007/s002280050719. [DOI] [PubMed] [Google Scholar]
- 32.Agteresch HJ, Burgers SA, van der GA, Wilson JHDagnelie PC. Randomized clinical trial of adenosine 5′-triphosphate on tumor growth and survival in advanced lung cancer patients. Anticancer Drugs. 2003;14:639–644. doi: 10.1097/00001813-200309000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Beijer S, Hupperets PS, van den Borne BE, Eussen SR, van Henten AM, van den Beuken-van Everdingen, de Graeff A, Ambergen TA, van den Brandt PADagnelie PC. Effect of adenosine 5′-triphosphate infusions on the nutritional status and survival of preterminal cancer patients. Anticancer Drugs. 2009;20:625–633. doi: 10.1097/CAD.0b013e32832d4f22. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Tan S, Paintsil E, Dutschman GE, Gullen EA, Chu ECheng YC. Analysis of deoxyribonucleotide pools in human cancer cell lines using a liquid chromatography coupled with tandem mass spectrometry technique. Biochem Pharmacol. 2011;82:411–417. doi: 10.1016/j.bcp.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mishra S, Tang Y, Wang L, DeGraffenried L, Yeh IT, Werner S, Troyer D, Copland JASun LZ. Blockade of transforming growth factor-beta (TGFbeta) signaling inhibits osteoblastic tumorigenesis by a novel human prostate cancer cell line. Prostate. 2011;71:1441–1454. doi: 10.1002/pros.21361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


