Abstract
The purpose of this study was to examine the influence of fat-free adipose tissue mass (FFAT) on association between dual-energy X-ray absorptiometry (DXA)-derived lean soft tissue mass and skeletal muscle mass (TMM). Forty-one middle-aged and older women were recruited for this study. Percent body fat, total and appendicular fat mass (tFM and aFM, respectively), and total and appendicular lean soft tissue mass (tLM and aLM, respectively) were measured using a DXA. FFAT was calculated based on the methods of a previous study. TMM was estimated from the ultrasound-derived prediction equation. The subjects were separated into three groups based on DXA-determined percent fat: low (n = 12, <25 %), middle (n = 15, ≥25 and <35 %), and high (n = 14, ≥35 %). DXA-derived aLM was greater in high than in middle or low, although ultrasound-estimated TMM was similar among the three groups. There was a strong correlation between aLM and TMM (r = 0.905, p < 0.001). The difference between aLM and TMM was correlated (p < 0.001) with aFM (r = 0.599) and tFM (r = 0.587). After adjusting for FFAT, aLM minus appendicular FFAT was similar among the three groups. aLM minus appendicular FFAT was strongly associated with TMM (r = 0.912, p < 0.001). Our results suggest that DXA-derived aLM accurately predicts TMM when subjects have moderate or lower adipose tissue mass. However, FFAT may falsely inflate the DXA-derived aLM measurement in individuals with a relatively high amount of adipose tissue mass (>35 % of body fat). Therefore, in this population, it is advisable to use DXA-derived aLM minus FFAT when evaluating age-related loss of skeletal muscle mass.
Keywords: Skeletal muscle, Fat-free mass, Body composition, Ultrasound
Introduction
Dual-energy X-ray absorptiometry (DXA) is widely viewed as the preferred method to assess body composition, especially bone mineral content and appendicular lean soft tissue mass (aLM). The DXA-derived aLM, as a major criterion for characterizing age-related loss of skeletal muscle mass, is commonly used and reported previously in many studies (Baumgartner et al. 1998; Cruz-Jentoft et al. 2010; Bijlsma et al. 2013). Lean soft tissue mass measured by DXA, however, contains nonskeletal muscle tissue components such as adipose tissue (fat-free adipose tissue: FFAT) (Heymsfield et al. 2002; Pietrobelli et al. 1996). A study reported that adipose tissue mass in middle-aged women (body mass index 25.1 ± 5.2 kg/m2) is up to 40 kg, and the FFAT component accounted for 15 % of the total adipose tissue (Heymsfield et al. 2002). Therefore, the calculated FFAT is ~5.3 kg. We hypothesized that after adjusting for the FFAT component, DXA-derived aLM minus FFAT would be more closely correlated to total skeletal muscle mass (TMM) compared to aLM itself. Although several studies have reported the relationship between DXA-derived aLM and TMM (Kim et al. 2002, 2006), the influence of FFAT on the association between aLM and TMM has not been investigated. In order to test our hypothesis, we examined the relationship between aLM minus FFAT and ultrasound-estimated TMM in middle-aged and older women.
Methods
Participants
Forty-one women aged 50 to 78 years volunteered for the study. The participants were recruited from the university campus and surrounding area. Prior to obtaining informed consent, a written description of the purpose of the study and its safety was distributed to potential subjects. All subjects were free of overt chronic disease (e.g., neuromuscular, diabetes, angina, myocardial infarction, cancer, stroke) as assessed by self-report. The subjects were not taking any medications known to affect muscle, such as angiotensin II receptor blockers, statins, steroids, or anti-diabetic drugs. Subjects being treated for mild hypertension with β-blockers or diuretics were allowed to participate in the study. Approximately 40 % of the subjects (17 women) reported participation in regular sports activity (at least twice a week) including running and cycling exercise. This study was conducted according to the Declaration of Helsinki and was approved by the university’s Institutional Review Board, and written informed consent was obtained from participants.
DXA-determined body composition
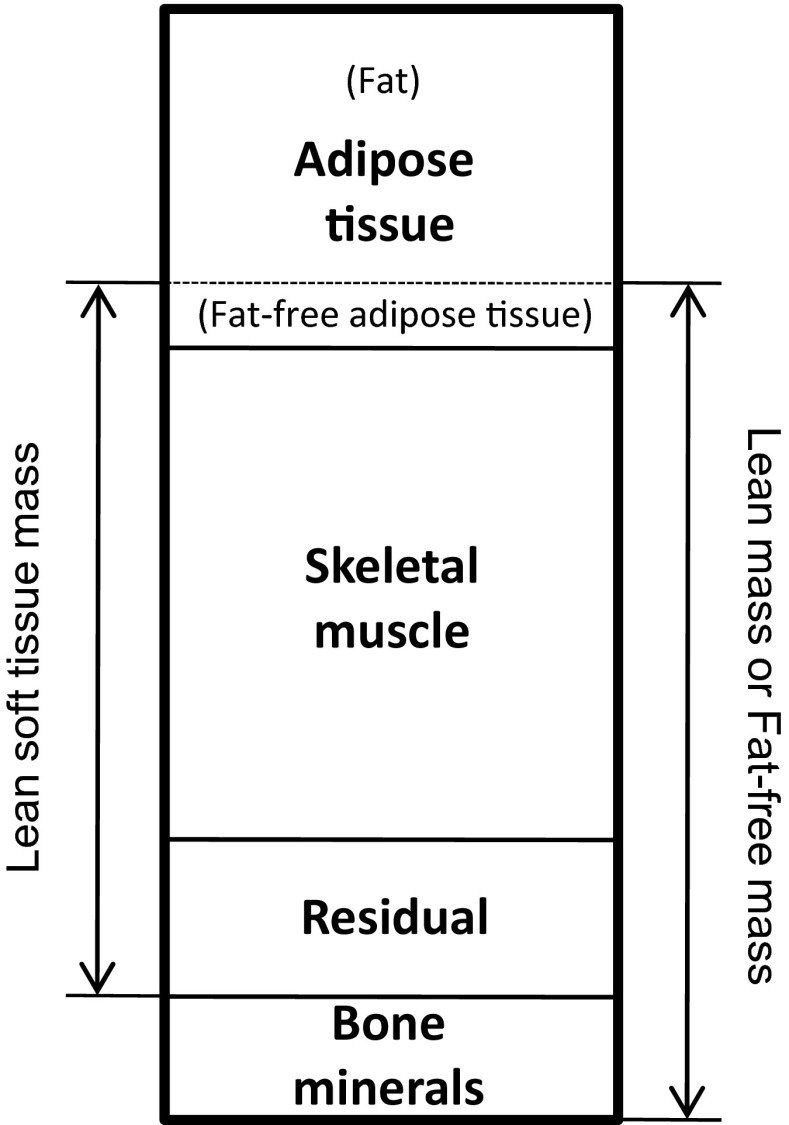
Subjects underwent dual-energy X-ray absorptiometry (DXA) scans (Discovery A, Hologic Inc., Bedford, MA, USA) to determine percent body fat (%fat), total body and appendicular fat mass (tFM and aFM, respectively), and total and appendicular lean soft tissue mass (tLM and aLM, respectively). FFAT was calculated based on the methods of Heymsfield et al. (Heymsfield et al. 2002) where they reported that 85 % of adipose tissue is fat and 15 % of adipose tissue is the remaining calculated fat-free component (Fig. 1). FFAT can then be calculated as FFAT = fat mass ÷ 0.85 × 0.15. Adjusting for the influence of FFAT on DXA-determined lean soft tissue mass, we calculated an index: aLM minus appendicular FFAT. Quality assurance testing and calibration was performed the morning of data collection days to ensure that the DXA was operating properly. Subjects were asked to refrain from eating for at least 4 h prior to scans and were offered water ad libitum. Also, subjects were asked to refrain from moderate/vigorous exercise for at least 48 h prior to the scans. DXA scans were conducted immediately before or after ultrasound measurements. Test–retest reliability using intraclass correlation coefficient (ICC3,1), standard error of measurement (SEM), and minimal difference to be considered real were previously determined from 17 subjects scanned twice for aLM (0.99, 0.21 kg, and 0.58 kg), tLM (0.99, 0.36 kg, and 0.71 kg), and %fat (0.99, 0.49 %, and 0.95 %) (Abe et al. 2014b).
Fig. 1.
Relations between DXA-determined lean soft tissue mass and skeletal muscle mass. DXA dual-energy X-ray absorptiometry
Ultrasound-estimated skeletal muscle mass
TMM was estimated from the ultrasound-derived prediction equation developed by Sanada et al. (Sanada et al. 2006). The sum of muscle thickness (MT) at nine sites was used to estimate TMM: TMM (kg) = 0.594 × sum MT (cm) × height (m) − 11.32 (R2 = 0.83, standard error of estimate (SEE) = 2.75 kg). MT was measured using B-mode ultrasound (SSD-500, Aloka, Tokyo, Japan) at nine sites (lateral forearm [at 30 % proximal between the styloid process and the head of the radius], anterior and posterior upper arm [at 60 % distal between the lateral epicondyle of the humerus and the acromial process of the shoulder], anterior and posterior thigh [midway between the lateral condyle of the femur and greater trochanter], anterior and posterior lower leg [at 30 % proximal between the lateral malleolus of the fibula and the lateral condyle of the tibia], abdomen [about 3 cm lateral to the umbilicus], and subscapula [about 5 cm below to the inferior angle of the scapula]) on the right side of the body as previously described (Abe et al. 1994). After measurement of limb length using anatomical landmarks described above, all measurement sites were marked with a marker pen. The measurements were taken while the women stood quietly with their elbows and knees extended and relaxed (Sanada et al. 2006). A linear transducer with a 5-MHz scanning head was coated with water-soluble transmission gel to provide acoustic contact and reduce pressure by the scanning head to achieve a clear image. The scanning head was placed on the skin surface of the measurement site using the minimum pressure required, and cross-sections of each muscle were imaged. Images from each site were printed (SONY UP-897MD, Tokyo, Japan), and values of each site were used for estimating TMM. The subcutaneous adipose tissue–muscle interface and muscle–bone interface were identified from the ultrasonic image, and the distance between the two interfaces was accepted as MT for limb muscles. For measurements in trunk, MT was defined as the distance between the adipose tissue–muscle interface and the deep muscle fascia interface. Test–retest reliability of MT measurements using ICC3,1, SEM, and minimum difference was previously determined from 15 middle-aged subjects for anterior (0.88, 0.08 cm, and 0.22 cm) and posterior (0.96, 0.08 cm, and 0.22 cm) upper arm and anterior (0.98, 0.07 cm, and 0.19 cm) and posterior (0.95, 0.10 cm, and 0.28 cm) thigh (Abe et al. 2014b).
Statistical analysis
All data are presented as mean and standard deviation (SD). The participants were separated into three groups based on DXA-determined percent body fat: low (n = 12, less than 25 %), middle (n = 15, between 25 and 35 %), and high (n = 14, over 35 %), and the differences among the three groups were tested for significance using a one-way analysis of variance (ANOVA). Pearson product correlation coefficients were carried out to determine the relationships between ultrasound estimated TMM and DXA-determined aLM or aLM minus appendicular FFAT, and between aLM minus TMM and tFM or aFM. Significance was set at p < 0.05.
Results
Age and height were similar among the three groups. As might be expected, the high group was heavier and had more body fat mass (both tFM and aFM) than the low and middle groups. tFM and aFM were also higher in the middle compared to the low group. There was a strong correlation between tFM and aFM (r = 0.976, p < 0.0001, n = 41). Significant group differences were also observed for appendicular FFAT, with high having greater FFAT compared to both middle and low and middle being significantly higher than low (1.1 kg in low, 1.8 kg in middle, and 3.1 kg in high group). With regard to lean mass variables, the high group had significantly greater tLM and aLM compared to the middle and low groups, with no significant differences between the middle and low groups. In contrast, ultrasound-estimated TMM was similar among the three groups (Table 1).
Table 1.
DXA-determined appendicular lean soft tissue mass and ultrasound-estimated total skeletal muscle mass of lower, middle, and higher body fat percentage groups in middle-aged and older women
| Percent body fat by DXA | ||||
|---|---|---|---|---|
| Lower, <25 % | Middle, ≥25 and <35 % | Higher, ≥35 % | Overall | |
| N | 12 | 15 | 14 | 41 |
| Age (year) | 58 (4) | 57 (7) | 60 (8) | 58 (7) |
| Height (m) | 1.63 (0.04) | 1.63 (0.05) | 1.64 (0.07) | 1.63 (0.05) |
| Body mass (kg) | 54.4 (6.5) | 63.4 (10.0) | 86.3 (17.5)a,b | 68.6 (18.0) |
| Body mass index (kg/m2) | 20.5 (2.2) | 23.9 (3.7) | 32.1 (5.5)a,b | 25.7 (6.3) |
| Body fatDXA (%) | 21.6 (3.1) | 31.2 (2.3)a | 39.3 (3.5)a,b | 31.0 (7.8) |
| FMDXA (kg) | 11.5 (2.3) | 19.7 (3.7)a | 33.9 (9.4)a,b | 22.1 (10.9) |
| aFMDXA (kg) | 6.2 (1.7) | 10.1 (2.2)a | 17.4 (5.1)a,b | 11.4 (5.7) |
| FFATappendicular (kg) | 1.1 (0.3) | 1.8 (0.4)a | 3.1 (0.9)a,b | 2.0 (1.0) |
| LMDXA (kg) | 40.5 (4.9) | 41.2 (6.4) | 49.3 (8.4)a,b | 43.7 (7.7) |
| aLMDXA (kg) | 17.4 (2.4) | 17.3 (3.1) | 20.6 (3.6)a,b | 18.5 (3.4) |
| aLM minus FFATappendicular (kg) | 16.3 (2.3) | 15.6 (2.9) | 17.4 (3.0) | 16.5 (2.8) |
| SMUS (kg) | 16.5 (2.3) | 16.2 (2.9) | 18.6 (2.7) | 17.2 (2.8) |
FM total fat mass, aFM appendicular fat mass, LM total lean soft tissue mass, aLM appendicular lean soft tissue mass, FFAT fat-free adipose tissue mass, SM skeletal muscle mass, DXA dual-energy x-ray absorptiometry, US ultrasound
aSignificant group difference from the lower group
bSignificant group difference from the middle group
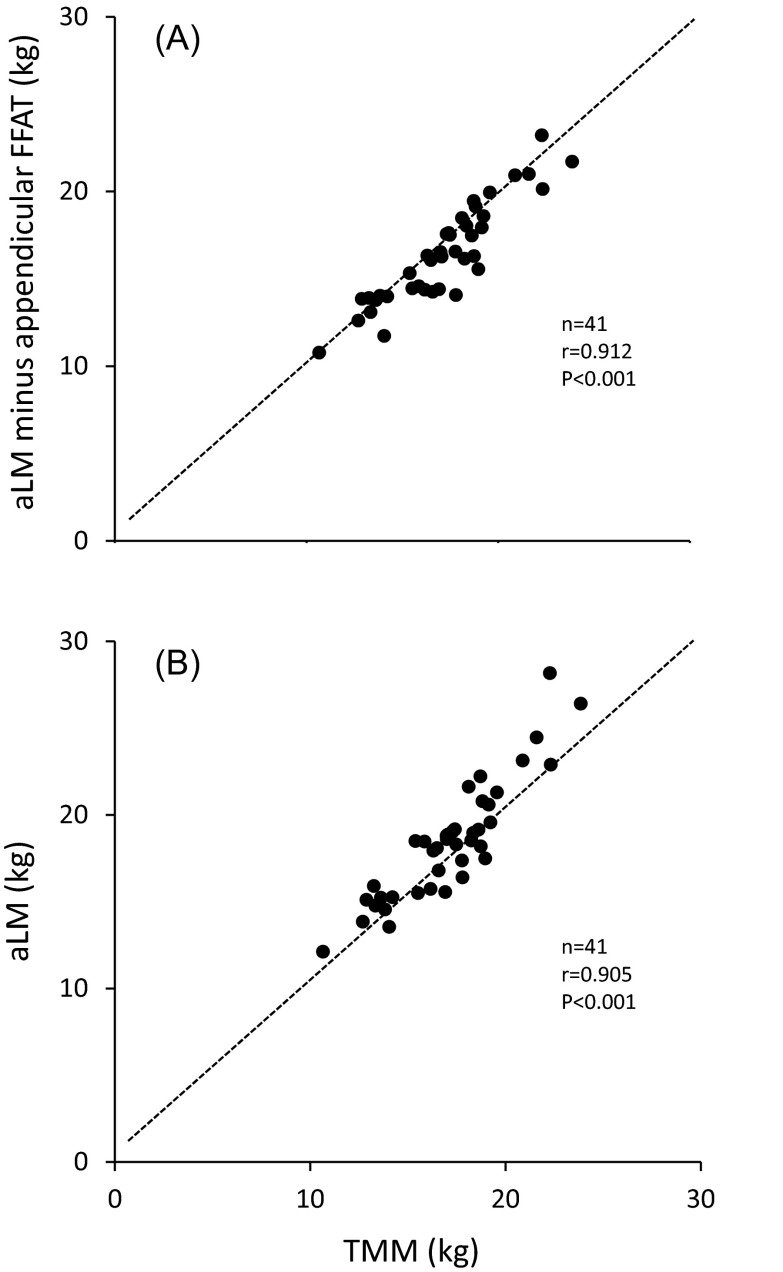
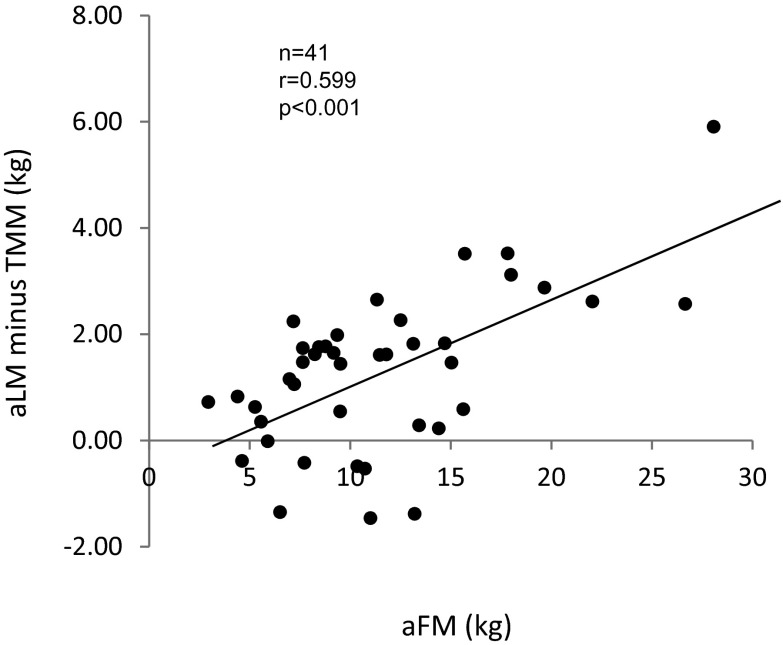
There was a strong positive correlation between aLM and TMM (r = 0.905, p < 0.001; Fig. 2b). A regression analysis between aLM and TMM resulted in aLM (kg) = 0.754 × TMM (kg) + 3.23 (SEE = 1.07 kg, n = 41). The difference between aLM and TMM was correlated with aFM (r = 0.599, p < 0.001; Fig. 3) and tFM (r = 0.587, p < 0.001). After adjusting for the influence of FFAT, aLM minus appendicular FFAT was similar among the three groups (Table 1). There was a strong association between aLM minus appendicular FFAT and TMM (r = 0.912, p < 0.001; Fig. 2a).
Fig. 2.
Relationships between ultrasound-estimated total skeletal muscle mass (TMM) and appendicular lean soft tissue mass (aLM) or aLM minus appendicular FFAT. FFAT fat-free adipose tissue mass
Fig. 3.
Relationship between DXA-determined appendicular fat mass (aFM) and aLM minus FFAT. aLM appendicular lean soft tissue mass, FFAT fat-free adipose tissue mass
Discussion
In the present study, aLM was higher in the high group than in the other two groups, although ultrasound-determined TMM was similar. A large proportion of skeletal muscle mass is found in the arms and legs including the shoulders and glutei (Abe et al. 2003). DXA-derived aLM does not include muscle mass of the trunk region. However, muscle mass in the shoulder and hip region are included in the DXA-determined aLM measurement. If resistance training induced an increase in skeletal muscle mass in the arms and legs as well as the shoulder and hip joint muscles, the aLM measurement would be sensitive to this change. However, the same would not hold true for the iliopsoas muscle (Abe et al. 2014a). The DXA-derived aLM measurement also includes skin and connective tissue mass of the extremities (Heymsfield et al. 2002; Kim et al. 2002). Conversely, the ultrasound-derived prediction of TMM used in this study includes muscle mass of the trunk, but does not include the skin and a large portion of connective tissue found in the extremities. Furthermore, the ultrasound-derived prediction equation of TMM is a valid method to predict TMM and an alternative to magnetic resonance imaging (MRI) in healthy adults and children (Sanada et al. 2006; Midorikawa et al. 2009). These methodological differences between the two techniques may explain the results observed here in both the low and middle groups that aLM by DXA is approximated by ultrasound-predicted TMM. However, the reasons described above cannot explain the observation that aLM was greater in the high group compared to the other groups. In this study, aLM minus appendicular FFAT was similar among all the three groups. Thus, based on these data, we believe the reason for the higher aLM observed in the high group is mainly due to the large amount of adipose tissue mass in the extremities.
Our findings suggest that the difference in appendicular FFAT between the low and high groups is 2.0 kg in these women. Baumgartner et al. (Baumgartner et al. 1998) proposed a definition for sarcopenia such that severe sarcopenia is a DXA-derived aLM index (aLM relative to height squared, kg/m2) value of 2 standard deviations (SD) below the mean for young adult women with a reference value of 5.45 kg/m2. This cutoff value for severe sarcopenia was calculated using mean and SD (7.3 ± 0.9 kg/m2) of the Rosetta Study (Gallagher et al. 1997) reference data for young women (1.63 m in height) aged 18–40 years (Baumgartner et al. 1998). In order to determine the influence of appendicular FFAT, we calculated a value relative to height squared of the difference in appendicular FFAT between the low and high groups, which was 2.0 kg. When we use a value of 1.63 m in height, the calculated relative value would be 0.75 kg/m2 (2.0/1.632). The value 0.75 is closely related to 1 SD of the reference data for young women.
Another interesting aspect of the present study is the regression model between DXA-derived aLM and TMM. A regression model between MRI-measured TMM and DXA-derived aLM has been reported using a sample of healthy men and women (TMM (kg) = 1.19 × aLM (kg) − 1.01; R2 = 0.96) (Kim et al. 2002). In the present study, our regression model in women is different (TMM (kg) = 0.75 × aLM + 3.23; R2 = 0.82), which may be due to the relatively small range of the variables. After adjusting for FFAT, however, the regression line between aLM minus appendicular FFAT and TMM is almost identical (TMM (kg) = 0.92 × aLM minus appendicular FFAT + 1.95; R2 = 0.83) with the line of Y = X. Therefore, we believe DXA-derived aLM adjusted for FFAT is a better parameter to evaluate age-related loss of skeletal muscle mass in older populations.
In conclusion, our results suggest that DXA-derived aLM accurately predicts TMM when subjects have moderate or lower adipose tissue mass. However, FFAT may falsely inflate the DXA-derived aLM measurement in individuals with a relatively high amount of adipose tissue mass (>35 % of body fat). Therefore, in this population, it is advisable to use either DXA-derived aLM minus FFAT or alternative method such as ultrasound-estimated TMM when evaluating age-related loss of skeletal muscle mass.
Acknowledgments
Conflict of interest
None of the authors had financial or personal conflict of interest with regard to this study.
References
- Abe T, Kondo M, Kawakami Y, Fukunaga T. Prediction equations for body composition of Japanese adults by B-mode ultrasound. Am J Hum Biol. 1994;6:161–170. doi: 10.1002/ajhb.1310060204. [DOI] [PubMed] [Google Scholar]
- Abe T, Kearns CF, Fukunaga T. Sex differences in whole body skeletal muscle mass measured by magnetic resonance imaging and its distribution in young Japanese adults. Br J Sports Med. 2003;37:436–440. doi: 10.1136/bjsm.37.5.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe T, Loenneke JP, Kojima K, Thiebaud RS, Fahs CA, Sekiguchi O. Influence of strength training on distribution of trunk and appendicular muscle mass. J Aging Res Clin Pract. 2014;3:28–30. [Google Scholar]
- Abe T, Patterson KM, Stover CD, Geddam DA, Tribby AC, Lajza DG, Young KC. Site-specific thigh muscle loss as an independent phenomenon for age-related muscle loss in middle-aged and older men and women. Age (Dordr) 2014;36:1353–1358. doi: 10.1007/s11357-014-9634-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross R, Garry PJ, Linderman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bijlsma AY, Meskers CG, Ling CH, Narici M, Kurrie SE, Cameron ID, Westendorp RG, Maler AB. Defining sarcopenia: the impact of different diagnostic criteria on the prevalence of sarcopenia in a large middle aged cohort. Age (Dordr) 2013;35:871–881. doi: 10.1007/s11357-012-9384-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher D, Visser M, De Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- Kim J, Shen W, Gallagher D, Jones A, Jr, Wang Z, Wang J, Heshka S, Heymsfield SB. Total-body skeletal muscle mass: estimation by dual-energy X-ray absorptiometry in children and adolescents. Am J Clin Nutr. 2006;84:1014–1020. doi: 10.1093/ajcn/84.5.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midorikawa T, Yoshitomi A, Sanada K, Abe T. Is the use of ultrasound-derived prediction equations for adults useful for estimating total and regional skeletal muscle mass in Japanese children? Br J Nutr. 2009;101:72–78. doi: 10.1017/S000711450899440X. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271:E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- Sanada K, Kearns CF, Midorikawa T, Abe T. Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol. 2006;96:24–31. doi: 10.1007/s00421-005-0061-0. [DOI] [PubMed] [Google Scholar]