Abstract
Stepdown beds provide an intermediate level of care for patients with requirements somewhere between that of the general ward and the intensive care unit. Models of care include incorporation of stepdown beds into intensive care units, stand-alone units, or incorporation of beds into standard wards. Stepdown beds may be used to provide a higher level of care for patients deteriorating on a ward (“step-up”), a lower level of care for patients transitioning out of intensive care (“stepdown”) or a lateral transfer of care from a recovery room for postoperative patients. These units are one possible strategy to improve critical care cost-effectiveness and patient flow without compromising quality, but these potential benefits remain primarily theoretical as few patient-level studies provide concrete evidence. This narrative review provides a general overview of the theory of stepdown beds in the care of hospitalized patients and a summary of what is known about their impact on patient flow and outcomes and highlights areas for future research.
Keywords: critical care, intermediate care, intensive care unit, continuity of patient care
Improvements in healthcare have contributed to longer life expectancy in developed countries. But with an escalation in healthcare delivery, the demands and costs of healthcare have risen dramatically. Intensive care provision contributes to these costs: from 2000 to 2005 in the United States, the number of critical care beds increased more than 6.5%, and critical care spending is now estimated to account for almost 1% of the Gross Domestic Product (1). The increasing availability of intensive care unit (ICU) beds is costly, but the alternative option of foregoing this expansion raises the concern of potential delays in admission of patients from wards and emergency departments (EDs) (2) and in elective surgery (3). In 2005 the Joint Commission on Accreditation of Healthcare Organizations (JCAHO) approved a new standard calling on U.S. hospital leadership to implement plans that identify and mitigate barriers to efficient patient flow across the continuum of care (4). Stepdown beds, also referred to as intermediate care beds or high-dependency beds, are one possible approach to providing higher levels of care while improving the efficiency of patient flow. The concept of a stepdown unit (SDU) is not a new one: in 1995 a study of 40 U.S. hospitals reported that 63% had at least one such unit (5), and the number of respiratory SDUs in Italy nearly doubled from 1997 to 2007 (6). Yet SDUs and their role in hospital care receive little focus.
Definition of a Stepdown Unit Bed
Gotsman and Schrire introduced the concept of SDUs in 1968. They proposed a patient-care area with specialized monitoring and nursing care for cardiac patients no longer requiring full intensive care but not ready for discharge to a regular ward (7). Since then, terminology and definitions of SDU beds remain diverse (Table 1) (5, 8–14), although implicit is the provision of a level of care that is intermediate between what is available in a ward bed and in an intensive care bed. Two themes emerge from attempts to define an SDU bed: (1) the nurse to patient ratio for these beds, and (2) the ability to provide specific organ support.
Table 1.
Examples of Terms and Definitions for Stepdown Units
| Reference | Term | Definition | Country |
|---|---|---|---|
| Nguyen et al., 2010 (8) | Stepdown unit | “… to allow for the care of patients who do not require full intensive care but cannot be safely cared for on a normal ward. These patient requirements may include (but are not limited to) specific organ support, nursing needs, vital sign monitoring, or ventilator weaning.” | Nonspecific |
| McIlroy et al., 2006 (9) | High dependency care unit | “… provides the capability for all the invasive monitoring of ICU but without the provision of mechanical ventilation. With a nursing ratio that is typically 1:2, HDU is believed to be a lower cost alternative to ICU in critically ill patients who do not require mechanical ventilation.” | Australia |
| Keenan et al., 1998 (10) | Transitional care unit | “… units were developed to provide varying levels of noninvasive monitoring with or without the capability to ventilate patients … [patients] require a lower nurse:patient ratio and may require fewer investigations when compared to patients in ICUs.” | Canada |
| Ambrosino et al., 2010 (11) | Respiratory intermediate unit | “Respiratory intermediate care units (RICUs) within acute care hospitals manage patients with ARF or ACRF with noninvasive ventilation … may also provide multidisciplinary rehabilitation and serve as a bridge to home care programs or long-term care facilities … may work also as 'step-down' units for difficult-to-wean patients …” | Italy |
| Comprehensive Critical Care, 2000 (14) | Level 2 care | “Patients requiring more detailed observation or intervention including support for a single failing organ system or post-operative care and those ‘stepping down’ from higher levels of care.” | UK |
| American Association of Critical-Care Nurses Progressive Care Task Force (12) | Progressive care unit | “… patients whose needs fall along the less acute end of [the patient care] continuum … moderately stable with less complexity, require moderate resources and require intermittent nursing vigilance or are stable with a high potential for becoming unstable and require increased intensity of care and vigilance.” | United States |
| Nasraway et al., 1998 (13) | Intermediate care unit | “ … does not require intensive care but needs more care than that provided on a general ward. These patients may require frequent monitoring of vital signs and/or nursing interventions, but usually do not require invasive monitoring.” | United States |
| Zimmerman et al., 1995 (5) | Intermediate care unit | “ … patients who received only monitoring and floor care services … and were at such low risk of receiving active life-supporting treatment that routine ICU admission might not be necessary.” | United States |
Definition of abbreviations: ACRF = acute on chronic respiratory failure; ARF = acute respiratory failure; HDU = high-density care unit; ICU = intensive care unit.
In England, patient care is stratified into levels ranging from 0 (general ward care) to 3 (full intensive care). In this system, level 2 corresponds to SDU care and is defined as: “Patients requiring more detailed observation or intervention including support for a single failing organ system or post-operative care and those ‘stepping down’ from higher levels of care” (14). The definition also explicitly excludes respiratory support in the form of invasive mechanical ventilation and states that this is only available in level 3 (full intensive care).
In the United States, critical care has remained more heterogeneous and classifications are less explicit. In studies of U.S. ICU use, rates of admission to ICU for all hospitalized patients ranged from 3 to 55% (15), and rates of ICU admission for patients with a diagnosis of diabetic ketoacidosis ranged from 0 to 100% (16). These findings suggest great heterogeneity in the patients who are cared for in U.S. ICU beds. Given this heterogeneity for intensive care, the definition of “stepdown care” becomes even more problematic, as it may range from additional tracking of vital signs to full organ support. The variation in terminology and definitions for stepdown care limits rigorous scientific comparisons. Additionally, “intermediate care” is often used to refer to long-term outpatient care or rehabilitation centers, which may cause confusion and complicates literature search strategies.
Patient Selection for SDU Care
Most SDU patients can be classified into three groups. The first is “stepdown” patients who were receiving intensive care (usually organ support) but who no longer have full intensive care needs. Patients may often be defined as “stepdown” by exclusion (i.e., that they no longer meet any criteria for full intensive care). These patients may still require frequent monitoring and/or nursing care and may also have some minimal organ support requirement. Patients who are critically ill rarely transition directly from full intensive care to ward-level care. Such patients (whether or not they receive care in an SDU-designated bed) all receive this level of care as they are readied for discharge to the ward. Only a minority of patients may bypass this stage. For example, a patient who requires mechanical ventilation due to a specific drug overdose may recover and, once extubated, have almost no additional nursing or monitoring requirements, allowing for immediate transition to ward-level care. Additionally, the most critically ill patients in an ICU may elect or be referred to comfort care services, including in a ward or hospice setting, bypassing the need for stepdown care.
The second group is “step-up” patients who are admitted to SDUs from an ED or regular ward with increased care requirements. Although diverse, this group of patients generally includes those with acute clinical changes, such as those with acute respiratory compromise requiring noninvasive ventilatory support or those requiring acute renal replacement therapy. Hilton and colleagues found that 33% of SDU admissions were step-up patients (8% from ED, 25% from wards) (17), and Lucena and colleagues found that almost 80% of SDU admissions were step-up patients (25% from ED, 52% from general wards) (18), suggesting that this group of patients does represent a substantial proportion of the individuals cared for in SDUs. However, this group may receive less focus in studies of stepdown-level care because of the term “stepdown unit” itself, which emphasizes the transition to a lower level of care; this confusion in nomenclature highlights the importance of developing better terminology for intermediate care in the hospital.
The third main category of SDU patients is postoperative patients who are admitted either directly from the operating room or after a short period of observation in a recovery room. These patients require an increased level of care because of some combination of underlying comorbidities, the effects of surgical and anesthetic interventions in the operating room, and postoperative nursing or other care needs. Some SDUs may be designed explicitly for the admission of postoperative patients, whereas others admit medical and surgical patients. After the development of a mixed medical-surgical SDU at a single center, Hilton and colleagues found that 16% of admissions were postoperative patients (17).
Guidelines published in Critical Care Medicine in 1998 based on expert consensus concluded that patients appropriate for admission to SDU included those with severe but stable organ dysfunction who remained hemodynamically stable, patients with diabetic ketoacidosis, and patients in the early postoperative period after major surgery with significant fluid shifts. Patients who should not be admitted to SDUs included those with complicated myocardial infarctions, acute respiratory failure, status epilepticus, or catastrophic brain injury; those requiring heavy nursing care; and those who have been triaged to comfort care (13). Although guidelines may be helpful, there is still a very large gray area regarding appropriate SDU admissions. Moreover, studies suggest that ICUs are still often used for SDU-level care; a large percentage of ICU beds are occupied at any given time by intermediate-care–level patients (5, 17, 19–21).
Location of SDU Beds
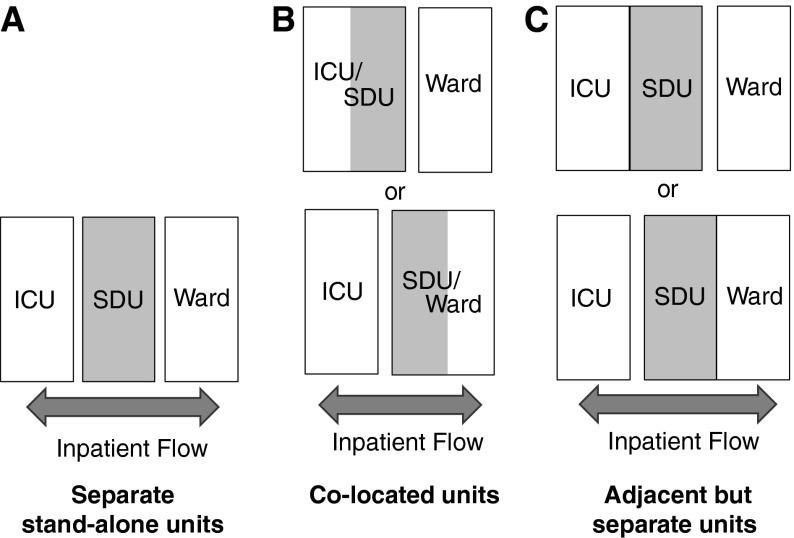
SDU beds can be specific stand-alone units, adjacent to but physically distinct from an ICU or general ward, or designated beds colocated within ICUs or general wards. SDU beds colocated within ICUs or wards can be separate beds reserved for only intermediate care or “flexible” beds that change designation based on patient needs (Figure 1).
Figure 1.
Schematic representation of potential stepdown unit (SDU) locations and the relation to nearby units. ICU = intensive care unit.
Colocation of SDU beds within an ICU is the predominant model of care in the UK. Colocation of SDU beds may allow for bed use and nursing intensity to change with patient needs on a fluid basis, without physical transfers, but there are no published studies examining different models. Moreover, although the colocation model may contribute to continuity of care, it requires flexible staffing (3, 22), which implies that all nursing staff must be trained to provide ICU-level care even while they may primarily be providing lower levels of care; the economic implications of this are unclear. Additionally, this model requires more beds to be equipped to accommodate changes to higher levels of care (such as monitoring systems for arterial lines, Swan-Ganz catheters, or ventilators). Nevertheless, some advocate for the ICU colocation model because larger units can more easily handle sudden influxes of patients and the separation of patients into smaller units reduces overall efficiency (23, 24). Colocation could be associated with a decrease in patient stress due to improved continuity of care, but data on this topic are lacking (25). They may also be associated with successful integration of new nurses into ICUs because they care for intermediate-level patients while still gaining exposure to the critically ill (3).
Separate SDUs located adjacent to ICUs may allow for some of the advantages of colocated SDU/ICU beds (overlapping intensivist/nursing coverage, ease of critical care outreach) while providing patients a calm environment free from the noise of a busy ICU. This model has been used in creating specialty postoperative units (i.e., for cardiac surgery patients) in the UK and United States, but studies have not evaluated the specific impact of location (3, 19, 26, 27). Separate SDUs located adjacent to or within regular wards may be located near the nursing station to improve monitoring, but patients in these units may distract nurses from other ward patients (25).
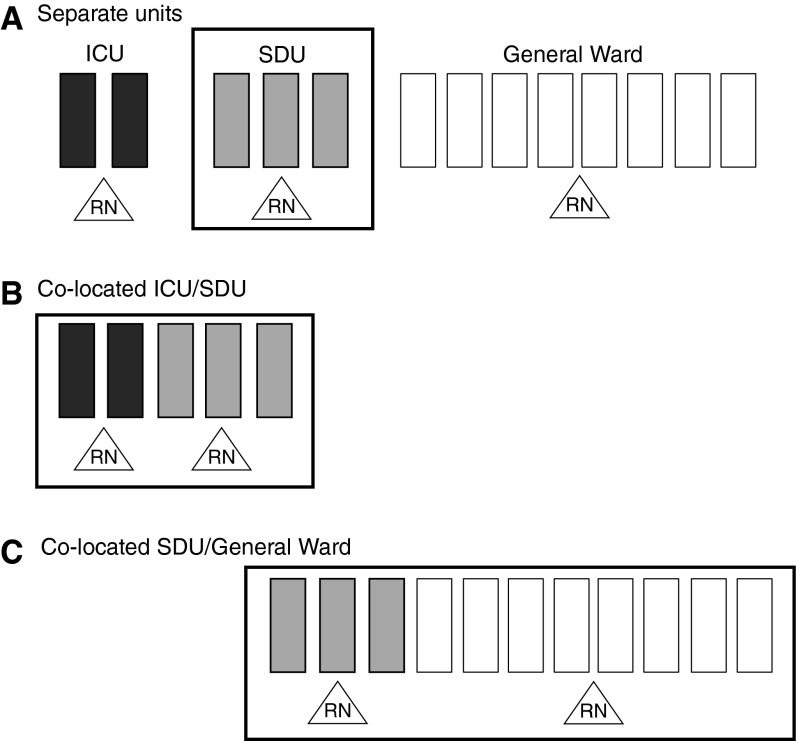
It is important to note that given differences in staffing of ICUs, SDUs, and general wards, the decision to place an SDU within or adjacent to an ICU versus a general ward will have an impact on the cumulative nursing ratio available to patients (Figure 2). For example, an SDU colocated within an ICU might result in a nurse to patient ratio of 2:5, whereas an SDU colocated within a general ward may result in a nurse to patient ratio of 2:11. These variations may not generally matter, as nurses are assigned to care for specific patients. However, these ratios may mean that a deteriorating SDU patient colocated within an ICU may receive additional nursing care more easily.
Figure 2.
Schematic representation of the effects of stepdown unit (SDU) location on nurse to patient ratios. Solid rectangles represent intensive care unit (ICU) beds; shaded rectangles represent SDU beds; open rectangles represent ward beds; triangles represent nursing staff (RN).
Staffing
Establishment of an SDU implies a need for specific stepdown-level staffing. The role of physician coverage for SDUs has not been explored. Location may influence choice in some cases, with intensivist-led teams assuming care of stepdown patients in or near the ICU and primary teams caring for stepdown patients on or near the general floor. Nonphysician coverage may also aid in the care of these patients (28, 29). Although multiple studies have demonstrated a mortality benefit for ICUs covered by full-time physician intensivists (30–33), there are very few studies on any staffing of SDUs (34, 35). Moreover, in the United States, with a relative shortage of intensivists, the use of intensivists to staff SDU beds may not be a practical approach and may contribute to the perception of a staffing shortage within the specialty. Nonphysician coverage may also aid in the care of these patients, potentially even ameliorating the perceived shortages of intensivists. Studies have focused on their role in acute care and critical care settings (36), and these data may be extrapolated to stepdown care. However, studies have not explicitly focused on the role of nonphysician coverage in caring for stepdown populations.
Studies suggest that most SDU beds are staffed with a nurse to patient ratio of 1:2 to 1:4 (3, 19, 23, 27, 37–39) (compared with ICU nurse to patient ratios of 1:1 or 1:2 and ward ratios of 1:6 up to 1:10). However, few studies have systematically evaluated SDU nurse to patient ratios or the need for advanced training. Garfield and colleagues performed a prospective observational study in the UK and recommended an SDU nurse to patient ratio of 2:3 (40). The authors noted that patients in UK SDUs had Therapeutic Intervention Scoring System (TISS) scores “only marginally lower” than most ICU patients across Europe, which accounted for their high recommended staffing ratio.
SDU Care and Patient Outcomes
Studies on the impact of SDUs are primarily limited to observational, single-center before/after reports with varying conclusions (9, 38, 41–47). The majority of studies to date provide data on SDUs as a safe postoperative care option, with a particular emphasis on cardiothoracic surgery patients and safe reduction in time to extubation, reducing the need for full intensive care (39, 43, 44, 48–51). Single-center studies of SDUs for abdominal surgery and medical patients have compared SDU care to general ward care and found reductions in postoperative complications (42) and a suggestion of improvements in patient pain control (41).
Data regarding the impact of availability of SDU care on mortality have been inconsistent. Franklin and colleagues found a decrease in general medical ward mortality after establishment of an adjacent SDU (52), and a prospective study of patients with peptic ulcer disease by Aga and colleagues found an association between SDU-level care and decreased mortality (in this narrow population) (53). Other studies have failed to find a mortality benefit attributable to the establishment of an SDU or SDU-level care itself (9, 38, 42, 45). The ongoing InCare trial in Denmark is explicitly focused on mortality associated with postoperative SDU care versus ward care after major abdominal emergency surgery (54) and will be a major multicenter trial evaluating outcomes attributable to different levels of postoperative care.
Outcomes for medical patients admitted to SDUs specifically as an alternative to full intensive care have also not been well researched. Analysis of a U.S. national database by Herring and colleagues demonstrated that from 2001 to 2009, the percentage of emergency room visits resulting in ICU admission increased 79%, from 1.2 to 2.2 million (2). The study also showed that among all ED patients admitted to the hospital, 15.1% were admitted to an ICU, but did not address whether some proportion of these patients could have been admitted to an SDU rather than ICU (2). In a multicenter trial, Fiebach and colleagues found no difference in outcomes for low-risk patients with acute coronary syndrome monitored in SDU versus ICU, suggesting that SDU may be an appropriate option for this population with monitoring-only requirements (55).
One of the factors limiting rigorous outcomes research on SDU patients is that patient severity scores (i.e., Acute Physiology and Chronic Health Evaluation score, Simplified Acute Physiology Score, Therapeutic Intervention Scoring System) are validated for ICU patients but not universally applied or validated for SDU patients (56). In a prospective observational study addressing this question, Auriant and colleagues assessed the performance of Simplified Acute Physiology Score (SAPS) II among 433 patients admitted to an SDU and found a standardized mortality ratio of 0.93, with area under the receiver operating characteristic curve of 0.85 ± 0.04 (57). A more recent prospective single-center study of 607 patients admitted to a single SDU found a standardized mortality ratio of 0.87 for SAPS II and 0.56 for SAPS 3, with area under the receiver operating characteristic curve of 0.76 and 0.75, respectively (18). These studies suggest that both scores overestimated mortality risk for SDU patients. This overestimation may be due to general poor performance of the scores in this population, but it is also possible that stepdown patient outcomes are better than expected due to improvements in care; such improvements in outcome have been seen in select ICU populations over time (58). With either scenario, recalibration is needed; continued research in this arena will contribute to development of patient severity scores specifically validated for SDU patients, to characterize the burden of illness in SDUs, and to allow for comparison of outcomes across different units.
Economics of SDUs
SDU care has the potential to affect hospital throughput and ICU use and therefore may be a prime focus for hospitals looking to restructure their care to be more efficient. Single-center studies have shown that the establishment of an SDU (1) may allow for more overall critical care admissions without an increase in mortality (27), (2) may shorten ICU length of stay without increasing ICU readmissions (19, 39), and (3) may decrease the proportion of stepdown patients residing in ICU beds (19, 21, 39, 52). Whether or not these improvements are cost-effective is unclear; a systematic review of the literature by Keenan and colleagues was unable to confirm improved cost-effectiveness with the use of SDUs (10).
SDUs may decrease ICU length of stay for some patients by providing a reservoir for patients discharged from ICU. However, this also creates available ICU beds. In a single-center cost-analysis study, Solberg and colleagues found that after the establishment of an SDU, cost of care and ICU length of stay actually increased because the hospital was able to admit more patients with a high severity of illness (23). Keegan and colleagues also found that after the establishment of an SDU, the burden of illness in the parent ICU increased, with concomitant increases in cost of care and ICU length of stay (26). Reducing the number of SDU-level patients residing in ICUs may have little effect on overall critical care spending if the costs are then increased by an influx of higher-acuity patients. SDU bed availability may also fail to contribute to cost savings if it results in increases in admissions of low-acuity patients to intermediate care, a demand-elasticity phenomenon that has been put forward in discussions of ICU bed availability but has not yet been addressed in studies of SDU use (59). Luce and Rubenfeld concluded in a 2002 editorial that reducing length of stay for critically ill patients is a poor target for cost reduction and that to really reduce costs, one would need to close intensive care beds (60). Combined or colocated SDU-ICUs may improve resource use by taking advantage of the economy of scale through sharing of both physical resources and staff responsibilities (61). In British ICUs and combined intensive care/high dependency units, the units’ predicted average cost-per-patient-day of a seven-bed unit was 96% that of a six-bed unit (62). The study found that the declining cost–scale relationship was not only due to distribution of fixed costs over a larger scale but also variable cost distribution. Applying this concept to the implementation of combined ICU-SDUs may save overall healthcare costs and improve patient care capacity. Finally, the provision of SDU care has been associated with an increased capacity for elective surgeries (3, 37, 39). As in-hospital delays of elective surgery for inpatients are linked to higher hospital admission costs and worse perioperative outcomes (i.e., infection rates, mortality) (63), this may represent a key reason for expansion of SDU availability.
Conclusions
Stepdown units are not a new concept, but understanding their role is difficult because of the large variations in definitions and delivery of care. The diversity of patients admitted to stepdown beds creates challenges for understanding SDU use at a systems level; these units are used both as a reservoir for patients stepping down from intensive care and stepping up from ward care. SDU design currently relies heavily on the ICU literature, and most published studies are single center, limiting generalizability. Furthermore, many of the studies specific to SDUs are more than 15 years old, underscoring the need for more research in this area.
Rigorous comparisons of different SDU care models are needed to establish best systems design, optimize critical care capacity, and manage healthcare costs. Standardization of terms for this level of care should be prioritized to limit errors in data comparison, and validated patient-severity scoring systems are needed to assess objective risk assessment and outcomes benefits.
Footnotes
Supported by National Institute on Aging award K08AG038477 (H.W.)
Originally Published in Press as DOI: 10.1164/rccm.201406-1117PP on August 27, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Halpern NA, Pastores SM. Critical care medicine in the United States 2000-2005: an analysis of bed numbers, occupancy rates, payer mix, and costs. Crit Care Med. 2010;38:65–71. doi: 10.1097/CCM.0b013e3181b090d0. [DOI] [PubMed] [Google Scholar]
- 2.Herring AA, Ginde AA, Fahimi J, Alter HJ, Maselli JH, Espinola JA, Sullivan AF, Camargo CA., Jr Increasing critical care admissions from U.S. emergency departments, 2001-2009. Crit Care Med. 2013;41:1197–1204. doi: 10.1097/CCM.0b013e31827c086f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heller J, Murch P. Development in service provision. Making major elective surgery happen. The development of a postoperative surgical unit. Nurs Crit Care. 2008;13:97–104. doi: 10.1111/j.1478-5153.2007.00245.x. [DOI] [PubMed] [Google Scholar]
- 4.JCAHO. New leadership standard on managing patient flow for hospitals. Jt Comm Perspect. 2004;24:13–14. [PubMed] [Google Scholar]
- 5.Zimmerman JE, Wagner DP, Knaus WA, Williams JF, Kolakowski D, Draper EA. The use of risk predictions to identify candidates for intermediate care units. Implications for intensive care utilization and cost. Chest. 1995;108:490–499. doi: 10.1378/chest.108.2.490. [DOI] [PubMed] [Google Scholar]
- 6.Scala R, Corrado A, Confalonieri M, Marchese S, Ambrosino N Scientific Group on Respiratory Intensive Care of the Italian Association of Hospital Pneumologists. Increased number and expertise of Italian respiratory high-dependency care units: the Second National Survey. Respir Care. 2011;56:1100–1107. doi: 10.4187/respcare.01157. [DOI] [PubMed] [Google Scholar]
- 7.Gotsman MS, Schrire V. Acute myocardial infarction—an ideal concept of progressive coronary care. S Afr Med J. 1968;42:829–832. [PubMed] [Google Scholar]
- 8.Nguyen Y, Wunsch H, Angus DC. Critical care: the impact of organization and management on outcomes. Curr Opin Crit Care. 2010;16:487–492. doi: 10.1097/MCC.0b013e32833d9180. [DOI] [PubMed] [Google Scholar]
- 9.McIlroy DR, Coleman BD, Myles PS. Outcomes following a shortage of high dependency unit beds for surgical patients. Anaesth Intensive Care. 2006;34:457–463. doi: 10.1177/0310057X0603400403. [DOI] [PubMed] [Google Scholar]
- 10.Keenan SP, Massel D, Inman KJ, Sibbald WJ. A systematic review of the cost-effectiveness of noncardiac transitional care units. Chest. 1998;113:172–177. doi: 10.1378/chest.113.1.172. [DOI] [PubMed] [Google Scholar]
- 11.Ambrosino N, Gabbrielli L. The difficult-to-wean patient. Expert Rev Respir Med. 2010;4:685–692. doi: 10.1586/ers.10.58. [DOI] [PubMed] [Google Scholar]
- 12.American Association of Critical Care Nurses Progressive Care Task Force: Progressive care fact sheet [accessed 2014 Sept 19]. Available from: http://www.aacn.org/wd/practice/content/progressivecarefactsheet.pcms?menu=practice
- 13.Nasraway SA, Cohen IL, Dennis RC, Howenstein MA, Nikas DK, Warren J, Wedel SK American College of Critical Care Medicine of the Society of Critical Care Medicine. Guidelines on admission and discharge for adult intermediate care units. Crit Care Med. 1998;26:607–610. doi: 10.1097/00003246-199803000-00039. [DOI] [PubMed] [Google Scholar]
- 14.Department of Health. London: Department of Health; 2000. Comprehensive critical care: a review of adult critical care services. [Google Scholar]
- 15.Seymour CW, Iwashyna TJ, Ehlenbach WJ, Wunsch H, Cooke CR. Hospital-level variation in the use of intensive care. Health Serv Res. 2012;47:2060–2080. doi: 10.1111/j.1475-6773.2012.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gershengorn HB, Iwashyna TJ, Cooke CR, Scales DC, Kahn JM, Wunsch H. Variation in use of intensive care for adults with diabetic ketoacidosis. Crit Care Med. 2012;40:2009–2015. doi: 10.1097/CCM.0b013e31824e9eae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilton G, Madayag M, Shagoury C. Development of a surgical/trauma intermediate care unit. Clin Nurse Spec. 1993;7:274–279. doi: 10.1097/00002800-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 18.Lucena JF, Alegre F, Martinez-Urbistondo D, Landecho MF, Huerta A, García-Mouriz A, García N, Quiroga J. Performance of SAPS II and SAPS 3 in intermediate care. PLoS ONE. 2013;8:e77229. doi: 10.1371/journal.pone.0077229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fox AJ, Owen-Smith O, Spiers P. The immediate impact of opening an adult high dependency unit on intensive care unit occupancy. Anaesthesia. 1999;54:280–283. doi: 10.1046/j.1365-2044.1999.00715.x. [DOI] [PubMed] [Google Scholar]
- 20.Henning RJ, McClish D, Daly B, Nearman H, Franklin C, Jackson D. Clinical characteristics and resource utilization of ICU patients: implications for organization of intensive care. Crit Care Med. 1987;15:264–269. doi: 10.1097/00003246-198703000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Pappachan JV, Millar BW, Barrett DJ, Smith GB. Analysis of intensive care populations to select possible candidates for high dependency care. J Accid Emerg Med. 1999;16:13–17. doi: 10.1136/emj.16.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaboyer W, James H, Kendall M. Transitional care after the intensive care unit: current trends and future directions. Crit Care Nurse. 2005;25:16–28. [PubMed] [Google Scholar]
- 23.Solberg BC, Dirksen CD, Nieman FH, van Merode G, Poeze M, Ramsay G. Changes in hospital costs after introducing an intermediate care unit: a comparative observational study. Crit Care. 2008;12:R68. doi: 10.1186/cc6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vincent JL, Burchardi H. Do we need intermediate care units? Intensive Care Med. 1999;25:1345–1349. doi: 10.1007/s001340051077. [DOI] [PubMed] [Google Scholar]
- 25.Beard H. Does intermediate care minimize relocation stress for patients leaving the ICU? Nurs Crit Care. 2005;10:272–278. doi: 10.1111/j.1362-1017.2005.00131.x. [DOI] [PubMed] [Google Scholar]
- 26.Keegan MT, Brown DR, Thieke MP, Afessa B. Changes in intensive care unit performance measures associated with opening a dedicated thoracic surgical progressive care unit. J Cardiothorac Vasc Anesth. 2008;22:347–353. doi: 10.1053/j.jvca.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Eachempati SR, Hydo LJ, Barie PS. The effect of an intermediate care unit on the demographics and outcomes of a surgical intensive care unit population. Arch Surg. 2004;139:315–319. doi: 10.1001/archsurg.139.3.315. [DOI] [PubMed] [Google Scholar]
- 28.D’Agostino R, Halpern NA. Acute care nurse practitioners in oncologic critical care: the memorial Sloan-Kettering Cancer Center experience. Crit Care Clin. 2010;26:207–217. doi: 10.1016/j.ccc.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Kapu AN, Thomson-Smith C, Jones P. NPs in the ICU: the Vanderbilt initiative. Nurse Pract. 2012;37:46–52. doi: 10.1097/01.NPR.0000413485.97744.11. [DOI] [PubMed] [Google Scholar]
- 30.Pollack MM, Katz RW, Ruttimann UE, Getson PR. Improving the outcome and efficiency of intensive care: the impact of an intensivist. Crit Care Med. 1988;16:11–17. doi: 10.1097/00003246-198801000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Brown JJ, Sullivan G. Effect on ICU mortality of a full-time critical care specialist. Chest. 1989;96:127–129. doi: 10.1378/chest.96.1.127. [DOI] [PubMed] [Google Scholar]
- 32.Carson SS, Stocking C, Podsadecki T, Christenson J, Pohlman A, MacRae S, Jordan J, Humphrey H, Siegler M, Hall J. Effects of organizational change in the medical intensive care unit of a teaching hospital: a comparison of ‘open’ and ‘closed’ formats. JAMA. 1996;276:322–328. [PubMed] [Google Scholar]
- 33.Ghorra S, Reinert SE, Cioffi W, Buczko G, Simms HH. Analysis of the effect of conversion from open to closed surgical intensive care unit. Ann Surg. 1999;229:163–171. doi: 10.1097/00000658-199902000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucena JF, Alegre F, Rodil R, Landecho MF, Garcia-Mouriz A, Marques M, Aquerreta I, Garcia N, Quiroga J. Results of a retrospective observational study of intermediate care staffed by hospitalists: impact on mortality, co-management, and teaching. J Hosp Med. 2012;7:411–415. doi: 10.1002/jhm.1905. [DOI] [PubMed] [Google Scholar]
- 35.Harding T, Wright M.Unequal staffing: a snapshot of nurse staffing in critical care units in New South Wales, Australia Contemp Nurse[online ahead of print] 3 Feb 2014; DOI: 10.5172/conu.2013.4260 [DOI] [PubMed] [Google Scholar]
- 36.Garland A, Gershengorn HB. Staffing in ICUs: physicians and alternative staffing models. Chest. 2013;143:214–221. doi: 10.1378/chest.12-1531. [DOI] [PubMed] [Google Scholar]
- 37.Cady N, Mattes M, Burton S. Reducing intensive care unit length of stay. A stepdown unit for first-day heart surgery patients. J Nurs Adm. 1995;25:29–35. doi: 10.1097/00005110-199512000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart G, Opdam H, Silvester W, Doolan L, Gutteridge G. A before and after trial of the effect of a high-dependency unit on post-operative morbidity and mortality. Crit Care Resusc. 2005;7:16–21. [PubMed] [Google Scholar]
- 39.Byrick RJ, Power JD, Ycas JO, Brown KA. Impact of an intermediate care area on ICU utilization after cardiac surgery. Crit Care Med. 1986;14:869–872. doi: 10.1097/00003246-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Garfield M, Jeffrey R, Ridley S. An assessment of the staffing level required for a high-dependency unit. Anaesthesia. 2000;55:137–143. doi: 10.1046/j.1365-2044.2000.055002137.x. [DOI] [PubMed] [Google Scholar]
- 41.Armstrong K, Young J, Hayburn A, Irish B, Nikoletti S. Evaluating the impact of a new high dependency unit. Int J Nurs Pract. 2003;9:285–293. doi: 10.1046/j.1440-172x.2003.00439.x. [DOI] [PubMed] [Google Scholar]
- 42.Jones HJ, Coggins R, Lafuente J, de Cossart L. Value of a surgical high-dependency unit. Br J Surg. 1999;86:1578–1582. doi: 10.1046/j.1365-2168.1999.01318.x. [DOI] [PubMed] [Google Scholar]
- 43.Crosby DL, Rees GA. Post operative care: the role of the high dependency unit. Ann R Coll Surg Engl. 1983;65:391–393. [PMC free article] [PubMed] [Google Scholar]
- 44.Chong JL, Pillai R, Fisher A, Grebenik C, Sinclair M, Westaby S. Cardiac surgery: moving away from intensive care. Br Heart J. 1992;68:430–433. doi: 10.1136/hrt.68.10.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davies J, Tamhane R, Scholefield C, Curley P. Does the introduction of HDU reduce surgical mortality? Ann R Coll Surg Engl. 1999;81:343–347. [PMC free article] [PubMed] [Google Scholar]
- 46.Peacock JE, Edbrooke DL. Rationing intensive care. Data from one high dependency unit supports their effectiveness. BMJ. 1995;310:1413. doi: 10.1136/bmj.310.6991.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richards BF, Fleming JB, Shannon CN, Walters BC, Harrigan MR. Safety and cost effectiveness of step-down unit admission following elective neurointerventional procedures. J Neurointerv Surg. 2012;4:390–392. doi: 10.1136/neurintsurg-2011-010058. [DOI] [PubMed] [Google Scholar]
- 48.Silbert B, Santamaria JD, O’Brien JL, Blyth CM, Kelly WJ, Moinar RR. Early extubation following coronary artery bypass surgery: a prospective randomized controlled trial. The Fast Track Cardiac Care Team. Chest. 1998;113:1481–1488. doi: 10.1378/chest.113.6.1481. [DOI] [PubMed] [Google Scholar]
- 49.Royse CF, Royse AG, Soeding PF. Routine immediate extubation after cardiac operation: a review of our first 100 patients. Ann Thorac Surg. 1999;68:1326–1329. doi: 10.1016/s0003-4975(99)00829-2. [DOI] [PubMed] [Google Scholar]
- 50.Svircevic V, Nierich AP, Moons KG, Brandon Bravo Bruinsma GJ, Kalkman CJ, van Dijk D. Fast-track anesthesia and cardiac surgery: a retrospective cohort study of 7989 patients. Anesth Analg. 2009;108:727–733. doi: 10.1213/ane.0b013e318193c423. [DOI] [PubMed] [Google Scholar]
- 51.Cheng DC, Karski J, Peniston C, Asokumar B, Raveendran G, Carroll J, Nierenberg H, Roger S, Mickle D, Tong J, et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: a prospective randomized controlled trial. J Thorac Cardiovasc Surg. 1996;112:755–764. doi: 10.1016/S0022-5223(96)70062-4. [DOI] [PubMed] [Google Scholar]
- 52.Franklin C, Rackow EC, Mamdani B, Nightingale S, Burke G, Weil MH. Decreases in mortality on a large urban medical service by facilitating access to critical care. An alternative to rationing. Arch Intern Med. 1988;148:1403–1405. [PubMed] [Google Scholar]
- 53.Aga H, Readhead D, Maccoli G, Thompson A. Fall in peptic ulcer mortality associated with increased consultant input, prompt surgery, and use of high dependency care identified through peer-review audit. BMJ Open. 2012;2:e000271. doi: 10.1136/bmjopen-2011-000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vester-Andersen M, Waldau T, Wetterslev J, Møller MH, Rosenberg J, Jørgensen LN, Gillesberg I, Jakobsen HL, Hansen EG, Poulsen LM, et al. Effect of intermediate care on mortality following emergency abdominal surgery. The InCare trial: study protocol, rationale and feasibility of a randomised multicentre trial. Trials. 2013;14:37. doi: 10.1186/1745-6215-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fiebach NH, Cook EF, Lee TH, Brand DA, Rouan GW, Weisberg M, Goldman L. Outcomes in patients with myocardial infarction who are initially admitted to stepdown units: data from the Multicenter Chest Pain Study. Am J Med. 1990;89:15–20. doi: 10.1016/0002-9343(90)90091-q. [DOI] [PubMed] [Google Scholar]
- 56.Vincent JL, Moreno R. Clinical review: scoring systems in the critically ill. Crit Care. 2010;14:207. doi: 10.1186/cc8204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Auriant I, Vinatier I, Thaler F, Tourneur M, Loirat P. Simplified acute physiology score II for measuring severity of illness in intermediate care units. Crit Care Med. 1998;26:1368–1371. doi: 10.1097/00003246-199808000-00023. [DOI] [PubMed] [Google Scholar]
- 58.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311:1308–1316. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 59.Gooch RA, Kahn JM. ICU bed supply, utilization, and health care spending: an example of demand elasticity. JAMA. 2014;311:567–568. doi: 10.1001/jama.2013.283800. [DOI] [PubMed] [Google Scholar]
- 60.Luce JM, Rubenfeld GD. Can health care costs be reduced by limiting intensive care at the end of life? Am J Respir Crit Care Med. 2002;165:750–754. doi: 10.1164/ajrccm.165.6.2109045. [DOI] [PubMed] [Google Scholar]
- 61.Fordham R, Field DJ, Hodges S, Normand C, Mason E, Burton P, Yates J, Male S. Cost of neonatal care across a regional health authority. J Public Health Med. 1992;14:127–130. [PubMed] [Google Scholar]
- 62.Jacobs P, Rapoport J, Edbrooke D. Economies of scale in British intensive care units and combined intensive care/high dependency units. Intensive Care Med. 2004;30:660–664. doi: 10.1007/s00134-003-2123-2. [DOI] [PubMed] [Google Scholar]
- 63.Vogel TR, Dombrovskiy VY, Lowry SF. In-hospital delay of elective surgery for high volume procedures: the impact on infectious complications. J Am Coll Surg. 2010;211:784–790. doi: 10.1016/j.jamcollsurg.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]