Abstract
Within the past decade, pulmonary arteriovenous malformations (PAVMs) have evolved from rare curiosities to not uncommon clinical states, with the latest estimates suggesting a prevalence of ∼1 in 2,600. PAVMs provide anatomic right-to-left shunts, allowing systemic venous blood to bypass gas exchange and pulmonary capillary bed processing. Hypoxemia and enhanced ventilatory demands result, although both are usually asymptomatic. Paradoxical emboli lead to strokes and cerebral abscesses, and these commonly occur in individuals with previously undiagnosed PAVMs. PAVM hemorrhage is rare but is the main cause of maternal death in pregnancy. PAVM occlusion by embolization is the standard of care to reduce these risks. However, recent data demonstrate that currently recommended management protocols can result in levels of radiation exposure that would be classified as harmful. Recent publications also provide a better appreciation of the hematologic and cardiovascular demands required to maintain arterial oxygen content and oxygen consumption in hypoxemic patients, identify patient subgroups at higher risk of complications, and emphasize the proportion of radiologically visible PAVMs too small to treat by embolization. This review, therefore, outlines medical states that exacerbate the consequences of PAVMs. Chief among these is iron deficiency, which is commonly present due to concurrent hereditary hemorrhagic telangiectasia: iron deficiency impairs hypoxemia compensations by restricting erythropoiesis and increases the risk of ischemic strokes. Management of periodontal disease, dental interventions, pulmonary hypertension, and pregnancy also requires specific consideration in the setting of PAVMs. The review concludes by discussing to what extent previously recommended protocols may benefit from modification or revision.
Keywords: embolization, HHT, hypoxemia/hypoxia, iron deficiency, radiation
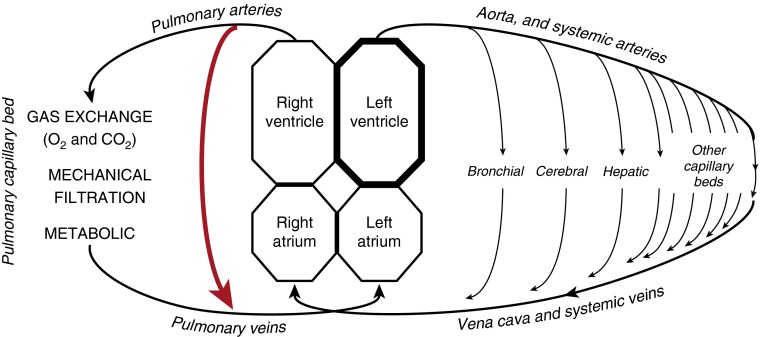
Pulmonary arteriovenous malformations (PAVMs) are structurally abnormal vessels that provide direct capillary-free communication between the pulmonary and systemic circulations (Figure 1), and hence an anatomic “right-to-left” shunt (Figure 2). Gas exchange, filtration, and other processing of systemic venous blood are impaired. The dilated, thin-wall structures rarely hemorrhage, although this is the most common contributor to the 1% rate of maternal death in pregnancy.
Figure 1.
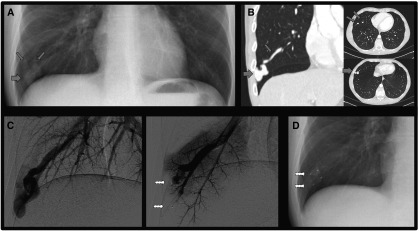
(A) Chest X-ray indicating a pulmonary arteriovenous malformation (PAVM) (thick arrow) and the subtle appearance of associated artery and vein (thin arrows) in a patient with resting SaO2 of 94%. (B) Thoracic computerized tomography scan images. (C) Angiographic appearances before (left) and after (right) embolization. Images, courtesy of Dr. James Jackson, were taken minutes apart. Preferential blood flow through the PAVM led to reduced perfusion of non-PAVM–associated arteries and to dilatation/early filling of the draining vein. After embolization with two Amplatzer plugs (arrows), arterial blood flow to normal pulmonary arteries was restored. (D) Chest radiographs of the same region 8 months after embolization, when the SaO2 was 97%. PAVM sac, feeding artery, and draining vein have regressed. The tips and bodies of the Amplatzer plugs are visible.
Figure 2.
Drawing indicating the major functions of the pulmonary capillary bed that are bypassed in the setting of pulmonary arteriovenous malformations (red arrow). Adapted with permission from Reference 20.
PAVMs are substantially more prevalent than suggested by autopsy studies of the 1950s and now barely meet the definition of a rare disease. Population-wide cancer screening programs using thoracic computed tomography (CT) suggest that PAVMs sufficiently large for detection by CT affect 1 in 2,630 (95% confidence intervals, 1 in 1,315 to 1 in 5,555) (1).
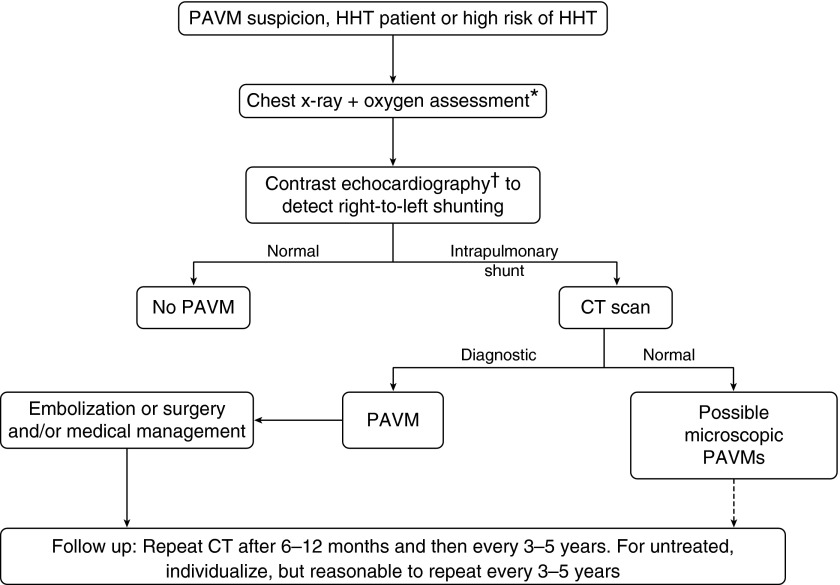
Previous review articles summarize the pathophysiology and management recommendations using evidence available in 1998 (2, 3), 2006 (4, 5), and 2011 to 2013 (6–11). As first proposed 60 years ago (12), treatment of asymptomatic patients with PAVMs is recommended to prevent later complications, with embolization now the standard of care (Figure 3).
Figure 3.
Typical 2013 flow chart. *At rest; †on exercise in Reference 8. Medical management to include use of supplemental oxygen when needed, prophylactic antibiotics (including for group with possible microscopic pulmonary arteriovenous malformations), and avoidance of SCUBA diving (8). Dashed arrow: As indicated in international guidelines (see Reference 5). HHT = hereditary hemorrhagic telangiectasia; PAVM = pulmonary arteriovenous malformation. Adapted from Reference 8.
However, the last 12 months have witnessed the publication of many PAVM data manuscripts that challenge concepts used in current management practice. Most importantly, new data demonstrate that currently used protocols can result in levels of radiation exposure that would be classified as harmful (13). It therefore seems imperative to reconsider risk–benefit considerations. The remainder of this review places the new PAVM evidence base in context for the practicing pulmonologist before considering to what extent previously recommended protocols may benefit from modification or revision.
Etiology of PAVMs
Hereditary Hemorrhagic Telangiectasia
The most common cause of PAVMs is hereditary hemorrhagic telangiectasia (HHT), a condition discussed in detail in HHT guidelines (5) and in more recent HHT review articles (14–17). Some brief comments are appropriate here due to the HHT-bias of the PAVM evidence base and the impact of HHT features on PAVM presentation patterns.
HHT affects ∼1 in 5 to 8,000 people, is transmitted from parent to child as an autosomal dominant trait, and is most commonly caused by mutations in ENG, endoglin (HHT1), ACVLI/ALK1 (HHT2), or Smad4 (HTJP). Arteriovenous malformations (AVMs) and smaller telangiectatic vessels develop at multiple sites, including nasal, mucocutaneous, pulmonary, hepatic, gastrointestinal, and cerebrovascular beds. Patients with HHT usually present with recurrent nosebleeds, iron deficiency anemia, and/or complications attributable to previously silent AVMs. There is also an increased risk of pulmonary hypertension.
The prevalence of PAVMs differs according to HHT genotype, although screening is recommended across all genotypes (5–7, 14–17). One series of 199 patients with HHT demonstrated CT-evident PAVMs in 58% with ENG mutations, compared with 18% with mutations in ACVLI/ALK1 (P < 0.001) (18). Conversely, pulmonary arterial hypertension and pulmonary venous hypertension attributed to hepatic AVMs and high output states are more common in HHT2 (19) but are only clinically significant in <5% of patients with HHT (20).
The diagnosis of HHT can be difficult and is further influenced by socioeconomic and geographical factors (21). With enhanced awareness of HHT, the reported proportion of PAVM cases with HHT rose from ∼70% before 1998 (2, 3) to 93.6% in one series (22), with 59% (121/205) of patients with PAVM/HHT having been previously unaware they had HHT (22).
Non-HHT PAVMs
In a broad PAVM referral practice, the second most common etiology of single PAVMs appears to be sporadic, although this diagnosis should not be considered until HHT has been formally sought in the proband and family members. The respective HHT/PAVM prevalences suggest that the proportion of sporadic PAVMs will rise as more asymptomatic PAVMs are detected in the general population through the increasing use of CT scans in medical practice.
Treatment of cyanotic congenital heart disease by surgical generation of a cavo-pulmonary shunt frequently leads to PAVM development in the lung not receiving hepatic venous effluent (23, 24). Rare etiological causes include gestational trophoblastic disease and arteriovenous fistulae induced by trauma. The case report–biased literature (27% of PubMed retrievals for “pulmonary arteriovenous malformations” from 2003 to 2012 were single case reports) suggests other causes that may reflect chance associations or alternate diagnoses.
Clinical Features of PAVMs
Presentation Patterns
Clinical features observed in patients with PAVMs vary widely and are summarized in Table 1.
Table 1.
Major Physiologic and Clinical Features of Pulmonary Arteriovenous Malformations
| Physiologic Compensations | Clinical Features* | |
|---|---|---|
| Right-to-left shunting reduces | Increased | |
| PaO2, SaO2, and CaO2 | Hemoglobin (secondary erythrocytosis) | Cyanosis. Often asymptomatic |
| Cardiac output (stroke volume; heart rate) | Dyspnea, palpitations, cardiac failure | |
| CO2 clearance | Ventilation (e/co2 slope) | Different ventilation patterns† |
| Capillary filtration/processing | Not known | Paradoxical embolic stroke, MI |
| Cerebral and/or peripheral abscesses | ||
| Migraine | ||
| Clubbing | ||
| Fragile PAVM walls | Not known | Hemoptysis; hemothorax |
| Endothelial cell changes | Not known | Speculative |
Definition of abbreviations: MI = myocardial infarction; PAVM = pulmonary arteriovenous malformation.
Vascular bruits may be audible.
Often not appreciated until after embolization.
Dyspnea and asymptomatic hypoxemia
Hypoxemia due to right-to-left shunting may be severe, but the most striking clinical finding for this patient group is the presence of asymptomatic hypoxemia (26).
Relatively few patients present because of dyspnea or describe dyspnea on exertion. Dyspnea was the reason for presentation in 51% (25/49) of cases by 1949 (25) and 47% (201/427) by 1998 (3), but in only 14.6% (32/219) of a more recent series (22). In 165 consecutive patients, MRC dyspnea grades did not exceed 3 (dyspnea after ∼1 mile on the flat) unless there was significant coexisting cardiopulmonary disease (27). Significantly hypoxemic patients with SaO2 < 85% were able to pursue sporting activities to a very high level (26, 28). Formal cardiopulmonary exercise tests (CPETs) of 21 patients with PAVMs with SaO2 80 to 96% demonstrated that Borg scale dyspnea, maximal work-rates, and peak oxygen consumption (o2) did not differ according to hypoxemia severity (28).
Patients with PAVM frequently exhibit orthodeoxia (27), attributed to basal predominance of PAVMs. Although the cardiac literature might suggest platypnea (dyspnea relieved by lying down) should be evident (29), in 257 consecutive patients with PAVMs, platypnea was not described by any of the 75 patients whose SaO2 fell by at least 2% on standing (27).
Only 6 of 95 patients completing a retrospective questionnaire of flight tolerance reported in-flight dyspnea, and there was no difference in sea-level SaO2 between those reporting dyspnea and those who did not (30).
Most strikingly, 86 of 98 (87.8%) consecutive patients reported no improvement in dypsnea/exercise capacity after embolization had corrected their hypoxemia (26). Those who did note an improvement were more likely to have coexisting concurrent diseases, such as airflow obstruction and/or elevated pulmonary artery pressure (26, 27). CPET studies in five patients in whom median SaO2 increased from 90% before embolization to 96% after embolization (P = 0.009) demonstrated no difference in peak work-rate or peak o2 (medians, 119 W, 1.69 L/min preembolization; 113 W, 1.72 L/min postembolization) (28). Four patients reset their peak oxygen pulse (oxygen consumption per heart beat) to values almost identical to their preembolization values (28).
Hemorrhage
PAVM hemorrhage leading to hemoptysis or hemothorax can be fatal but is a relatively rare feature of PAVMs. The two main exceptions are if the patient is pregnant (31, 32) or if PAVMs are perfused at systemic pressures, for example due to pulmonary hypertension or systemic arterial supply (from bronchial or nonbronchial systemic arteries) to PAVM sacs. Systemic arterial supplies may be spontaneous but more commonly develop after PAVM embolization (33–36). In patients with coexisting HHT, hemoptysis also results from nasopharyngeal and endobronchial telangiectasia.
Chest pain
Pleuritic chest pain has been described in up to 10% of patients with PAVMs. In our experience, and as reported elsewhere (25, 37), for untreated PAVMs, chest pain is much more commonly a reason for the diagnostic imaging to be performed rather than attributable to the PAVM itself.
Neurological risks due to compromised capillary bed filtration
Neurological PAVM-associated risks are common and remain poorly recognized. Even in recent series, the majority of PAVM-induced ischemic strokes or cerebral abscess occurred in patients who had not yet received their diagnosis of PAVMs (22, 38). The median delay from cerebral event to PAVM diagnosis was 2 years (22).
Cerebral abscess
A Danish epidemiological study demonstrated cerebral abscess rates of 0.4/100,000/yr in the general population but 155/100,000/yr in patients with HHT/PAVMs (38). Cross-sectional rates of 7.8 to 19.0% are reported in different series (2–11, 22), including 9.05% in 219 consecutive patients with PAVM/HHT, adjusting for ascertainment bias (22).
Ischemic strokes
Cross-sectional studies indicate ischemic stroke (2) rates of 9 to 18% (2–11, 22) and 11.3% when adjusting for ascertainment bias (22). Modeling suggests that by 65 years, at least 25% of untreated patients PAVM will have a clinical stroke (symptoms lasting >24 h) (20, 22), whereas a 1999 imaging study identified cortical or subcortical infarcts in 34/67 (51%) patients, at a median age of 41 years (39). Ischemic stroke risk was reduced after embolization of PAVMs (22).
Migraine headaches
Multiple studies demonstrate the risk of migraine in patients with HHT is approximately doubled if they have pulmonary AVMs (40–44) and suggest that migraines improve after PAVM treatment (40, 43). Migraine features and precipitants appear indistinguishable to migraines in the general population (44).
Myocardial infarctions
Myocardial infarctions also occur as a result of paradoxical emboli (45) and may have similar risk factors as ischemic strokes (20).
Pregnancy-related deaths
Pregnancy is a hazardous period for women with PAVMs. Absolute risks were quantified in two separate series of 487 (31) and 244 (32) pregnancies. In one series, 1.0% of pregnancies (95% confidence interval, 0.1–1.9) resulted in a major PAVM bleed (31), and the maternal death rate was 1.00% (95% confidence interval, 0.13–1.92%) (31). There are also enhanced risks of pulmonary emboli and myocardial infarction with normal coronary arteries (31). In women experiencing a life-threatening event, prior awareness of the PAVM of HHT diagnosis was associated with improved survival (P = 0.041) (31).
Longevity
PAVM development is usually completed by the end of puberty, but, despite the clear risks, cross-sectional series include large proportions of older patients. In our recent series of 497 consecutive patients, a quarter of patients were 60 years or older at presentation, and it was not uncommon for patients describing cyanosis in childhood to become grandparents and even great-grandparents in their lifetime (20).
Diagnosis and Assessment of PAVMs
Radiological methods
Imaging modalities define PAVM number, size, nature, and suitability for embolization (36, 46). “Simple” lesions consist of an aneurysmal venous sac communicating with a dilated feeding artery and draining vein (Figure 1A). Other PAVMs are complex plexiform masses with multiple afferent and efferent vessels. Diffuse PAVMs have been defined as multiple small PAVMs affecting a single segment, or every segment of one or more lobes (35, 47). Distinguishing features of possible “PAVM mimics,” such as a pulmonary varix or bronchocele, are presented elsewhere (46).
Although PAVMs may be clearly visible on chest radiographs, many are not, and the chest radiograph has been reported as being normal in 10 to 40% of instances where clinically significant PAVMs were present (7). CT is generally considered the gold standard investigation for diagnosing PAVMs and demonstrating their size and extent before therapy. CT will detect lesions far below the size for which embolization is feasible. This modality is preferred to magnetic resonance imaging because of its greater resolution. Elegant CT demonstration of angiographic anatomy means that diagnostic angiograms are very rarely required: in our center, they are performed at the same session as therapeutic embolization and are limited to the lung where PAVMs are sited (6, 7, 46, 48).
Evaluation of right-to-left shunts
PAVMs provide an anatomic right-to-left shunt; pulmonary arterial blood passing through PAVMs is not exposed to ventilated alveoli. This contrasts to the hepatopulmonary syndrome, wherein shunting is considered to arise as a result of perfusion-diffusion defects in dilated microvascular channels.
Gas exchange
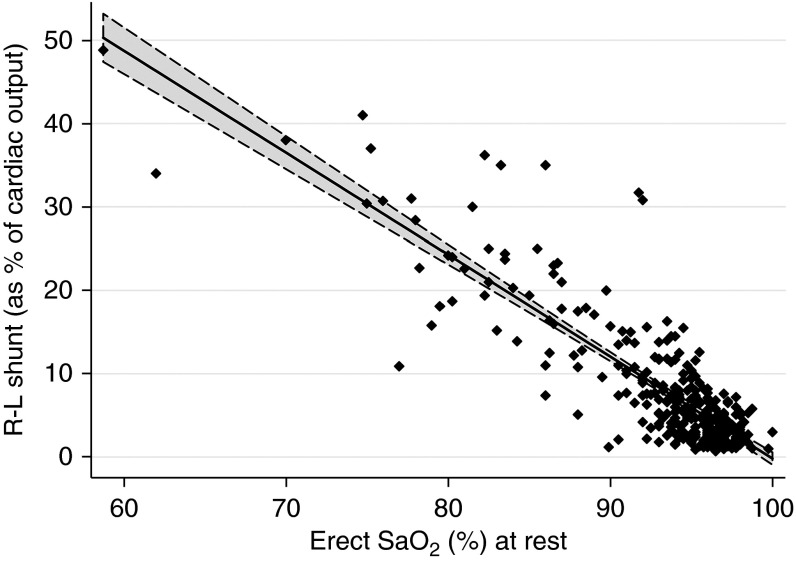
Classically, shunts were measured by arterial PaO2 breathing 100% oxygen. More commonly, arterial PaO2 and/or oxygen saturation (SaO2) are measured on air; each is inversely related to the proportion of the cardiac output flowing through the shunt (Figure 4). Other methods, such as the multiple inert gas technique (MIGET), have not been used in large PAVM series.
Figure 4.
Inverse relationship between right-to-left shunt fraction and oxygen saturation. Shown are 309 paired same-day values of right-to-left (R-L) shunt (quantified by nuclear medicine perfusion scans using Tc-labeled albumin macroaggregates) and the mean SaO2 after 7, 8, 9, and 10 minutes of standing. Data are from 198 individuals. Regression coefficient, −1.22 (95% confidence interval, −1.31 to −1.14; P < 0.0001). All subjects would be predicted to have grade 3 shunts by contrast echocardiography. Adapted with permission from Reference 20.
Technetium perfusion scans
Perfusion scans using technetium-labeled albumin macroaggregates permit precise shunt quantification (Figure 4). As for 100% oxygen quantifications, this method is no longer in general use for diagnosis or follow-up of PAVMs.
Contrast echocardiography
More commonly in PAVM practice, the circulatory transit of microbubbles generated by intravenously injected echocontrast material is detected by imaging bubbles arriving in the left heart or systemic circulation (Figure 2). Microbubbles of gas behave differently from albumin particles on pulmonary capillary transit because the gas rapidly diffuses into the alveolus down the concentration gradient, leading to microbubble shrinkage and then collapse (7). With the sensitivity to detect single microbubble transits, such studies should detect all anatomic shunts, which is the reason why transthoracic contrast echocardiography (CE) was recommended by the international HHT guidelines committee as the initial PAVM screening test (5).
CE studies are also frequently positive in normal lungs without any detectable impairment of gas exchange (49–52). Approximately 8% of the general population exhibits this phenomenon at rest, rising to up to 90% on exercise and in other high cardiac output states (49–52). There is interest and debate regarding the implications and basis of these findings in the general population. The proportion attributable to “alveolar transit” bubbles (bubbles that have passed through the capillaries but have not fully collapsed) (7) is not known.
One study demonstrated positive CEs in 85% of patients with HHT1 and in 35% with HHT2 (18). However, in HHT1, a positive CE study only provided a 36.3% positive predictive value (PPV) for a treatable PAVM (18). The remainder likely reflects PAVMs below the limits of CT scan detection, and normal physiological responses in a state characterized by high cardiac outputs (compensating for anemia [53], hypoxemia [26–28, 54, 55], and hepatic and/or other systemic AVMs [56]).
Careful grading of the number of bubbles visible in the left ventricle can facilitate distinction between PAVMs and more normal variation. In a combined Dutch–Italian study, grade 3 shunts (>100 bubbles per frame) had a PPV for CT-evident PAVMs of 92.5%, compared with a PPV of 13.4% for the lowest positive contrast shunt grade (grade 1, <30 bubbles per frame) (57).
New Insights into PAVM Physiology and Clinical Consequences
Mechanisms to Preserve Oxygen Delivery to the Tissues
During chronic compensation, a graded secondary erythrocytotic (polycythemic) response operates to maintain arterial oxygen content (CaO2). CaO2 is determined by oxygenation and hemoglobin: CaO2 breathing room air approximates to SaO2 × hemoglobin × (1.34/100), where hemoglobin is expressed in g/dl, and SaO2 as a percentage (58). In 165 consecutive patients with PAVMs with SaO2 varying from 78.5 to 99% (median, 95%), the hemoglobin was 0.82 g/dl higher for every 1% fall in SaO2 (P < 0.0001) (26). Thus, healthy patients with PAVMs appeared to preserve CaO2 at just over 18 ml/dl on air (26–28), as reported at altitude (59). Self-reported exercise tolerance was worse in patients with lower CaO2 (P < 0.0001) (26). After correction of hypoxemia by PAVM embolization, secondary eythrocytotic responses were lost, hemoglobin fell, and CaO2 returned to preembolization values within months (26).
Patients with PAVMs have high cardiac outputs at rest and on exercise (54, 55, 60). On exercise, one study demonstrated an excessive increase in total pulmonary blood flow (142% of predicted) in relation to the observed oxygen consumption (o2), resulting in higher-than-predicted tissue oxygen delivery on exercise (54). Data from two studies suggest that on exercise, hypoxemic patients use increased stroke volumes, with similar heart rates to control subjects (28, 54), and this may be regulated through maintenance of the oxygen pulse (oxygen delivered/used per heart beat) (28). Cardiac output and stroke volume fall after embolization (55).
Apparently separately, acute falls in SaO2/CaO2 (e.g., on standing) appear to be compensated by increased heart rate: orthostatic tachycardia was more pronounced in 257 patients with PAVMs than in 40 control subjects without orthodeoxia. The age-adjusted pulse rate was 0.79/min higher for every 1% fall in SaO2 on standing (P < 0.001) (27). Unlike postural orthostatic tachycardia syndrome, for patients with PAVMs, more exuberant postural tachycardias were associated with better exercise tolerance (27).
Understanding of these findings, and multiple PAVM case reports (61), may be improved using the analogy of a train line delivering oxygen to the tissues (62): hypoxemia leads to an increased number of train carriages (hemoglobin), and/or cardiac work such that blood hypoxemia does not lead to tissue hypoxia. The train line analogy also provides an intuitive understanding of why compensation may be less successful for hypoxemic patients who have concurrent cardiac disease or airways/alveolar pathologies, particularly in the setting of iron-restricted erythropoeisis (62).
Exercise adaptations
Studies published recently (26, 28) suggest that patients with PAVMs who participate in intense sporting activity may enhance compensatory mechanisms, facilitating oxygen delivery. A CPET study of 21 patients suggested the strongest predictors of maximal work rate were lower body mass index and higher anerobic threshold, both of which reflect better aerobic conditioning (28). Of 117 consecutive patients with PAVMs with normal exercise tolerance, a predefined athletic group displayed higher hemoglobin for their degree of hypoxemia (resulting in higher CaO2) (26), mirroring general population data that exercise training can stimulate bone marrow erythropoiesis, increasing hemoglobin and red cell mass (63).
One PAVM study (26) suggests mechanisms may include modified iron handling: the median serum ferritin was 38 μg/L in athletes with PAVMs (IQR, 19–77 μg/L) and in nonathletic nondyspneic subjects with PAVMs (IQR, 16–73 μg/L), but serum iron was higher in athletes with PAVMs (median, 19.5; IQR, 13–28 μmol/L) than in nonathletic nondyspneic subjects with PAVMs (median, 14; IQR, 9–17; μmol/L; P = 0.01) (26). This difference could be predicted if intense physical activity enhanced skeletal muscle production of erythroferrone (64). Erythroferrone was recently identified as an erythroblast-synthesized hormone that suppresses hepcidin production (64) and hence promotes gastrointestinal iron absorption and mobilization of iron from stores (65). However, the same protein appears to be synthesized by skeletal muscle (66), providing a plausible mechanism for an aerobically advantageous adaptation to exercise.
Impaired CO2 Clearance
The impairment of gas exchange by right-to-left shunting through PAVMs also impairs H+/CO2 clearance (5, 8), resulting in abnormally high ventilatory drives (28, 54, 60). On exercise, patients with PAVMs increase minute ventilation (e) more than control subjects for a given increase in CO2 production (co2) (28), with a steeper e/co2 slope in patients with lower SaO2 (28). Recent (28) and earlier (54, 60) studies indicate that the ventilatory stimulus from the combined O2 and CO2 shunts not only maintains normocapnia but appears to cause an overshoot, resulting in lower end-tidal Pco2 and venous bicarbonate (28, 54, 60). After successful embolization, end-tidal Pco2, e/co2 slopes, and lower ventilatory equivalent for CO2 at nadir normalize (28).
The increased ventilation was not captured as dyspnea by Borg scale testing during CPET (28). In a large observational series, a small group of individuals did notice that ventilatory patterns they had experienced during yoga, swimming, and singing resolved after embolization (26).
Mechanisms of Paradoxical Emboli
Ischemic stroke
The 2011 American Heart Association Stroke Guidelines recommended use of antiplatelet agents for secondary prophylaxis of ischemic strokes due to PAVMs (67). Recent data highlight that patients with HHT/PAVMs with nosebleeds often tolerate antiplatelet and anticoagulant therapies better than expected (68).
In the PAVM population, ischemic strokes are not attributable to conventional vascular risk factors, such as hypertension, hypercholesterolemia, diabetes, or arrhythmias (20, 22). It was expected that risks would increase with the severity of PAVM-induced shunting. An earlier proposed “3 mm rule” for a feeding artery luminal diameter of clinical consequence was withdrawn (69), although 3 mm is still referred to as a possible threshold for PAVMs requiring embolization (9).
Paradoxical embolic events are more common in patients with the higher-grade contrast echocardiography shunts that are more likely to be associated with visible PAVMs seen on CT (70). In one study, grade 3 shunts (PPV for CT-evident PAVMs, 92.5% [57]) were associated with a 10.4-fold increase in stroke/abscess, whereas patients with grade 1 shunts (PPV for CT-evident PAVMs, 13.4% [57]) had no enhanced risk (70). Such findings are reassuring for the members of general and HHT populations with low-grade intrapulmonary shunts by contrast echocardiography and no evidence of PAVMs on a formal CT scan.
Once PAVMs are sufficiently large for CT detection or for grade 3 shunts, there is little evidence that stroke risk is substantially influenced by further increase in shunt size. A prospective series of 219 consecutive PAVM cases provided no evidence of increased stroke risk with PAVM severity judged by six different biomarkers (22), and only a marginal association was seen with low SaO2 in an extended series of 497 patients (20). The strongest stroke risk factors identified to date are low serum iron (20) (common due to inadequate iron intake for HHT hemorrhagic losses [71]); low pulmonary artery pressure (proposed to facilitate the paradoxical embolic process [20, 22]); and serum fibrinogen (20), which is the predominant circulating plasma protein for platelet adhesion (72). Iron deficiency (73) and serum fibrinogen (74) are emerging as risk factors for ischemic stroke in the general population, a third of whom have a patent foramen ovale that can also lead to paradoxical emboli at specific times (67, 75).
The low hemoglobin caused by iron deficiency has been suggested to contribute to stroke risk through impaired oxygen delivery (73). However, PAVM-induced ischemic strokes are not global hypoxic insults but rather are conventional, focal infarcts, resulting in partial anterior or posterior circulation syndromes (20). Iron deficiency is associated with increased blood viscosity (76), higher factor VIII (77), and venous thrombemboli (77). Conventional venous thrombemboli can lead to ischemic strokes after paradoxical embolism of a pulmonary embolus (78), but this is uncommon in patients with PAVMs (20, 22). Given the AHA recommended antiplatelet agents for prevention of ischemic strokes in patients with PAVMs (67), the recently rediscovered exuberant platelet aggregation in iron deficiency seems a more plausible mechanistic link (20). Platelets from iron-deficient patients (with and without HHT) (20, 79) demonstrate enhanced ex vivo aggregation to 5HT (serotonin), providing potential new therapeutic targets (20, 79).
Cerebral abscess
The organisms most commonly isolated from PAVM-associated cerebral abscesses are anaerobic or facultative anaerobic organisms of periodontal origin (22, 80). Risk factors identified for PAVM-associated cerebral abscess include male gender (22); dental hygiene and interventions (22); and, once adjusted for male gender, a lower SaO2 (22). A genetic predisposition is suspected due to the frequent recurrence in siblings.
Migraines
Modified pulmonary metabolism of vasoactive amines is usually proposed as a plausible mechanistic link between PAVMs and migraines. The precipitation of migraines after contrast injection for nuclear medicine scans (20) suggests to the current author that paradoxical emboli may contribute to migraine pathogenesis.
Impact of Concurrent Cardiopulmonary Disease
Airflow obstruction
Asthma and chronic obstructive pulmonary disease emerged as a surprising predictor of dyspnea and improvement in exercise tolerance after embolization (26). Suggested reasons why patients with airflow obstruction may be less able to compensate for PAVMs include impaired ability to achieve the supranormal ventilation required for CO2 clearance (6).
Pulmonary hypertension
Significant pulmonary hypertension results in enhanced risk of PAVM growth and rupture/hemoptysis, greater dyspnea (with attendant greater likelihood of symptomatic improvement after embolization) (26), and reduced risk of paradoxical ischemic stroke (20, 22). PAVM embolization in the presence of severe pulmonary hypertension is more hazardous and can be fatal, and temporary balloon occlusion does not predict subsequent rises in pulmonary artery pressure (81).
Higher cardiac outputs
Physiological and pathological states associated with higher cardiac outputs also appear to affect symptomatic status (Table 1) and often modify risks of PAVM treatment. Such states include pregnancy, iron deficiency, HHT-related hepatic AVMs (56), and sepsis. Formal data are required, but it is this author’s impression that more athletically trained individuals fare better when they encounter such stresses.
Altered Barometric Pressure
Theoretical concerns that the reduced barometric pressure and relative immobility associated with flying might be detrimental were not substantiated in a retrospective, questionnaire-based study of 3,950 flights in 145 patients with HHT, including 95 patients with PAVMs (30). Scuba divers with right-to-left shunts, including PAVMs, are at increased risk of decompression illness, through paradoxical gas embolism, leading to vascular obstruction and resultant tissue ischemia (7).
Management Strategies
Embolization
PAVM embolization is recommended for first-line treatment of PAVMs amenable to treatment (10). Detailed descriptions of embolization techniques are beyond the scope of this study, and the reader is referred to recent studies (6–8, 10, 34, 36, 46, 48, 81). Amplatzer vascular plugs have rapidly become the preferred agent for embolization compared with coils (36, 48, 82, 83). Advantages include the ability to occlude large-diameter feeding arteries with single plugs (thus reducing procedure time and radiation exposure), easier occlusion at the neck of the sac, and occlusion over a shorter length of vessel, thereby reducing the likelihood of occluding vessels supplying normal lung (6, 7, 48).
Benefits of embolization
After successful embolization, assuming feeding arteries remain occluded and the PAVM does not recruit a new vascular supply from neighboring pulmonary and/or systemic arteries (34, 36), organization and remodeling leads to regression of the PAVM sac and normalization of diameters of former feeding arteries/draining veins (Figure 1) (28). Multiple series have demonstrated anatomic resolution of PAVMs, reduction in right-to-left shunting, and improvement in oxygenation after embolization (reviewed in References 2, 3, 6–11). More recent publications highlight reduction in strokes (20), migraines (40, 43), and reduced hematologic (hemoglobin) (26), cardiac (28, 55), and ventilatory (28) demands.
Limitations of embolization
There are technical limitations to embolization feasibility. Feeding arteries with luminal diameters <2 to 3 mm are technically untreatable using current catheterization methods. This may be why more proximal occlusions (with attendant risks) have been used in some centers. The now obsolete “3 mm rule” means that PAVMs purely supplied by such vessels were not included in many of the efficacy series. Patients with HHT often have multiple PAVMs, only a proportion of which are suitable for embolization. In these cases, improvement in shunting, as opposed to complete obliteration of PAVMs, is the most realistic outcome that can be offered.
As detailed elsewhere (36), PAVM sac persistence after embolization occurred in up to 25% of cases in the pre-Amplatzer plug era. One series with an overall persistence rate of 5% demonstrated recanalization through previously embolized feeding arteries accounted for 91% (48/53) of persistent PAVMs (36). A separate series indicated recanalization was more likely if coils were used singly or placed >1 cm from the PAVM sac (84), and there are suggestions in the literature that recanalization rates may be higher in childhood (11). A recent study demonstrated no recanalization 6 to 40 months after embolization of 28 PAVMs with Amplatzer plugs and coils (82).
PAVM sacs that persist after embolization may acquire new pulmonary, and more hazardously systemic, arterial feeders (34, 36). This mirrors findings reported for spontaneous pulmonary embolic occlusions of normal pulmonary arteries (85). The neovascular supplies are less amenable than recanalized feeding arteries to subsequent reembolization (36). With appropriate evaluations of systemic arteries, the development of a systemic arterial supply appears to be frequent (13/32 in Reference 34).
Risks
Risks appear to be low in experienced hands where long-term follow-up of patients is used, but risks are not zero. Generally, the most common complication of embolization is transient pleurisy, which is reported in approximately 10% of patients and can occur in the absence of pulmonary infarction. Rates appear higher with peripheral or diffuse PAVMs (35). Paradoxical embolism of devices, thrombi, or air bubbles (leading to angina, strokes, or the need for retrieval) are rare and seem to have been reduced by technical advances reported in later series. Potential massive hemoptysis from systemic arterial collaterals that develop in the months after embolization remains a concern, requiring dual systemic (bronchial) and pulmonary angiographic approaches for embolization (7, 9, 36).
Specific considerations apply in the setting of preexisting pulmonary hypertension when occlusion of PAVMs can result in acute and fatal increases in PAP. A detailed discussion using data available in 2008 was presented by Shovlin and colleagues (86). Our current interpretation of the evidence base is that severe pulmonary hypertension remains a relative, if not absolute, contraindication to elective embolization. However, further data are needed, particularly because patients with higher PAP are more likely to report a symptomatic benefit if PAVMs can be safely occluded (26).
Radiation Limitation Strategies
The management approaches recommended in Figure 3 have recently been shown to result in potentially harmful radiation burdens (13). In a single-center study of 246 patients with PAVMs (mean age, 53 yr), the mean cumulative effective dose (CED) over an 11-year period was 51.7 mSv, and the CED exceeded 100 mSv in 26 patients (11%) (13). CT scans accounted for 46% of the CED, compared with 51% from interventional procedures (13). A survey with predominantly North American respondents had reported an excess of breast cancer (but no other cancer) in patients with HHT (87). It therefore seems imperative to reconsider radiation exposure at multiple time points, particularly in younger patients.
Initial PAVM diagnostic investigations
In the HHT community, there have been major efforts to limit radiation exposure at the point of screening of HHT family members, with contrast echocardiography recommended (5). If genuinely negative or grade 1, this can spare individuals with no evidence of shunting from a single thoracic CT scan.
More widely, initial diagnoses often rely on a single thoracic CT scan, which in our view is reasonable given the risks associated with PAVMs. However, the initial CT may then be followed by repeat CT scans, and sometimes diagnostic angiography before referral for formal embolization treatment. “Stop and refer after first CT” seems an appropriate simple paradigm that could be used.
There seems to be some confusion about the likelihood of development of PAVMs in a patient with HHT with a previously negative CT. In our experience, carefully performed CT scans, if negative, are unlikely to require repeating in adulthood unless clinical circumstances change substantially; thus, alternate methods of patient evaluation can be used (see below).
Angiographic treatments
Treatment-associated exposures are incorporated in risk–benefit considerations. Careful consideration is given to whether additional embolization treatment of multiple small (<2 mm) feeding vessels is likely to carry overall treatment benefit for patients. Where patients appear to be at high risk of complications (e.g., those experiencing recurrent ischemic strokes), surgical resection is considered (6, 7). Because children are at higher risk from ionizing radiation, where possible we avoid CT scans and angiography until after puberty (6, 7, 20, 22).
Follow-up
Current international guidelines recommend postembolization CT scans at 6 to 12 months, then every 3 years thereafter, with CT scans every 1 to 5 years for other patients with CT-evident PAVMs or positive contrast echocardiographic studies (5). These recommendations were not adopted universally due to radiation concerns (6, 7, 20, 22, 88, 89), and in the light of data from Reference 13, are likely to be revised in the future. Alternate follow-up strategies used include magnetic resonance imaging, noting resolution is currently limiting (88, 89); exercise testing (90); and SaO2 plus chest X-ray (Figure 1; further images in Reference 26). At our institution, CT scans are reserved for patients demonstrating new symptoms or deteriorating SaO2 or with failure to obliterate a PAVM sac, which would have been predicted based on angiographic appearances (6, 7).
Medical Management of Patients with PAVMs
As an adjunct to embolization treatments, a number of relatively simple lifestyle and medical management recommendations may be used.
Oxygen for hypoxemic patients
Supplementary oxygen can be prescribed for symptomatic patients, but there is no rationale for prophylactic use to prevent hypoxic pulmonary hypertension because there is no alveolar hypoxia (26, 28, 30).
Venesection for polycythemia
Secondary polycythemia is an adaptive response. Venesection is not without risk, particularly the induction of iron deficiency (76, 91), which will increase cardiac demands to maintain tissue oxygen delivery and is a strong risk factor for paradoxical embolic stroke in patients with PAVMs (20). The pathology most resembling PAVMs is cyanotic congenital heart disease, for which United Kingdom polycythemia guidelines (91) only recommend venesection if the patient describes symptoms of hyperviscosity.
Lung transplantation
Lung transplantation has been performed for patients with PAVMs but is rarely indicated because of the life expectancy of even severely hypoxemic patients. The three patients in our practice (20) who were referred for lung transplantation assessment in the late 1980s/early 1990s (and elected not to proceed) remain stable 20, 22, and 25 years later. A separate retrospective study of 36 patients with diffuse PAVMs followed up 8.5 years (0.12–26 yr) later indicated 24 were working or studying full time, but in one patient an attempted lung transplant resulted in an operative death (35).
Pregnancy
To date, it has not been possible to identify women at higher risk of pregnancy-related complications (31). Unfortunately, maternal deaths and life-threatening pulmonary hemorrhage do occur in previously treated patients (31). Anglo-French management strategies emphasize the need to inform women of the 1% rate of maternal death before pregnancy and to manage the cases as high-risk pregnancies (31). Further specific management details are beyond the scope of this text (see References 31 and 32)
Dental
Links between oral bacteria, dental procedures, and PAVM-associated cerebral abscess have been recognized for many years and were the reason why antibiotic prophylaxis was recommended for dental and surgical procedures. This was originally based on the endocarditis paradigm, noting that the absolute risk for patients with PAVM is several orders of magnitude higher and that mechanisms are likely to differ (92). Antibiotic prophylaxis is still recommended for patients with PAVMs (92). Judicious dental hygiene is also recommended (92).
Iron deficiency
Optimization of iron status is emerging as a core principle for the management of patients with PAVMs, particularly those with HHT and substantial iron losses from nosebleeds (71), when conventional iron-rich diets are insufficient to meet the enhanced iron demands. Iron deficiency restricts hemoglobin synthesis/erythrocytosis, and this is an even greater problem for patients with hypoxemia requiring supranormal hemoglobins to maintain CaO2 (26) and/or for patients needing to achieve enhanced cardiac outputs (28, 55, 56, 60). Iron deficiency is also associated with enhanced risks of venous (77) and arterial (20) thromboses in the HHT/PAVM population.
Conclusions
New data offer opportunities for a balanced, informed approach to PAVM risk management. An “advise and embolize” approach is recommended, weighing the benefits and risks of specific treatments on an individual basis (Figure 5), before determining a mutually agreed management plan with the patient. Using this approach in our practice, embolization is almost always recommended, together with adjunct medical measures and radiation-limiting follow-up strategies. Comparative outcome/exposure data and formal updated management guidance are urgently required. The terms hypoxemia and hypoxia should not be used interchangeably, as is commonly observed in publications and clinical practice.
Figure 5.
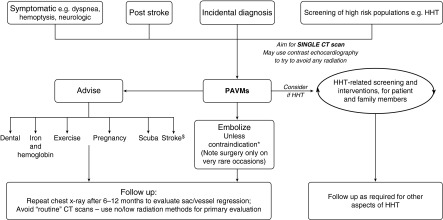
2014 proposed management flowchart. Irrespective of why pulmonary arteriovenous malformations (PAVMs) are identified, all should be considered for embolization if technically feasible and suitable expertise is available. *Caution required if preexisting severe pulmonary hypertension is present when risk benefit considerations change (increased risk of procedural related mortality; reduced benefit form prevention of paradoxical embolic strokes [90]). $Advise of risks of brain abscess and hemorrhagic strokes that may modify stroke management (early MRI scan, caution with thrombolysis). CT = computerized tomography; HHT = hereditary hemorrhagic telangiectasia.
Acknowledgments
Acknowledgment
The author thanks colleagues and students for helpful discussions and Dr. James Jackson for angiography/embolization insights and critical manuscript review.
Footnotes
This work was supported by European Respiratory Society: Rare Disease Achievement Award (C.L.S.), NIHR: London (NW) Comprehensive Local Research Network, and HHT patient donations.
CME will be available for this article at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201407-1254CI on November 24, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Nakayama M, Nawa T, Chonan T, Endo K, Morikawa S, Bando M, Wada Y, Shioya T, Sugiyama Y, Fukai S. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med. 2012;51:1677–1681. doi: 10.2169/internalmedicine.51.7305. [DOI] [PubMed] [Google Scholar]
- 2.Gossage JR, Kanj G. Pulmonary arteriovenous malformations: a state of the art review. Am J Respir Crit Care Med. 1998;158:643–661. doi: 10.1164/ajrccm.158.2.9711041. [DOI] [PubMed] [Google Scholar]
- 3.Shovlin CL, Letarte M. Hereditary haemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax. 1999;54:714–729. doi: 10.1136/thx.54.8.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottin V, Chinet T, Lavolé A, Corre R, Marchand E, Reynaud-Gaubert M, Plauchu H, Cordier JF Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM”O”P) Pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: a series of 126 patients. Medicine (Baltimore) 2007;86:1–17. doi: 10.1097/MD.0b013e31802f8da1. [DOI] [PubMed] [Google Scholar]
- 5.Faughnan ME, Palda VA, Garcia-Tsao G, Geisthoff UW, McDonald J, Proctor DD, Spears J, Brown DH, Buscarini E, Chesnutt MS, et al. HHT Foundation International - Guidelines Working Group. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet. 2011;48:73–87. doi: 10.1136/jmg.2009.069013. [DOI] [PubMed] [Google Scholar]
- 6.Shovlin CL, Jackson JE.Pulmonary arteriovenous malformations and other vascular abnormalities Mason RJ, Broaddus VC, Martin T.et al. editorsMurray and Nadel’s textbook of respiratory medicine. 5th editionPhiladelphia, PA: Elsevier-Saunders; 20101261–1282. [Google Scholar]
- 7.Shovlin CL, Wilmshurst P, Jackson JE. Pulmonary arteriovenous malformations and other pulmonary aspects of HHT. Eur Respir Monogr. 2011;54:218–245. [Google Scholar]
- 8.Cartin-Ceba R, Swanson KL, Krowka MJ. Pulmonary arteriovenous malformations. Chest. 2013;144:1033–1044. doi: 10.1378/chest.12-0924. [DOI] [PubMed] [Google Scholar]
- 9.Lacombe P, Lacout A, Marcy PY, Binsse S, Sellier J, Bensalah M, Chinet T, Bourgault-Villada I, Blivet S, Roume J, et al. Diagnosis and treatment of pulmonary arteriovenous malformations in hereditary hemorrhagic telangiectasia: an overview. Diagn Interv Imaging. 2013;94:835–848. doi: 10.1016/j.diii.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CC, Kwan GN, Thompson SA, Evans-Barns H, van Driel ML. Embolisation for pulmonary arteriovenous malformation. Cochrane Database Syst Rev. 2012;8:CD008017. doi: 10.1002/14651858.CD008017.pub3. [DOI] [PubMed] [Google Scholar]
- 11.Circo S, Gossage JR. Pulmonary vascular complications of hereditary haemorrhagic telangiectasia. Curr Opin Pulm Med. 2014;20:421–428. doi: 10.1097/MCP.0000000000000076. [DOI] [PubMed] [Google Scholar]
- 12.Lindskog GE, Liebow A, Kausel H, Janzen A. Pulmonary arteriovenous aneurysm. Ann Surg. 1950;132:591–610. doi: 10.1097/00000658-195010000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanneman K, Faughnan ME, Prabhudesai V. Cumulative radiation dose in patients with hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations. Can Assoc Radiol J. 2014;65:135–140. doi: 10.1016/j.carj.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 14.Govani FS, Shovlin CL. Hereditary haemorrhagic telangiectasia: a clinical and scientific review. Eur J Hum Genet. 2009;17:860–871. doi: 10.1038/ejhg.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shovlin CL. Hereditary haemorrhagic telangiectasia: pathophysiology, diagnosis and treatment. Blood Rev. 2010;24:203–219. doi: 10.1016/j.blre.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Dupuis-Girod S, Bailly S, Plauchu H. Hereditary hemorrhagic telangiectasia: from molecular biology to patient care. J Thromb Haemost. 2010;8:1447–1456. doi: 10.1111/j.1538-7836.2010.03860.x. [DOI] [PubMed] [Google Scholar]
- 17.McDonald J, Bayrak-Toydemir P, Pyeritz RE. Hereditary hemorrhagic telangiectasia: an overview of diagnosis, management, and pathogenesis. Genet Med. 2011;13:607–616. doi: 10.1097/GIM.0b013e3182136d32. [DOI] [PubMed] [Google Scholar]
- 18.van Gent MW, Post MC, Snijder RJ, Westermann CJ, Plokker HW, Mager JJ. Real prevalence of pulmonary right-to-left shunt according to genotype in patients with hereditary hemorrhagic telangiectasia: a transthoracic contrast echocardiography study. Chest. 2010;138:833–839. doi: 10.1378/chest.09-1849. [DOI] [PubMed] [Google Scholar]
- 19.Girerd B, Montani D, Coulet F, Sztrymf B, Yaici A, Jaïs X, Tregouet D, Reis A, Drouin-Garraud V, Fraisse A, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med. 2010;181:851–861. doi: 10.1164/rccm.200908-1284OC. [DOI] [PubMed] [Google Scholar]
- 20.Shovlin CL, Chamali B, Santhirapala V, Livesey JA, Angus G, Manning R, Laffan MA, Meek J, Tighe HC, Jackson JE. Ischaemic strokes in patients with pulmonary arteriovenous malformations and hereditary hemorrhagic telangiectasia: associations with iron deficiency and platelets. PLoS ONE. 2014;9:e88812. doi: 10.1371/journal.pone.0088812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Donaldson JW, McKeever TM, Hall IP, Hubbard RB, Fogarty AW. The UK prevalence of hereditary haemorrhagic telangiectasia and its association with sex, socioeconomic status and region of residence: a population-based study. Thorax. 2014;69:161–167. doi: 10.1136/thoraxjnl-2013-203720. [DOI] [PubMed] [Google Scholar]
- 22.Shovlin CL, Jackson JE, Bamford KB, Jenkins IH, Benjamin AR, Ramadan H, Kulinskaya E. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax. 2008;63:259–266. doi: 10.1136/thx.2007.087452. [DOI] [PubMed] [Google Scholar]
- 23.Kopf GS, Laks H, Stansel HC, Hellenbrand WE, Kleinman CS, Talner NS.Thirty-year follow-up of superior vena cava-pulmonary artery (Glenn) shunts J Thorac Cardiovasc Surg 1990100662–670.discussion 670–671 [PubMed] [Google Scholar]
- 24.Field-Ridley A, Heljasvaara R, Pihlajaniemi T, Adatia I, Sun C, Keller RL, Gong WH, Datar S, Oishi P, Fineman JR. Endostatin, an inhibitor of angiogenesis, decreases after bidirectional superior cavopulmonary anastamosis. Pediatr Cardiol. 2013;34:291–295. doi: 10.1007/s00246-012-0441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sluiter-Eringa H, Orie NGM, Sluiter HJ. Pulmonary arteriovenous fistula. Diagnosis and prognosis in noncomplainant patients. Am Rev Respir Dis. 1969;100:177–188. doi: 10.1164/arrd.1969.100.2.177. [DOI] [PubMed] [Google Scholar]
- 26.Santhirapala V, Williams LC, Tighe HC, Jackson JE, Shovlin CL. Arterial oxygen content is precisely maintained by graded erythrocytotic responses in settings of high/normal serum iron levels, and predicts exercise capacity: an observational study of hypoxaemic patients with pulmonary arteriovenous malformations. PLoS ONE. 2014;9:e90777. doi: 10.1371/journal.pone.0090777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santhirapala V, Chamali B, McKernan H, Tighe HC, Williams LC, Springett JT, Bellenberg HR, Whitaker AJ, Shovlin CL. Orthodeoxia and postural orthostatic tachycardia in patients with pulmonary arteriovenous malformations: a prospective 8-year series. Thorax. 2014;69:1046–1047. doi: 10.1136/thoraxjnl-2014-205289. [DOI] [PubMed] [Google Scholar]
- 28.Howard LS, Santhirapala V, Murphy K, Mukherjee B, Busbridge M, Tighe HC, Jackson JE, Hughes JM, Shovlin CL. Cardiopulmonary exercise testing demonstrates maintenance of exercise capacity in patients with hypoxemia and pulmonary arteriovenous malformations. Chest. 2014;146:709–718. doi: 10.1378/chest.13-2988. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues P, Palma P, Sousa-Pereira L. Platypnea-orthodeoxia syndrome in review: defining a new disease? Cardiology. 2012;123:15–23. doi: 10.1159/000339872. [DOI] [PubMed] [Google Scholar]
- 30.Mason CG, Shovlin CL. Flight-related complications are infrequent in patients with hereditary haemorrhagic telangiectasia/pulmonary arteriovenous malformations, despite low oxygen saturations and anaemia. Thorax. 2012;67:80–81. doi: 10.1136/thoraxjnl-2011-201027. [DOI] [PubMed] [Google Scholar]
- 31.Shovlin CL, Sodhi V, McCarthy A, Lasjaunias P, Jackson JE, Sheppard MN. Estimates of maternal risks of pregnancy for women with hereditary haemorrhagic telangiectasia (Osler-Weber-Rendu syndrome): suggested approach for obstetric services. BJOG. 2008;115:1108–1115. doi: 10.1111/j.1471-0528.2008.01786.x. [DOI] [PubMed] [Google Scholar]
- 32.de Gussem EM, Lausman AY, Beder AJ, Edwards CP, Blanker MH, Terbrugge KG, Mager JJ, Faughnan ME. Outcomes of pregnancy in women with hereditary hemorrhagic telangiectasia. Obstet Gynecol. 2014;123:514–520. doi: 10.1097/AOG.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 33.Sagara K, Miyazono N, Inoue H, Ueno K, Nishida H, Nakajo M. Recanalization after coil embolotherapy of pulmonary arteriovenous malformations: study of long-term outcome and mechanism for recanalization. AJR Am J Roentgenol. 1998;170:727–730. doi: 10.2214/ajr.170.3.9490963. [DOI] [PubMed] [Google Scholar]
- 34.Brillet PY, Dumont P, Bouaziz N, Duhamel A, Laurent F, Remy J, Remy-Jardin M. Pulmonary arteriovenous malformation treated with embolotherapy: systemic collateral supply at multidetector CT angiography after 2-20-year follow-up. Radiology. 2007;242:267–276. doi: 10.1148/radiol.2421041571. [DOI] [PubMed] [Google Scholar]
- 35.Pierucci P, Murphy J, Henderson KJ, Chyun DA, White RI., Jr New definition and natural history of patients with diffuse pulmonary arteriovenous malformations: twenty-seven-year experience. Chest. 2008;133:653–661. doi: 10.1378/chest.07-1949. [DOI] [PubMed] [Google Scholar]
- 36.Woodward CS, Pyeritz RE, Chittams JL, Trerotola SO. Treated pulmonary arteriovenous malformations: patterns of persistence and associated retreatment success. Radiology. 2013;269:919–926. doi: 10.1148/radiol.13122153. [DOI] [PubMed] [Google Scholar]
- 37.Yater W, Finnegan J, Giffin H. Pulmonary arteriovenous fistula (varix) JAMA. 1949;141:581–589. doi: 10.1001/jama.1949.02910090007002. [DOI] [PubMed] [Google Scholar]
- 38.Kjeldsen AD, Tørring PM, Nissen H, Andersen PE. Cerebral abscesses among Danish patients with hereditary haemorrhagic telangiectasia. Acta Neurol Scand. 2014;129:192–197. doi: 10.1111/ane.12167. [DOI] [PubMed] [Google Scholar]
- 39.Moussouttas M, Fayad P, Rosenblatt M, Hashimoto M, Pollak J, Henderson K, Ma TY, White RI. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology. 2000;55:959–964. doi: 10.1212/wnl.55.7.959. [DOI] [PubMed] [Google Scholar]
- 40.Thenganatt J, Schneiderman J, Hyland RH, Edmeads J, Mandzia JL, Faughnan ME. Migraines linked to intrapulmonary right-to-left shunt. Headache. 2006;46:439–443. doi: 10.1111/j.1526-4610.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 41.Marziniak M, Jung A, Guralnik V, Evers S, Prudlo J, Geisthoff UW. An association of migraine with hereditary haemorrhagic telangiectasia independently of pulmonary right-to-left shunts. Cephalalgia. 2009;29:76–81. doi: 10.1111/j.1468-2982.2008.01703.x. [DOI] [PubMed] [Google Scholar]
- 42.Post MC, van Gent MW, Plokker HW, Westermann CJ, Kelder JC, Mager JJ, Overtoom TT, Schonewille WJ, Thijs V, Snijder RJ. Pulmonary arteriovenous malformations associated with migraine with aura. Eur Respir J. 2009;34:882–887. doi: 10.1183/09031936.00179008. [DOI] [PubMed] [Google Scholar]
- 43.Post MC, Thijs V, Schonewille WJ, Budts W, Snijder RJ, Plokker HW, Westermann CJ. Embolization of pulmonary arteriovenous malformations and decrease in prevalence of migraine. Neurology. 2006;66:202–205. doi: 10.1212/01.wnl.0000194257.75559.b0. [DOI] [PubMed] [Google Scholar]
- 44.Elphick A, Shovlin CL. Relationships between epistaxis, migraines, and triggers in hereditary hemorrhagic telangiectasia. Laryngoscope. 2014;124:1521–1528. doi: 10.1002/lary.24526. [DOI] [PubMed] [Google Scholar]
- 45.Clark K, Pyeritz RE, Trerotola SO. Angina pectoris or myocardial infarctions, pulmonary arteriovenous malformations, hereditary hemorrhagic telangiectasia, and paradoxical emboli. Am J Cardiol. 2013;112:731–734. doi: 10.1016/j.amjcard.2013.04.052. [DOI] [PubMed] [Google Scholar]
- 46.Gill SS, Roddie ME, Shovlin CL, Jackson JE. Pulmonary arteriovenous malformations and their mimics: a pictorial review. Clin Radiol. In press doi: 10.1016/j.crad.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Faughnan ME, Lui YW, Wirth JA, Pugash RA, Redelmeier DA, Hyland RH, White RI., Jr Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest. 2000;117:31–38. doi: 10.1378/chest.117.1.31. [DOI] [PubMed] [Google Scholar]
- 48.Hart JL, Aldin Z, Braude P, Shovlin CL, Jackson J. Embolization of pulmonary arteriovenous malformations using the Amplatzer vascular plug: successful treatment of 69 consecutive patients. Eur Radiol. 2010;20:2663–2670. doi: 10.1007/s00330-010-1851-2. [DOI] [PubMed] [Google Scholar]
- 49.Hopkins SR, Olfert IM, Wagner PD. Exercise-induced intrapulmonary shunting is imaginary. J Appl Physiol. 2009;107:993–994. doi: 10.1152/japplphysiol.91489.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mélot C, Naeije R. Pulmonary vascular diseases. Compr Physiol. 2011;1:593–619. doi: 10.1002/cphy.c090014. [DOI] [PubMed] [Google Scholar]
- 51.Gazzaniga P, Buscarini E, Leandro G, Reduzzi L, Grosso M, Pongiglione G, Pedrinazzi C, Lanzarini L, Portugalli V, Blotta P, et al. Contrast echocardiography for pulmonary arteriovenous malformations screening: does any bubble matter? Eur J Echocardiogr. 2009;10:513–518. doi: 10.1093/ejechocard/jen317. [DOI] [PubMed] [Google Scholar]
- 52.Laurie SS, Elliott JE, Goodman RD, Lovering AT. Catecholamine-induced opening of intrapulmonary arteriovenous anastomoses in healthy humans at rest. J Appl Physiol. 2012;113:1213–1222. doi: 10.1152/japplphysiol.00565.2012. [DOI] [PubMed] [Google Scholar]
- 53.Hébert PC, Van der Linden P, Biro G, Hu LQ. Physiologic aspects of anemia. Crit Care Clin. 2004;20:187–212. doi: 10.1016/j.ccc.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 54.Whyte MK, Hughes JM, Jackson JE, Peters AM, Hempleman SC, Moore DP, Jones HA. Cardiopulmonary response to exercise in patients with intrapulmonary vascular shunts. J Appl Physiol (1985) 1993;75:321–328. doi: 10.1152/jappl.1993.75.1.321. [DOI] [PubMed] [Google Scholar]
- 55.Vorselaars VM, Velthuis S, Mager JJ, Snijder RJ, Bos WJ, Vos JA, van Strijen MJ, Post MC. Direct haemodynamic effects of pulmonary arteriovenous malformation embolisation. Neth Heart J. 2014;22:328–333. doi: 10.1007/s12471-014-0539-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buscarini E, Leandro G, Conte D, Danesino C, Daina E, Manfredi G, Lupinacci G, Brambilla G, Menozzi F, De Grazia F, et al. Natural history and outcome of hepatic vascular malformations in a large cohort of patients with hereditary hemorrhagic teleangiectasia. Dig Dis Sci. 2011;56:2166–2178. doi: 10.1007/s10620-011-1585-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Velthuis S, Buscarini E, Mager JJ, Vorselaars VM, van Gent MW, Gazzaniga P, Manfredi G, Danesino C, Diederik AL, Vos JA, et al. Predicting the size of pulmonary arteriovenous malformations on chest computed tomography: a role for transthoracic contrast echocardiography. Eur Respir J. 2014;44:150–159. doi: 10.1183/09031936.00133713. [DOI] [PubMed] [Google Scholar]
- 58.Pittman RN.Oxygen transport. In: Regulation of tissue oxygenation. San Rafael, CA: Morgan & Claypool Life Sciences; 2011 [accessed 2014 Nov 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK54103 [PubMed]
- 59.Grocott MP, Martin DS, Levett DZ, McMorrow R, Windsor J, Montgomery HE Caudwell Xtreme Everest Research Group. Arterial blood gases and oxygen content in climbers on Mount Everest. N Engl J Med. 2009;360:140–149. doi: 10.1056/NEJMoa0801581. [DOI] [PubMed] [Google Scholar]
- 60.Terry PB, White RI, Jr, Barth KH, Kaufman SL, Mitchell SE. Pulmonary arteriovenous malformations. Physiologic observations and results of therapeutic balloon embolization. N Engl J Med. 1983;308:1197–1200. doi: 10.1056/NEJM198305193082005. [DOI] [PubMed] [Google Scholar]
- 61.Shovlin CL. Curable hypoxia in an octogenarian with an undiagnosed inherited condition: a case commentary. Breathe 2014:10;153–156.
- 62.Shovlin CL. The oxygen train [accessed 2014 Nov 18]. Available from: www.theoxygentrain.net
- 63.Hu M, Lin W. Effects of exercise training on red blood cell production: implications for anemia. Acta Haematol. 2012;127:156–164. doi: 10.1159/000335620. [DOI] [PubMed] [Google Scholar]
- 64.Kautz L, Jung G, Valore EV, Rivella S, Nemeth E, Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism. Nat Genet. 2014;46:678–684. doi: 10.1038/ng.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seldin MM, Peterson JM, Byerly MS, Wei Z, Wong GW. Myonectin (CTRP15), a novel myokine that links skeletal muscle to systemic lipid homeostasis. J Biol Chem. 2012;287:11968–11980. doi: 10.1074/jbc.M111.336834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, et al. American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Clinical Cardiology, and Interdisciplinary Council on Quality of Care and Outcomes Research. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 68.Devlin HL, Hosman AE, Shovlin CL. Antiplatelet and anticoagulant agents in hereditary hemorrhagic telangiectasia. N Engl J Med. 2013;368:876–878. doi: 10.1056/NEJMc1213554. [DOI] [PubMed] [Google Scholar]
- 69.Pollak JS, Saluja S, Thabet A, Henderson KJ, Denbow N, White RI., Jr Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol. 2006;17:35–44, quiz 45. doi: 10.1097/01.RVI.0000191410.13974.B6. [DOI] [PubMed] [Google Scholar]
- 70.Velthuis S, Buscarini E, van Gent MW, Gazzaniga P, Manfredi G, Danesino C, Schonewille WJ, Westermann CJ, Snijder RJ, Mager JJ, et al. Grade of pulmonary right-to-left shunt on contrast echocardiography and cerebral complications: a striking association. Chest. 2013;144:542–548. doi: 10.1378/chest.12-1599. [DOI] [PubMed] [Google Scholar]
- 71.Finnamore H, Le Couteur J, Hickson M, Busbridge M, Whelan K, Shovlin CL. Hemorrhage-adjusted iron requirements, hematinics and hepcidin define hereditary hemorrhagic telangiectasia as a model of hemorrhagic iron deficiency. PLoS ONE. 2013;8:e76516. doi: 10.1371/journal.pone.0076516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9:92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 73.Chang YL, Hung SH, Ling W, Lin HC, Li HC, Chung SD. Association between ischemic stroke and iron-deficiency anemia: a population-based study. PLoS ONE. 2013;8:e82952. doi: 10.1371/journal.pone.0082952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao Z, Liu M, Counsell C, Wardlaw JM, Lin S, Zhao X. Fibrinogen depleting agents for acute ischaemic stroke. Cochrane Database Syst Rev. 2012;3:CD000091. doi: 10.1002/14651858.CD000091.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shovlin CL. Iron deficiency, ischaemic strokes, and right-to-left shunts: From pulmonary arteriovenous malformations to patent foramen ovale? Intractable Rare Dis Res. 2014;3:60–64. doi: 10.5582/irdr.2014.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van de Pette JE, Guthrie DL, Pearson TC. Whole blood viscosity in polycythaemia: the effect of iron deficiency at a range of haemoglobin and packed cell volumes. Br J Haematol. 1986;63:369–375. doi: 10.1111/j.1365-2141.1986.tb05562.x. [DOI] [PubMed] [Google Scholar]
- 77.Livesey JA, Manning RA, Meek JH, Jackson JE, Kulinskaya E, Laffan MA, Shovlin CL. Low serum iron levels are associated with elevated plasma levels of coagulation factor VIII and pulmonary emboli/deep venous thromboses in replicate cohorts of patients with hereditary haemorrhagic telangiectasia. Thorax. 2012;67:328–333. doi: 10.1136/thoraxjnl-2011-201076. [DOI] [PubMed] [Google Scholar]
- 78.Roked F, Jackson JE, Fuld J, Basheer FT, Chilvers ER, Beattie S, Shovlin CL. Pulmonary thromboemboli modifying the natural history of pulmonary arteriovenous malformations. Am J Respir Crit Care Med. 2011;183:828–829. doi: 10.1164/ajrccm.183.6.828. [DOI] [PubMed] [Google Scholar]
- 79.Woods HF, Youdim MBH, Boullin D, Callender S.Monoamine metabolism and platelet function in iron-deficiency anaemia. Ciba Found Symp 1976;51:227–248. [DOI] [PubMed] [Google Scholar]
- 80.Mathis S, Dupuis-Girod S, Plauchu H, Giroud M, Barroso B, Ly KH, Ingrand P, Gilbert B, Godenèche G, Neau JP. Cerebral abscesses in hereditary haemorrhagic telangiectasia: a clinical and microbiological evaluation. Clin Neurol Neurosurg. 2012;114:235–240. doi: 10.1016/j.clineuro.2011.10.036. [DOI] [PubMed] [Google Scholar]
- 81.Shovlin CL, Tighe HC, Davies RJ, Gibbs JSR, Jackson JE. Embolisation of pulmonary arteriovenous malformations: no consistent effect on pulmonary artery pressure. Eur Respir J. 2008;32:162–169. doi: 10.1183/09031936.00126207. [DOI] [PubMed] [Google Scholar]
- 82.Trerotola SO, Pyeritz RE. Does use of coils in addition to amplatzer vascular plugs prevent recanalization? AJR Am J Roentgenol. 2010;195:766–771. doi: 10.2214/AJR.09.3953. [DOI] [PubMed] [Google Scholar]
- 83.Letourneau-Guillon L, Faughnan ME, Soulez G, Giroux MF, Oliva VL, Boucher LM, Dubois J, Prabhudesai V, Therasse E. Embolization of pulmonary arteriovenous malformations with amplatzer vascular plugs: safety and midterm effectiveness. J Vasc Interv Radiol. 2010;21:649–656. doi: 10.1016/j.jvir.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 84.Milic A, Chan RP, Cohen JH, Faughnan ME. Reperfusion of pulmonary arteriovenous malformations after embolotherapy. J Vasc Interv Radiol. 2005;16:1675–1683. doi: 10.1097/01.RVI.0000182163.25493.BB. [DOI] [PubMed] [Google Scholar]
- 85.Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax. 1963;18:225–237. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shovlin CL, Gibbs JS, Jackson JE. Management of pulmonary arteriovenous malformations in pulmonary hypertensive patients: a pressure to embolise? Eur Respir Rev. 2009;18:4–6. doi: 10.1183/09059180.00011102. [DOI] [PubMed] [Google Scholar]
- 87.Hosman AE, Devlin HL, Silva BM, Shovlin CL. Specific cancer rates may differ in patients with hereditary haemorrhagic telangiectasia compared to controls. Orphanet J Rare Dis. 2013;8:195. doi: 10.1186/1750-1172-8-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schneider G, Uder M, Koehler M, Kirchin MA, Massmann A, Buecker A, Geisthoff U. MR angiography for detection of pulmonary arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. AJR Am J Roentgenol. 2008;190:892–901. doi: 10.2214/AJR.07.2966. [DOI] [PubMed] [Google Scholar]
- 89.Boussel L, Cernicanu A, Geerts L, Gamondes D, Khouatra C, Cottin V, Revel D, Douek P. 4D time-resolved magnetic resonance angiography for noninvasive assessment of pulmonary arteriovenous malformations patency. J Magn Reson Imaging. 2010;32:1110–1116. doi: 10.1002/jmri.22384. [DOI] [PubMed] [Google Scholar]
- 90.Li W, Niu B, Henderson K, Northrup V, Pollak JS, Trow T, Fahey J, White RI., Jr Reproducibility of oxygen saturation monitoring during six-minute walk test and exercise stress test in patients with pulmonary arteriovenous malformations associated with hereditary hemorrhagic telangiectasia. Pediatr Cardiol. 2011;32:590–594. doi: 10.1007/s00246-011-9917-8. [DOI] [PubMed] [Google Scholar]
- 91.McMullin MF, Bareford D, Campbell P, Green AR, Harrison C, Hunt B, Oscier D, Polkey MI, Reilly JT, Rosenthal E, et al. General Haematology Task Force of the British Committee for Standards in Haematology. Guidelines for the diagnosis, investigation and management of polycythaemia/erythrocytosis. Br J Haematol. 2005;130:174–195. doi: 10.1111/j.1365-2141.2005.05535.x. [DOI] [PubMed] [Google Scholar]
- 92.Shovlin C, Bamford K, Wray D. Post-NICE 2008: Antibiotic prophylaxis prior to dental procedures for patients with pulmonary arteriovenous malformations (PAVMs) and hereditary haemorrhagic telangiectasia. Br Dent J. 2008;205:531–533. doi: 10.1038/sj.bdj.2008.978. [DOI] [PubMed] [Google Scholar]