Abstract
Rationale: Although current resuscitation guidelines are rescuer focused, the opportunity exists to develop patient-centered resuscitation strategies that optimize the hemodynamic response of the individual in the hopes to improve survival.
Objectives: To determine if titrating cardiopulmonary resuscitation (CPR) to blood pressure would improve 24-hour survival compared with traditional CPR in a porcine model of asphyxia-associated ventricular fibrillation (VF).
Methods: After 7 minutes of asphyxia, followed by VF, 20 female 3-month-old swine randomly received either blood pressure–targeted care consisting of titration of compression depth to a systolic blood pressure of 100 mm Hg and vasopressors to a coronary perfusion pressure greater than 20 mm Hg (BP care); or optimal American Heart Association Guideline care consisting of depth of 51 mm with standard advanced cardiac life support epinephrine dosing (Guideline care). All animals received manual CPR for 10 minutes before first shock. Primary outcome was 24-hour survival.
Measurements and Main Results: The 24-hour survival was higher in the BP care group (8 of 10) compared with Guideline care (0 of 10); P = 0.001. Coronary perfusion pressure was higher in the BP care group (point estimate +8.5 mm Hg; 95% confidence interval, 3.9–13.0 mm Hg; P < 0.01); however, depth was higher in Guideline care (point estimate +9.3 mm; 95% confidence interval, 6.0–12.5 mm; P < 0.01). Number of vasopressor doses before first shock was higher in the BP care group versus Guideline care (median, 3 [range, 0–3] vs. 2 [range, 2–2]; P = 0.003).
Conclusions: Blood pressure–targeted CPR improves 24-hour survival compared with optimal American Heart Association care in a porcine model of asphyxia-associated VF cardiac arrest.
Keywords: asphyxia, heart arrest, vascular access devices
At a Glance Commentary
Scientific Knowledge on the Subject
Current treatment algorithms for cardiac arrest recommend provider-centric resuscitation that varies little from patient to patient, rather than patient-centric care targeted to hemodynamics during cardiopulmonary resuscitation.
What This Study Adds to the Field
This porcine investigation establishes that individualized, prospective titration of cardiopulmonary resuscitation to blood pressure improves 24-hour survival compared with optimal American Heart Association Guideline care.
The current paradigm for optimal resuscitation is “rescuer focused” in that the rescuer is coached to provide an optimal rate and depth of compression (1, 2). With this approach, all victims end up getting the same treatment. We propose an alternative strategy, which is more “patient centered,” and requires monitoring the actual hemodynamic response of the individual to cardiopulmonary resuscitation (CPR), and then seeks to optimize that response in the hopes of improving survival (3).
Although in-hospital cardiac arrest has been poorly studied, it remains a significant public health problem with 200,000 patients receiving CPR at some time during their hospitalization (4). Presumably because of the successful implementation of medical emergency teams, most of these arrests now occur in highly monitored intensive care units (ICUs) where patients often have invasive monitoring in place at the time of arrest (5, 6). Therefore, the opportunity exists to develop a patient-centered resuscitation strategy that incorporates the hemodynamic response of the individual into the ongoing resuscitation effort. Such an individualized approach would be a major paradigm shift in the field of resuscitation.
Survival following CPR for cardiac arrest depends on provision of adequate myocardial blood flow during CPR (7–9). The primary determinant of myocardial blood flow during CPR is coronary perfusion pressure (CPP), the mathematical difference between arterial and right atrial diastolic pressures (10). Although obtaining measurements of myocardial blood flow during CPR is not practical, both CPP and arterial diastolic pressure, the primary driving force of CPP, are readily available to many ICU rescuers (5, 6) and may be useful clinical surrogates to guide resuscitation quality. The American Heart Association recommends monitoring resuscitation efforts using arterial blood pressure (11); however, no study has demonstrated that prospectively targeting such goals during resuscitation leads to improved survival.
Building on previous work (12, 13), this randomized investigation compares 24-hour survival with “patient-centric” blood pressure–targeted care (BP care) intended to attain systolic blood pressures greater than 100 mm Hg and CPP greater than 20 mm Hg versus “provider-centric” optimal American Heart Association Guideline care (Guideline care; absolute depth-guided CPR to 51 mm with standard Advanced Cardiac Life Support [ACLS] vasopressor dosing [1]) in a porcine model of asphyxia-associated ventricular fibrillation (VF) cardiac arrest. We hypothesized that the blood pressure–targeted care would improve 24-hour survival compared with optimal American Heart Association Guideline care.
Methods
Animal Preparation
The experimental protocol was approved by The Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee. Twenty healthy 3-month-old female domestic swine were anesthetized and mechanically ventilated using a Datex Ohmeda anesthesia machine (Modulus SE) on a mixture of room air and titrated isoflurane (∼1.0–2.5%) with a tidal volume of 10–12 ml/kg, positive end-expiratory pressure of 6 cm H2O, and titration of rate to maintain end-tidal carbon dioxide (ETco2) at 38–42 mm Hg (NICO; Novametrix Medical Systems Inc., Wallingford, CT).
Vascular introducer sheaths (6–7F catheter; Cordis Corp., Fremont, CA) were placed percutaneously under sterile conditions into an internal and external jugular vein and a femoral artery and vein. High-fidelity, solid-state, micromanometer-tipped catheters (MPC-500; Millar Instruments, Houston, TX) were then advanced through the femoral artery and internal jugular access sites into thoracic locations to measure continuous aortic and right atrial pressures, respectively. A Swan-Ganz Thermodilution catheter (Edwards Lifesciences, Irvine, CA) was placed in the pulmonary artery and a bipolar pacing catheter (Edwards Lifesciences) was placed in the right ventricle via the femoral vein and external jugular access sites. All catheter placements were confirmed with fluoroscopy. Unfractionated heparin 200 U/kg was provided to prevent catheter clotting. Before obtaining any baseline measurements, all animals received 0.9% normal saline intravenously titrated to left atrial wedge pressure to have similar volume status across animals before cardiac arrest (<16 mm Hg, 20 cc/kg; 16–18 mm Hg, 10 cc/kg; >18 mm Hg, no bolus).
Measurements
Thermodilution cardiac outputs (ICU monitor: model HP66; Hewlett Packard, Palo Alto, CA) were obtained at baseline. Arterial blood gas specimens were obtained from the thoracic aorta at baseline (before endotracheal tube clamping); at 2, 4, and 6.5 minutes after endotracheal tube clamping; and then 2.5 and 6 minutes after VF induction and start of CPR.
To ensure rigorous adherence to CPR quality goals, the Heart Start MRx defibrillator with Q-CPR option, jointly designed by Philips Health Care (Andover, MA) and the Laerdal Medical Corporation (Stavanger, Norway), and a metronome were deployed during the experimental protocol. This defibrillator records CPR quality and provides audiovisual feedback to the chest compression provider for rate (min−1), depth (millimeters), and incomplete chest wall recoil (residual leaning force [grams]).
Experimental Protocol
Overview
The protocol used in this experiment was designed to address resuscitation provided in an ICU for an asphyxia-associated cardiac arrest (Figure 1). A porcine model was chosen because of the documented similarities between swine and humans in regards to anterior-posterior chest depth and compression characteristics (e.g., chest stiffness) (14). These similarities provide justification for using a treatment strategy recommended in humans as our control group (i.e., Guideline care with a depth of 51 mm). In both groups, after 7 minutes of endotracheal tube clamping, VF was induced to ensure a standard 10-minute period of CPR that was consistently terminated by defibrillation, because excellent CPR for experimental asphyxia-associated cardiac arrest often results in return of spontaneous circulation (ROSC) within 2–4 minutes (15, 16), which would have precluded our evaluation of the different resuscitation approaches for longer periods (i.e., up to 10 min). This model has clinical relevance because nearly 40% of in-hospital adult arrests have acute respiratory insufficiency as a cause of arrest (17), of which one-fifth have VF or pulseless ventricular tachycardia as their initial documented rhythm (17). Ten minutes of CPR was performed before the first defibrillation attempt to provide further clinical relevance to our model because most in-hospital CPR is greater than or equal to 10 minutes in duration for survivors and nonsurvivors (17, 18).
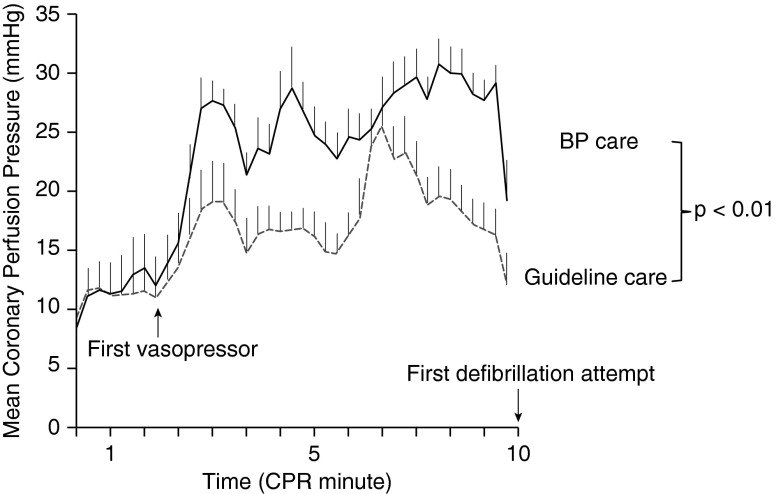
Figure 1.

Protocol design. During protocol resuscitation period, animals were randomized to receive one of two resuscitation strategies. Guideline care refers to target depth of 51 mm and standard advanced cardiac life support epinephrine dosing. BP care refers to cardiopulmonary resuscitation (CPR) directed to attain a systolic blood pressure (SBP) of 100 mm Hg and a coronary perfusion pressure (CPP) greater than 20 mm Hg. BP = blood pressure; ETT = endotracheal tube; ROSC = return of spontaneous circulation; VF = ventricular fibrillation.
In both groups, compressions were provided with a target rate of 100 min−1 and ventilations at 6 min−1 with 100% oxygen. Brief interruptions in CPR every 2 minutes mimicked American Heart Association recommended pulse checks and rhythm analysis. Animals randomly received either blood pressure–targeted care (BP care), consisting of compression depth titrated to a target systolic blood pressure of 100 mm Hg and titration of vasopressors to maintain CPP greater than 20 mm Hg; or optimal American Heart Association Guideline care (Guideline care), consisting of target depth of 51 mm with standard ACLS epinephrine dosing. ACLS dosing was intravenous epinephrine (0.02 mg/kg) every 4 minutes starting at minute 9 of the protocol (2 min after CPR was started). Animals in the BP care group received intravenous vasopressor if and only if the CPP was less than 20 mm Hg, also starting at minute 9 of the protocol. The order of drug administration in BP care was epinephrine (0.02 mg/kg), epinephrine (0.02 mg/kg), and vasopressin (0.4 U/kg). The minimal dosing interval was 1 minute between epinephrine doses, and if vasopressin was given, the minimal dosing interval was 2 minutes before the cycle was restarted. After 10 minutes of CPR, the initial 200-J biphasic waveform defibrillation attempt was provided. Resuscitation according to treatment strategy continued until sustained ROSC or after an additional 10 minutes of resuscitation postinitial defibrillation attempt. If ROSC was attained, the animals received protocolized postarrest care (discussed next). Any animals that died received a post-mortem examination for detection of visceral injuries.
Postarrest care
Animals that achieved ROSC received protocolized intensive care treatment which included (1) titration of oxygen concentration to maintain oxygen saturation 92–96%, (2) titration of ventilation (tidal volume [6–10 cc/kg] and ventilation rate) to maintain ETco2 between 38 and 42 mm Hg, and (3) intravenous infusions of dopamine (up to 20 μg/kg/min) and/or epinephrine boluses (20 μg) to maintain mean arterial pressure greater than 55 mm Hg. Anesthesia was maintained with inhaled isoflurane (∼1.0–2.5%) during this time period. All animals received bupernorphine (0.02 mg/kg intramuscularly every 6 h) for pain control. By 4 hours post-ROSC, vasopressor support was weaned off and the animals were extubated. At 24 hours, all animals were neurologically assessed using the previously validated swine cerebral performance category (CPC) scale (19, 20), performed by two experienced research technicians, who agreed on the final score.
Data Analysis and Outcomes
The primary outcome of the study was 24-hour survival. Secondary outcomes included (1) any ROSC, (2) 45-minute survival, (3) favorable neurologic outcome at 24 hours (defined as a CPC of 1–2), (4) hemodynamic measures (specifically mean diastolic CPP), and (5) CPR quality variables. To calculate the mean diastolic CPP, the diastolic period of each heartbeat was identified by locating extrema in a wavelet transform (ricker wavelet, period 0.5–3 s) of the aortic pressure signal. After identification of the diastolic periods in each heartbeat, the average pressure difference between the aortic pressure and the right atrial pressure was calculated for each diastolic period. Normality of continuous variables was assessed using the Skewness-Kurtosis test. Normally distributed continuous variables were described as mean ± SEM and compared by Student t test. Continuous variables that were not normally distributed were described as median (25%, 75%) and evaluated by Wilcoxon rank sum. Comparisons of dichotomous variables were evaluated by Fisher exact test. Differences in CPR quality variables and in CPPs over time and between treatment groups and between survivors and nonsurvivors were assessed using generalized estimating equations with an identity link (21). A robust variance estimator with an exchangeable correlation structure was used to account for longitudinal correlation (i.e., each experiment was analyzed using multiple 15-s epochs). For our primary outcome, 10 animals were randomly assigned to each treatment to give us 80% power to detect a difference in 24-hour survival of 70% (10% in the Guideline care group and 80% in the BP care group), conservative estimates chosen from our short-term survival studies (12, 13). Statistical analysis was completed using the Stata-IC statistical package (Version 12.0; StataCorp, College Station, TX).
Results
The primary outcome variable of 24-hour survival and the secondary survival outcomes of any ROSC, 45-minute survival, and survival with good neurologic outcome were all higher in the BP care group compared with Guideline care (Table 1). Of the seven animals surviving to 24 hours, five (71%) had a CPC score of 1, one (14%) had a CPC score of 2, and one (14%) had a CPC score of 3. In a model using generalized estimating equations, CPP was significantly higher in the BP care group compared with Guideline care (point estimate +8.5 mm Hg; 95% confidence interval, 3.9–13.0 mm Hg; P < 0.01) (Figure 2), and higher in 24-hour survivors compared with nonsurvivors irrespective of treatment group (point estimate +8.7 mm Hg; 95% confidence interval, 3.9–13.4 mm Hg; P < 0.01).
Table 1.
Rates of Survival across Treatment Groups
| Guideline Care (n = 10) | BP Care (n = 10) | P Value | |
|---|---|---|---|
| Any ROSC, n (%) | 3 (33) | 10 (100) | 0.003 |
| 45-minute ICU survival, n (%) | 1 (10) | 9 (90) | 0.001 |
| 24-hour survival, n (%) | 0 (0) | 8 (80) | 0.001 |
| Favorable neurologic outcome, n (%) | 0 (0) | 7 (70) | 0.003 |
Definition of abbreviations: BP = blood pressure; ICU = intensive care unit; ROSC = return of spontaneous circulation.
Guideline care refers to target depth of 51 mm and standard advanced cardiac life support epinephrine dosing. BP care refers to cardiopulmonary resuscitation directed to attain a systolic blood pressure of 100 mm Hg and a coronary perfusion pressure greater than 20 mm Hg. Favorable neurologic outcome indicates swine cerebral performance category of 1 or 2.
Figure 2.
Mean diastolic coronary perfusion pressure during each minute of cardiopulmonary resuscitation (CPR) across treatment groups. Error bars represent SEM. Guideline care refers to target depth of 51 mm and standard advanced cardiac life support epinephrine dosing. BP care refers to CPR directed to attain a systolic blood pressure of 100 mm Hg and a coronary perfusion pressure greater than 20 mm Hg. BP = blood pressure.
Resuscitation Variables
Chest compression depth was greater in Guideline care compared with BP care (point estimate +9.3 mm; 95% confidence interval, 6.0–12.5 mm; P < 0.01). Other CPR quality variables were not different in the two groups (rate = 100 ± 0.1 min−1; chest compression fraction = 96 ± 0.3%; no delivered compressions had leaning). Number of vasopressor doses administered before first defibrillation attempt after 10 minutes of resuscitation was greater in BP care compared with Guideline care (median, 3 [range, 0–3] vs. 2 [range, 2–2]; P = 0.003). However, the total number of vasopressor doses provided to BP care was lower compared with Guideline care when doses after the first defibrillation attempts were included (median, 3 [range, 0–4] vs. 5, [range, 2–5]; P = 0.001). Median number of defibrillation attempts to terminate VF and lead to sustained ROSC in survivors was 1 (range, 1–3). Of the 10 animals achieving 45-minute survival, six (60%) required vasopressor support with either a dopamine continuous infusion (mean peak dose, 13 ± 2 μg/kg/min) or intermittent epinephrine boluses (20 μg; three animals received one dose). In the BP care group (n = 8), post-ROSC mean systolic blood pressure was 102.9 ± 0.92 mm Hg; mean diastolic blood pressure was 72.0 ± 0.6 mm Hg. In the Guideline care group (n = 1), post-ROSC mean systolic blood pressure was 98.7 ± 1.9 mm Hg, and mean diastolic blood pressure was 63.1 ± 1.3 mm Hg; however, this animal was unable to be successfully weaned from vasopressor support at the end of the ICU period and died. Of note, animals with “any ROSC” (two in Guideline care, one in BP care) are not included in the post-ROSC hemodynamic summary statistics because they had immediate recurrent VF (<1 min) and were not successfully resuscitated for more than 1 minute.
Hemodynamics and Arterial Blood Gases
Hemodynamic variables (Table 2) were not different at prearrest baseline or at the end of the asphyxial period before VF induction. At the end of the resuscitation period (16:15 to 16:30), the BP care group compared with Guideline care had significantly higher systolic blood pressure (110 ± 3 vs. 83 ± 11 mm Hg; P = 0.04), diastolic blood pressure (45 ± 4 vs. 28 ± 3 mm Hg; P = <0.01), and mean diastolic CPP (29 ± 2 vs. 16 ± 2 mm Hg; P < 0.001). There were no differences in ETCO2 between the groups during this time period. There were no differences in arterial blood gases (Table 3).
Table 2.
Hemodynamic Variables
| Guideline Care (n = 10) | BP Care (n = 9) | P Value | |
|---|---|---|---|
| Baseline | |||
| Weight, kg | 30.5 (0.6) | 29.8 (0.6) | 0.44 |
| CO, L/min | 2.5 (0.2) | 2.4 (0.2) | 0.68 |
| AoS, mm Hg | 110 (91–119) | 116 (107–119) | 0.46 |
| AoD, mm Hg | 82 (68–86) | 77 (76–88) | 0.87 |
| RAD, mm Hg | 11 (8–12) | 10 (9–11) | 0.81 |
| CPP, mm Hg | 73 (62–76) | 71 (58–82) | 0.99 |
| End of asphyxial period* | |||
| AoS, mm Hg | 57 (9) | 76 (10) | 0.15 |
| AoD, mm Hg | 34 (4) | 41 (3) | 0.20 |
| RAD, mm Hg | 15 (12–16) | 15 (14–17) | 0.57 |
| CPP, mm Hg | 21 (4) | 26 (5) | 0.45 |
| End of resuscitation period† | |||
| AoS, mm Hg | 83 (11) | 110 (3) | 0.04 |
| AoD, mm Hg | 28 (3) | 45 (4) | <0.01 |
| RAD, mm Hg | 5 (3) | 10 (3) | 0.23 |
| CPP, mm Hg | 16 (2) | 29 (2) | <0.001 |
| ETCO2, mm Hg | 37 (3) | 33 (3) | 0.31 |
Definition of abbreviations: AoD = aortic diastolic pressure; AoS = aortic systolic pressure; BP = blood pressure; CPP = coronary perfusion pressure; ETco2, = end tidal carbon dioxide; RAD = right atrial diastolic pressure.
Guideline care refers to target depth of 51 mm and standard advanced cardiac life support epinephrine dosing. BP care refers to cardiopulmonary resuscitation directed to attain a systolic blood pressure of 100 mm Hg and a CPP greater than 20 mm Hg. Data presented as mean (SEM) or median (interquartile range).
15-second epoch during asphyxial period from 6:30 to 6:45.
15-second epoch during protocol resuscitation period from 16:15 to 16:30.
Table 3.
Arterial Blood Gases
| Guideline Care (n = 10) | BP Care (n = 10) | P Value | |
|---|---|---|---|
| Baseline | |||
| pH | 7.52 (0.01) | 7.50 (0.03) | 0.44 |
| PaCO2, mm Hg | 37 (1) | 40 (1) | 0.19 |
| PaO2, mm Hg | 110 (6) | 114 (10) | 0.70 |
| End of asphyxial period* | |||
| pH | 7.26 (0.02) | 7.25 (0.04) | 0.81 |
| PaCO2, mm Hg | 69 (6) | 54 (9) | 0.21 |
| PaO2, mm Hg | 9 (2) | 14 (2) | 0.08 |
| After 6 minutes of CPR† | |||
| pH | 7.23 (0.04) | 7.29 (0.03) | 0.34 |
| PaCO2, mm Hg | 49 (7) | 38 (4) | 0.18 |
| PaO2, mm Hg | 144 (45) | 208 (32) | 0.25 |
Definition of abbreviations: BP = blood pressure; CPR = cardiopulmonary resuscitation.
Guideline care refers to target depth of 51 mm and standard advanced cardiac life support epinephrine dosing. BP care refers to CPR directed to attain a systolic blood pressure of 100 mm Hg and a coronary perfusion pressure greater than 20 mm Hg.
Sample drawn at 6 minutes 30 seconds during asphyxial period.
Sample drawn at 6 minutes during protocol resuscitation period.
Discussion
This porcine investigation establishes that individualized, prospective titration of CPR to blood pressures improves 24-hour survival compared with uniform optimal American Heart Association Guideline care for asphyxia-associated cardiac arrest. Favorable neurologic outcome was common in the blood pressure–targeted care group (70%), despite an injury that was universally fatal with Guideline-recommended care. Importantly, animals in the BP care group survived despite receiving less vasopressors overall compared with the Guideline care group, thereby highlighting the “patient” level variability in cardiac arrest and CPR physiology and need for the development of a treatment strategy using individualized care.
This animal model was intended to address treatment for a VF cardiac arrest associated with an acute respiratory deterioration. Clinically relevant, nearly 40% of adult in-hospital cardiac arrests have acute respiratory insufficiency as the immediate cause of arrest, and of these arrests, the initial documented rhythm is VF or ventricular tachycardia in one-fifth (6, 17). The median duration of in-hospital CPR for survivors and nonsurvivors is at least 10 minutes; therefore, 10 minutes of CPR was provided before the first defibrillation attempt (17). Although waiting to provide the first defibrillation attempt in an in-hospital model is somewhat artificial, in previous studies of “asphyxial” cardiac arrest without VF, ROSC occurred during the first few minutes of CPR (15, 16). Such models do not provide the opportunity to evaluate the experimental resuscitation strategies during a more prolonged cardiac arrest.
Given the recent emphasis on providing high-quality CPR (11), the poor survival rate in the Guideline care group deserves comment. This apparent failure of Guideline-compliant care is explained by the severity of the insult provided in our model. First, to mimic respiratory deterioration, there was profound arterial hypoxemia (Table 3) before VF induction. Our insult was designed to mimic “subsequent” (rather than initial) VF, a clinical entity known to have far worse outcome compared with either primary VF or even asystole without subsequent VF (17, 22). The addition of the myocardial energy-consuming process of VF on a severe asphyxial background is indeed a severe insult. Furthermore, defibrillation attempts were delayed until 10 minutes of CPR was provided to evaluate the different BP versus Guideline resuscitation strategies. Because such delayed defibrillation is associated with poor survival (23), it is likely that the survival was decreased even further in this model. In summary, because this model mimics asphyxia-induced “subsequent” VF with delayed defibrillation, the low survival rates with optimal Guideline management in the control group are not unexpected.
Most in-hospital cardiac arrests now take place in ICUs, likely the result of increasing implementation of medical emergency teams (6, 17, 24–26). Many of these patients have invasive hemodynamic monitoring in situ at the time of the arrest, yet current treatment paradigms focus on a nonindividualized “provider-centric” approach that varies little from patient to patient (1, 2). This provider-centric approach is attractive for mass education of rescuers and for unmonitored patients, but is not necessary for patients with hemodynamic monitoring in place during CPR. The relationship between diastolic blood pressure, CPP, myocardial blood flow, and in turn resuscitation success is well established (7–9, 27–33). The American Heart Association recommends monitoring resuscitation efforts using arterial blood pressure (11) based on the known association between blood pressure and survival, but no prospective study has demonstrated that targeting such goals during resuscitation improves survival. Therefore, this investigation fills an important gap in the resuscitation knowledge base and highlights a potential new therapeutic strategy that warrants further study, possibly in human trials.
A surprising result of our study was that the Guideline care animals received deeper compressions but had far worse outcome. Previous investigations have demonstrated that deeper compressions are associated with better outcomes (34–37), presumably because deeper compressions improve blood pressure and CPP. In apparent contrast to those investigations, the blood pressure–targeted care group was able to achieve better CPPs, with depths that were almost 10 mm less than Guideline care. There was also a wide variability in the amount of vasopressor needed to maintain CPPs in the arterial blood pressure–targeted group, with some animals receiving no medication during the entire CPR period. Although speculative, the success of the individualized approach may be that it allows rescuers to avert potential harm of overly deep compressions and/or vasopressor use when they are not required. By titrating CPR effect to blood pressure, compressions that were too deep for a given “patient” were avoided (38) and unnecessary vasopressors that could adversely affect myocardial afterload after ROSC (39–43) were avoided unless they were needed to maintain CPP during CPR.
During actual resuscitation attempts, the American Heart Association recommends monitoring of resuscitation quality with arterial blood pressure and/or continuous ETco2 (11). In this investigation, the ETco2 levels were as high or higher in the Guideline care group with deeper compressions compared with the blood pressure–targeted care group with less deep compressions. This apparent discrepancy should not be surprising. ETco2 correlates better with pulmonary blood flow than with CPP or myocardial blood flow (44–49). Our data support that among the patient physiologic variables during CPR, CPP is a better predictor of outcome than ETco2 measurements, and in line with the American Heart Association recommendation to use arterial blood pressure first tier and ETco2 second tier to monitor CPR effect (11).
This laboratory study has notable limitations. First, the authors acknowledge that the biggest limitation of this study is that our findings are most applicable to patients who already have invasive monitoring in place at the time of arrest. Although not impossible, placing invasive monitors during resuscitation is difficult and may limit generalizability of our findings more broadly. Second, these were previously healthy animals with no coronary artery disease, and the number of animals was not large. The effect of physiologic-directed resuscitation in humans remains an unanswered question. However, one of the strengths of this study lies in the choice of a longer-term outcome (24-h survival) with a validated neurologic assessment. Next, in the blood pressure–targeted care group, the overall median number of vasopressors before first defibrillation attempt was greater than in the Guideline care group. However, some arterial blood pressure–targeted animals survived without need for any vasopressor. Third, drug regimens differed between groups. Some of the blood pressure–targeted care animals received vasopressin when epinephrine alone did not achieve our CPP goals. We chose to alter our vasopressor choice, instead of continuing to administer an ineffective therapy, so as to evaluate a dynamic treatment algorithm that was targeted to subject hemodynamics. Improved outcomes from cardiac arrest with vasopressin administration in the clinical setting have not been observed (50); however, in these studies, vasopressin was not actively titrated to CPP. Finally, because the investigators needed to be aware of the resuscitation strategy during the experiment, this study was not blinded. However, the statistical differences in chest compression depths between the groups, although other CPR quality variables were similar, provide evidence that there was strict adherence to the study protocol and that this potential bias was mitigated.
Conclusions
In a porcine model of asphyxia-associated VF cardiac arrest, blood pressure–targeted CPR improved 24-hour survival compared with optimal American Heart Association care. This individualized treatment approach adjusted therapy according to patient-centric hemodynamics in contrast to the usual provider-centric “one-size-fits-all” strategy. These findings highlight a promising new therapeutic strategy that could be used during the resuscitation of invasively monitored ICU patients.
Acknowledgments
Acknowledgment
The authors thank Drs. Heather A. Wolfe, Thomas Conlon, and Utpal Bhalala who have supported our cardiopulmonary resuscitation laboratory.
Footnotes
Supported by the Laerdal Foundation for Acute Care Medicine, the National Institute of Child Health and Human Development (K23, R.M.S.), the National Institute of Neurological Disorders and Stroke (K08, S.H.F.), and CHOP CCM Endowed Chair Funds.
Author Contributions: All authors made substantial contributions to the conception and design of the study, acquisition and analysis of the data, drafting and revision of the manuscript, and approved the final version submitted.
Originally Published in Press as DOI: 10.1164/rccm.201407-1343OC on October 16, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Neumar RW, Otto CW, Link MS, Kronick SL, Shuster M, Callaway CW, Kudenchuk PJ, Ornato JP, McNally B, Silvers SM, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18) Suppl. 3:S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 2.Kleinman ME, Chameides L, Schexnayder SM, Samson RA, Hazinski MF, Atkins DL, Berg MD, de Caen AR, Fink EL, Freid EB, et al. Part 14: pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Circulation 2010122;18Suppl. 3S876–S908. [DOI] [PubMed] [Google Scholar]
- 3.Sutton RM, Friess SH, Maltese MR, Naim MY, Bratinov G, Weiland TR, Garuccio M, Bhalala U, Nadkarni VM, Becker LB, et al. Hemodynamic-directed cardiopulmonary resuscitation during in-hospital cardiac arrest. Resuscitation. 2014;85:983–986. doi: 10.1016/j.resuscitation.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merchant RM, Yang L, Becker LB, Berg RA, Nadkarni V, Nichol G, Carr BG, Mitra N, Bradley SM, Abella BS, et al. American Heart Association Get With The Guidelines-Resuscitation Investigators. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406. doi: 10.1097/CCM.0b013e3182257459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg RA, Sutton RM, Holubkov R, Nicholson CE, Dean JM, Harrison R, Heidemann S, Meert K, Newth C, Moler F, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Collaborative Pediatric Critical Care Research Network and for the American Heart Association’s Get With the Guidelines-Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Ratio of PICU versus ward cardiopulmonary resuscitation events is increasing. Crit Care Med. 2013;41:2292–2297. doi: 10.1097/CCM.0b013e31828cf0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS American Heart Association Get with the Guidelines–Resuscitation Investigators. Trends in survival after in-hospital cardiac arrest. N Engl J Med. 2012;367:1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern KB, Ewy GA, Voorhees WD, Babbs CF, Tacker WA. Myocardial perfusion pressure: a predictor of 24-hour survival during prolonged cardiac arrest in dogs. Resuscitation. 1988;16:241–250. doi: 10.1016/0300-9572(88)90111-6. [DOI] [PubMed] [Google Scholar]
- 8.Ralston SH, Voorhees WD, Babbs CF. Intrapulmonary epinephrine during prolonged cardiopulmonary resuscitation: improved regional blood flow and resuscitation in dogs. Ann Emerg Med. 1984;13:79–86. doi: 10.1016/s0196-0644(84)80566-1. [DOI] [PubMed] [Google Scholar]
- 9.Halperin HR, Lee K, Zviman M, Illindala U, Lardo A, Kolandaivelu A, Paradis NA. Outcomes from low versus high-flow cardiopulmonary resuscitation in a swine model of cardiac arrest. Am J Emerg Med. 2010;28:195–202. doi: 10.1016/j.ajem.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sanders AB, Ewy GA, Taft TV. Prognostic and therapeutic importance of the aortic diastolic pressure in resuscitation from cardiac arrest. Crit Care Med. 1984;12:871–873. doi: 10.1097/00003246-198410000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Meaney PA, Bobrow BJ, Mancini ME, Christenson J, de Caen AR, Bhanji F, Abella BS, Kleinman ME, Edelson DP, Berg RA, et al. CPR Quality Summit Investigators, the American Heart Association Emergency Cardiovascular Care Committee, and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Cardiopulmonary resuscitation quality: [corrected] improving cardiac resuscitation outcomes both inside and outside the hospital: a consensus statement from the American Heart Association. Circulation. 2013;128:417–435. doi: 10.1161/CIR.0b013e31829d8654. [DOI] [PubMed] [Google Scholar]
- 12.Friess SH, Sutton RM, Bhalala U, Maltese MR, Naim MY, Bratinov G, Weiland TR, III, Garuccio M, Nadkarni VM, Becker LB, et al. Hemodynamic directed cardiopulmonary resuscitation improves short-term survival from ventricular fibrillation cardiac arrest. Crit Care Med. 2013;41:2698–2704. doi: 10.1097/CCM.0b013e318298ad6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutton RM, Friess SH, Bhalala U, Maltese MR, Naim MY, Bratinov G, Niles D, Nadkarni VM, Becker LB, Berg RA. Hemodynamic directed CPR improves short-term survival from asphyxia-associated cardiac arrest. Resuscitation. 2013;84:696–701. doi: 10.1016/j.resuscitation.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neurauter A, Nysaether J, Kramer-Johansen J, Eilevstjønn J, Paal P, Myklebust H, Wenzel V, Lindner KH, Schmölz W, Pytte M, et al. Comparison of mechanical characteristics of the human and porcine chest during cardiopulmonary resuscitation. Resuscitation. 2009;80:463–469. doi: 10.1016/j.resuscitation.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Berg RA, Hilwig RW, Kern KB, Babar I, Ewy GA. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27:1893–1899. doi: 10.1097/00003246-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 16.Berg RA, Hilwig RW, Kern KB, Ewy GA. “Bystander” chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless “cardiac arrest”. Circulation. 2000;101:1743–1748. doi: 10.1161/01.cir.101.14.1743. [DOI] [PubMed] [Google Scholar]
- 17.Meaney PA, Nadkarni VM, Kern KB, Indik JH, Halperin HR, Berg RA. Rhythms and outcomes of adult in-hospital cardiac arrest. Crit Care Med. 2010;38:101–108. doi: 10.1097/CCM.0b013e3181b43282. [DOI] [PubMed] [Google Scholar]
- 18.Goldberger ZD, Chan PS, Berg RA, Kronick SL, Cooke CR, Lu M, Banerjee M, Hayward RA, Krumholz HM, Nallamothu BK American Heart Association Get With The Guidelines—Resuscitation (formerly National Registry of Cardiopulmonary Resuscitation) Investigators. Duration of resuscitation efforts and survival after in-hospital cardiac arrest: an observational study. Lancet. 2012;380:1473–1481. doi: 10.1016/S0140-6736(12)60862-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg RA, Chapman FW, Berg MD, Hilwig RW, Banville I, Walker RG, Nova RC, Sherrill D, Kern KB. Attenuated adult biphasic shocks compared with weight-based monophasic shocks in a swine model of prolonged pediatric ventricular fibrillation. Resuscitation. 2004;61:189–197. doi: 10.1016/j.resuscitation.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 20.Berg RA, Sanders AB, Kern KB, Hilwig RW, Heidenreich JW, Porter ME, Ewy GA. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104:2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 21.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 22.Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA American Heart Association National Registry of CPR Investigators. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354:2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 23.Chan PS, Krumholz HM, Nichol G, Nallamothu BK American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Delayed time to defibrillation after in-hospital cardiac arrest. N Engl J Med. 2008;358:9–17. doi: 10.1056/NEJMoa0706467. [DOI] [PubMed] [Google Scholar]
- 24.Nadkarni VM, Larkin GL, Peberdy MA, Carey SM, Kaye W, Mancini ME, Nichol G, Lane-Truitt T, Potts J, Ornato JP, et al. National Registry of Cardiopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 25.Chan PS, Jain R, Nallmothu BK, Berg RA, Sasson C. Rapid response teams: a systematic review and meta-analysis. Arch Intern Med. 2010;170:18–26. doi: 10.1001/archinternmed.2009.424. [DOI] [PubMed] [Google Scholar]
- 26.Chan PS, Khalid A, Longmore LS, Berg RA, Kosiborod M, Spertus JA. Hospital-wide code rates and mortality before and after implementation of a rapid response team. JAMA. 2008;300:2506–2513. doi: 10.1001/jama.2008.715. [DOI] [PubMed] [Google Scholar]
- 27.Paradis NA, Martin GB, Rivers EP, Goetting MG, Appleton TJ, Feingold M, Nowak RM. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263:1106–1113. [PubMed] [Google Scholar]
- 28.Kern KB, Lancaster L, Goldman S, Ewy GA. The effect of coronary artery lesions on the relationship between coronary perfusion pressure and myocardial blood flow during cardiopulmonary resuscitation in pigs. Am Heart J. 1990;120:324–333. doi: 10.1016/0002-8703(90)90076-a. [DOI] [PubMed] [Google Scholar]
- 29.Niemann JT, Criley JM, Rosborough JP, Niskanen RA, Alferness C. Predictive indices of successful cardiac resuscitation after prolonged arrest and experimental cardiopulmonary resuscitation. Ann Emerg Med. 1985;14:521–528. doi: 10.1016/s0196-0644(85)80774-5. [DOI] [PubMed] [Google Scholar]
- 30.Niemann JT, Rosborough JP, Ung S, Criley JM. Coronary perfusion pressure during experimental cardiopulmonary resuscitation. Ann Emerg Med. 1982;11:127–131. doi: 10.1016/s0196-0644(82)80236-9. [DOI] [PubMed] [Google Scholar]
- 31.Sanders AB, Kern KB, Atlas M, Bragg S, Ewy GA. Importance of the duration of inadequate coronary perfusion pressure on resuscitation from cardiac arrest. J Am Coll Cardiol. 1985;6:113–118. doi: 10.1016/s0735-1097(85)80261-8. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care. 2010;14:78–84. doi: 10.3109/10903120903349796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crile G, Dolley DH. An experimental research into the resuscitation of dogs killed by anesthetics and asphyxia. J Exp Med. 1906;8:713–725. doi: 10.1084/jem.8.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stiell IG, Brown SP, Christenson J, Cheskes S, Nichol G, Powell J, Bigham B, Morrison LJ, Larsen J, Hess E, et al. Resuscitation Outcomes Consortium (ROC) Investigators. What is the role of chest compression depth during out-of-hospital cardiac arrest resuscitation? Crit Care Med. 2012;40:1192–1198. doi: 10.1097/CCM.0b013e31823bc8bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton RM, French B, Niles DE, Donoghue A, Topjian AA, Nishisaki A, Leffelman J, Wolfe H, Berg RA, Nadkarni VM, et al. 2010 American Heart Association recommended compression depths during pediatric in-hospital resuscitations are associated with survival. Resuscitation. 2014;85:1179–1184. doi: 10.1016/j.resuscitation.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TL, Steen PA, Becker LB. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71:137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Kramer-Johansen J, Myklebust H, Wik L, Fellows B, Svensson L, Sørebø H, Steen PA. Quality of out-of-hospital cardiopulmonary resuscitation with real time automated feedback: a prospective interventional study. Resuscitation. 2006;71:283–292. doi: 10.1016/j.resuscitation.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 38.Miller AC, Rosati SF, Suffredini AF, Schrump DS. A systematic review and pooled analysis of CPR-associated cardiovascular and thoracic injuries. Resuscitation. 2014;85:724–731. doi: 10.1016/j.resuscitation.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prengel AW, Lindner KH, Ensinger H, Grünert A. Plasma catecholamine concentrations after successful resuscitation in patients. Crit Care Med. 1992;20:609–614. doi: 10.1097/00003246-199205000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Rivers EP, Wortsman J, Rady MY, Blake HC, McGeorge FT, Buderer NM. The effect of the total cumulative epinephrine dose administered during human CPR on hemodynamic, oxygen transport, and utilization variables in the postresuscitation period. Chest. 1994;106:1499–1507. doi: 10.1378/chest.106.5.1499. [DOI] [PubMed] [Google Scholar]
- 41.Stiell IG, Hebert PC, Weitzman BN, Wells GA, Raman S, Stark RM, Higginson LA, Ahuja J, Dickinson GE. High-dose epinephrine in adult cardiac arrest. N Engl J Med. 1992;327:1045–1050. doi: 10.1056/NEJM199210083271502. [DOI] [PubMed] [Google Scholar]
- 42.Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350:1722–1730. doi: 10.1056/NEJMoa032440. [DOI] [PubMed] [Google Scholar]
- 43.Olasveengen TM, Sunde K, Brunborg C, Thowsen J, Steen PA, Wik L. Intravenous drug administration during out-of-hospital cardiac arrest: a randomized trial. JAMA. 2009;302:2222–2229. doi: 10.1001/jama.2009.1729. [DOI] [PubMed] [Google Scholar]
- 44.Weil MH, Bisera J, Trevino RP, Rackow EC. Cardiac output and end-tidal carbon dioxide. Crit Care Med. 1985;13:907–909. doi: 10.1097/00003246-198511000-00011. [DOI] [PubMed] [Google Scholar]
- 45.Gudipati CV, Weil MH, Bisera J, Deshmukh HG, Rackow EC. Expired carbon dioxide: a noninvasive monitor of cardiopulmonary resuscitation. Circulation. 1988;77:234–239. doi: 10.1161/01.cir.77.1.234. [DOI] [PubMed] [Google Scholar]
- 46.Sanders AB, Ewy GA, Bragg S, Atlas M, Kern KB. Expired PCO2 as a prognostic indicator of successful resuscitation from cardiac arrest. Ann Emerg Med. 1985;14:948–952. doi: 10.1016/s0196-0644(85)80235-3. [DOI] [PubMed] [Google Scholar]
- 47.Ornato JP, Garnett AR, Glauser FL. Relationship between cardiac output and the end-tidal carbon dioxide tension. Ann Emerg Med. 1990;19:1104–1106. doi: 10.1016/s0196-0644(05)81512-4. [DOI] [PubMed] [Google Scholar]
- 48.Ornato JP, Levine RL, Young DS, Racht EM, Garnett AR, Gonzalez ER. The effect of applied chest compression force on systemic arterial pressure and end-tidal carbon dioxide concentration during CPR in human beings. Ann Emerg Med. 1989;18:732–737. doi: 10.1016/s0196-0644(89)80005-8. [DOI] [PubMed] [Google Scholar]
- 49.Levine RL, Wayne MA, Miller CC. End-tidal carbon dioxide and outcome of out-of-hospital cardiac arrest. N Engl J Med. 1997;337:301–306. doi: 10.1056/NEJM199707313370503. [DOI] [PubMed] [Google Scholar]
- 50.Stiell IG, Hébert PC, Wells GA, Vandemheen KL, Tang AS, Higginson LA, Dreyer JF, Clement C, Battram E, Watpool I, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358:105–109. doi: 10.1016/S0140-6736(01)05328-4. [DOI] [PubMed] [Google Scholar]