Abstract
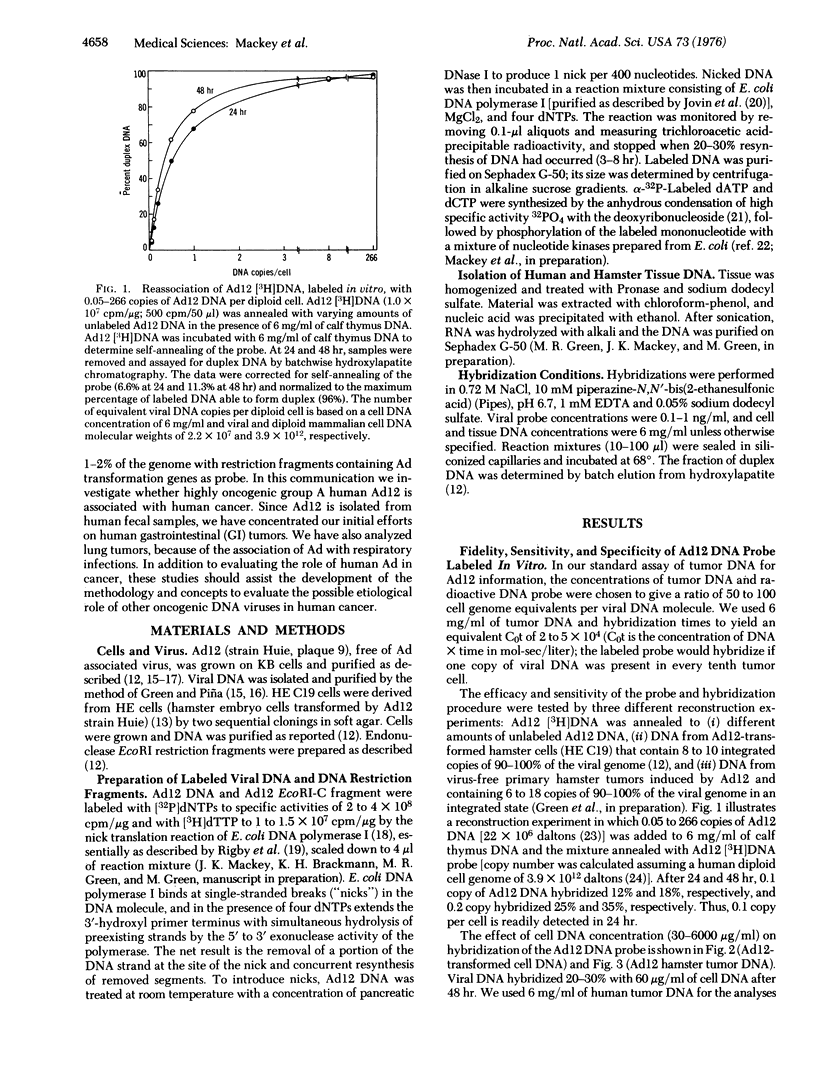
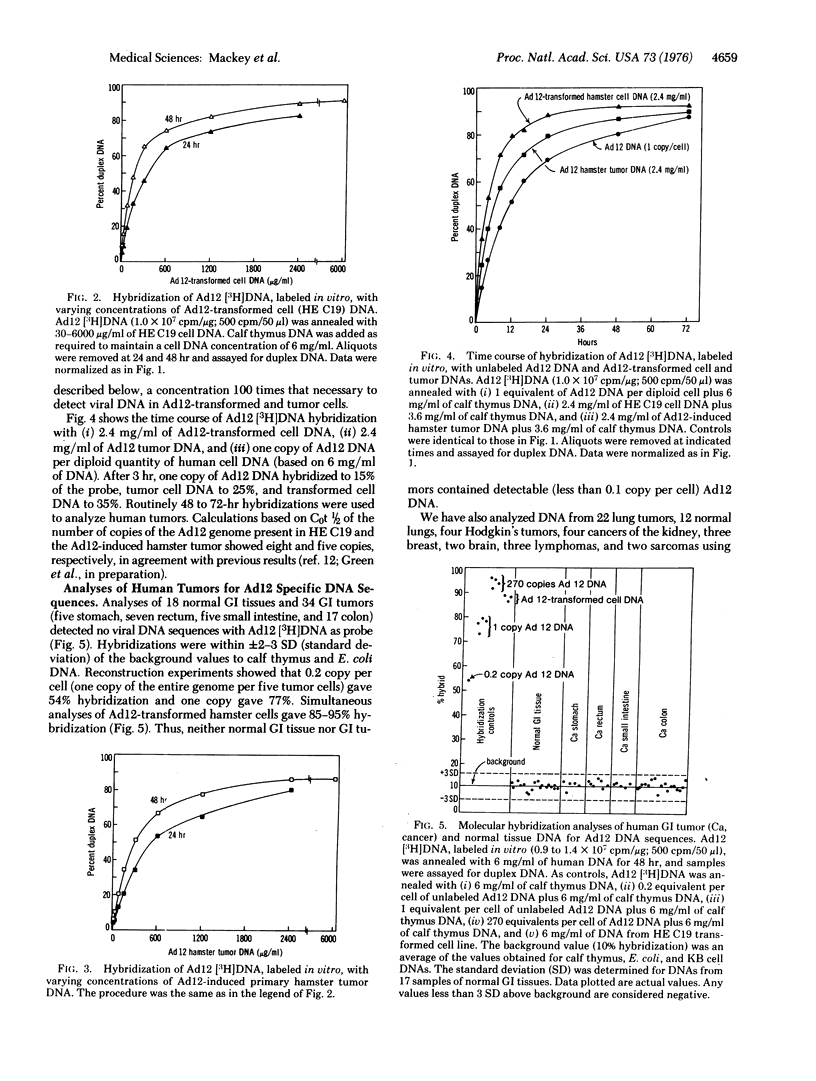
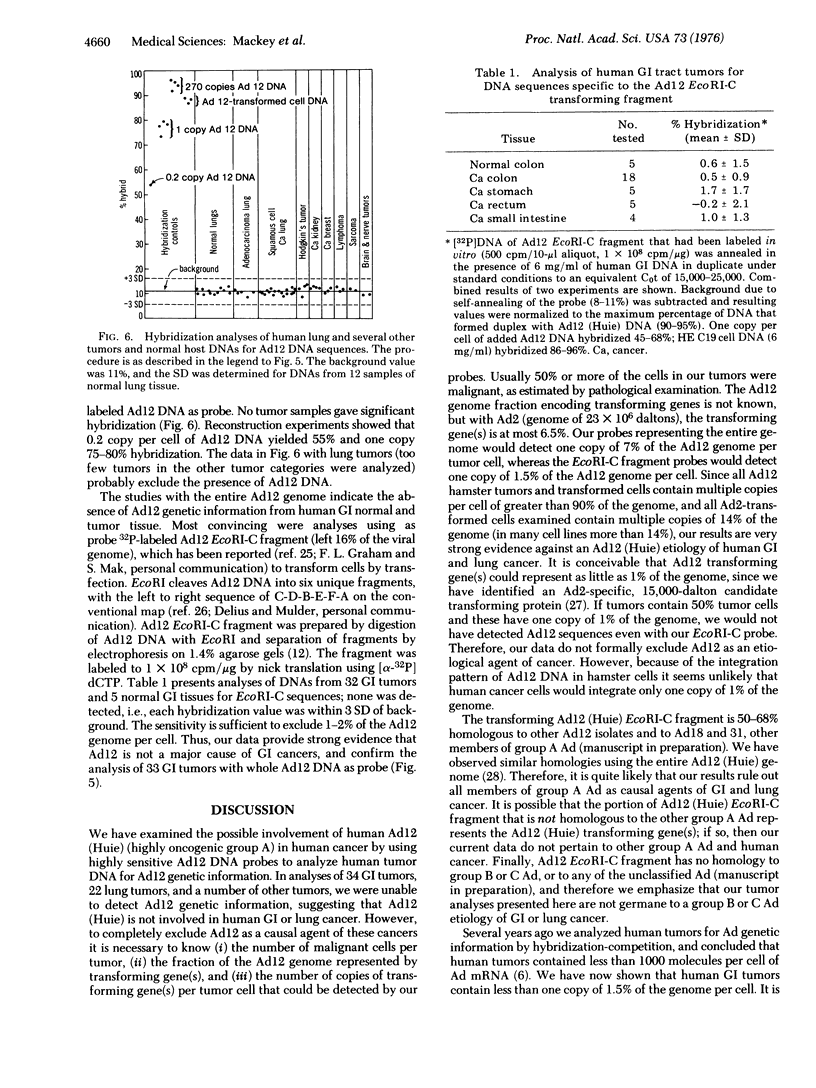
Adenovirus 12 (Ad12) (Huie) (highly oncogenic group A) readily induces tumors in newborn rodents. Since Ad12 is isolated from human fecal samples, we investigated whether it plays a role in the etiology of human gastrointestinal cancer. If Ad12 is a causal agent of human cancer, then human tumors should contain Ad12 transforming genes, as indicated by studies of cells transformed in vitro and in vivo by oncogenic viruses. Ad12 DNA and the Ad12 transforming restriction fragment (EcoRI-C fragment, left 16% of the viral genome) were labeled in vitro to 10(7) to 4 X 10(8) cpm/mug by the nick translation reaction of DNA polymerase of Escherichia coli. The fidelity and sensitivity of these probes were established by (i) analysis of DNA from Ad12-transformed cells and from hamsters with tumors induced by Ad12, (ii) reconstruction experiments with added Ad12 DNA and EcoRI restriction fragments, and (iii) comparison of annealing characteristics with Ad12 probes labeled in vivo. With Ad12 [3H]DNA as probe, no viral DNA sequences were detected in 18 normal gastrointestinal tissues and 34 gastrointestinal tumors, including cancers of the colon, rectum, small intestine, and stomach, under conditions that would detect 0.1 copy of the Ad12 genome per tumor cell. Similar analyses of Ad12-transformed hamster cells and Ad12 primary hamster tumors indicated 6-18 copies per cell of over 90% of the viral genome. With the Ad12 EcoRI-C transforming fragment as probe, no hybridization was detected with 32 human gastrointestinal tumors and five normal tissues; this result excludes 1-2% of the Ad12 genome per tumor cell. Our date are strong evidence that Ad12 is not a major cause of human gastrointestinal cancer. The Ad12 transforming EcoRI-C fragment hybridized (50-68% efficiency) with other Ad12 isolates and with Ad18 and 31 (members of oncogenic group A), but not at all with 28 other human Ad serotypes (manuscript in preparation). Thus other group A members probably are also not involved in human gastrointestinal cancer. No viral DNA sequences were detected in 12 normal lungs and 22 lung tumors, suggesting that respiratory cancer does not involve an Ad12 etiology.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. H., Obijeski J. F., Simpson R. W. Transcription of the influenza ribonucleic acid genome by a virion polymerase. I. Optimal conditions for in vitro activity of the ribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1971 Jul;8(1):66–73. doi: 10.1128/jvi.8.1.66-73.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Green M. The mechanism of viral carcinogenesis by DNA mammalian viruses: viral-specific RNA in polyribosomes of adenovirus tumor and transformed cells. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1567–1574. doi: 10.1073/pnas.55.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga K., Sekikawa K., Yamazaki H., Green M. Analysis of multiple viral genome fragments in adenovirus 7-transformed hamster cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):633–636. doi: 10.1101/sqb.1974.039.01.076. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., HILLEMAN M. R., ZWICKEY R. E. TESTS IN HAMSTERS FOR ONCOGENIC QUALITY OF ORDINARY VIRUSES INCLUDING ADENOVIRUS TYPE 7. Proc Soc Exp Biol Med. 1964 Apr;115:1141–1150. doi: 10.3181/00379727-115-29138. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Similarity of DNAs isolated from tumor-inducing viruses of human and animal origin. Proc Natl Acad Sci U S A. 1963 Jul;50:44–46. doi: 10.1073/pnas.50.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallimore P. H. Viral DNA in transformed cells. II. A study of the sequences of adenovirus 2 DNA IN NINE LINES OF TRANSFORMED RAT CELLS USING SPECIFIC FRAGMENTS OF THE VIRAL GENOME;. J Mol Biol. 1974 Oct 15;89(1):49–72. doi: 10.1016/0022-2836(74)90162-4. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Abrahams P. J., Mulder C., Heijneker H. L., Warnaar S. O., De Vries F. A., Fiers W., Van Der Eb A. J. Studies on in vitro transformation by DNA and DNA fragments of human adenoviruses and simian virus 40. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):637–650. doi: 10.1101/sqb.1974.039.01.077. [DOI] [PubMed] [Google Scholar]
- Green M. R., Chinnadurai G., Mackey J. K., Green M. A unique pattern of integrated viral genes in hamster cells transformed by highly oncogenic human adenovirus 12. Cell. 1976 Mar;7(3):419–428. doi: 10.1016/0092-8674(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Green M. Molecular basis for the attack on cancer. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1036–1041. doi: 10.1073/pnas.69.4.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. Oncogenic viruses. Annu Rev Biochem. 1970;39:701–756. doi: 10.1146/annurev.bi.39.070170.003413. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S. Oncogenic DNA viruses--replication, tumor gene expression, and role in human cancer. Semin Oncol. 1976 Mar;3(1):65–79. [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., LANE W. T. Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2051–2058. doi: 10.1073/pnas.48.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Kelly R. B., Cozzarelli N. R., Deutscher M. P., Lehman I. R., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem. 1970 Jan 10;245(1):39–45. [PubMed] [Google Scholar]
- Mulder C., Sharp P. A., Delius H., Pettersson U. Specific fragmentation of DNA of adenovirus serotypes 3, 5, 7, and 12, and adeno-simian virus 40 hybrid virus Ad2+ND1 by restriction endonuclease R.EcoRI. J Virol. 1974 Jul;14(1):68–77. doi: 10.1128/jvi.14.1.68-77.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEREIRA M. S., PERIERA H. G., CLARKE S. K. HUMAN ADENOVIRUS TYPE 31. A NEW SEROTYPE WITH ONCOGENIC PROPERTIES. Lancet. 1965 Jan 2;1(7375):21–23. doi: 10.1016/s0140-6736(65)90925-6. [DOI] [PubMed] [Google Scholar]
- Piña M., Green M. Biochemical studies on adenovirus multiplication. XIV. Macromolecule and enzyme synthesis in cells replicating oncogenic and nononcogenic human adenovirus. Virology. 1969 Aug;38(4):573–586. doi: 10.1016/0042-6822(69)90178-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Botchan M., Gallimore P., Ozanne B., Pettersson U., Williams J., Sharp P. A. Viral DNA sequences in cells transformed by simian virus 40, adenovirus type 2 and adenovirus type 5. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):615–632. doi: 10.1101/sqb.1974.039.01.075. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Preparation of [alpha-32P]nucleoside and deoxynucleoside 5'-triphosphates from 32Pi and protected and unprotected nucleosides. Biochim Biophys Acta. 1969 Oct 22;190(2):548–550. doi: 10.1016/0005-2787(69)90105-1. [DOI] [PubMed] [Google Scholar]



