Abstract
Introduction: A device has been developed to apply freezing temperatures to temporarily impede nerve conduction, resulting in inhibition of voluntary skeletal muscle contraction. This device was designed as an alternative to the neurotoxins usually used to treat movement disorders. Methods: We evaluated the effects of single and 3 repeat treatments with a cryoprobe device (−55°C) on a sciatic nerve rat model. Long-term effects of repeated treatment were evaluated through assessments of physiological function and histological analysis. Results: There was consistent weakening of physiological function after each treatment, with recovery of normal function by 8 weeks posttreatment. Histological findings showed axonal degeneration with no disruption to the epineurial or perineurial structures. Progressive axonal regeneration was followed by normal recovery by 24 weeks post-treatment. Conclusions: Low-temperature treatment of motor nerves did not result in permanent or long-term changes to nerve function or structure. Muscle Nerve 51: 268–275, 2015
Keywords: cryoprobe, cryotherapy, denervation, movement disorder, peripheral nerve, reinnervation
Local application of low temperatures for medical therapy has been used for centuries and is documented as far back as Hippocrates in 460 B.C., when he described the use of cold for its analgesic and anti-inflammatory properties.1 Advancements in cryosurgical technology since then have allowed for the use of much colder temperatures, which, when applied directly to cells, can induce apoptotic cell death (with temperatures in the range of −5° to −15°C) or necrotic cell death at lower than −20°C.2,3 A significant difference between peripheral nerves and most cell types is that the application of freezing temperatures to the nerve axon causes axonal degeneration distal to the treatment site, whereas the proximal portion of the axon and the nerve body remain viable. With this unique feature, peripheral nerve has the ability to regenerate its axon. In addition, freezing temperatures do not disrupt the acellular epineurial or perineurial structures, which are important for support of axonal growth.4 The use of freezing temperatures on sensory nerves, known as cryoanalgesia, has been practiced with good success for pain management,5 and the ability to treat within specific temperature ranges has enabled treatment of motor nerves as well, a method known as cryoneuromodulation. Cryoanalgesia has been used with reasonably good success in the medical field since the mid-1970s,6,7 with reports of functional recovery and axon regeneration rates of about 1–4 mm/day.5,8 Although sensory and motor nerves differ in physiological function, they have very similar capabilities for axon regeneration.9
Focused cold therapy (FCT) is the direct application of low temperatures to inhibit signaling of peripheral nerves. Temporary inactivation of the nerve begins near +10°C, whereas temperatures slightly colder than −5°C can produce a conduction block lasting from several hours to days.10,11 This temporary conduction block is believed to be due to disruption of the channels and/or pumps involved in transfer of sodium and potassium ions across the cell membrane. FCT on motor nerves at colder temperatures (∼−20°C and below) leads to axonal and myelin degeneration, also known as Wallerian degeneration.12 This is in contrast to more traumatic nerve injuries, such as transection and thermal heat lesioning, which disrupt structural proteins and can lead to neuroma formation.12 In the case of FCT, the epineurium and perineurium resist freeze damage, which allows the structural scaffold to remain intact. This allows normal axonal regeneration and remyelination to occur.7
Research into the behavioral, electrophysiological, and pathological recovery of peripheral nerves after cryogenic injury has been conducted in a variety of animal models.13–15 Historical data has indicated that peripheral nerves recover their structure and function within months of direct contact with a cryoprobe, delivering temperatures as cold as −120°C.16,17 Still, as this body of data concerns primarily sensory nerves, there is a distinct need for more studies to identify the effects of FCT on peripheral motor nerves.
In this study we compare the long-term effects of a single treatment versus 3 repeat treatments at 2-month intervals. Use of FCT on motor nerves can potentially be employed for various medical conditions, including movement disorders, muscle spasms, muscle hyperactivity, and/or any condition in which reduced muscle movement is desired. Proof-of-concept studies are currently underway to explore potential application of this technology for upper limb spasticity.
Methods
Animal Model
Studies were performed at ISIS Services (San Carlos, California), and the study protocol was approved by the ISIS Institutional Animal Care and Use Committee. ISIS staff were trained by Myoscience staff to support the surgical procedure and perform the physiological evaluation. Study treatment was performed by Myoscience staff. Assessments were performed by 2 independent reviewers (ISIS personnel trained by Myoscience) who were blinded to the study and each other. Data analysis and study design were performed by Myoscience staff. Study design and data analysis were performed by Myoscience staff. Ninety female Sprague-Dawley rats, aged 12 weeks and weighing 215−308 g, were used in this study.18 Animals were divided randomly into 3 treatment groups. Group A animals were sham and untreated negative controls (n = 24). Group B animals received a single treatment in a randomly selected hindlimb (n = 26). Group C animals received a total of 3 treatments to a randomly selected hindlimb at 8-week intervals (n = 38). Previous unpublished studies from our laboratory have shown that muscle function of animals recovers to nearly normal by 8 weeks.
Animals were housed 2 to a cage with standard chow and water available ad libitum. Housing was maintained on a 12-hour light cycle for the duration of the study.
Focused Cold Therapy
Animals were anesthetized using isoflurane at 2−4% with 2.5 L/min oxygen delivered in an induction chamber. The treatment leg was selected randomly, while the contralateral limb was left as an untreated control. Under aseptic conditions, the sciatic nerve was accessed surgically through the thigh, targeting the trifurcation of the sciatic nerve. A Cryo-Touch III device (Myoscience, Inc., Redwood City, California) was used to deliver a 60-second treatment to the sciatic nerve proximal to its trifurcation into the tibial, sural, and fibular nerves. The cryoprobe configuration consisted of a linear array of 3 equally spaced stainless-steel needles, 27G × 6-mm in length. The delivery temperature of −54.9 ± 6.2°C was monitored with a type T bifilar (0.0015-in. diameter) thermocouple wire soldered to the tip of the probe. After treatment delivery, the cryoprobe was removed, and the incision was closed using the standard 2-layer technique. The animal was allowed to recover from anesthesia and then returned to its standard housing.
Physiological Toe Spread Assessment
Physiological toe spread was assessed twice weekly up to 224 days after the start of the study. Assessments were performed by 2 independent reviewers (ISIS personnel trained by Myoscience, Inc.) who were blinded to the study and each other. A modified toe spread test was performed to determine change in muscle function.19 Toe spread was determined by lifting the rat by the base of the tail with the legs hanging freely. The toe spread reflex was observed with the following ratings: normal reflex with all toes spreading out received a score of 0; weak reflex with some toes spreading apart partially received a score of 1; and lack of reflex with all toes clubbed together received a score of 2. Statistical significance was determined using single-factor analysis of variance (ANOVA).
Physiological Motor Function Assessment
Physiological motor function was also assessed at the same time as the toe spread assessment by the same blinded independent reviewers. They assessed the walking behavior of the animal and assigned a score as follows20: normal walking function received a score of 0; normal dorsiflexion ability while walking with curled toes received a 1; moderate dorsiflexion ability while walking with curled toes received a 2; and no dorsiflexion ability while walking with curled toes received a 3. Statistical significance was determined using single-factor ANOVA.
Histological Assessment
Animals were killed at 1, 8, 16, 24, 32, 40, and 48 weeks after final treatment. Hindlimb nerves were harvested en bloc with the surrounding tissue and immersed in 10% buffered formalin. After fixation, the tissues were trimmed at approximately 1-mm intervals and processed by standard formalin-fixed paraffin-embedded slide preparation. Slides were stained by standard hematoxylin and eosin (H&E) staining. Slides were assessed and scored by a board-certified veterinary pathologist blinded to the study under standard light microscopy with a digital capture camera (Olympus; Tokyo, Japan). Injury to tissue was scored on a 1−4 scale where: 1 = minimal (0–25% of cells affected); 2 = mild (25–50% affected); 3 = moderate (50–75% affected); and 4 = severe (75–100% affected).
Immunofluorescence Staining
Immunofluorescence was performed on unstained formalin-fixed paraffin-embedded slides. Slides were first deparaffinized through standard xylene wash followed by standard rehydration. Antigen retrieval was performed in sodium citrate buffer (10 mM sodium citrate, 0.05% Tween-20, pH 6.0) at 95°−100°C for 10 minutes. Samples were blocked in 1% bovine serum albumin (BSA) in 1× phosphate-buffered saline (PBS) with 0.05% Tween-20. Serial antibody staining was performed with S-100 (AB941; Chemicon; 1:60) primary antibody and Alexa Fluor 546 goat anti-rabbit (A-11010; Life Technologies; 1:1000) secondary antibody, followed by neurofilament (sc-58561; Santa Cruz Biotechnologies; 1:60) primary antibody and Alexa Fluor 488 goat anti-mouse (A-11001; Life Technologies; 1:1000) secondary antibody. All antibody steps were diluted in 1× PBS and performed for 1 hour at room temperature. Nuclei were stained with DAPI at 1:1000 dilution of 1× PBS for 10 minutes. Slides were imaged on a Zeiss Axioskop fluorescent microscope with associated Zeiss AxioVision software.
Results
Physiological Weakening and Recovery after Single Cryotreatment
Group B animals received a single treatment at the start of the study and were followed for up to 32 weeks. The treated hindlimbs showed a significant (P < 0.05) reduction in both toe splaying (score 1.6 ± 0.1) and motor function (score 1.5 ± 0.1) ability at 3 days after treatment, followed by a gradual recovery of ability (Fig. 1). Functional recovery was determined to be the point when the mean score of the treated limb was not significantly different from that of the untreated limb. The recovery of toe splay was determined to have occurred by 56 days posttreatment (P = 0.41 as compared with untreated), whereas motor function demonstrated recovery by 31 days posttreatment (P = 1 as compared with untreated; Fig. 1). There were no significant differences in physiological function between sham, naive, and contralateral untreated control animals.
Figure 1.

(A) Toe spread and (B) motor function assessment scores of single-treated versus untreated contralateral limbs. *Statistically significant (P < 0.05) when compared with control hindlimb. All other points are not significant (P > 0.05). Error bars represent mean ± SEM.
Histology Assessment Following a Single Cryotreatment
Treated nerves at the site of treatment and 1−2-mm intervals distal to the treatment were collected en bloc with the surrounding tissue at 1, 8, 16, 24, and 32 weeks posttreatment.
At 1 week posttreatment, the nerves showed marked acute to subacute and diffuse axonal degeneration and edema affecting the entire cross-section of nerve fascicles (Fig. 2A). Endoneural swelling with and without fragmented cellular debris was observed. The perineurium and epineurium remained intact architecturally. In addition, there was intense phagocytosis with concurrent endoneurial hypercellularity (Schwann cell proliferation). Wallerian degeneration of the axons was also observed in the tributaries distal to the treatment site.
Figure 2.
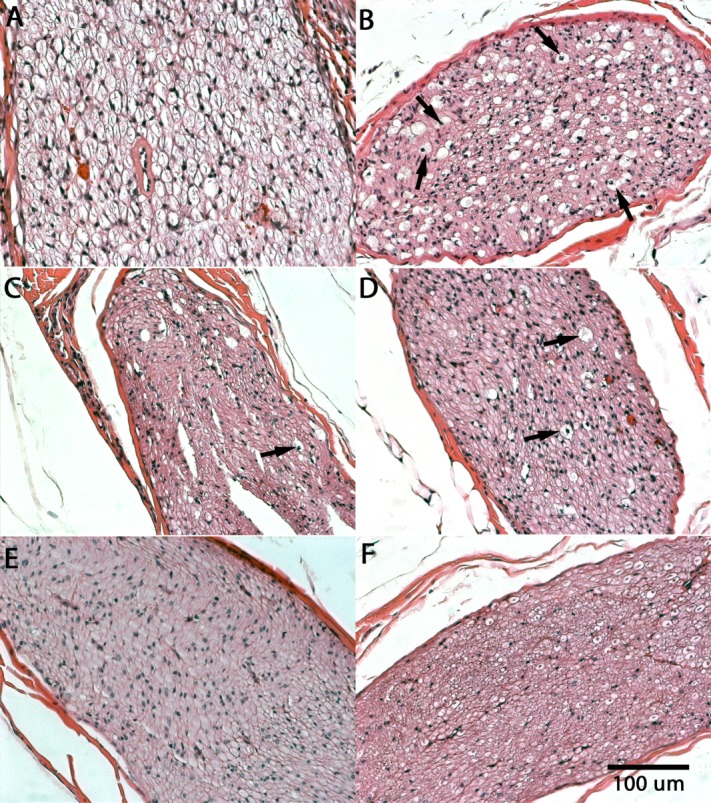
Samples of (A) 1-week, (B) 8-week, (C) 16-week, (D) 24-week, and (E) 32-week posttreatment H&E-stained cross-sections of single-treatment sciatic nerves. (F) Untreated controls are shown for comparison. Arrows indicate degenerated axons.
At 8 weeks post-treatment, sections of the sciatic nerve and its tributaries continued to have approximately 50% axonal degeneration concurrent with areas that demonstrated regeneration of myelinated axons (Fig. 2B). Approximately 50% of the axons had sprouted within the endoneurium supported by the reactive Schwann cells. Phagocytic activity persisted throughout the affected regions.
At 16 weeks, the nerves in the single-treatment group continued to heal, with substantial cellular restoration as compared with healing at 8 weeks (Fig. 2C). Approximately 75% of the myelinated axons had regenerated, whereas phagocytic activity had subsided considerably with persisting Schwann cell proliferation.
By 24 and 32 weeks posttreatment, nerves had uninterrupted regeneration and were estimated to have near 100% axonal restoration (Fig. 2D and E). Remyelination was further confirmed with immunofluorescence double staining for neurofilaments (neuron) and S-100 (Schwann cells) (Fig. 3). Tissue hypercellularity was observed due to continued Schwann cell proliferation, whereas a few sites of slightly swollen axonal fibers were noted, suggesting delayed regeneration and/or axonal artifacts due to tissue orientation and plane of sectioning. Similar artifacts were also noted in untreated control nerve sections (Fig. 2F).
Figure 3.

Immunofluorescence staining for neurofilaments (green, neuron), S-100 (red, Schwann cell), and nuclei (blue, DAPI) of (A) untreated control, (B) 1-week, and (C) 32-week posttreatment for repeat-treated nerves. Arrows indicate myelinated neurons.
Physiological Weakening and Recovery after Repeated Cryotreatment
The animals in group C (repeat treatment; n = 38) received a total of 3 treatments at 8-week intervals. The treated hindlimbs showed a significant (P < 0.05) reduction in both toe splaying and motor function ability after each treatment, followed by gradual recovery (Fig. 4A). Significant weakening in toe splay ability was observed consistently 3 days posttreatment with average toe spread scores of 1.7 ± 0.1 (P = 1.38 × 10−34), 1.8 ± 0.1 (P = 3.73 × 10−40), and 1.0 ± 0.03 (P = 3.35 × 10−45) after the first, second, and third treatments, respectively. Functional recovery of toe splay was determined to have occurred 56 days after the first and third treatments. Toe splay remained significantly different for the treated hindlimb after the second treatment during the recovery period of 56 days, reaching the time of the third treatment. Although function after the second treatment was not normal, the trend of the graph indicates that normal function was nearly complete.
Figure 4.
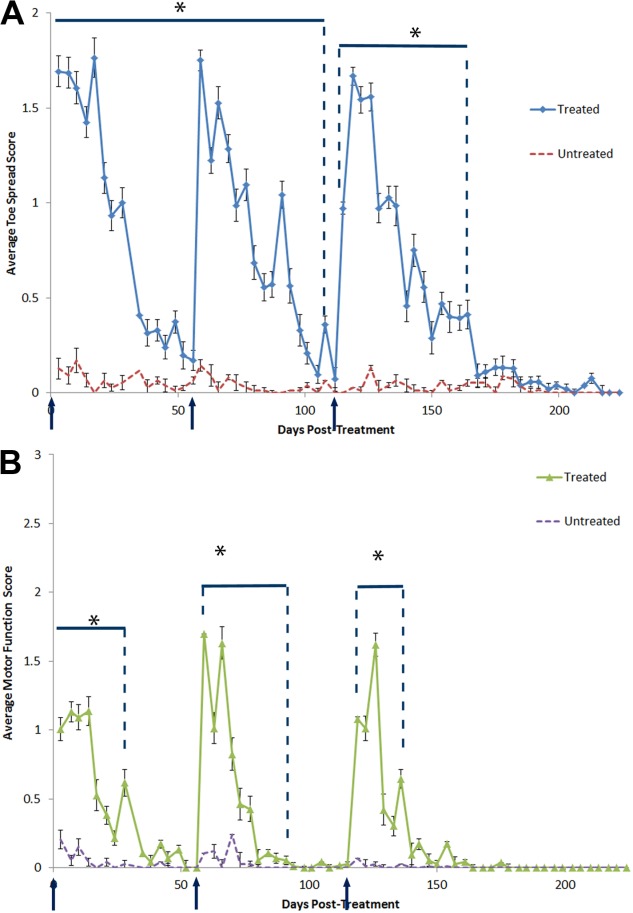
(A) Toe spread and (B) motor function assessment scores of repeat-treatment versus untreated contralateral limbs. *Statistically significant (P < 0.05) when compared with control hindlimb. All other points are not significant (P > 0.05). Error bars represent mean ± SEM.
Motor function scores after the first, second, and third treatment were 1.0 ± 0.1 (P = 1.29 × 10−11), 1.7 ± 0.1 (P = 2.05 × 10−26), and 0.0 ± 0.1 (P = 0.16), respectively, at 3 days posttreatment (Fig. 4B). The average motor function scores, 1.1 ± 0.1 (P = 1.22 × 10−21), 1.0 ± 0.1 (P = 1.14 × 10−10), and 1.1 ± 0.1 (P = 6.80 × 10−19) were measured at 7 days after the first, second, and third treatments (Fig. 4B). Although hindlimb motor function was normal at 3 days after third treatment, the toe spread indicated weakening of distal muscle groups. In addition, the motor function demonstrated weakening at day 7 after the third treatment. Full recovery of motor function was determined to be 38, 38, and 35 days after first, second, and third treatments, respectively (recovery defined as the day when motor score becomes P > 0.05 when compared with untreated limb). Motor function scores demonstrated a lesser degree and duration of weakening when compared with the toe spread assay.
Overlay of the repeated treatments shows similar weakening and recovery patterns of toe function and motor function (Fig. 5). Consistent patterns were observed with each successive treatment.
Figure 5.

Overlay of (A) toe spread and (B) motor function scores to compare weakening and recovery after first, second, and third treatment in the repeat-treatment group. Error bars represent mean ± SEM.
Histologic Assessment of Peripheral Nerves Receiving Repeated Cryotreatments
Tissues were explanted and stained with H&E at 8, 16, 24, and 32 weeks after the third treatment. Similar to the results seen in the single-treatment group, the repeat-treatment group showed about 50% axonal degeneration in nerve cross-sections at 8 weeks posttreatment (Fig. 6A). Schwann cell activity within the endoneurium supported axonal regrowth. Hypercellularity and phagocytic activity were observed throughout the nerve cross-section.
Figure 6.
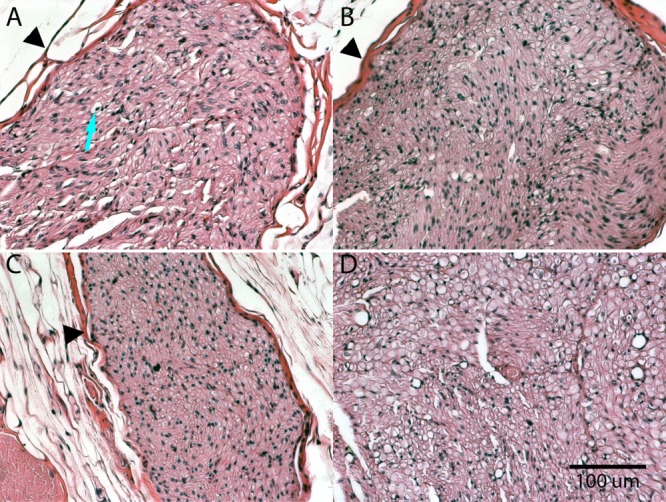
Samples of (A) 8-week, (B) 16-week, (C) 24-week, and (D) 32-week posttreatment H&E-stained cross-sections of repeat-treatment sciatic nerves. Samples were collected at the indicated time intervals after the third cryotreatment. Arrow indicates degenerated axon. Arrowheads indicate epineurial structure of the nerve.
At 16 weeks after third treatment, the histology continued to be comparable to the single-treatment 16-week samples. Approximately 75% axonal regeneration was observed (Fig. 6B) with tissue hypercellularity, due to persistence of Schwann cell proliferation and phagocytic activity. A few populations of slightly swollen axonal fibers indicated continued axonal degeneration.
By 24 and 32 weeks after the third treatment, nerve sections showed significant axonal regeneration (Fig. 6C and D). Slight hypercellularity was observed, indicating continued Schwann cell activity.
Untreated control limbs showed results comparable to those in sham and naive control groups at all time-points.
Histology Assessment of Surrounding Tissue Post Single and Repeated Cryotreatment Groups
Tissues surrounding the treatment site were collected en bloc at 1 week posttreatment (single-treatment group only). Adipose tissue adjacent to the treatment site sustained a minimal degree of necrosis attended by a few macrophages and fibrocytes (Fig. 2A). Small arterioles in proximity to the treatment site had rare fibrinoid degeneration, whereas their lumens remained patent.
In addition, at 1 week posttreatment (single treatment group only), adjacent skeletal muscle fibers had a narrow band of minimal coagulative necrosis and cellular condensation, consistent with cold thermal damage from the periphery of the cold zone (refer to Figure S1 in Supplementary Material, available online). Thick linear tracks of muscle necrosis infiltrated by macrophages radiated outward from the treated sites, representing the initial surgical pathway. Distal muscle tissue, innervated by the treated nerves, showed no observable histological changes at any of the explant time-points. Skeletal muscle in the treated legs had normal structural similarity to untreated muscle in the control leg.
At 8−32 weeks posttreatment (single and repeat groups), blood vessels, fat tissue, and surrounding muscles appeared normal (Figs. 2 and 6).
Discussion
The results demonstrate that FCT treatment of the rat sciatic nerve produces temporary interruption of hindlimb function. The interruption is associated with axonal degeneration at and distal to the treatment site. Physiological and histological data also show that the nerve is able to regenerate and allow for return of normal function after multiple treatments.
At 1 week posttreatment, physiological weakening is associated with histological evidence of axonal degeneration. When compared with the various negative controls, these results demonstrate that direct cryoprobe application to the nerve results in complete axonal degeneration while allowing the acellular nerve structure (epineurium and perineurium) to remain intact. Keeping the nerve structure intact plays a significant role in allowing for normal axonal regeneration to its original target site.21 In contrast, disruption of the nerve structure via transection results in incomplete regeneration.22
The toe spread score comparison between single- and repeat-treatment groups resulted in consistent weakening followed by consistent recovery times, suggesting a consistent rate of nerve regeneration and functional reinnervation after each treatment. Motor function scoring was somewhat more variable. The single-treatment group demonstrated an earlier recovery to normal as compared with the first treatment in the retreatment group (31 vs. 38 days). The single- and repeat-treated groups reached a score of <0.25 at 24 days and, therefore, any difference in recovery time is insignificant.
Due to the focused nature of this treatment, there is denervation in distal muscle groups, whereas proximal muscle groups of the hindlimb are not affected. With typical peripheral nerve injuries, the affected nerve does not undergo retrograde axonal degeneration.23 The specificity of the treatment is reflected by the difference in weakening and recovery observed between the toe spread and motor function assays. The toe function assay measures the function of distal muscle groups, whereas the motor function assay measures both proximal and distal muscle groups, consistent with their locations relative to the treatment site. Although the motor function assay is less specific than the toe spread, it is a general indicator of the effect of weakening on total hindlimb function. In addition, the difference in toe spread and motor function assays demonstrates that the treatment is focused on the treated nerve and its target muscle groups, and the effect does not disperse to other hindlimb areas.
A change in motor function was observed 3 days posttreatment for the first 2 treatments, but it was not observed until 7 days after the third treatment. Given that there was not more resolution in the follow-up period (animals were not assessed at 4, 5, or 6 days posttreatment), it is possible that, after the third treatment, animals showed weakened motor function as early as 4 days post-treatment. However, the trend of motor function weakening after each treatment is the same over the recovery period, which suggests that this minor delay is of little significance to the outcome of the treatment.
The accumulation of any altered tissue can decrease the ability of the treated hindlimb to return to normal function. In particular, fibrosis of skeletal muscle or neuroma formation in the nerve may prevent recovery of normal motor movement of the hindlimb. However, histological analysis showed no fibrotic activity or scar tissue in the treated region at 32 weeks posttreatment (both single- and repeat-treatment groups) (see Fig. S2 in Supplementary Material, available online). In addition, the repeat treatment motor function and toe splay results showed repeatable weakening and recovery after each treatment. The results from that portion of the study demonstrate that the animals returned to normal function in a repeatable fashion, suggesting that the treatment did not alter any tissue structures. Further histological analyses of the repeat-treatment animals confirmed complete regeneration of all tissue in and around the treatment area. Finally, none of the animals from any treatment group exhibited a change of behavior or any complications due to treatment.
These findings have implications for any disorders involving involuntary muscle contractions. Blepharospasm, spasmodic dysphonia, upper limb spasticity, and lower limb spasticity are some examples of disorders that could be treated with FCT. Many movement disorders are currently treated using a neurotoxin, which, although effective in many instances, involves diffusion to unintended muscle groups owing to the use of high doses for larger tissue areas, such as upper limb spasticity.24 Movement disorders are also treated by sclerosing the nerve, which is painful and can lead to damage of the surrounding structures, or by an intrathecal baclofen injection, which can produce systemic side effects. FCT, however, only treats the nerve that innervates the target muscle when directed with electrical nerve stimulation before treatment. In addition, FCT has no systemic side effects regardless of the size of the muscle affected.
Glossary
- FCT
focused cold therapy
- H&E
hematoxylin and eosin
Supporting Information
Additional Supporting Information may be found in the online version of this article.
References
- 1.Cooper SM, Dawber RP. The history of cryosurgery. J R Soc Med. 2001;94:196–201. doi: 10.1177/014107680109400416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson A, Dawber RPR, Colver G. Cutaneous cryosurgery: principles and clinical practice. London: Taylor & Francis; 2005. [Google Scholar]
- 3.Tatsutani KN, Joye JD, Virmani R, Taylor MJ. In vitro evaluation of vascular endothelial and smooth muscle cell survival and apoptosis in response to hypothermia and freezing. Cryo Lett. 2005;26:55–64. [PubMed] [Google Scholar]
- 4.Feng Y, Yan T, He Z, Zhai Q. Wld(S), Nmnats and axon degeneration―progress in the past two decades. Protein Cell. 2010;1:237–245. doi: 10.1007/s13238-010-0021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trescot AM. Cryoanalgesia in interventional pain management. Pain Phys. 2003;6:345–360. [PubMed] [Google Scholar]
- 6.Lloyd JW, Barnard JD, Glynn CJ. Cryoanalgesia. A new approach to pain relief. Lancet. 1976;2:932–934. doi: 10.1016/s0140-6736(76)90893-x. [DOI] [PubMed] [Google Scholar]
- 7.Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001;20:502–507. doi: 10.1016/s1010-7940(01)00815-6. [DOI] [PubMed] [Google Scholar]
- 8.Trojaborg W. Rate of recovery in motor and sensory fibres of the radial nerve: clinical and electrophysiological aspects. J Neurol Neurosurg Psychiatry. 1970;33:625–638. doi: 10.1136/jnnp.33.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. J Clin Invest. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg PH, Heavner JE. Temperature-dependent nerve-blocking action of lidocaine and halothane. Acta Anaesthesiol Scand. 1980;24:314–320. doi: 10.1111/j.1399-6576.1980.tb01555.x. [DOI] [PubMed] [Google Scholar]
- 11.Gage AA, Baust JM, Baust JG. Experimental cryosurgery investigations in vivo. Cryobiology. 2009;59:229–243. doi: 10.1016/j.cryobiol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos NA, Chiles JH, Plunkett AR. Ultrasound-guided cryoablation of genitofemoral nerve for chronic inguinal pain. Pain Physician. 2009;12:997–1000. [PubMed] [Google Scholar]
- 13.Willenbring S, DeLeo JA, Coombs DW. Sciatic cryoneurolysis in rats: a model of sympathetically independent pain. Part 2: Adrenergic pharmacology. Anesth Analg. 1995;81:549–554. doi: 10.1097/00000539-199509000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Popken F, Land M, Bosse M, Erberich H, Meschede P, Konig DP. Cryosurgery in long bones with new miniature cryoprobe: an experimental in vivo study of the cryosurgical temperature field in sheep. Eur J Surg Oncol. 2003;29:542−547. doi: 10.1016/s0748-7983(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 15.Jiang J, Goel R, Schmechel S, Vercellotti G, Forster C, Bischof J. Pre-conditioning cryosurgery: cellular and molecular mechanisms and dynamics of TNF-alpha enhanced cryotherapy in an in vivo prostate cancer model system. Cryobiology. 2010;61:280–288. doi: 10.1016/j.cryobiol.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard D, Lloyd J, Evans J. Cryoanalgesia in the management of chronic facial pain. J Maxillofac Surg. 1981;9:101–102. doi: 10.1016/s0301-0503(81)80024-0. [DOI] [PubMed] [Google Scholar]
- 17.Zakrzewska JM, Nally FF, Flint SR. Cryotherapy in the management of paroxysmal trigeminal neuralgia. Four year follow up of 39 patients. J Maxillofac Surg. 1986;14:5–7. doi: 10.1016/s0301-0503(86)80248-x. [DOI] [PubMed] [Google Scholar]
- 18.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 19.Varejão A, Melo-Pinto P, Meek M, Filipe V, Bulas-Cruz J. Methods for the experimental functional assessment of rat sciatic nerve regeneration. Neurol Res. 2004;26:186–194. doi: 10.1179/016164104225013833. [DOI] [PubMed] [Google Scholar]
- 20.Brummett CM, Norat MA, Palmisano JM, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008;109:502–511. doi: 10.1097/ALN.0b013e318182c26b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tos P, Ronchi G, Papalia I, Sallen V, Legagneux J, Geuna S. Chapter 4: Methods and protocols in peripheral nerve regeneration experimental research: part I―experimental models. Int Rev Neurobiol. 2009;87:47–79. doi: 10.1016/S0074-7742(09)87004-9. [DOI] [PubMed] [Google Scholar]
- 22.Geuna S, Raimondo S, Ronchi G, Di Scipio F, Tos P, Czaja K. Chapter 3: Histology of the peripheral nerve and changes occurring during nerve regeneration. Int Rev Neurobiol. 2009;87:27–46. doi: 10.1016/S0074-7742(09)87003-7. [DOI] [PubMed] [Google Scholar]
- 23.Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas AM, Simpson DM. Contralateral weakness following botulinum toxin for poststroke spasticity. Muscle Nerve. 2012;46:443–448. doi: 10.1002/mus.23492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


