Abstract.
Diffuse reflectance spectroscopy (DRS) can be used to noninvasively measure skin properties. To extract skin properties from DRS spectra, you need a model that relates the reflectance to the tissue properties. Most models are based on the assumption that skin is homogenous. In reality, skin is composed of multiple layers, and the homogeneity assumption can lead to errors. In this study, we analyze the errors caused by the homogeneity assumption. This is accomplished by creating realistic skin spectra using a computational model, then extracting properties from those spectra using a one-layer model. The extracted parameters are then compared to the parameters used to create the modeled spectra. We used a wavelength range of 400 to 750 nm and a source detector separation of . Our results show that use of a one-layer skin model causes underestimation of hemoglobin concentration [Hb] and melanin concentration [mel]. Additionally, the magnitude of the error is dependent on epidermal thickness. The one-layer assumption also causes [Hb] and [mel] to be correlated. Oxygen saturation is overestimated when it is below 50% and underestimated when it is above 50%. We also found that the vessel radius factor used to account for pigment packaging is correlated with epidermal thickness.
Keywords: diffuse reflectance spectroscopy, Monte Carlo, two-layer
1. Introduction
Diffuse reflectance spectroscopy (DRS) is an optical technique that has been widely used to noninvasively measure skin optical properties.1–9 Typically, a DRS probe consists of a group of fibers that are placed in contact with the skin. The most common fiber orientation is the six-around-one geometry where a central fiber connected to a light source injects light into the tissue, and the six peripheral fibers collect the light that has travelled through the tissue and returned to the surface. This light contains quantitative information about the tissue that it has passed through, and this information can be used to assess the tissue’s physiological state. A model that relates the diffuse reflectance to physiological properties of tissue is used to extract physiological parameters from the DRS spectra. Many models based on the diffusion approximation have been developed to extract properties from DRS spectra; however, this technique requires source detector separations (SDSs) of at least 1 mm.2,10,11 Because the thickness of the epidermis is on the order of ,12 SDSs much less than 1 mm are necessary in order to probe the epidermis properties, meaning the assumptions required for the diffusion approximation are invalid. To overcome this problem, recently developed models for extracting physiological properties from DRS spectra have used Monte Carlo (MC) simulations to model the transport of photons through tissue.13–15 Most of these models are based on the assumption that skin is homogeneous and that its properties are independent of depth. In reality, skin is composed of multiple layers with different properties. For example, melanin is primarily located in the epidermis, whereas hemoglobin is only located in the dermis. Additionally, the thickness of the epidermis varies with anatomical location. Assuming that skin is homogenous can lead to errors in the extracted physiological properties because variations in epidermal thickness can make the measurement of chromophore concentrations difficult by changing the sensitivity of the probe to each layer. Some multilayered inverse models of skin have been developed to overcome this problem.16–21 While depth-dependent heterogeneities were analyzed in this study, heterogeneities that are spatially in the plane of detection were not considered. Such heterogeneities would include the border of a nevus or other concentrations of pigment in the skin as well as the localization of hemoglobin in vessels which was investigated by Fredriksson et al.20 Additionally, Fredriksson et al. generated a spectra using a two-layer model of skin with individual blood vessels and fit those spectra with a one-layer model. They found that using the one-layer model led to much greater errors in extracted parameters when compared to using a three-layer model to fit the spectra.20
In this study, we analyze the specific errors that are caused by the one-layer assumption of skin. This is accomplished by first creating modeled DRS spectra using a two-layered forward diffuse reflectance skin model in the 400 to 750 nm wavelength range with an SDS of . Next, parameters are extracted from the spectra using an inverse one-layer, or homogenous, skin model. The extracted parameters can then be compared to the parameters used to generate the modeled two-layer spectra, and this allows for a quantitative and systematic analysis of the errors that arise from the homogeneity assumption for skin.
2. Materials and Methods
2.1. Two-Layer Forward Model
Modeled spectra were created using a two-layer skin model based on an MC lookup table (MCLUT) approach.16 A four-dimensional MCLUT was created using a two-dimensional MC code written in ANSI C22 implemented on an NVIDIA GTX 560 Ti GPU on 386 parallel threads.23 The refractive index above the tissue was set to 1.45 to match the refractive index of an optical fiber, and the refractive index of the medium was set to 1.4 to match the refractive index of tissue. Spatially resolved diffuse reflectance was calculated by convolving the impulse response using a Gaussian-shaped beam profile with a radius of , and reflectance was calculated at a center-to-center SDS of with a radius collection fiber.24 This geometry was chosen because of its common use in skin applications.25 Each entry in the MCLUT contains a reflectance value for a given top layer thickness (), epidermal absorption (), dermal absorption (), and reduced scattering coefficient (), which is assumed to be equal in both layers. In the MCLUT, ranges from 0 to , ranges from 0 to , ranges from 0 to , and ranges from 0 to . Ten evenly spaced increments were used for each of the parameters, giving a total of 10,000 separate MC simulations. A total of photons were used for each MC simulation. The Henyey–Greenstein phase function was used for sampling scattering angles. Scattering anisotropy was set to 0.85 for all simulations.
The MCLUT-based forward model for diffuse reflectance is based on a two-layer skin model where a reference absorption spectrum of melanin26 is used for the top layer and oxy- and deoxy- hemoglobin27 spectra are used for the bottom layer with a wavelength range of 400 to 750 nm. Spectra are generated by first selecting the following properties: (1) epidermal thickness (), (2) hemoglobin concentration ([Hb]), (3) oxygen saturation (), (4) melanin concentration ([mel]), and (5) . Reduced scattering at all wavelengths is calculated using Eq. (1), which is commonly used in tissue optics,13–16
| (1) |
where is the reduced scattering coefficient at wavelength , , and is the scattering exponent, which is related to the size of the scattering particles. Absorption in the top layer at each wavelength is calculated using Eq. (2)
| (2) |
where is the epidermal absorption coefficient at wavelength , is the extinction coefficient of melanin at wavelength , and [mel] is the concentration of melanin. Absorption in the bottom layer at each wavelength is calculated using Eq. (3),
| (3) |
where is the dermal absorption coefficient at wavelength , [Hb] is the total concentration of hemoglobin, is the extinction coefficient of oxygenated hemoglobin at wavelength , is the extinction coefficient of deoxygenated hemoglobin at wavelength , and is the oxygen saturation. Once the optical properties are determined at each wavelength, the MCLUT is used to determine the reflectance at each wavelength. Cubic splines are used to interpolate between values in the MCLUT.
2.2. One-Layer Inverse Model
A one-layer inverse skin model was used to extract the parameters from the two-layer spectra. The same code used to generate the two-layer MCLUT was also used to create the one-layer MCLUT. Refractive indices and probe geometry parameters were also the same. In the one-layer MCLUT, ranges from 0 to and ranges from 0 to to cover the range of optical properties present in skin.28 Ten evenly spaced increments were used for each parameter. In the one-layer inverse model, the first step is to set initial values to the following parameters: (1) , (2) [mel], (3) [Hb], (4) , and (5) vessel radius (). Next, is calculated using Eq. (1) and is determined using the following equation:
| (4) |
where [mel] represents the concentration of melanin and is the wavelength dependent absorption due to hemoglobin that has been corrected for the inhomogeneous distribution. Because hemoglobin is confined to very small volumes in blood vessels, we account for this inhomogeneous distribution in tissue by using the corrections described by van Veen et al. to calculate a corrected absorption coefficient of blood.29 The correction factor can be calculated as
| (5) |
where is the absorption coefficient of whole blood and is assumed to be the mean vessel radius in the tissue volume sampled. The packaging corrected absorption coefficient of blood in tissue can now be written as
| (6) |
where
| (7) |
where [Hb] is the hemoglobin concentration, is the extinction coefficient for oxygenated hemoglobin at wavelength , is the extinction coefficient for deoxygenated hemoglobin at wavelength , and is the oxygen saturation. After Eqs. (1), (4) to (7) are used to calculate and , the one-layer MCLUT is used to generate a reflectance spectrum. The root-mean-sqared error between this spectrum and the modeled two-layer spectrum is then calculated. The parameters are then iteratively updated until the error is minimized. An interior-point nonlinear optimization routine provided in the MATLAB® optimization toolbox (Mathworks, Natick, Massachusetts) was used as the optimization algorithm. In order to avoid converging to a local minima, the optimization algorithm was run three times with three different sets of initialization parameters and then we used the solution that gave the smallest error. We are confident that the global minimum was found because the three different initialization parameters led to very similar solutions.
2.3. Extracting One-Layer Properties from Two-Layer Spectra
By fixing some parameters and changing others in the two-layer forward model and then fitting the resulting two-layer spectra with the one-layer model, we are able to systematically determine the errors that arise from the homogeneity assumption of skin. The following experiments were performed:
-
1.
changing [mel] and fixing all other two-layer parameters,
-
2.
changing [Hb] and fixing all other two-layer parameters,
-
3.
changing and fixing all other two-layer parameters,
-
4.
changing and fixing all other two-layer parameters to analyze the relationship between and , and
-
5.
selecting random pairs of [mel] and [Hb] and fixing all other two-layer parameters.
3. Results
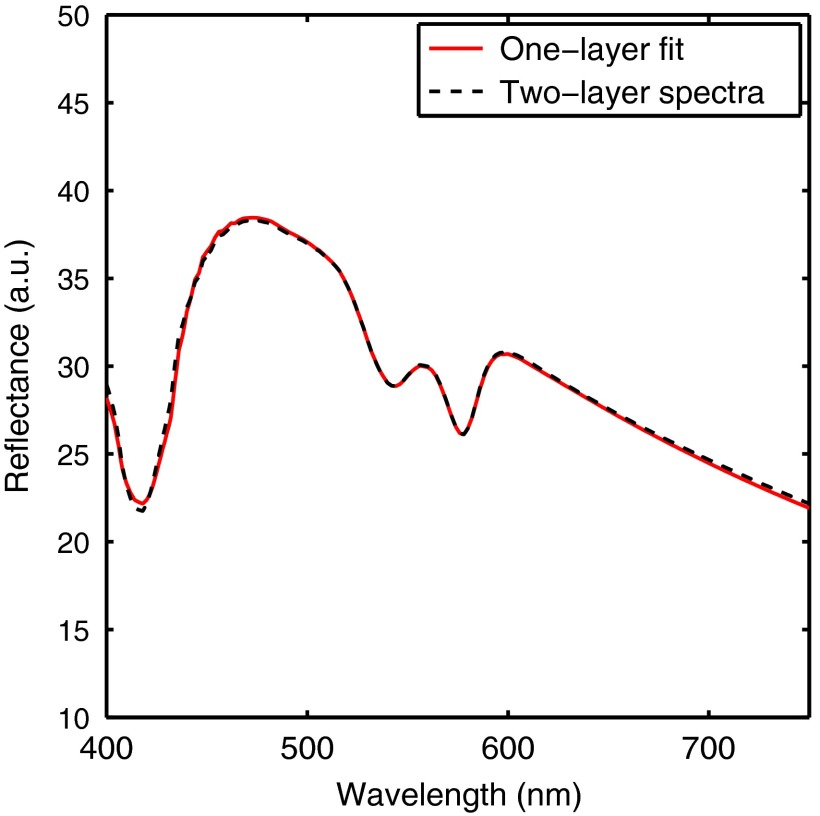
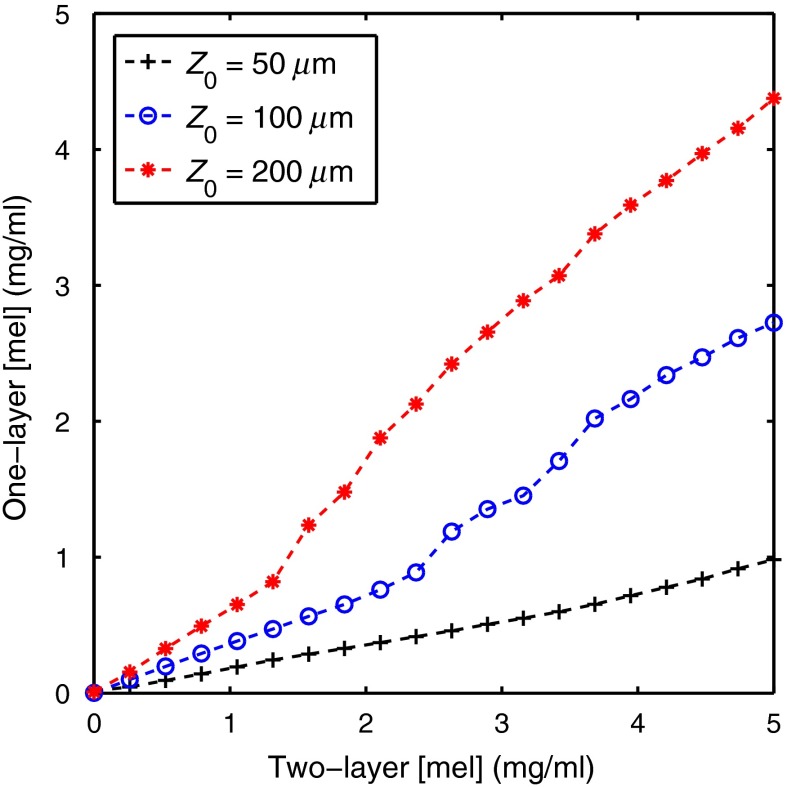
Spectra based on a two-layer skin model were generated and then parameters from the spectra were extracted using a one-layer inverse skin model. Figure 1 shows a representative fit and illustrates the good agreement between the two-layer and one-layer spectra. Because the same scattering value was used for both layers in the two-layer model, the error in extracted scattering values was always less than 1.7%. Figure 2 shows the two-layer [mel] versus the one-layer extracted [mel]. This plot was created by varying the two-layer [mel] used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [Hb] was fixed at , was fixed at , was fixed at 100%, and was fixed at . [mel] ranged from 0 to in 20 increments. The one-layer inverse model was then used to extract [mel] from each spectra.
Fig. 1.
A representative fit showing the good agreement between the two-layer and one-layer spectra.
Fig. 2.
Two-layer [mel] versus the one-layer extracted [mel]. This plot was created by varying the two-layer [mel] used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [Hb] was fixed at , was fixed at , was fixed at 100%, and was fixed at . [mel] ranged from 0 to in 20 increments.
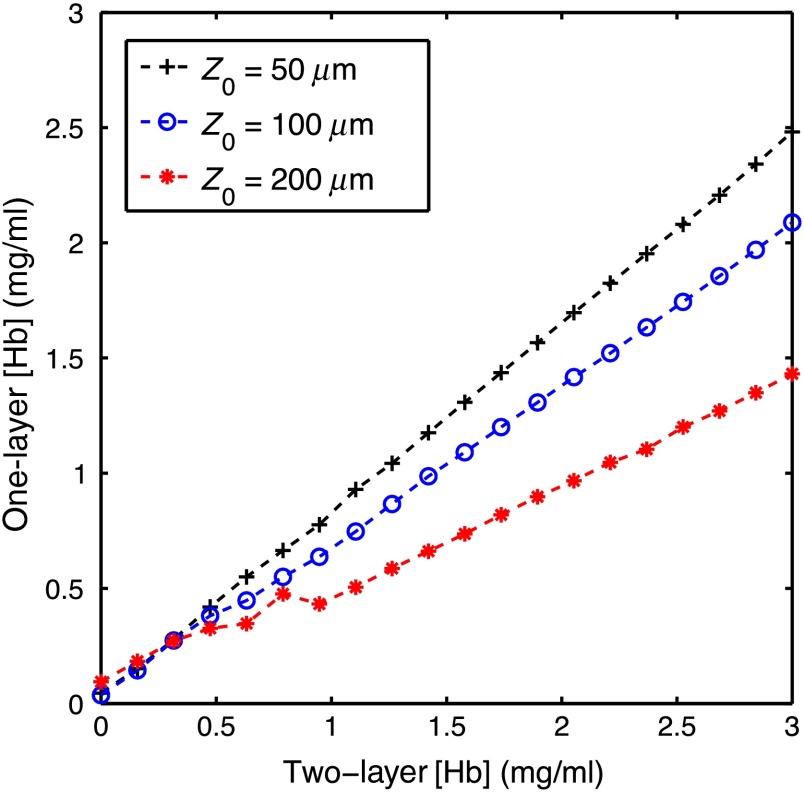
Figure 3 shows the two-layer [Hb] versus the one-layer extracted [Hb]. This plot was created by varying the two-layer [Hb] used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [mel] was fixed at , was fixed at , was fixed at 100%, and was fixed at . [Hb] ranged from 0 to in 20 increments. The one-layer inverse model was then used to extract [Hb] from each spectra.
Fig. 3.
Two-layer [Hb] versus the one-layer extracted [Hb]. This plot was created by varying the two-layer [Hb] used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [Hb] was fixed at , was fixed at , was fixed at 100%, and was fixed at . [mel] ranged from 0 to in 20 increments.
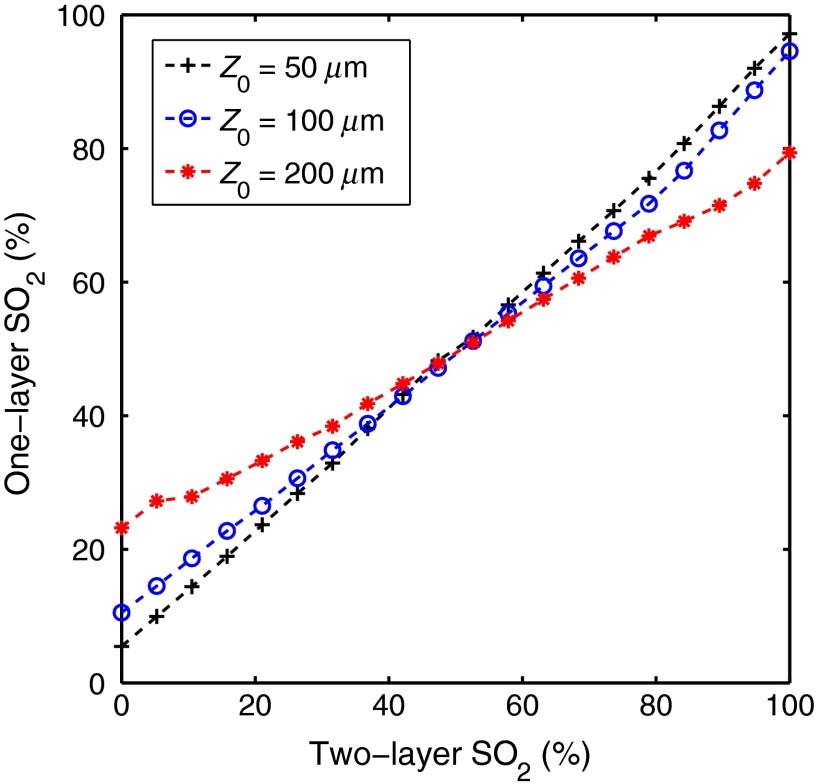
Figure 4 shows the two-layer versus the one-layer extracted . This plot was created by varying the two-layer used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [mel] was fixed at , was fixed at , [Hb] was fixed at , and was fixed at . ranged from 0 to 100% in 20 increments. The one-layer inverse model was then used to extract from each spectra.
Fig. 4.
Two-layer versus the one-layer extracted . This plot was created by varying the two-layer used to create the spectra and fixing all other parameters at three different values for (50, 100, and ). [mel] was fixed at , was fixed at , [Hb] was fixed at , and was fixed at . ranged from 0 to 100% in 20 increments.
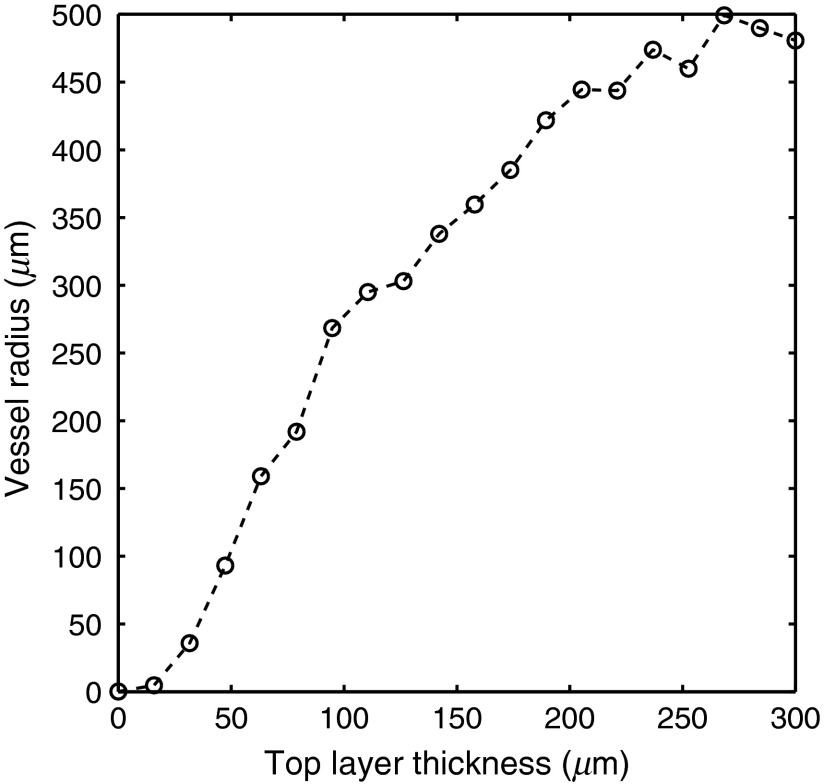
Figure 5 shows versus the vessel radius parameter used in the one-layer inverse model. This plot was created by varying in the two-layer model used to create the spectra and fixing all other parameters. [mel] was fixed at , [Hb] was fixed at , was fixed at , was fixed at 100%, and was fixed at . ranged from 0 to in 20 increments. The one-layer inverse model was then used to extract vessel radius from each spectra. To illustrate the relationship between the pigment packaging factor in the one-layer model and the epidermal thickness, a pigment packaging factor was not included in the two-layer model.
Fig. 5.
versus the vessel radius parameter used in the one-layer inverse model. This plot was created by varying in the two-layer model used to create the spectra and fixing all other parameters. [mel] was fixed at , [Hb] was fixed at , was fixed at , was fixed at 100%, and was fixed at . ranged from 0 to in 20 increments.
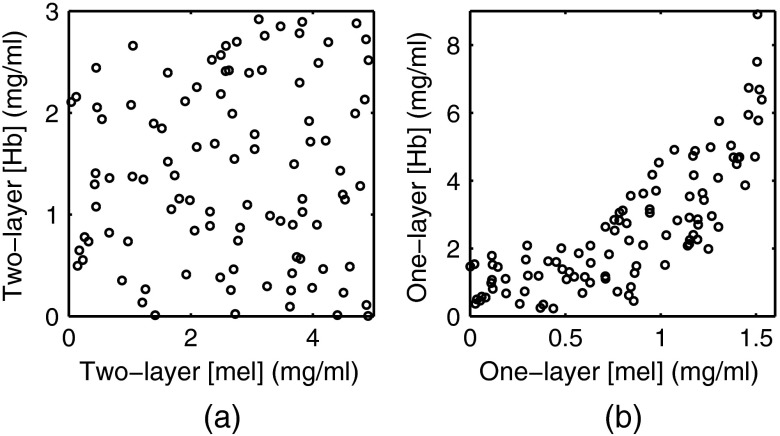
Figures 6(a) and 6(b) were created by generating 100 random pairs of [mel] and [Hb] to generate two-layer spectra while all other parameters were fixed. was fixed at , was fixed at , was fixed at 100%, and was fixed at . The random pairs of [Hb] and [mel] used to generate the two-layer spectra are plotted in Fig. 6(a) and the extracted one-layer values for [Hb] and [mel] are plotted in Fig. 6(b). In Fig. 6(a), [Hb] and [mel] have a Pearson correlation coefficient(PCC) of . In Fig. 6(b), [Hb] and [mel] have a PCC of .
Fig. 6.
(a) Random pairs of [Hb] and [mel] used to generate the two-layer spectra. (b) Extracted one-layer values for [Hb] and [mel].
4. Discussion and Conclusions
In this study, we investigated errors caused by using a one-layer assumption for skin when using diffuse reflectance spectroscopy to measure optical properties. This was accomplished by first creating spectra using a two-layer skin model and then extracting the properties from the modeled spectra using a one-layer inverse skin model. The parameters used to generate the two-layer spectra were then compared to the parameters extracted with the one-layer inverse model.
Figure 2 shows the extracted one-layer [mel] versus the two-layer [mel] for three different epidermal thicknesses. Notice that the one-layer model underestimates [mel]; however, this would be expected since the one-layer inverse model is extracting a volume average for [mel], and the melanin is located only in a thin top layer. Additionally, the magnitude of the error is dependent on epidermal thickness, with an underestimation by a factor of 5 when the epidermal thickness is and by a factor of approximately 1.25 when the epidermal thickness is . If the epidermal thickness is unknown, it would not be possible to interpret a [mel] value extracted with a one-layer skin model. Similarly, Fig. 3 shows the extracted one-layer [Hb] versus the two-layer [Hb] for three different epidermal thicknesses. [Hb] is also underestimated when a one-layer skin model is used; however, the errors are smaller than the ones for [mel] and the relationship of the error to epidermal thickness is the opposite with an underestimation of a factor of 1.2 when the epidermal thickness is and by a factor of 2 when the epidermal thickness is . Figures 2 and 3 show that [Hb] and [mel] will be underestimated when a one-layer skin model is used and that the magnitude of the underestimation is a function of epidermal thickness. If the epidermal thickness was known, it could be possible to correct for these errors; however, in many clinically realistic scenarios, the epidermal thickness will be unknown.
Figure 4 shows the extracted one-layer versus the two-layer for three different epidermal thicknesses. For , the one-layer model overestimates , and for , the one-layer model underestimates . The magnitude of the errors is directly proportional to epidermal thickness, meaning the error will be larger when the epidermis is thicker with error levels reaching 20% when the epidermal thickness is . Similar to the problem with using a one-layer model to extract [mel] and [Hb], it will be difficult to interpret values that are extracted using a one-layer skin model when the epidermal thickness is unknown.
To account for inhomogeneously distributed blood in skin, many one-layer models have incorporated a pigment packaging factor. This factor, often calculated as the average vessel radius, accounts for the flattening of the hemoglobin absorption spectra that is caused by the reduced path length of photons at wavelengths where the absorption is high. We noticed a similar flattening phenomenon is caused by increasing the epidermal thickness. Figure 5 was created in order to further investigate the relationship between the vessel radius factor in a one-layer model and the epidermal thickness in a two-layer model. Figure 5 shows that there is a strong positive correlation between epidermal thickness and the vessel radius factor. Because of this, we believe the pigment packaging factor is influenced by both the localization of blood in vessels and the localization of blood under the epidermis.
In Fig. 6, we investigate if the one-layer assumption would have any effect on the correlation between [mel] and [Hb]. First, random pairs of [mel] and [Hb] were selected and used to generate two-layer spectra. These random pairs are plotted in Fig. 6(a) and are essentially uncorrelated with a PCC of . Figure 6(b) plots the pairs of [mel] and [Hb] that were extracted using the one-layer model and shows that they are highly correlated with a PCC of . This correlation is due to the wavelength dependence of photon sampling depth. At shorter wavelengths, both scattering and absorption in skin are higher, therefore, photons with shorter wavelengths have shallower sampling depths and are more heavily weighted toward the properties of the epidermis.30 This means that the effect of melanin is larger at shorter wavelengths in a two-layer model. In a one-layer model, this does not occur because hemoglobin and melanin are evenly distributed. When you attempt to fit a one-layer model to two-layer data, the one-layer model will underestimate the absorption due to melanin at shorter wavelengths. To compensate for this, the optimization routine can increase the hemoglobin concentration since hemoglobin absorbs strongly at shorter wavelengths. This allows the optimization routine to minimize the error, but causes the artificial correlation between melanin concentration and hemoglobin concentration. We believe that this is the biggest limitation of using a one-layer model since there is no way to correct for the artificial correlation between [mel] and [Hb]. Additionally, correlation between extracted parameters can decrease the performance of a classifier. For example, if the extracted parameters were used to train a classifier for the diagnosis of skin cancer, we would expect an inferior performance from the classifier because of the artificial correlation between [mel] and [Hb] that is caused by the one-layer assumption of skin.
We have demonstrated evidence that using a one-layer model for skin to extract properties from DRS spectra leads to errors in the extracted properties. By generating modeled spectra with a more physiologically realistic two-layer model, and then extracting properties from those spectra using a one-layer inverse skin model, we were able to quantitatively and systematically analyze the errors that arise from the one-layer assumption for skin. All of our simulations were performed using a 400 to 750 nm wavelength range and an SDS of since these values are common for DRS in skin. At longer wavelengths where the absorption due to hemoglobin and melanin is negligible, a one-layer model could be sufficient. Additionally, a one-layer model could also be sufficient for much larger SDSs where the effect of the epidermis is greatly diminished. The main disadvantage of using a two-layer model is the increased computational complexity; however, through the use of an LUT method and advances in GPU computing, this is no longer a major issue. Our results can be used to aid in the interpretation of extracted one-layer parameters; but more importantly, these results provide evidence showing that a one-layer model is inadequate for extracting optical properties from a two-layered tissue.
Acknowledgments
The funding for this project was provided by the NIH (R21EB015892), the NSF (DGE-1110007), and the Cancer Prevention and Research Institute of Texas (RP130702).
Biographies
Ricky Hennessy is an NSF graduate research fellow in the Department of Biomedical Engineering at the University of Texas at Austin. He is advised by Drs. James W. Tunnell and Mia K. Markey. He earned his BS and MS degrees in biomedical engineering from California Polytechnic State University in San Luis Obispo. He will graduate with his PhD in February 2015.
Mia K. Markey is a full-time professor of biomedical engineering at the University of Texas at Austin and and adjunct professor of imaging physics at the University of Texas at MD Anderson Cancer Center. A 1994 graduate of the Illinois Mathematics and Science Academy, she received her BS degree in computational biology (1998) from Carnegie Mellon University and a PhD degree in biomedical engineering (2002), along with a certificate in bioinformatics, from Duke University.
James W. Tunnell is an associate professor of biomedical engineering at the University of Texas at Austin. He earned his BS degree in electrical engineering from the University of Texas at Austin and his PhD degree in bioengineering from Rice University. He was awarded a National Research Service award from the NIH to fund his postdoctoral fellowship at MIT from 2003 to 2005. He joined the faculty of the University of Texas at Austin in the fall of 2005.
References
- 1.Rajaram N., et al. , “Pilot clinical study for quantitative spectral diagnoisis of non-melanoma skin cancer,” Laser Surg. Med. 42(10), 716–727 (2010). 10.1002/lsm.21009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zonios G., Bykowski J., Kollias N., “Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy,” J. Invest. Dermatol. 117, 1452–1457 (2001). 10.1046/j.0022-202x.2001.01577.x [DOI] [PubMed] [Google Scholar]
- 3.Stamatas G. N., et al. , “In vivo measurement of skin erythema and pigmentation: new means of implementation of diffuse reflectance spectroscopy with a commercial instrument,” Brit. J. Dermatol. 159(3), 683–690 (2008). 10.1111/bjd.2008.159.issue-3 [DOI] [PubMed] [Google Scholar]
- 4.Lim L., et al. , “Probe pressure effects on human skin diffuse reflectance and fluorescence spectroscopy measurements,” J. Biomed. Opt. 16(1), 011012 (2011). 10.1117/1.3525288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng S., Grant A., Durkin A. J., “In vivo determination of skin near-infrared optical properties using diffuse optical spectroscopy,” J. Biomed. Opt. 13(1), 014016 (2008). 10.1117/1.2829772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy B. W., et al. , “Toward the discrimination of early melanoma from common and dysplastic nevus using fiber optic diffuse reflectance spectroscopy,” J. Biomed. Opt. 10(6), 064020 (2005). 10.1117/1.2135799 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Uribe A., et al. , “In vivo characterization of optical properties of pigmented skin lesions including melanoma using oblique incidence diffuse reflectance spectroscopy,” J. Biomed. Opt. 16(2), 020501 (2011). 10.1117/1.3536509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zonios G., et al. , “Melanin absorption spectroscopy: new method for noninvasive skin investigation and melanoma detection,” J. Biomed. Opt. 13(1), 014017 (2008). 10.1117/1.2844710 [DOI] [PubMed] [Google Scholar]
- 9.Borisova E., et al. , “Diagnostics of pigmented skin tumors based on laser-induced autofluorescence and diffuse reflectance spectroscopy,” Quantum Electron. 38(6), 597–605 (2008). 10.1070/QE2008v038n06ABEH013891 [DOI] [Google Scholar]
- 10.Zonios G., et al. , “Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo,” Appl. Optics 38(31), 6628–6637 (1999). 10.1364/AO.38.006628 [DOI] [PubMed] [Google Scholar]
- 11.Doornbos R. M. P., et al. , “The determination of in vivo human tissue optical properties and absolute chromophore concentrations using spatially resolved steady-state diffuse reflectance spectroscopy,” Phys. Med. Biol. 44(4), 967–981 (1999). 10.1088/0031-9155/44/4/012 [DOI] [PubMed] [Google Scholar]
- 12.Gambichler T., et al. , “In vivo data of epidermal thickness evaluated by optical coherence tomography: effects of age, gender, skin type, and anatomic site,” J. Dermatol. Sci. 44(3) 145–152 (2006). 10.1016/j.jdermsci.2006.09.008 [DOI] [PubMed] [Google Scholar]
- 13.Zhu C., et al. , “Diagnosis of breast cancer using diffuse reflectance spectroscopy: comparison of a Monte Carlo versus partial least squares analysis based feature extraction technique,” Laser Surg. Med. 38(7), 714–724 (2006). 10.1002/(ISSN)1096-9101 [DOI] [PubMed] [Google Scholar]
- 14.Palmer G. M., Ramanujam N., “Monte Carlo-based inverse model for calculating tissue optical properties. Part I: theory and validation on synthetic phantoms,” Appl. Opt. 45(5), 1062–1071 (2006). 10.1364/AO.45.001062 [DOI] [PubMed] [Google Scholar]
- 15.Hennessy R., et al. , “Monte Carlo lookup table-based inverse model for extracting optical properties from tissue-simulating phantoms using diffuse reflectance spectroscopy,” J. Biomed. Opt. 18(3) 037003 (2013). 10.1117/1.JBO.18.3.037003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma M., et al. , “Verification of a two-layer inverse Monte Carlo absorption model using multiple source-detector separation diffuse reflectance spectroscopy,” Biomed. Opt. Express 5(1), 40–53 (2014). 10.1364/BOE.5.000040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Q., Ramanujam N., “Sequential estimation of optical properties of a two-layered epithelial tissue model from depth-resolved ultraviolet-visible diffuse reflectance spectra,” Appl. Opt. 45(19), 4776–4790 (2006). 10.1364/AO.45.004776 [DOI] [PubMed] [Google Scholar]
- 18.Kienle A., et al. , “Noninvasive determination of the optical properties of two-layered turbid media,” Appl. Opt. 37(4), 779–791 (1998). 10.1364/AO.37.000779 [DOI] [PubMed] [Google Scholar]
- 19.Mantis G., Zonios G., “Simple two-layer reflectance model for biological tissue applications,” Appl. Opt. 48(18), 3490–3496 (2009). 10.1364/AO.48.003490 [DOI] [PubMed] [Google Scholar]
- 20.Fredriksson I., Larsson M., Strömberg T., “Inverse Monte Carlo method in a multilayered tissue model for diffuse reflectance spectroscopy,” J. Biomed. Opt. 17(4) 047004 (2012). 10.1117/1.JBO.17.4.047004 [DOI] [PubMed] [Google Scholar]
- 21.Zhong X., Wen X., Zhu D., “Lookup-table-based inverse model for human skin reflectance spectroscopy: two-layered Monte Carlo simulations and experiments,” Opt. Express 22(2), 1852–1864 (2014). 10.1364/OE.22.001852 [DOI] [PubMed] [Google Scholar]
- 22.Wang L., Jacques S. L., Zheng L., “MCML – Monte Carlo modeling of light transport if multi-layered tissues,” Comput. Methods Programs Biomed. 47(2), 131–146 (1995). 10.1016/0169-2607(95)01640-F [DOI] [PubMed] [Google Scholar]
- 23.Alerstam E., et al. , “Next-generation acceleration and code optimization for light transport in turbid media using GPUs,” Biomed. Opt. Express 1(2), 658–675 (2010). 10.1364/BOE.1.000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., Jacques S. L., Zheng L., “CONV—Convolution for responses to a finite diameter photon beam incident on multi-layered tissues,” Comput. Methods Programs Biomed. 54(3), 141–150 (1997). 10.1016/S0169-2607(97)00021-7 [DOI] [PubMed] [Google Scholar]
- 25.Utzinger U., Richards-Kortum R. R., “Fiber optic probes for biomedical optical spectroscopy,” J. Biomed. Opt. 8(1), 121–147 (2003). 10.1117/1.1528207 [DOI] [PubMed] [Google Scholar]
- 26.Jacques S. L., Glickman R. D., Schwartz J. A., “Internal absorption coefficient and threshold for pulsed laser distribution of melanosomes isolated from retinal pigment epithelium,” Proc. SPIE 2681, 468–477 (1996). 10.1117/12.239608 [DOI] [Google Scholar]
- 27.Prahl S. A., “Optical absorption of hemoglobin,” in Tabulated Hemoglobin Spectra, compiled by S. A. Prahl, Oregon Medical Laser Center, http://omlc.ogi.edu/spectra/hemoglobin/ (22 August 2014).
- 28.Lister T., Wright P. A., Chappell P. H., “Optical properties of human skin,” J. Biomed. Opt. 17(9) 090901 (2012). 10.1117/1.JBO.17.9.090901 [DOI] [PubMed] [Google Scholar]
- 29.van Veen R. L., Verkuysse W., Sterenborg H. J., “Diffuse-reflectance spectroscopy from 500 to 1060 nm by correction for inhomogeneously distributed absorbers,” Opt. Lett. 27(4), 246–248 (2002). 10.1364/OL.27.000246 [DOI] [PubMed] [Google Scholar]
- 30.Hennessy R., et al. , “Effect of probe geometry and optical properties on the sampling depth for diffuse reflectance spectroscopy,” J. Biomed. Opt. 19(10) 107002 (2014). 10.1117/1.JBO.19.10.107002 [DOI] [PMC free article] [PubMed] [Google Scholar]