Abstract
Discrepancies between adenosine 3′:5′-cyclic monophosphate (cAMP) and steroid production have been frequently observed in isolated target cells stimulated by low concentrations of trophic hormone. This dissociation is particularly marked in the interstitial cells of the testis, where testosterone production is elicited by gonadotropin concentrations in the picomolar range. Because of these observations, and a disparity between steroidogenesis and protein kinase (ATP: protein phosphotransferase, EC 2.7.1.37) activation in Leydig cells, the role of cAMP as a mediator of the acute steroidogenic response has been questioned. This problem has been further analyzed by assay of free and occupied cAMP-binding sites of the regulatory subunit of protein kinase in basal and hormone-stimulated cells. Free sites were measured by a [3H]-cAMP-binding assay, and occupied sites were measured by radioimmunoassay of endogenous cAMP eluted from receptor protein.
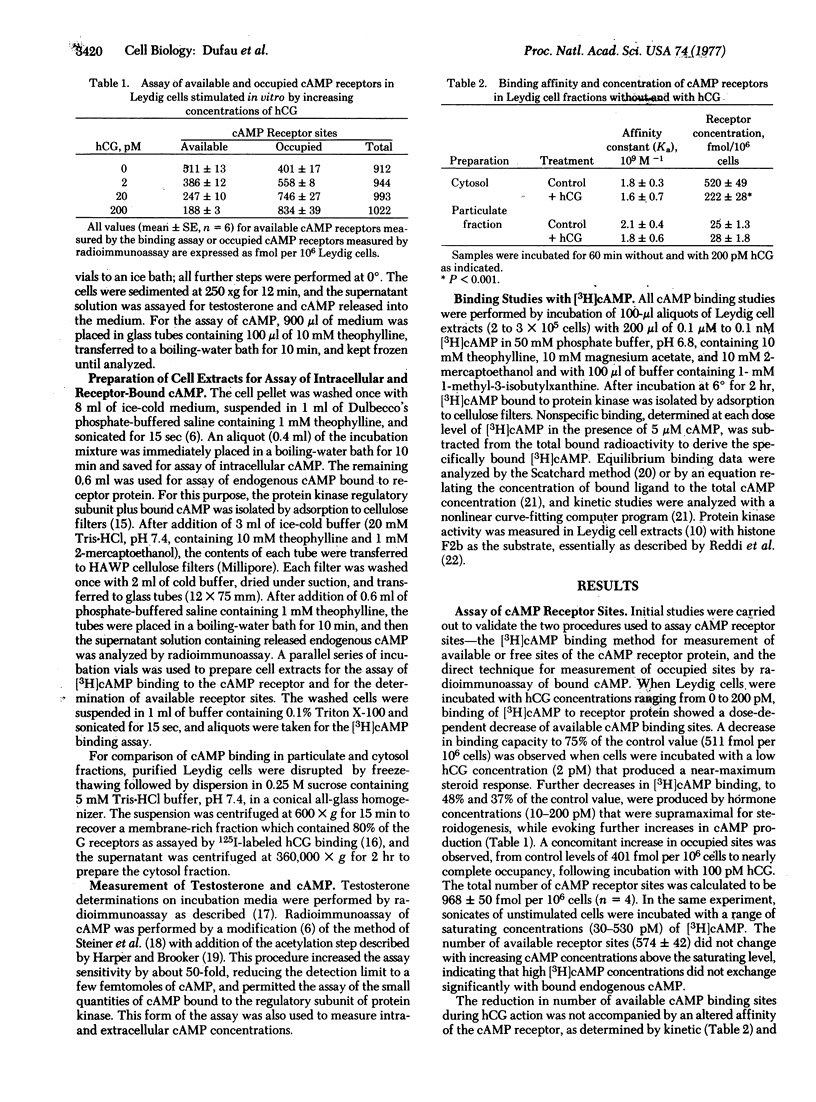
After stimulation of purified Leydig cells with 0.1-10 pM human chorionic gonadotropin, a dose-dependent decrease in available [3H]cAMP-binding sites was observed, with no change in binding affinity. The reduction in cAMP-binding sites was equivalent to the increase in occupancy of cAMP receptors by endogenous nucleotide formed during gonadotropin action. Fractional occupancy of cAMP receptors rose progressively from basal values of 0.2-0.40 to full saturation as intracellular cAMP rose 10- to 30-fold during hormone stimulation. The testosterone dose-response curve was coincident with the initial part of the cAMP-receptor occupancy curve. These changes in endogenous cAMP binding to the regulatory subunit were accompanied by a significant increase in protein kinase activity in gonadotropin-stimulated Leydig cells. These observations provide direct evidence for the role of cAMP and protein kinase during hormonal activation of steroidogenesis in the Leydig cell by low concentrations of gonadotropin.
Keywords: Leydig cells, cyclic AMP receptor, receptor occupancy, hormone action, testosterone
Full text
PDF
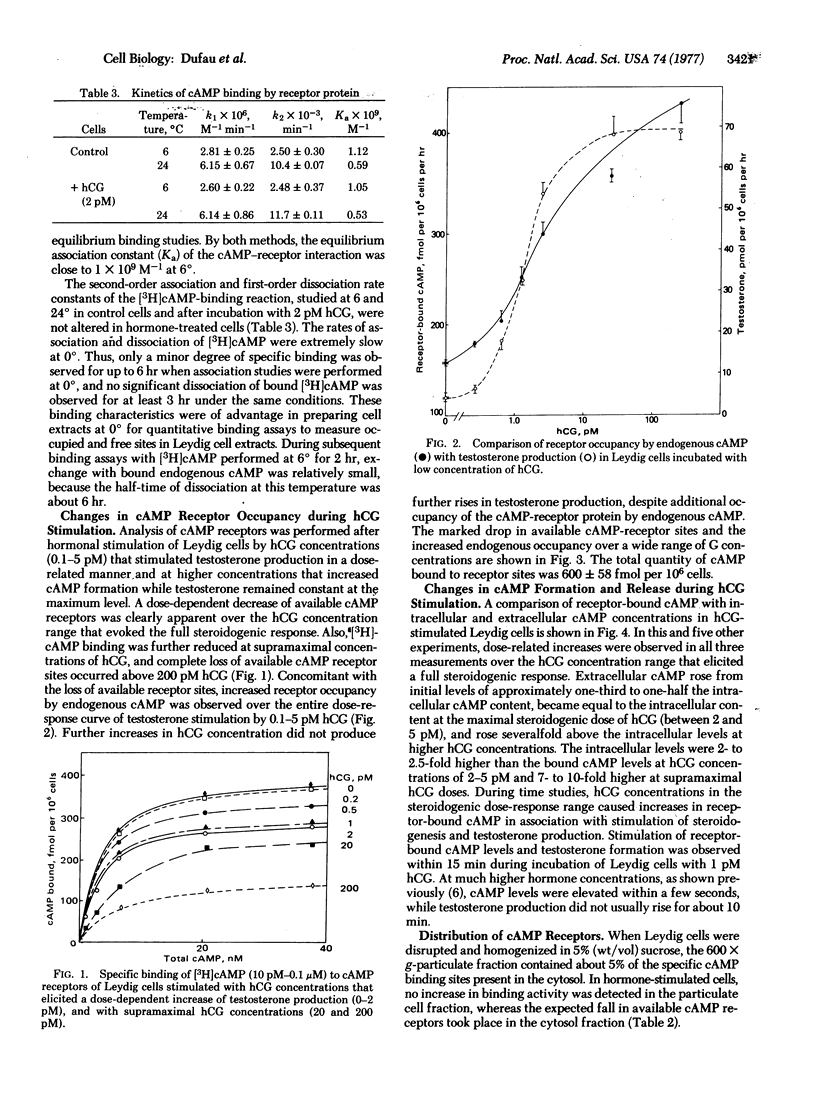

Selected References
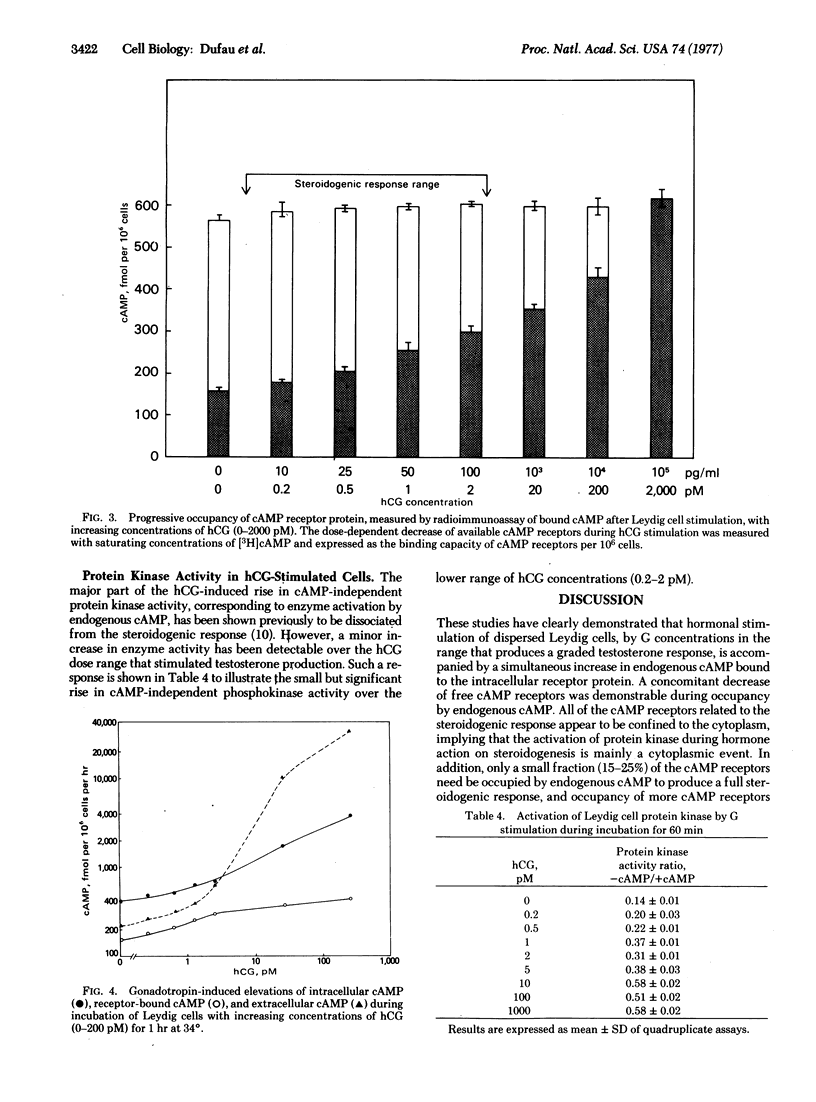
These references are in PubMed. This may not be the complete list of references from this article.
- Azhar S., Clark M. R., Menon K. M. Regulation of cyclic adenosine 3', 5' -mono-phosphate dependent protein kinase of rat ovarian cells by luteinizing hormone and human chorionic gonadotropin. Endocr Res Commun. 1976;3(2):93–104. doi: 10.3109/07435807609052925. [DOI] [PubMed] [Google Scholar]
- Beall R. J., Sayers G. Isolated adrenal cells: steroidogenesis and cyclic AMP accumulation in response to ACTH. Arch Biochem Biophys. 1972 Jan;148(1):70–76. doi: 10.1016/0003-9861(72)90116-6. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Dufau M. L. Spare gonadotrophin receptors in rat testis. Nat New Biol. 1973 Aug 15;244(137):219–221. doi: 10.1038/newbio244219a0. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Tsuruhara T., Dufau M. L. Gonadotrophin binding sites of the rat testis. Biochim Biophys Acta. 1972 Aug 18;279(1):194–201. doi: 10.1016/0304-4165(72)90254-1. [DOI] [PubMed] [Google Scholar]
- Conn P. M., Tsuruhara T., Dufau M., Catt K. J. Isolation of highly purified Leydig cells by density gradient centrifugation. Endocrinology. 1977 Aug;101(2):639–642. doi: 10.1210/endo-101-2-639. [DOI] [PubMed] [Google Scholar]
- Cooke B. A., Lindh M. L., Janszen F. H. Correlation of protein kinase activation and testosterone production after stimulation of Leydig cells with luteinizing hormone. Biochem J. 1976 Dec 15;160(3):439–446. doi: 10.1042/bj1600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufau M. L., Catt K. J., Tsuruhara T. A sensitive gonadotropin responsive system: radioimmunoassay of testosterone production by the rat testis in vitro. Endocrinology. 1972 Apr;90(4):1032–1040. doi: 10.1210/endo-90-4-1032. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Mendelson C. R., Catt K. J. A highly sensitive in vitro bioassay for luteinizing hormone and chorionic gonadotropin: testosterone production by dispersed Leydig cells. J Clin Endocrinol Metab. 1974 Sep;39(3):610–613. doi: 10.1210/jcem-39-3-610. [DOI] [PubMed] [Google Scholar]
- Dufau M. L., Watanabe K., Catt K. J. Stimulation of cyclic AMP production by the rat testis during incubation with hCG in vitro. Endocrinology. 1973 Jan;92(1):6–11. doi: 10.1210/endo-92-1-6. [DOI] [PubMed] [Google Scholar]
- Grahame-Smith D. G., Butcher R. W., Ney R. L., Sutherland E. W. Adenosine 3',5'-monophosphate as the intracellular mediator of the action of adrenocorticotropic hormone on the adrenal cortex. J Biol Chem. 1967 Dec 10;242(23):5535–5541. [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Ketelslegers J. M., Knott G. D., Catt K. J. Kinetics of gonadotropin binding by receptors of the rat testis. Analysis by a nonlinear curve-fitting method. Biochemistry. 1975 Jul 15;14(14):3075–3083. doi: 10.1021/bi00685a006. [DOI] [PubMed] [Google Scholar]
- Khac L. D., Harbon S., Clauser H. J. Intracellular titration of cyclic AMP bound to receptor proteins and correlation with cyclic-AMP levels in the surviving rat diaphragm. Eur J Biochem. 1973 Dec 3;40(1):177–185. doi: 10.1111/j.1432-1033.1973.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Korenman S. G., Bhalla R. C., Sanborn B. M., Stevens R. H. Protein kinase translocation as an early event in the hormonal control of uterine contraction. Science. 1974 Feb 1;183(4123):430–432. doi: 10.1126/science.183.4123.430. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Butcher R. W., Savard K., Sutherland E. W. The stimulatory effect of luteinizing hormone on adenosine 3',5'-monophosphate accumulation in corpus luteum slices. J Biol Chem. 1966 Nov 25;241(22):5436–5440. [PubMed] [Google Scholar]
- Mendelson C., Dufau M., Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3':5'-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975 Nov 25;250(22):8818–8823. [PubMed] [Google Scholar]
- Podesta E. J., Dufau M. L., Catt K. J. Adenosine 3',5'-monophosphate-dependent protein kinase of Leydig cells: in vitro activation and relationship to gonadotropin action upon cyclic AMP and steroidogenesis. FEBS Lett. 1976 Nov;70(1):212–216. doi: 10.1016/0014-5793(76)80760-0. [DOI] [PubMed] [Google Scholar]
- Podesta E. J., Dufau M. L., Catt K. J. Characterization of two forms of cyclic 3', 5'-adenosine monophosphate-dependent protein kinase in rat testicular interstitial cells. Mol Cell Endocrinol. 1976 Jun-Jul;5(1-2):109–122. doi: 10.1016/0303-7207(76)90074-5. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Ewing L. L., Williams-Ashman H. G. Protein phospholinase reactions in mammalian testis. Stimulatory effects of adenosine 3':5'-cyclic monophosphate on the phosphorylation of basic proteins. Biochem J. 1971 Apr;122(3):333–345. doi: 10.1042/bj1220333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tao M., Salas M. L., Lipmann F. Mechanism of activation by adenosine 3':5'-cyclic monophosphate of a protein phosphokinase from rabbit reticulocytes. Proc Natl Acad Sci U S A. 1970 Sep;67(1):408–414. doi: 10.1073/pnas.67.1.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. A. Cyclic AMP formation and thyroid secretion by incubated mouse thyroid lobes. Endocrinology. 1972 Dec;91(6):1411–1417. doi: 10.1210/endo-91-6-1411. [DOI] [PubMed] [Google Scholar]


