Abstract
Risk of relapse during the unrelated donor coordination period biases comparisons between allogeneic hematopoietic stem cell transplantation from an HLA 8 of 8 allele-matched unrelated donor (8/8 MUD) and that from a related donor with an HLA-1 antigen mismatch in the graft-versus-host (GVH) direction (RD/1AGMM-GVH). To reduce this bias, we performed a decision analysis focusing on acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in first complete remission (CR1). The primary outcome measure was 5-year survival probability with or without quality-of-life (QOL) adjustment. A baseline analysis showed that the decision to perform MUD transplantation was superior to that to perform RD/1AGMM-GVH transplantation for patients with AML or ALL. However, in the ALL cohort, the direction of superiority was reversed when the interval between CR1 and 8/8 MUD transplantation was >5.5 months (without QOL adjustment) or >6 months (after QOL adjustment) or when overall survival of RD/1AGMM-GVH transplantation improved by 1.3% without QOL adjustment and 2.1% after QOL adjustment. In conclusion, 8/8 MUD should be prioritized in transplantation for AML and ALL in CR1. However, the MUD coordination period and improvements in RD/1AGMM-GVH transplantation might change the donor selection priority in transplantation for ALL in CR1.
Introduction
An HLA allele-matched unrelated donor (MUD) is considered to be the most appropriate alternative donor in allogeneic hematopoietic stem cell transplantation (HSCT) for patients who lack an HLA-identical sibling. However, it is difficult to find an MUD for patients with rare HLA alleles or haplotypes. A partially HLA-mismatched related donor is an attractive potential alternative donor, as such donors are easier to access and transplantation can be performed at an appropriate time for disease control. The outcomes of HSCT from a related donor with a 1-antigen mismatch at the HLA-A, HLA-B or HLA-DR locus have previously been shown to be comparable to those of HSCT from a matched related donor (MRD) in patients with high-risk diseases.1, 2, 3 This is due to the reduction in the risk of relapse via a graft-versus-leukemia (GVL) effect with an acceptable risk of acute graft-versus-host disease (GVHD). The outcome of HSCT from a 1-antigen mismatched related donor was also comparable to that of HSCT from an antigen-MUD in patients with either standard- or high-risk disease.1 In our recent retrospective comparison, however, we showed that the outcomes with an 8 of 8 allele-MUD (8/8 MUD) were superior to those with a related donor with an HLA-1 antigen mismatch in the graft-versus-host (GVH) direction (RD/1AGMM-GVH) in transplantation for leukemia.4
The coordination period from the start of donor search application until the actual receipt of grafts takes a median of 5 months in Japan (3.3 months in the US). The risk of relapse during the unrelated donor coordination period considerably biases comparisons between unrelated and related transplantation, even if such confounding factors are considered in multivariate analyses. Patients who have an early relapse after achieving a first complete remission (CR1) during the coordination period are excluded from analyses of HSCT for leukemia in CR1. Thus, patients with leukemia in CR1 in the MUD group are patients with good disease control who have maintained CR for several months. Although randomized trials would be ideal for controlling such bias, it is practically difficult to perform prospective clinical trials in which patients with leukemia in CR1 are randomly assigned to receive HSCT from an 8/8 MUD or RD/1AGMM-GVH. To reduce the bias, inherent to donor selection, such as that owing to the donor coordination period we performed a ‘decision analysis' in allogeneic HSCT focusing on acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in CR1. A decision analysis is a statistical technique that aids the clinical decision-making process under conditions of uncertainty. With this method, we can consider various factors including the risk of relapse during the donor coordination period and the decrease in quality of life (QOL) resulting from chronic GVHD.
Materials and methods
Model structure
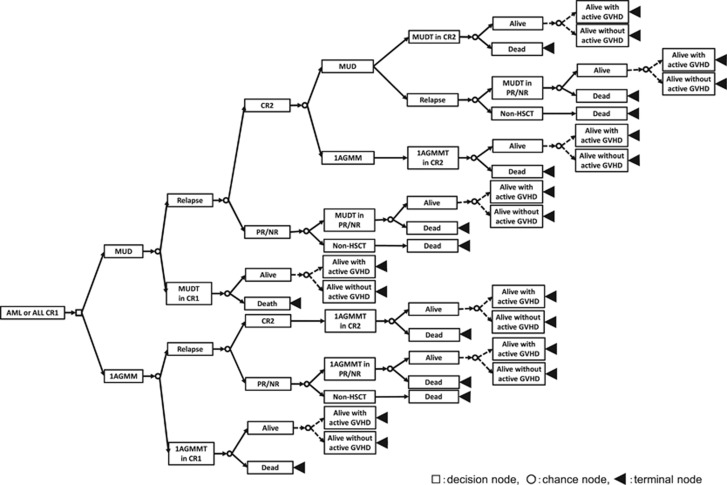
We constructed a decision tree to identify the optimal donor selection for adult patients with AML and ALL in CR1 who lack an HLA-matched sibling, but have a candidate 8/8 MUD (HLA-A, HLA-B, HLA-C and HLA-DRB1 alleles) and RD/1AGMM-GVH (HLA-A, HLA-B and HLA-DR antigens) (Figure 1). At the decision node, we can decide to proceed to HSCT either from an 8/8 MUD or RD/1AGMM-GVH. Each decision is followed by chance nodes that have possible outcomes with transition probabilities and every branch finally ends with terminal nodes that have utilities according to different health states. The sum of the products of the transition probabilities and the utilities of all branches following each chance node become the expected value of each chance node and the expected value of each decision is calculated as the sum of the expected values in all of the chance nodes following each decision. The analyses were performed using TreeAge Pro 2009 software (Williamstown, MA, USA) and Stata version 12 (Stata Corp., College Station, TX, USA). The study design was approved by the Transplant Registry Unified Management Program (TRUMP) Data Management committee and the Institutional Review Board of Saitama Medical Center, Jichi Medical University, where this study was organized.
Figure 1.
Decision tree: donor selection for HLA 8 of 8 allele-MUD or a related donor with an HLA-1 antigen mismatch for AML or ALL in CR1. In analyses with QOL adjustments, ‘Alive' after transplantation was followed by two branches with or without active chronic GVHD. CR, complete remission; HSCT, hematopoietic stem cell transplantation; MUD, HLA 8 of 8 allele-matched unrelated donor; MUDT, MUD transplantation; NR, non remission; PR, partial remission; 1AGMM, a related donor with an HLA-1 antigen mismatch in the graft-versus-host direction; 1AGMMT, 1AGMM transplantation.
Data sources
Data for adult non-M3 AML and Philadelphia chromosome (Ph)-negative ALL patients (⩾16 years) who had received their first allogeneic HSCT from an 8/8 MUD or RD/1AGMM-GVH between 1997 and 2010 were obtained from TRUMP.5 Transplantations that used ex vivo or in vivo T-cell depletion were excluded. Grafts from 8/8 MUDs were exclusively bone marrow, as the donation of peripheral blood by unrelated volunteers was not permitted in Japan until 2011. The characteristics of the patients with AML and ALL in CR1 included in this study are summarized in Table 1. A reduced-intensity conditioning regimen was more frequently used in the RD/1AGMM-GVH group than in the 8/8 MUD group. There was no difference in patient age, sex, GVHD prophylaxis or year at transplantation between the 8/8 MUD and RD/1AGMM-GVH groups. To determine the following transition probabilities, overall survival was estimated using the Kaplan–Meier method. Probabilities that we could not estimate from these data were estimated from the literature as described below.
Table 1. Characteristics of patients with acute leukemia in CR1.
| Variable |
AML CR1 |
P-value |
ALL CR1 |
P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
8/8 MUD |
RD/1AGMM-GVH |
8/8 MUD |
RD/1AGMM-GVH |
|||||||
| n=458 | % | n=86 | % | n=381 | % | n=57 | % | |||
| Age at transplant, median (range) | 40 | 16–69 | 43 | 16–67 | 0.706 | 35 | 16–65 | 37 | 16–63 | 0.926 |
| Recipient sex | ||||||||||
| Female | 198 | 43 | 36 | 42 | 0.814 | 161 | 42 | 28 | 49 | 0.329 |
| Male | 260 | 57 | 50 | 58 | — | 220 | 58 | 29 | 51 | — |
| Source of stem cells | ||||||||||
| Bone marrow | 458 | 100 | 51 | 59 | — | 381 | 100 | 34 | 60 | — |
| Peripheral blood | — | — | 35 | 41 | — | — | — | 23 | 40 | |
| HLA compatibility in the host-versus-graft direction | ||||||||||
| Matched | 458 | 100 | 11 | 13 | — | 381 | 100 | 6 | 11 | — |
| One-antigen mismatch | — | — | 65 | 76 | — | — | — | 41 | 72 | — |
| Two-antigen mismatches | — | — | 9 | 10 | — | — | — | 6 | 11 | — |
| Three-antigen mismatches | — | — | 1 | 1 | — | — | 4 | 7 | — | |
| HLA mismatch in graft-versus-host direction | ||||||||||
| HLA-A or HLA-DR | — | — | 69 | 80 | — | — | — | 41 | 72 | — |
| HLA-B | — | — | 17 | 20 | — | — | — | 16 | 28 | — |
| Conditioning regimen | ||||||||||
| Myeloablative | 376 | 82 | 51 | 59 | <0.001 | 339 | 89 | 41 | 72 | <0.001 |
| Reduced intensity | 68 | 15 | 23 | 27 | — | 39 | 10 | 8 | 14 | — |
| Unclassifiable | 14 | 3 | 12 | 14 | — | 3 | 1 | 8 | 14 | — |
| GVHD prophylaxis | ||||||||||
| Cyclosporine based | 164 | 36 | 31 | 36 | 0.966 | 158 | 41 | 24 | 42 | 0.928 |
| Tacrolimus based | 294 | 64 | 55 | 64 | — | 223 | 59 | 33 | 58 | — |
| Year at transplant | ||||||||||
| 1997–2004 | 188 | 41 | 37 | 43 | 0.733 | 170 | 45 | 23 | 40 | 0.545 |
| 2005–2010 | 270 | 59 | 49 | 57 | — | 211 | 55 | 34 | 60 | — |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CR1, first complete remission; RD/1AGMM-GVH, related donor with an HLA-1 antigen mismatch in the graft-versus-host direction; 8/8 MUD, HLA 8 of 8 allele-matched unrelated donor.
Transition probabilities and utilities
Transition probabilities were determined as summarized in Table 2. Each transition probability has a baseline value and a plausible range. Baseline decision analyses were performed using baseline values.
Table 2. Transition probabilities of the overall population for AML and ALL.
| Transition probabilities |
Baseline value (plausible range) |
|
|---|---|---|
| AML | ALL | |
| Achievement of CR2 with chemotherapy after relapse in CR1 | 0.50 (0.47–0.53) | 0.53 (0.46–0.59) |
| DFS after CR1 during RD/1AGMM coordination | 0.94 (0.92–0.96) | 0.95 (0.91–0.97) |
| Alive at 5 years following HSCT from RD/1AGMM in CR1 | 0.44 (0.32–0.56) | 0.56 (0.41–0.68) |
| Alive at 5 years following HSCT from RD/1AGMM in CR2 | 0.44 (0.26–0.61) | 0.42 (0.17–0.65) |
| Alive at 5 years HSCT from RD/1AGMM in non-CR | 0.15 (0.07–0.26) | 0.12 (0.03–0.27) |
| Choice of HSCT from RD/1AGMM in non-CR | 0.40 (0.36–0.45) | 0.40 (0.36–0.45) |
| DFS after CR1 during MUD coordination | 0.85 (0.75–0.92) | 0.86 (0.77–0.91) |
| Alive at 5 years following HSCT from MUD in CR1 | 0.64 (0.58–0.69) | 0.61 (0.55–0.67) |
| Alive at 5 years following HSCT from MUD in CR2 | 0.53 (0.45–0.60) | 0.41 (0.29–0.52) |
| Alive at 5 years following HSCT from MUD in non-CR | 0.17 (0.12–0.23) | 0.13 (0.07–0.21) |
| Choice of HSCT from MUD in non-CR | 0.40 (0.36–0.45) | 0.40 (0.36–0.45) |
| Choice of HSCT from RD/1AGMM following relapse and CR2 after the decision of HSCT from MUD | 0.50 (0.40–0.60) | 0.50 (0.40–0.60) |
| DFS after CR2 during MUD coordination | 0.85 (0.75–0.92) | 0.86 (0.77–0.91) |
| active chronic GVHD following HSCT from RD/1AGMM | 0.23 (0.12–0.35) | 0.23 (0.12–0.35) |
| active chronic GVHD following HSCT from MUD | 0.19 (0.09–0.28) | 0.19 (0.09–0.28) |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CR, complete remission; DFS, disease-free survival; HSCT, hematopoietic stem cell transplantation; MUD, matched unrelated donor; RD/1AGMM, related donor with HLA-1 antigen mismatch in the graft-versus-host direction; 8/8 MUD, HLA 8 of 8 allele-MUD.
Patients may have been precluded from undergoing HSCT in CR1 due to early relapse or comorbidities even if they had decided to undergo HSCT and therefore the transition probability of actually undergoing HSCT in CR1 after the decision branch to undergo HSCT was determined as follows. First, the median duration between the achievement of CR1 and related or unrelated HSCT without relapse was set as 2 months or 5 months, respectively, based on the unrelated donor coordination period reported from the Japan Marrow Donor Program. Next, disease-free survival (DFS) rates after achieving CR1 during the coordination period for 8/8 MUD or RD/1AGMM-GVH were estimated using published data for AML and ALL patients who achieved CR1 in studies by the Japan Adult Leukemia Study Group.6, 7 As DFS for Ph-negative and Ph-positive ALL patients were not reported separately in the Japan Adult Leukemia Study Group's study,6 DFS for Ph-negative ALL patients was estimated from that for the combined patients, referring to the DFS rates for Ph-negative and Ph-positive ALL patients during the same era in another study.8 The rates of achievement of CR2 with chemotherapy after relapse in CR1 were retrieved from another nationwide study.9, 10 As DFS, after achieving CR2 during the coordination period for 8/8 MUD was not available in the registry or published data, we applied the DFS after achieving CR1 during the coordination period for 8/8 MUD. The transition probability values for ‘Alive at 5 years' following HSCT from an 8/8 MUD or RD/1AGMM-GVH for AML/ALL in various disease statuses were determined based on the TRUMP data. We assigned 95% confidence intervals (CIs) to the plausible ranges for the sensitivity analyses. The probability of proceeding to HSCT for AML/ALL in non-CR was assigned a baseline value of 0.40 and 95% CI to the plausible range after referencing a previous study on AML.9 We could not obtain a transition probability of selecting an 8/8 MUD or RD/1AGMM-GVH for AML/ALL in CR2 after relapse following an initial decision of 8/8 MUD. Therefore, a baseline value of 0.5 was assigned with a wide plausible range (0.30–0.70).
Utilities were calculated based on a 5-year survival probability, which was the primary outcome measure, with or without adjusting for QOL. The survival curve nearly reaches a plateau after 5 years, and therefore ‘Alive at 5 years' reflects ‘Cure of leukemia', which is the primary goal of HSCT. In an analysis without an adjustment for QOL, we considered only two kinds of health states, ‘Alive at 5 years' and ‘Dead', and assigned utility values of 100 to the former and 0 to the latter. On the other hand, in an analysis with an adjustment for QOL, ‘Alive without active chronic GVHD' and ‘Alive with active chronic GVHD' were considered different health states. No data were available on the rates of active GVHD at 5 years in either the 8/8 MUD or RD/1AGMM-GVH groups. To estimate the rate of active GVHD, first we calculated the cumulative incidence of chronic GVHD for patients in the 8/8 MUD and RD/1AGMM-GVH groups. The cure rate of chronic GVHD was set at 0.60 with a wide plausible range (0.40–0.80). We assigned a value of 100 to the utility for being alive without active chronic GVHD, and a value of 0 to the utility for being dead. We assigned a value of 70 to the utility for being alive with active chronic GVHD, with a plausible range of 0–100. Accordingly, we calculated a total of utilities for alive after HSCT from an 8/8 MUD or RD/1AGMM-GVH.
Subgroup analyses were performed in patients aged <40 years and ⩾40 years, and in patients who received a myeloablative conditioning regimen. A subgroup analysis for patients who received a reduced-intensity conditioning regimen was not performed because of the small number of such patients in the registry data. The RD/1AGMM-GVH group was also stratified according to the mismatch antigen. Transition probabilities were recalculated using the registry data for patients in each subgroup (Supplementary table 1).
Sensitivity analyses
To evaluate the robustness of the decision model, we performed one-way sensitivity analyses for all transition probabilities, in which the decision tree was recalculated by varying each transition probability value across its plausible range and confirmed whether the decision of the baseline analyses changed. In analyses with an adjustment for QOL, the utility for being alive after HSCT from an 8/8 MUD or RD/1AGMM-GVH was also subjected to a one-way sensitivity analysis.
We also performed a probabilistic sensitivity analysis using a Monte Carlo simulation,11 in which the uncertainties of all transition probabilities were considered simultaneously. The distribution of the random variables for each transition probability was determined to follow a normal distribution, with 95% of the random variables included in the plausible range. One thousand simulations were performed based on the decision tree, and the mean and s.d. of the differences of expected value between two decisions were calculated.
Results
Baseline analysis
AML in CR1
The baseline analysis in the AML population without adjusting for QOL revealed that the expected 5-year survival probability for the decision to perform HSCT from an 8/8 MUD was superior to that for the decision to perform HSCT from an RD/1AGMM-GVH (60.2% vs 43.6%, Table 3). This superiority was unchanged after adjusting for QOL (56.7% vs 40.6%).
Table 3. Expected 5-year survival probabilities with and without adjustment for QOL.
|
Expected survival probability without a QOL adjustment |
Expected survival probability with a QOL adjustment |
|||
|---|---|---|---|---|
| MUD | RD/1AGMM-GVH | MUD | RD/1AGMM-GVH | |
| AML | ||||
| All patients | 60.2 | 43.6 | 56.7 | 40.6 |
| HLA-A/-DR mismatch | 60.5 | 43.8 | 57.1 | 40.7 |
| HLA-B mismatch | 59.7 | 44.4 | 56.3 | 41.3 |
| Myeloablative conditioning | 59.4 | 44.7 | 56.0 | 41.6 |
| <40 years old | 61.8 | 59.2 | 58.3 | 55.1 |
| ⩾40 years old | 58.7 | 32.0 | 55.3 | 29.8 |
| ALL | ||||
| All patients | 55.7 | 54.4 | 52.5 | 50.7 |
| HLA-A/-DR mismatch | 55.8 | 61.1 | 52.6 | 56.9 |
| HLA-B mismatch | 55.1 | 35.1 | 51.9 | 32.6 |
| Myeloablative conditioning | 56.5 | 52.4 | 53.2 | 48.8 |
| <40 years old | 57.6 | 60.7 | 54.3 | 56.5 |
| ⩾40 years olda | — | — | — | — |
Abbreviations: ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; MUD, matched unrelated donor; QOL, quality of life; RD/1AGMM-GVH, related donor with an HLA-1 antigen mismatch in the graft-versus-host direction; 8/8 MUD, HLA 8 of 8 allele-MUD.
Expected survival probability could not be calculated due to insufficient information for outcomes of transplantation for ALL in CR2 and non-CR.
ALL in CR1
Similar to AML in CR1, the expected 5-year survival probability for the decision to perform HSCT from an 8/8 MUD was superior to that for the decision to perform HSCT from an RD/1AGMM-GVH, regardless of whether QOL adjustment was performed (55.7% vs 54.4% without QOL adjustment and 52.5% vs 50.7% after QOL adjustment, Table 3).
Sensitivity analysis
AML in CR1
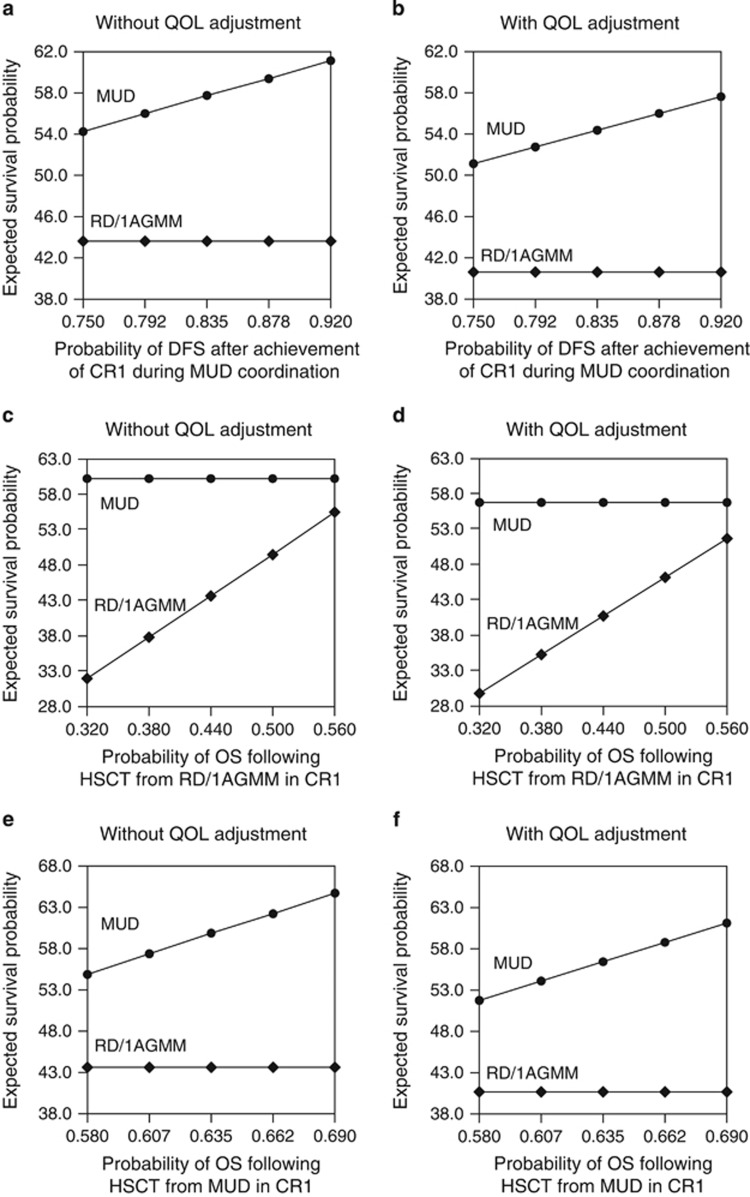
We performed one-way sensitivity analyses for all transition probabilities in the decision model of AML in CR1. Regardless of QOL adjustment, the expected survival probability for the decision to perform HSCT from an 8/8 MUD was consistently superior in transition probabilities within the plausible ranges of various factors, including the interval between achieving CR1 and actually receiving transplantation (Figure 2).
Figure 2.
One-way sensitivity analysis for the acute myeloid leukemia cohort. The results of the one-way analysis are shown without or with quality of life (QOL) adjustments according to disease-free survival (DFS) after the achievement of first complete remission (CR1) during HLA 8 of 8 allele-matched unrelated donor (8/8 MUD) coordination (a and b), 5-year overall survival (OS) following hematopoietic stem cell transplantation (HSCT) from the related donor with an HLA-1 antigen mismatch in the graft-versus-host direction (RD/1AGMM) group (c and d) and the 8/8 MUD group (e and f).
In the probabilistic sensitivity analysis, the expected survival probabilities for the decision to perform HSCT from an 8/8 MUD was higher than HSCT from an RD/1AGMM-GVH in 997 of 1000 simulations without QOL adjustment (mean of increments 16.7% and s.d. 6.1%) and in 997 of 1000 simulations with QOL adjustment (mean of differences 16.3% and s.d. 5.9%) (Figure 3).
Figure 3.
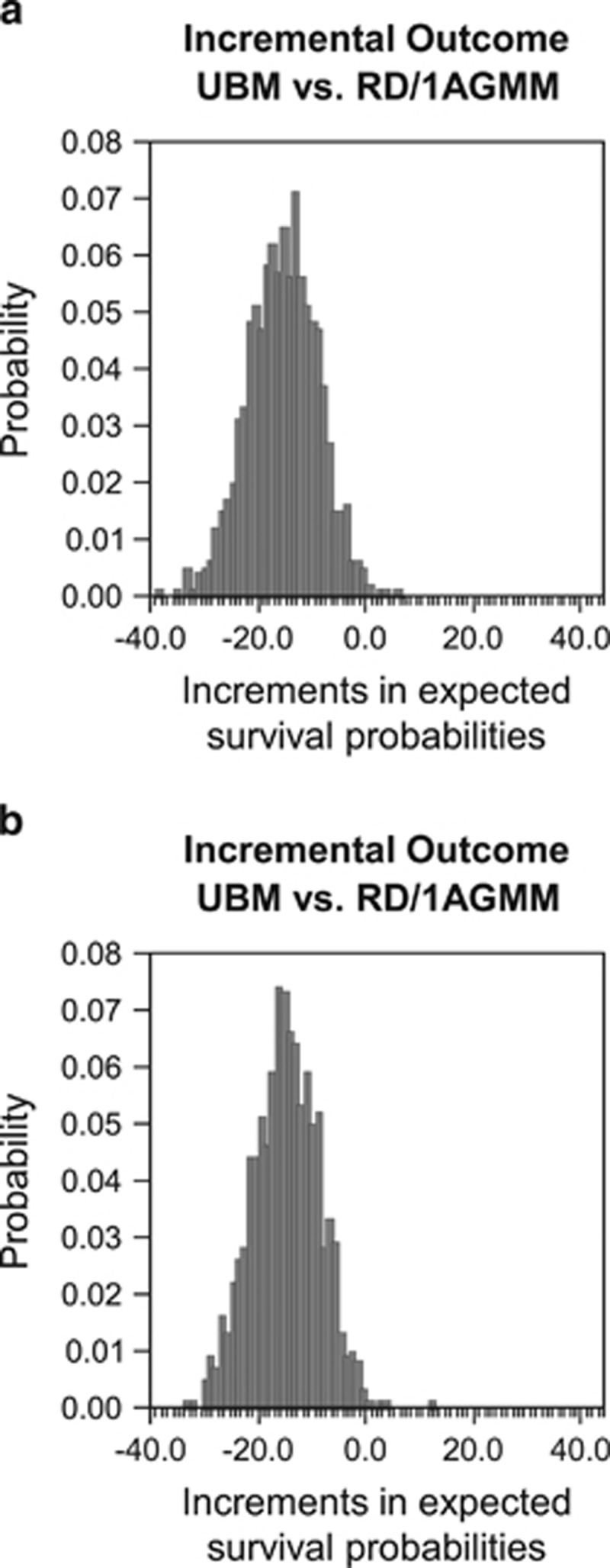
Probabilistic sensitivity analysis using a Monte Carlo simulation for the AML cohort. A probabilistic sensitivity analysis was performed for AML without (a) or with QOL adjustments. (b) Distributions of increments in the expected survival probabilities (MUD vs RD/1AGMM) in all simulations are shown. MUD, HLA 8 of 8 allele-matched unrelated donor; MV, mean value; RD/1AGMM, related donor with an HLA-1 antigen mismatch in the GVH direction.
ALL in CR1
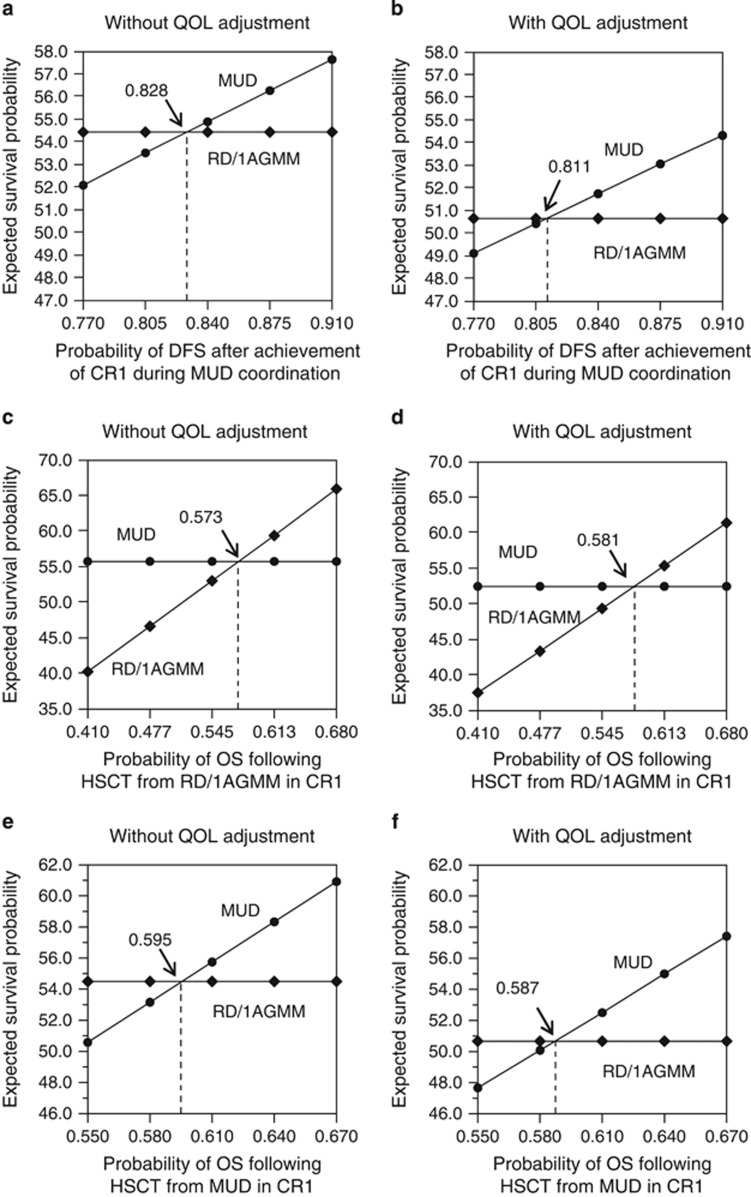
In the one-way sensitivity analyses, the decision models both with and without adjusting for QOL were sensitive to the interval between CR1 and 8/8 MUD transplantation and the 5-year survival rate in transplantation from an 8/8 MUD and an RD/1AGMM-GVH. A superior expected survival probability for the decision to perform HSCT from an RD/1AGMM-GVH was obtained when DFS after CR1 at MUD transplantation was <82.8% (synonymous with when the interval between CR1 and 8/8 MUD transplantation was >5.5 months) in the analysis without QOL adjustment and <81.1% (synonymous with when the interval was >6 months) after QOL adjustment (Figure 4a and b). The direction of the superiority was also reversed in favor of RD/1AGMM-GVH selection when the 5-year survival rate in transplantation using an RD/1AGMM-GVH was improved by 1.3% (2.1% after QOL adjustment) (Figure 4c and d) or when the 5-year survival rate in transplantation using an 8/8 MUD was decreased by 1.5% (2.3% after QOL adjustment) (Figure 4e and f).
Figure 4.
One-way sensitivity analysis for the ALL cohort. The results of one-way analysis are shown without or with quality-of-life (QOL) adjustments according to disease-free survival (DFS) after the achievement of first complete remission (CR1) during HLA 8 of 8 allele-matched unrelated donor (MUD) coordination (a and b), 5-year overall survival (OS) following hematopoietic stem cell transplantation (HSCT) from a related donor with an HLA-1 antigen mismatch in the graft-versus-host direction (RD/1AGMM) group (c and d) and the 8/8 MUD group (e and f).
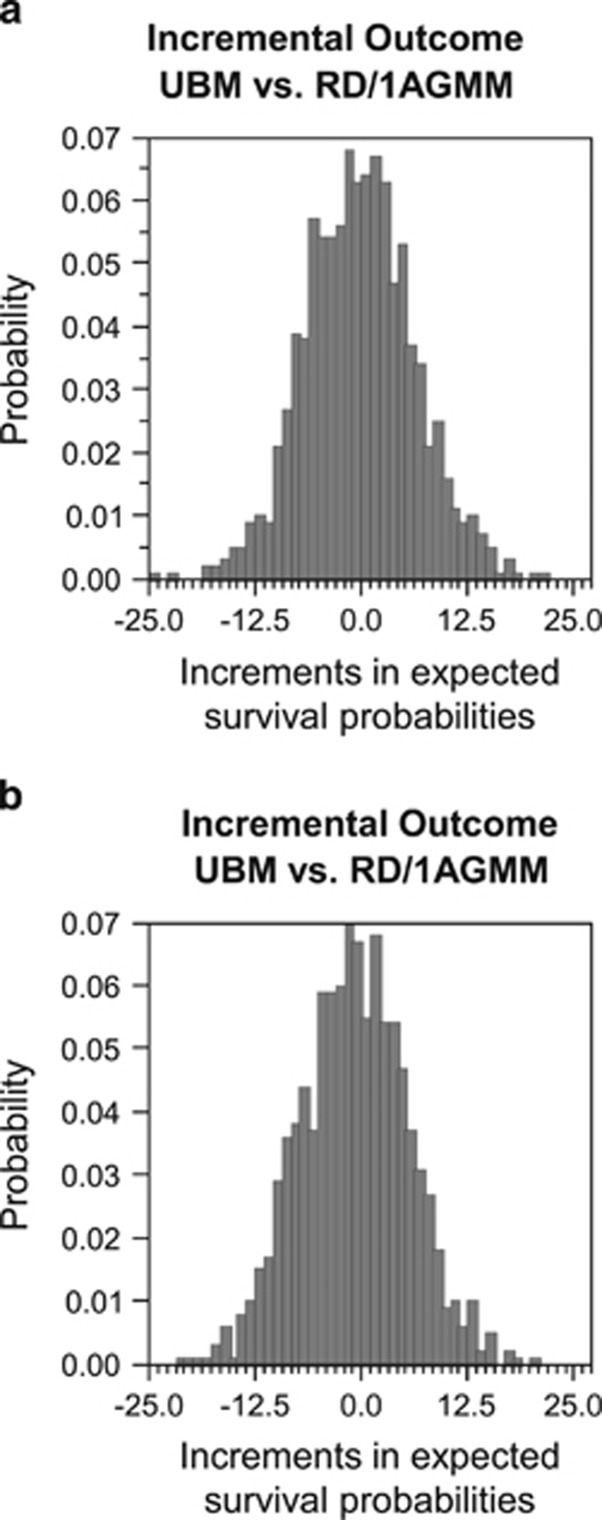
In the probabilistic sensitivity analysis, the expected survival probabilities for the decision to perform HSCT from an 8/8 MUD was higher than HSCT from an RD/1AGMM-GVH in 588 of 1000 simulations without QOL adjustment (mean of increments 1.3% and s.d. 6.1%) and in 618 of 1000 simulations with QOL adjustment (mean of differences 1.9% and s.d. 6.1%) (Figure 5).
Figure 5.
Probabilistic sensitivity analysis using a Monte Carlo simulation for the ALL cohort. A probabilistic sensitivity analysis was performed for ALL without (a) or with quality-of-life (QOL) adjustments (b). Distributions of increments in the expected survival probabilities (MUD vs RD/1AGMM) in all the simulations are shown. MUD, HLA 8 of 8 allele-matched unrelated donor; MV, mean value; RD/1AGMM, related donor with an HLA-1 antigen mismatch in the graft-versus-host direction.
Subgroup analysis
AML in CR1
The expected survival probability in the 8/8 MUD group was consistently superior to that in the RD/1AGMM-GVH group in all subgroup analyses (Table 3).
ALL in CR1
When the RD/1AGMM-GVH group was restricted to donors with an HLA-A or HLA-DR antigen mismatch, the expected survival probability in the RD/1AGMM-GVH group was superior to that in the 8/8 MUD group in the baseline analysis, regardless of QOL adjustment (Table 3). In patients aged <40 years, the expected survival probability in the RD/1AGMM-GVH group was also superior to that in the 8/8 MUD group, regardless of QOL adjustment (Table 3).
Discussion
An 8/8 MUD is considered to be the best alternative donor for patients without an HLA-identical sibling, as the outcomes after HSCT from an 8/8 MUD are almost comparable to those after HSCT from an HLA-identical sibling.4, 12, 13 However, the substantial risk of relapse that exists during the MUD coordination period is a disadvantage of MUD transplantation. Simple retrospective comparisons of outcomes after HSCT with 8/8 MUD and related donors for patients with leukemia in CR1 are biased, as patients who have an early relapse after achieving CR1 during the 8/8 MUD coordination period are excluded from the analyses. In the present study, to minimize the potential for this bias, we performed a decision analysis to determine the optimal donor choice for patients with AML and ALL in CR1 who lack an HLA-identical sibling but have a candidate 8/8 MUD and RD/1AGMM-GVH.
A baseline analysis showed that the decision to perform MUD transplantation was superior to the decision to perform RD1AGMM-GVH transplantation for patients with either AML or ALL in CR1. Further, sensitivity analyses supported the robustness of the superiority of the decision to perform MUD transplantation for patients with AML in CR1. On the other hand, this superiority was reversed in patients with ALL in CR1 when the interval between CR1 and 8/8 MUD transplantation was long (>5.5 months without QOL adjustment and >6 months after QOL adjustment). The decision model was also sensitive to the overall survival rates after transplantation from an MUD or RD/1AGMM-GVH in patients with ALL in CR1. At least a 1.3% improvement in overall survival after HSCT for ALL in CR1 is required to proceed to HSCT from an RD/1AGMM-GVH instead of an 8/8 MUD. In a previous study that compared RD/1AGMM-GVH and umbilical cord blood transplantation for leukemia,14 we showed that the occurrence of severe acute GVHD increased nonrelapse mortality after RD/1AGMM-GVH transplantation, whereas the use of in vivo T-cell depletion, mostly with antithymocyte globulin (ATG), significantly decreased the incidence of severe acute and extensive chronic GVHD, to a level comparable to that after umbilical cord blood transplantation. This provided the potential for an improvement in overall survival after RD/1AGMM-GVH transplantation. The establishment of a conditioning regimen that includes ATG for an RD/1AGMM-GVH transplantation might make the decision to perform HSCT from RD/1AGMM-GVH more favorable than the decision to perform HSCT from an 8/8 MUD. Furthermore, the use of ATG-containing regimens for HSCT from an RD1AGMM-GVH will lead to QOL outcomes comparable to those after HSCT from an 8/8 MUD.
We have previously shown that HSCT from an RD/1AGMM-GVH involving an HLA-B mismatch was associated with significantly higher nonrelapse mortality and lower overall survival in standard-risk leukemia, probably owing to an additional HLA-C antigen or allele mismatch in the HLA-B mismatched group.4 In the subgroup analysis according to the presence or absence of an HLA-B mismatch in patients with ALL in CR1, we showed that the decision to perform HSCT from an RD/1AGMM-GVH involving an HLA-A or HLA-DR mismatch was more favorable than the decision to perform HSCT from an MUD in the baseline analysis, regardless of QOL adjustment. We also performed separate analyses according to patient age, as age is a significant confounding factor associated with survival. In patients <40 years of age with ALL in CR1, the decision to perform HSCT from an RD/1AGMM-GVH was more favorable than the decision to perform HSCT from an MUD, regardless of QOL adjustment. Even in the AML cohorts, the expected 5-year survival probabilities for the decision to perform HSCT from an MUD or RD/1AGMM-GVH were almost comparable. This suggests that younger adults may better tolerate the adverse effect of severe acute GVHD and GVHD-related complications after RD/1AGMM-GVH transplantation.
The reason for the differences in the effects of donor selection between the AML and ALL populations is unclear. The differences may be partly owing to differences in treatment protocols for AML and ALL chemotherapy, or differences in the etiologies of disease relapse between AML and ALL. Prospective studies are needed to determine the exact reasons for these differences. Several limitations of this study should also be noted. First, the small number of patients undergoing HSCT from an RD/1AGMM-GVH and the use of heterogeneous conditioning regimens and GVHD prophylaxis regimens may have biased the results. Second, some transition probabilities could not be retrieved, although the percentages for transition probability were mostly obtained from large prospective published studies and national registry data in Japan. In the present study, we performed sensitivity analyses using wide plausible ranges to limit biases arising from the arbitrary assignment of transition probabilities.
In conclusion, an 8/8 MUD should be prioritized in transplantation for AML and ALL in CR1. However, in transplantation for ALL in CR1, an RD/1AGMM-GVH should be prioritized when the interval between CR1 and 8/8 MUD transplantation is expected to be long. The selection of RD/1AGMM-GVH involving HLA-A or HLA-DR mismatch or for patients aged <40 years would also provide superior outcomes. Improvements in outcomes after RD/1AGMM-GVH transplantation may also affect the decision in the direction to prefer HSCT from an RD/1AGMM-GVH donor. RD/1AGMM-GVH transplantation using ATG could potentially improve outcomes, and a prospective study of RD/1AGMM-GVH transplantation using low-dose ATG is ongoing (UMIN000011192).
Acknowledgments
We thank all of the physicians and data managers who provided valuable transplantation data to the Japan Society for Hematopoietic Cell Transplantation (JSHCT), the Japan Marrow Donor Program (JMDP) and the Transplant Registry Unified Management Program (TRUMP). We also thank the members of the Data Management Committees of JSHCT, JMDP and TRUMP for their assistance. We are grateful to Dr Shinichi Kako (Saitama Medical Center, Jichi Medical University) for his helpful advice on the decision analysis. This work was supported in part by the SENSHIN Medical Research Foundation (JK).
Author Contributions
JK and YK designed the research, analyzed the data and performed the statistical analysis. KO, TF, YO, HK, TE, NK, KI, YM, HS and YA gathered the data. JK wrote the first draft of the paper and all of the authors reviewed and approved the final manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Kanda Y, Chiba S, Hirai H, Sakamaki H, Iseki T, Kodera Y, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- Teshima T, Matsuo K, Matsue K, Kawano F, Taniguchi S, Hara M, et al. Impact of human leucocyte antigen mismatch on graft-versus-host disease and graft failure after reduced intensity conditioning allogeneic haematopoietic stem cell transplantation from related donors. Br J Haematol. 2005;130:575–587. doi: 10.1111/j.1365-2141.2005.05632.x. [DOI] [PubMed] [Google Scholar]
- Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990;29:79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- Kanda J, Saji H, Fukuda T, Kobayashi T, Miyamura K, Eto T, et al. Related transplantation with HLA-1 Ag mismatch in the GVH direction and HLA-8/8 allele-matched unrelated transplantation: a nationwide retrospective study. Blood. 2012;119:2409–2416. doi: 10.1182/blood-2011-08-372573. [DOI] [PubMed] [Google Scholar]
- Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, et al. Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP System. Int J Hematol. 2007;86:269–274. doi: 10.1532/IJH97.06239. [DOI] [PubMed] [Google Scholar]
- Miyawaki S, Ohtake S, Fujisawa S, Kiyoi H, Shinagawa K, Usui N, et al. A randomized comparison of 4 courses of standard-dose multiagent chemotherapy versus 3 courses of high-dose cytarabine alone in postremission therapy for acute myeloid leukemia in adults: the JALSG AML201 Study. Blood. 2011;117:2366–2372. doi: 10.1182/blood-2010-07-295279. [DOI] [PubMed] [Google Scholar]
- Jinnai I, Sakura T, Tsuzuki M, Maeda Y, Usui N, Kato M, et al. Intensified consolidation therapy with dose-escalated doxorubicin did not improve the prognosis of adults with acute lymphoblastic leukemia: the JALSG-ALL97 study. Int J Hematol. 2010;92:490–502. doi: 10.1007/s12185-010-0672-z. [DOI] [PubMed] [Google Scholar]
- Thomas X, Boiron JM, Huguet F, Dombret H, Bradstock K, Vey N, et al. Outcome of treatment in adults with acute lymphoblastic leukemia: analysis of the LALA-94 trial. J Clin Oncol. 2004;22:4075–4086. doi: 10.1200/JCO.2004.10.050. [DOI] [PubMed] [Google Scholar]
- Kurosawa S, Yamaguchi T, Miyawaki S, Uchida N, Sakura T, Kanamori H, et al. Prognostic factors and outcomes of adult patients with acute myeloid leukemia after first relapse. Haematologica. 2010;95:1857–1864. doi: 10.3324/haematol.2010.027516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kako S, Kanamori H, Kobayashi N, Shigematsu A, Nannya Y, Nakamae M, et al. Outcome after first relapse in adult patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia. Br J Haematol. 2013;161:95–103. doi: 10.1111/bjh.12225. [DOI] [PubMed] [Google Scholar]
- Doubilet P, Begg CB, Weinstein MC, Braun P, McNeil BJ. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- Kanda J, Ichinohe T, Kato S, Uchida N, Terakura S, Fukuda T, et al. Unrelated cord blood transplantation vs related transplantation with HLA 1-antigen mismatch in the graft-versus-host direction. Leukemia. 2013;27:286–294. doi: 10.1038/leu.2012.203. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.