Abstract
Group B streptococcus [(GBS or Streptococcus agalactiae)] is a leading cause of neonatal meningitis and septicaemia. Most clinical isolates express simultaneously a β-haemolysin/cytolysin and a red polyenic pigment, two phenotypic traits important for GBS identification in medical microbiology. The genetic determinants encoding the GBS haemolysin and pigment have been elucidated and the molecular structure of the pigment has been determined. The cyl operon involved in haemolysin and pigment production is regulated by the major two-component system CovS/R, which coordinates the expression of multiple virulence factors of GBS. Genetic analyses indicated strongly that the haemolysin activity was due to a cytolytic toxin encoded by cylE. However, the biochemical nature of the GBS haemolysin has remained elusive for almost a century because of its instability during purification procedures. Recently, it has been suggested that the haemolytic and cytolytic activity of GBS is due to the ornithine rhamnopolyenic pigment and not to the CylE protein. Here we review and summarize our current knowledge of the genetics, regulation and biochemistry of these twin GBS phenotypic traits, including their functions as GBS virulence factors.
Keywords: Streptococcus agalactiae, Group B streptococcus, GBS, hemolysin, pigment, granadaene
Introduction
Streptococcus agalactiae [Group B streptococcus, (GBS)] is a leading bacterial agent in neonatal infections, an emerging cause of life-threatening infections in adults and an important pathogen in veterinary medicine. In addition to its role as a pathogen, GBS asymptomatically colonizes the lower gastrointestinal and genitourinary tract of up to 30% of healthy human adults (Ewards & Baker, 2010). In the early 1930s, GBS was first recognized as an animal pathogen and the main cause of bovine mastitis (Sherman, 1937; Keefe, 1997). However, despite its isolation from a number of human sources, including the birth canal (Lancefield, 1933; Lancefield & Hare, 1935), GBS was only reported as a human pathogen by Fry (1938) with the description of three fatal cases of puerperal sepsis. Since the early 1960s (Hood et al., 1961) GBS has been considered a leading cause of neonatal infections, associated with sepsis, meningitis and pneumonia (Verani et al., 2010; Edwards & Nizet, 2011; Rodriguez-Granger et al., 2012). GBS emerged recently as a significant pathogen responsible for invasive infections in adults with predisposing underlying diseases such as diabetes and cancer (Farley, 2001; Ewards & Baker, 2010).
In addition to human and bovine infections, GBS has been isolated from animals such as chickens, camels, dogs, dolphins, horse, lizards, cats, fish, frogs, hamsters, mice and monkeys (Garcia et al., 2008). Thus, in contrast to other streptococcal species that display a fairly restricted host spectrum, GBS can cause infections in a wide range of cold- and warm-blooded animals (Kornblatt et al., 1983; Messier et al., 1995; Evans et al., 2008; Delannoy et al., 2012, 2013; Shuster et al., 2013). Although interspecies transmission of GBS strains among animals has not been demonstrated (Garcia et al., 2008), transmission between human and animal species has been suggested recently (Delannoy et al., 2013).
Streptococcal haemolysins
β-Haemolysins are potent exotoxins that play a key role in the virulence of pyogenic streptococci, such as Streptococcus pyogenes [Group A streptococcus, (GAS)], GBS and S. dysgalactiae spp. equisimilis. One of the most noticeable phenotypic characteristics of these species is a zone of β-haemolysis surrounding the colonies when grown on blood agar plates (Ayers & Rupp, 1922). Therefore, β-haemolysis as a phenotypic trait has been widely used for the preliminarily identification of pyogenic streptococcal species in the clinical laboratory (Facklam et al., 1979; Facklam, 2002; Spellerberg & Brandt, 2011). Despite the similar phenotypic appearance of β-haemolysis in most streptococci from the pyogenic group, the molecular details of these β-haemolysins differ considerably.
GAS produce two different haemolytic toxins, streptolysin S (SLS) and streptolysin O (SLO) (Todd, 1938). SLS is a 2.7-kDa bacteriocin-like peptide, oxygen-stable and nonimmunogenic, encoded by the sag gene cluster (Nizet et al., 2000) and responsible for β-haemolysis (Todd, 1938). Its cellular substrates include erythrocytes, leukocytes, platelets and subcellular organelles (Datta et al., 2005; Molloy et al., 2011). SLS-encoding gene clusters are not only present in many streptococcal species (Fuller et al., 2002; Humar et al., 2002; Rato et al., 2011), but have also been found in Staphylococcus aureus, Clostridium botulinum and Listeria monocytogenes (Cotter et al., 2008; Gonzalez et al., 2010) indicating that this toxin is widespread and conserved among gram-positive pathogens. SLO is a 57-kDa protein belonging to the group of thiol-activated cytolysins including listeriolysin O of L. monocytogenes, and perfringolysin O (PFO) of Clostridium perfringens. Based on the high overall degree of similarity in the primary structure, all members of the family are thought to share a common mechanism of action that involves binding to cholesterol-containing membranes (Billington et al., 2000) followed by insertion, oligomerization of 20–80 monomers, and formation of a pore of 20–30 nm diameter (Dramsi & Cossart, 2002). In contrast to SLS, SLO is an immunogenic protein, and antibodies against SLO are useful for documenting recent exposure to GAS (McCormick et al., 2006) or S. dysgalactiae spp. equisimilis (Jansen et al., 1999; Brandt & Spellerberg, 2009).
GBS exhibit two different cytolytic toxins, the β-haemolysin and the CAMP (Christie Atkins Munch-Petersen) factor. The CAMP factor is a heat-stable 226-aa protein which is independent of β-haemolysin and pigment production (Marchlewicz & Duncan, 1980; Tapsall & Phillips, 1987). The CAMP factor is not haemolytic per se, although it can lyse sheep erythrocytes pretreated with staphylococcal sphingomyelinase. It is used as a diagnostic tool in identifying GBS strains (Christie et al., 1944; Rühlmann et al., 1988; Hensler et al., 2008b). The GBS β-haemolysin accounts for the haemolytic phenotype on blood agar plates. Nevertheless, in spite of the wealth of literature referring to its role in virulence and its mechanism of action (Rajagopal, 2009), the biochemical nature of the GBS haemolysin has remained elusive until the publication of a recent report indicating that the haemolysin is not a pore-forming toxin but a rhamnolipid identical to the GBS pigment (Whidbey et al., 2013).
GBS β-haemolysin/cytolysin (β-h/c)
Most human GBS strains produce a surface-associated β-h/c, which plays a key role in GBS pathogenesis. It can target a wide spectrum of cells, and hyperproduction of this haemolysin is associated with fulminant disease in clinical GBS cases as well as severe cases of infection in animal models.
Biological characteristics
The prototypical phenotype of GBS clinical isolates displays a narrow zone of β-haemolysis on blood agar plates (Rotta, 1986). GBS haemolysin is primarily a broad-spectrum cytolysin capable of destroying many eukaryotic cells (Tapsall & Phillips, 1991; Nizet et al., 1996). It is therefore referred to as the GBS β-h/c (Doran et al., 2002). In contrast to other well-characterized streptococcal haemolysins (Nizet, 2002), such as SLS and SLO, not much is known about the molecular details responsible for the membrane alterations (Rajagopal, 2009). However, membrane defects observed as a result of exposure of erythrocytes with a haemolytic GBS wild-type strain appear irregular in shape and exhibit different sizes (Fig. 1), suggesting a mechanism different from a classical pore-forming toxin. The cytolytic activity of the GBS β-h/c was shown to be inhibited by phospholipids such as phosphatidylcholine and phosphatidylethanolamine (Marchlewicz & Duncan, 1980; Ferrieri, 1982; Dal & Monteil, 1983; Tapsall & Phillips, 1991; Fettucciari et al., 2011). This led to the hypothesis that GBS β-h/c could have a similar affinity for phospholipids in the eukaryotic cell membrane guiding the toxin to its site of action (Liu & Nizet, 2006).
Fig 1.
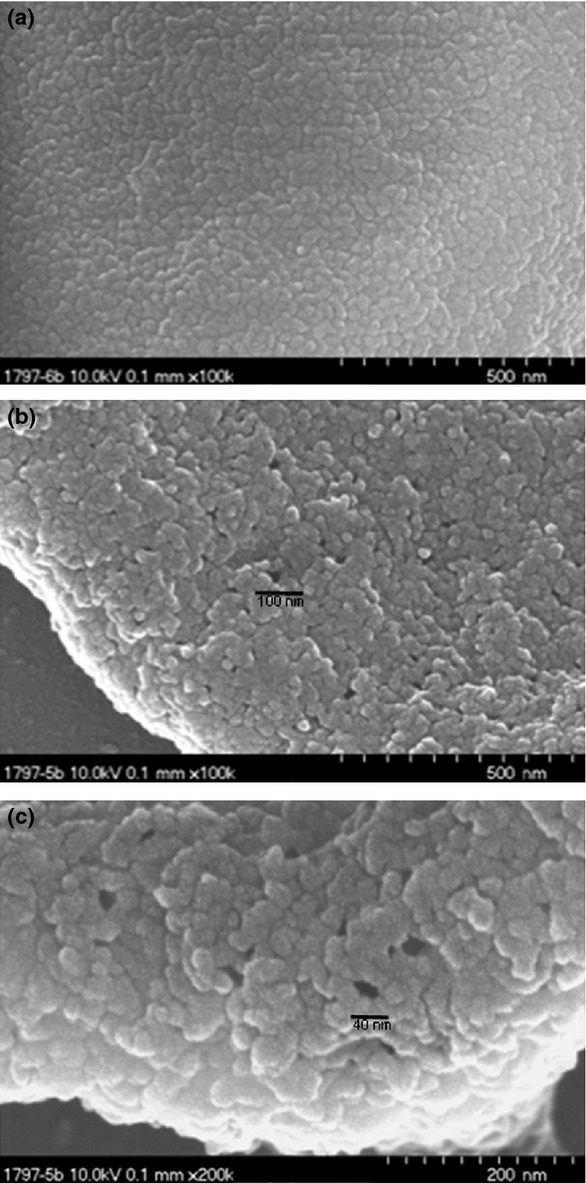
Electron micrographs of human erythrocytes incubated with haemolytic extracts of the β-haemolytic GBS wild-type strain AC450 (b, c) and a nonhaemolytic GBS mutant (a) carrying an ISS1 insertion in the acpC gene of the cyl gene cluster. Preparation of the haemolytic extracts and the generation of the nonhaemolytic mutant strain have been described previously (Spellerberg et al., 1999). A 4% solution of human erythrocytes was incubated with the respective haemolysin extract for 5 min at 17 °C to allow attachment of haemolysin to the erythrocyte membrane, at a temperature at which no haemolysis occurs. Following fixation of the erythrocytes the assay was incubated for 3 min at 37 °C to induce erythrocyte lysis. Images were taken with an Hitachi S 5200 scanning electron microscope at magnifications as indicated.
It has long been assumed that the GBS β-h/c could be a surface-associated protein requiring a direct contact with the target membrane to induce cell lysis (Marchlewicz & Duncan, 1980, 1981; Dal & Monteil, 1983; Platt, 1995; Nizet, 2002; Liu & Nizet, 2004). Haemolytic activity can be extracted from the bacterial surface using molecules such as starch, Tween or bovine serum albumin that act as stabilizer or carrier molecules (Marchlewicz & Duncan, 1980, 1981; Ferrieri, 1982Dal & Monteil, 1983). As the elution profiles of the carrier molecules in gel size-exclusion chromatography did not change in the presence of the haemolysin (Marchlewicz & Duncan, 1980; Tsaihong & Wennerstrom, 1983), it was assumed that GBS haemolysin is a small molecule. As attempts to produce specific antisera in rabbits with a haemolytic preparation obtained after gel exclusion chromatography of crude bacterial surface extracts had been unsuccessful (Dal & Monteil, 1983), the GBS haemolysin was characterized as being a nonimmunogenic substance.
Many attempts to purify and study this elusive cytolysin have failed, raising doubts about its proteinaceous nature (Marchlewicz & Duncan, 1980, 1981; Dal & Monteil, 1983; Tsaihong & Wennerstrom, 1983; Nizet et al., 1997a;). The main obstacles encountered in β-haemolysin purification include rapid loss of activity when stored at room temperature (Tapsall & Phillips, 1991), due to its high thermolability (Marchlewicz & Duncan, 1981; Dal & Monteil, 1983), and the loss of haemolytic activity upon detachment from the carrier molecule (Liu & Nizet, 2004). As most studies have been carried out using complex haemolysin-carriers, an accurate analysis has been hampered, leading to the production of confusing results about the proteinaceous nature of the molecule. The biological characteristics of the GBS haemolysin can be summarized as a broad-range surface-associated nonimmunogenic cytolysin of small molecular size displaying a rapid loss of activity.
The cyl gene cluster
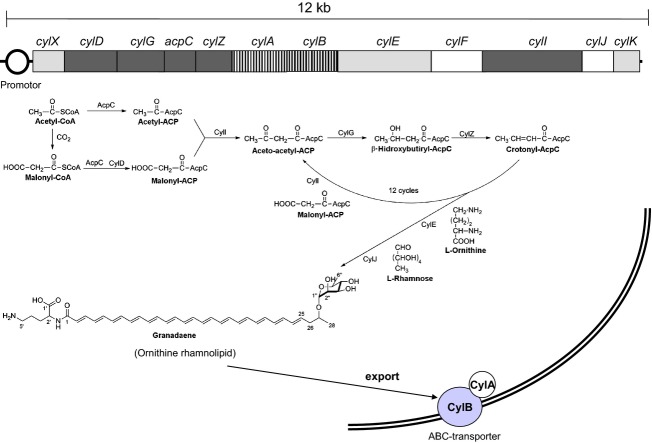
The genes responsible for β-haemolysis of GBS are encoded in the cyl gene cluster Spellerberg et al., 1999, 2000a, b; (Pritzlaff et al., 2001). They were identified by screening transposon mutant libraries of a serotype Ia and a serotype III GBS strain (Spellerberg et al., 1999). Nonhaemolytic mutants were shown to harbour various mutations in a cluster of genes, designated as cyl genes in reference to the cytolytic function of the toxin. The link between these genes and β-haemolysin production was substantiated by analysing naturally occurring nonhaemolytic GBS strains. 1–5% of human GBS isolates are nonhaemolytic (Merrit & Jacobs, 1976; Noble et al., 1983; Reardon et al., 1984; Brimil et al., 2006; Adler et al., 2008; Verani et al., 2010) and often harbour insertion sequences (ISs) in one of the cyl genes (Spellerberg et al., 1999, 2000b; Sigge et al., 2008). The cyl operon, which is made up of 12 genes (cylX, cylD, cylG, acpC, cylZ, cylA, cylB, cylE, cylF, cylI, cylJ, cylK) (Fig. 2), is unique to GBS. The cyl operon was initially linked to haemolytic activity (Spellerberg et al., 1999) and later to pigment production by genetic studies of nonpigmented mutants (Spellerberg et al., 2000b). CylD, CylG, ApcC, CylZ (Spellerberg et al., 1999) and CylI (Spellerberg et al., 2000a; Pritzlaff et al., 2001) display homologies with enzymes of prokaryotic fatty acid biosynthesis: CylD with a malonyl-CoA-ACP transacylase, CylG with a 3-ketoacyl-ACP-reductase, ApcC with an acyl carrier protein and CylZ with FabZ enzymes (fatty acid biosynthesis, 3R-hydroxymyristoyl ACP dehydratase). The genes cylA and cylB encode an ABC (ATP-binding cassette) transporter (Spellerberg et al., 1999). The CylAB transporter displays significant similarities to multidrug resistance (MDR) transporters and can export MDR substrates (Gottschalk et al., 2006): cylX has been predicted to encode an acetyl coenzyme A (CoA) carboxylase; the cylE gene product displays homologies with an N-acetyltransferase; cylF encodes a putative aminomethyltransferase; CylI displays homologies with a 3-ketoacyl-ACP synthase; CylJ displays homologies with with a glycosyltransferase; and cylK encodes a putative phosphopantetheinyl transferase (Spellerberg et al., 1999, 2000a; Pritzlaff et al., 2001; Whidbey et al., 2013). While initial studies found that mutations in different cyl genes led to a loss of haemolytic activity (Spellerberg et al., 1999), a subsequent publication demonstrated that only mutation of cylE invariably resulted in a nonhaemolytic phenotype that could be restored upon complementation (Pritzlaff et al., 2001). In addition, overexpression of cylE in Escherichia coli conferred to the recombinant bacteria the ability to lyse erythrocytes. These results suggested strongly that CylE represented the GBS haemolysin. Attempts to purify and characterize the cylE product, a protein of 78.3 kDa, were unsuccessful, and CylE did not show significant homology to any known pore-forming toxin (Pritzlaff et al., 2001).
Fig 2.
A representation of the 12 genes belonging to the cyl gene cluster of GBS and the theoretical biosynthetic steps toward granadaene formation. The biosynthesis of granadaene should take place by sequential condensation of malonyl-ACP blocs in an iterated cycle of condensation reduction and dehydratation similar to the fatty acid biosynthesis pathway. The cyl operon genes coding for the respective enzymes are shown.
Moreover, the reintroduction of cylE including the adjacent cylA/B in a GBS nonhaemolytic mutant harbouring a deletion of the cyl cluster did not lead to a restoration of the haemolytic phenotype (Whidbey et al., 2013). Today, sequence analysis supports that CylE is an acyl CoA acyltransferase involved in the biosynthesis of the rhamnolipid (Tettelin et al., 2005; Whidbey et al., 2013; http://www.uniprot.org/uniprot/Q3K232-). Furthermore, attempts to confirm the previous report that recombinant expression of cylE in E. coli results in a haemolytic phenotype have failed (Whidbey et al., 2013). These findings show that cylE is necessary but not sufficient for expression of the haemolysin (Gottschalk et al., 2006; Whidbey et al., 2013).
A recent study challenged the idea of CylE as a pore-forming toxin (Whidbey et al., 2013). This result is supported by the fact that no protein could be found in haemolytic cell-free extracts of GBS; extracts were analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) (Whidbey et al., 2013) as well as nuclear magnetic resonance (NMR) spectroscopy (M. Rosa-Fraile, unpublished results). The same study demonstrated also that the haemolytic and cytolytic activity of GBS is due to the ornithine rhamnolipid pigment. Interestingly, analogues of the cyl operon genes, which is absent in other streptococcal genomes, can be found in bacteria such as Bacillus spp., Actinomyces viscosus and Propionibacterium spp. (Whidbey et al., 2013).
Regulation of the cyl operon
The dual nature of GBS – with its ability to shift from a harmless commensal microorganism to a life-threatening pathogen – requires the appropriate regulation of virulence factors in response to different environmental conditions encountered in the host. Regulation of virulence factor expression in bacteria is primarily accomplished by two-component regulatory systems (TCSs) that allow bacteria to adapt to changing environmental conditions (Stock et al., 2000).
A typical TCS consists of a membrane-associated histidine kinase (HK) – with an extracellular input sensor domain and a corresponding cytoplasmic effector domain – and a cytoplasmic response regulator (RR). Specific environmental stimuli provoke a conformational change in the input domain of the HK that causes the activation of its cytoplasmic domain, which autophosphorylates at a specific histidine residue. The phosphate group is then transferred to a specific aspartate residue in the cognate RR. Phosphorylation of the RR controls its activity as a transcriptional activator or repressor of multiple genes and initiates the corresponding cellular responses.
Transcription of the cyl operon is tightly controlled by the TCS, CovS/R (control of virulence) also known as CsrR/S (Csr capsule synthesis regulator). Apart from regulating the expression of the cyl gene cluster, CovS/R controls several other virulence factors (Lamy et al., 2004; Jiang et al., 2005, 2008; Lembo et al., 2010; Cumley et al., 2012; Patras et al., 2013). CovS phosphorylates the regulator CovR at a conserved aspartate residue (Asp53), which allows binding of CovR to a conserved DNA motif in the cyl promoter region repressing cyl expression. Therefore, inactivation of the regulator CovR leads to constitutive overexpression of the cyl operon, resulting in a hyperhaemolytic and hyperpigmented mutant (Lamy et al., 2004; Jiang et al., 2005). These hyperhaemolytic GBS strains have been linked to fulminant GBS infections in humans (Sendi et al., 2009; Whidbey et al., 2013).
Additional regulatory elements were shown to allow the fine-tuning of CovS/R and therefore indirectly control β-haemolysin expression. GBS encodes a single eukaryotic-type membrane-associated serine/threonine kinase Stk1 and its cognate, soluble protein, serine/threonine phosphatase Stp1 (Rajagopal et al., 2003; Burnside et al., 2011). Stp1 phosphorylates CovR on threonine 65, which decreases the phosphorylation of CovR at Asp53 relieving the CovR-mediated repression of the cyl operon. Stk1 positively regulates transcription of β-h/c, which is critical for GBS virulence, and Stk1 mutants produce less β-h/c compared with wild-type strains (Rajagopal et al., 2006; Lin et al., 2009). Stp1 is the cognate phosphatase of Stk1, and indeed Stp1 mutants exhibit several phenotypes such as decreased haemolytic activity, increased autolysis and a reduction in the ability to cause systemic infections (Burnside et al., 2011). Abx1 was recently identified as the third partner of the CovS/R system in GBS through direct interaction with CovS (Firon et al., 2013). RovS, a stand-alone transcriptional regulator, activates the expression of cylE and other genes of the cyl operon through direct binding to the promoter region (Samen et al., 2006). Thus, multiple signals sensed through CovS, Stk1 and Abx1 are integrated via CovS/R to fine-tune haemolysin expression (Firon et al., 2013; Fig. 3).
Fig 3.
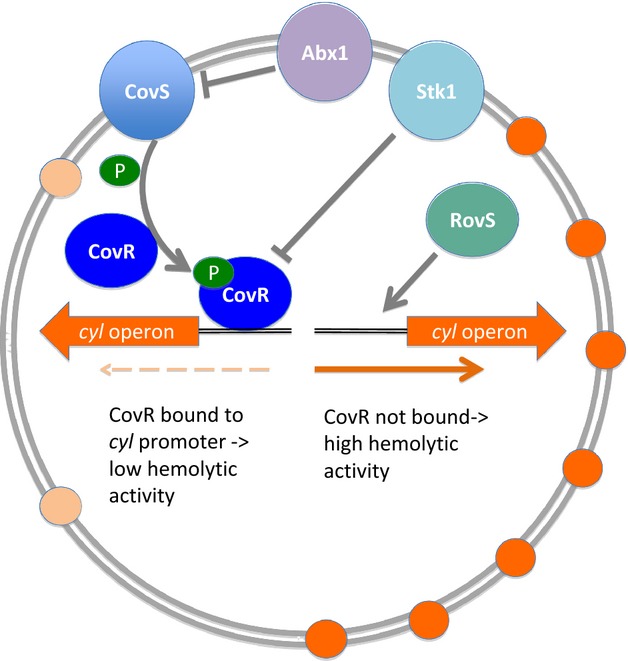
Regulators controlling transcription of the cyl operon. The two-component system CovS/R represents the major regulator of haemolysis and pigmentation in GBS. In wild-type strains the response regulator CovR is phosphorylated through CovS and bound to the cyl promotor region. Binding of CovR results in a repression of cyl gene transcription. This repression is modulated by an inhibition of CovR through the serine threonine kinase Skt1 and an inhibition of CovS through the Abi domain protein Abx1. In addition, the RovS regulator as a stand-alone system can increase haemolysin and pigment expression through binding to the cyl promotor region.
GBS pigment
Approximately 95% of all human GBS isolates produce a characteristic brick-red pigment that is unique among streptococci. Expression of the pigment is always linked to the expression of a key virulence factor, the GBS β-haemolysin encoded by a single genetic locus known as the cyl operon.
Biological characteristics
The production of a orange to brick-red pigment by human GBS strains is a characteristic phenotypic feature reported very early in the literature (Durand & Giraud, 1923; Sherman, 1937; Plummer, 1941). Fallon (1974) proposed the use of pigment detection as a diagnostic tool for GBS identification. This orange, brick or red pigment is unique and highly specific for GBS isolates and is used in the clinical laboratory for the identification of GBS (Fallon, 1974; Merrit & Jacobs, 1976; Merrit et al., 1976; Noble et al., 1983; Rosa-Fraile et al., 1999b; Spellerberg & Brandt, 2011). Nevertheless, pigment production can be variable among bovine strains and other animal species isolates (Mhalu, 1976; Merritt & Jacobs, 1978; Brglez, 1983; Lämmler et al., 1985; Garcia et al., 2008).
In early studies (Merritt & Jacobs, 1978), GBS pigment was shown to exhibit a three-peak UV–visible absorption spectrum at 525, 485 and 455 nm strongly resembling the characteristic spectrum of a carotene with 12 double conjugated bonds (Britton, 1995) and thus suggesting for the first time that the GBS pigment is a carotene (Merritt & Jacobs, 1978). Unlike isoprenoid carotenes, which are lipophilic substances (Schiedt & Liaaen-Jensen, 1995), the GBS pigment cannot be extracted from GBS cultures with organic solvents (Merritt & Jacobs, 1978). In addition, the GBS genome sequences (Glaser et al., 2002; Tettelin et al., 2002, 2005) do not contain homologues of phytoene synthases, phytoene or carotene dehydrogenases and lycopene cyclases, which are common enzymes of the carotene biosynthesis pathway (Sieiro et al., 2003). The characterization of GBS pigment structure was hampered for many years by its strong association to the cell wall (Merritt & Jacobs, 1978). It cannot be extracted using water, physiological saline, HCl, alcoholic KOH, methanol, ethanol, acetone, diethyl ether or petroleum ether (Haug & Soderlund, 1977; Merritt & Jacobs, 1978). Similar to β-haemolysin, the GBS pigment was shown to be released into the culture medium using starch (Merritt & Jacobs, 1978; Tapsall, 1987; Wennestrom et al., 1991).
The chemical structure of the chromophore in the GBS pigment was elucidated using NMR spectroscopy and MS. It was shown to be an ornithine rhamnopolyene named granadaene with a linear chain of 12 unsaturated conjugated bonds (Rosa-Fraile et al., 2006; Paradas et al., 2012) (Fig. 2). The granadaene molecule exhibits some characteristics of an acid–base indicator, and its UV–visible absorption spectrum shifts from a carotene-like spectrum, with three peaks at 525, 485 and 455 nm at low pH (red colour), to a one-peak spectrum of 420 nm (yellow colour) at high pH (Rosa-Fraile et al., 2006). Under certain conditions, for example in the presence of amylase or serum, GBS produces a one-peak pigment, which could explain previous reports suggesting the existence of two different pigments (Tapsall, 1986, 1987; Haug & Soderlund, 1977). However, it remains to be determined whether granadaene represents the full GBS pigment. It is possible that an additional part that attaches the pigment to the cell wall was removed during the stringent conditions [dimethylsulfoxide – trifluoroacetic acid (DMSO-TFA)] used for pigment extraction (Rosa-Fraile et al., 2006).
GBS pigment is a polyene, and the polyene biosynthesis pathway closely resembles that of fatty acid biosynthesis (Schweizer, 1989; Goel et al., 2002). The cyl chromosomal locus encoding haemolysin and pigment production includes several genes (cylD, cylG, cylZ, apcC and cylI) with homology to enzymes involved in fatty acid – and polyene – biosynthesis (Goel et al., 2002; Aparicio et al., 2004). Based on these homologies, a theoretical pathway for the biosynthesis of GBS pigment has been suggested (Whidbey et al., 2013; Fig. 2).
Inhibitors of the folate pathway trigger pigment production in human GBS strains (Rosa et al., 1983, 1992; Schaufuβ et al., 1985; Tapsall, 1987). Methylfolate is a key intermediate in the biosynthesis of thymidine and is the carrier of the hexose in the biosynthesis of rhamnolipids (Pazur & Shuey, 1961; Burger et al., 1963; Ochsner et al., 1994). CylJ displays homology to a glycosyltransferase (Pritzlaff et al., 2001) and presumably encodes the rhamnosyltransferase used in the biosynthesis of granadaene. However, the theoretical biochemical pathway proposed for the biosynthesis of granadaene (Whidbey et al., 2013; Fig. 2) does not account for the pigment-enhancing effect of folate inhibitors. Interestingly, the pigment-enhancing effect of folate antagonists is not seen in most bovine strains (Schaufuβ et al., 1985). This fact, together with the lack of pigment production in a high proportion of these strains (Mhalu, 1976; Merritt & Jacobs, 1978; Brglez, 1983; Lämmler et al., 1985; Garcia et al., 2008), prevents the use of Granada media (Rosa et al., 1992) for the detection of bovine GBS infections.
GBS haemolysin and pigment
More than 30 years ago, GBS pigment and haemolysin were identified as key determinants for GBS pathogenicity. A close link between these two phenotypic traits has been reported for some time but has never been fully explained, until a recent study demonstrating that GBS haemolysin and pigment appear to exhibit, Janus-like, the two faces of a single virulence factor.
Phenotypic linkage of GBS haemolysin and pigment production
A strong link exists between the β-haemolytic phenotype and the production of GBS pigment (Lancefield, 1934; Sherman, 1937; Plummer, 1941). Systematic studies could not identify nonhaemolytic pigmented GBS strains or nonpigmented haemolytic strains (Fallon, 1974; Noble et al., 1983; Tapsall, 1987; Wennestrom et al., 1991). Moreover, the amount of pigment produced by GBS wild-type isolates always correlates with the amount of haemolysin produced (Wennerstrom et al., 1985; Tapsall, 1987; Nizet et al., 1996). Nonhaemolytic mutants isolated from a large GBS mutant library were mapped in the cyl operon and were simultaneously altered in pigment production (Wennerstrom et al., 1985; Nizet et al., 1996; Spellerberg et al., 1999, 2000a; Forquin et al., 2007; Pritzlaff et al., 2001). As a proof that haemolysin and pigment activities are carried by the same molecule, it has been shown that a nonhaemolytic preparation of GBS pigment displays haemolytic activity after the addition of starch (Whidbey et al., 2013).
Pigmentation and haemolysis in veterinary GBS strains
In veterinary GBS isolates nonpigmented strains are frequently reported in addition to a lack of correlation between pigment and haemolysin production (Mhalu, 1976; Islam, 1977; Merritt & Jacobs, 1978; Brglez, 1983; Lämmler et al., 1985; Garcia et al., 2008). This discrepancy may have been caused by the diversity of culture media used to detect pigment and haemolysin (Merrit et al., 1976; Mhalu, 1976; Lämmler et al., 1985), given that media lacking proteose peptone 3 and folate pathway inhibitors (Rosa-Fraile et al., 1999a) may lead to an overestimation of nonpigmented strains. Therefore, a careful re-evaluation of these findings using quantitative methods, standard media for the detection of GBS pigment (e.g. Granada medium) and sequencing of the cyl gene cluster may solve previously reported discrepancies between pigment and haemolysin production.
Moreover, variations in the cyl gene cluster were reported in recently sequenced GBS strains from fish. Some strains harbour the complete cyl gene cluster and display β-haemolysis, while other strains are nonhaemolytic and contain mutations in the cyl locus (Liu et al., 2012, 2013; Pereira et al., 2013). Analysis of the nonhaemolytic STIR-CD-17 GBS strain isolated from tilapia (Oreochromis sp.) indicated that only parts of the cyl operon are present in this strain (Delannoy et al., 2012). Interestingly in another nonhaemolytic GBS strain isolated from tilapia an incomplete cyl operon was found containing cylE, cylA and cylB (Liu et al., 2013; Pereira et al., 2013), thus supporting the interpretation that these genes are necessary but not sufficient for GBS haemolysis. In fish pathogenic strains, most hypervirulent isolates are nonhaemolytic, indicating that the β-haemolysin is not an important virulence factor in these hosts (C.M.J. Delannoy, pers. commun.). Genome analysis of veterinary GBS strains reveals a considerable heterogeneity and shows that mechanisms of acquisition, duplication and reshuffling have permitted GBS to adapt to different environmental niches (Tettelin et al., 2005; Delannoy et al., 2012; Liu et al., 2012; Pereira et al., 2013; Rosinski-Chupin et al., 2013; Wang et al., 2013; Zubair et al., 2013).
The role of GBS haemolysin and pigment in virulence
Haemolysin
GBS, a commensal bacterium that asymptomatically colonizes human mucosal surfaces, can turn into a life-threatening pathogen in susceptible hosts (Ewards & Baker, 2010; Rodriguez-Granger et al., 2012). The molecular bases underlying GBS infections have been unveiled in the last decade and several reviews described the identification and importance of these virulence factors (Doran & Nizet, 2004; Nizet & Rubens, 2006; Maisey et al., 2009; Rajagopal, 2009).
Among them, the cell surface-associated β-haemolysin is thought to play a key role by promoting GBS penetration of host cell barriers such as the epithelial and endothelial cells of the lung and the blood–brain barrier. Furthermore, β-h/c was shown to induce host inflammatory responses (Doran et al., 2002; Bebien et al., 2012; Costa et al., 2012). The membrane-damaging effect of β-haemolysin is not restricted to erythrocytes; GBS β-haemolysin extracts exert a direct cytotoxicity against different eukaryotic cell types (Liu et al., 2004; Hensler et al., 2008a; Alkuwaity et al., 2012). Different studies suggest that GBS β-haemolysin has immunomodulatory properties that favour intracellular survival of GBS contributing to virulence (Doran et al., 2002; Liu et al., 2004; Bebien et al., 2012). Haemolysin-deficient mutants are attenuated in virulence in several animal models of GBS infections. By contrast, hyperhaemolytic GBS strains exhibit increased virulence (Wennerstrom et al., 1985; Tapsall & Phillips, 1991; Wennerstrom et al., 1991; Nizet et al., 1996, 1997b; Gibson et al., 1999; Puliti et al., 2000; Doran et al., 2002, 2003; Ring et al., 2002; Liu & Nizet, 2004; Hensler et al., 2005, 2008a; Forquin et al., 2007; Kaplan et al., 2008; Lembo et al., 2010; Fettucciari et al., 2011; Alkuwaity et al., 2012; Bebien et al., 2012; Whidbey et al., 2013). In addition, strong haemolytic GBS strains but not weak or nonhaemolytic strains are able to trigger macrophage apoptosis and to disrupt the macrophage cytoskeleton (Fettucciari et al., 2000, 2006, 2011; Liu et al., 2004; Liu & Nizet, 2006; Whidbey et al., 2013). Hyperhaemolytic GBS strains have been reported to be more frequently associated with women in preterm labour (Whidbey et al., 2013) and with cases of GBS streptococcal toxic shock syndrome and necrotizing fasciitis (Sendi et al., 2009). In line with these findings, it has also been reported that GBS nonhaemolytic strains are quite infrequent among GBS strains causing neonatal infections (Rodriguez-Granger et al., 2011). Moreover, the effect of intravenous administration of partially purified haemolysin in rabbits or rats produced dose-dependent hypotensive changes and deaths due to shock (Nizet et al., 1996). Nevertheless, controversial reports exist about the exact role of β-haemolysin in GBS infections (Sendi et al., 2009; Cumley et al., 2012; Sagar et al., 2013). Two reports indicate no differences between haemolytic and their nonhaemolytic counterparts in a mouse or a neonatal rat sepsis model (Wennerstrom et al., 1985; Weiser & Rubens, 1987). Surprisingly, GBS strains belonging to the hypervirulent clonal complex 17 that are responsible for the majority of invasive neonatal infections in Europe (Poyart et al., 2008; Fluegge et al., 2011) exhibit very low levels of haemolysis (Tazi et al., 2012). However, it remains possible that the amount of β-haemolysin production observed in vitro may not reflect the in vivo situation.
Pigment
It was first suggested in the 1980s that the pigment of GBS can neutralize superoxide and therefore pigment could confer resistance to radical oxygen species (Nemergut & Merritt, 1982, 1983). Indeed, it has been shown that a filtered extract of GBS pigment confers the ability to resist to the antimicrobial effects of reactive oxygen species during phagolysosomal killing (Liu & Nizet, 2004; Liu et al., 2004). Nevertheless, these studies assumed that GBS pigment was a carotene, whereas it is a polyene (nonisoprenoid) (Rosa-Fraile et al., 2006). Polyenic pigments share with isoprenoid carotenes a conjugated double-bond system with delocated electrons resulting in a characteristic carotene-like UV–visible spectrum (Britton, 1995). Polyenic pigments can also act – as do carotenes – as antioxidants (Krinsky, 1979; Krinsky & Yeum, 2003) and can protect membrane lipids against peroxidation, as described for some plant pathogenic bacteria, i.e. xantomonadin in Xantomonas orizae (Rajagopal et al., 1997; Goel et al., 2002). A recent study reported no difference in survival within mouse monocyte-derived macrophages between a nonpigmented cylE mutant and its wild-type counterpart, challenging the idea that pigment is a crucial virulence factor for resisting macrophage killing (Cumley et al., 2012).
Other bacterial components displaying similarity to GBS haemolysin and granadaene
While the GBS granadaene is unique among streptococci, it has been found in other bacterial species. Propionibacterium jensenii is a gram-positive, high-G + C-content bacterium that causes splitting and formation of red spots in Swiss-type cheeses. Propionibacterium jensenii is β-haemolytic and produces a red pigment identical to GBS granadaene. A genetic link between pigmentation and haemolytic activity in P. jensenii was suggested by chemical mutagenesis studies using nitrosoguanidine. Nonpigmented mutants of P. jensenii are nonhaemolytic, while mutants demonstrating a reduced pigmentation also display a diminished haemolytic activity (Vanberg et al., 2007). Pigment is also invariably linked with β-haemolysis in other species of Propionibacterium, such as P. thoenii and P. rubrum (Vedamuthu et al., 1971). As for P. jesenii, chemical mutagenesis in P. thoenii causes a simultaneous loss of haemolysis and pigment production (Vanberg et al., 2007). Nevertheless, to the best of our knowledge, the production of granadaene in P. rubrum and P. thoenii has not been investigated. Granadaene has been detected in P. jensenii, and it is interesting to note that a gene cluster with considerable similarities to the cyl genes has been identified in this bacterium. With the exception of cylK, homologues of all of the cyl genes are present in P. jensenii, but with a different gene organization (C. Vanberg C., Langsrud T., Nes I.F. & Holo H, unpublished data, P. jensenii strain LMG 2818 granadaene gene cluster, complete sequence GenBank: FJ617193.1).
With the increasing availability of bacterial genomes, orthologues and paralogues of the cyl gene cluster could be identified in a number of bacterial species distantly related to streptococci such as Bacillus cereus, propionibacterium spp., Arthrobacter aurescens and Actinomyces viscosus (Whidbey et al., 2013). Interestingly, all these species are gram-positive rods, some as in the case of Arthrobacter spp. are frequently found in environmental soil samples and these species do not represent classical human pathogens. This observation may indicate a function of the cyl genes that is not linked to human pathogenesis.
GBS pigment and haemolysin, identical or nonidentical twins?
Because of the close link between haemolysin and pigment production, it has been suggested that the pigment could be the natural carrier for the haemolysin or that GBS pigment and haemolysin are identical (Tapsall, 1987; Pritzlaff et al., 2001; Liu & Nizet, 2006; Whidbey et al., 2013). The addition of starch to a nonhaemolytic GBS pigment solution in DMSO-TFA results in a haemolytic and cytolytic preparation (Whidbey et al., 2013) leading to the conclusion that GBS pigment and haemolysin are identical molecules. Nevertheless, it remains possible that – although closely related – pigment and haemolysin could be slightly different molecules. For example, the pigment dissolved in DMSO-TFA and the haemolytic mixture of pigment and starch are stable, in contrast to the poor stability of GBS haemolysin–starch preparations (Dal & Monteil, 1983), and haemolytic colonies of GBS on blood agar plates are not pigmented. Different culture conditions can result in different haemolysin and pigment production (Rosa-Fraile et al., 1999a; Tapsall, 1987). Many early studies reporting differences between haemolysin and pigment production relied on qualitative visual detection of haemolysin and pigment, and this may explain the observed discrepancies between pigment and haemolysin to some extent. Some reports indicate that trimethoprim increases pigment production but does not have a stimulatory effect on haemolysin production (Schaufuβ et al., 1985; Tapsall, 1987). However, in contrast to these reports, on blood agar plates inoculated with a haemolytic GBS strain and with a sulfamethoxazole/trimethoprim (23.75/1.25 μg) antibiotic paper disc, an increased haemolysin production occurs in the GBS colonies surrounding the antibiotic disc (Fig. 4). This observation raises doubts about the validity of previous reports indicating that folate pathway inhibitors do not increase GBS haemolysin production. It is also worth pointing out that GBS pigment is stabilized by the same carrier molecules – albumin and starch – that are required to stabilize haemolysin (Tapsall, 1986), and both carrier substances can closely bind to long-chain fatty acids (Mikus et al., 1946; BeMiller, 1965; Spector et al., 1969; Spector, 1975; Blazek, 2008).
Fig 4.
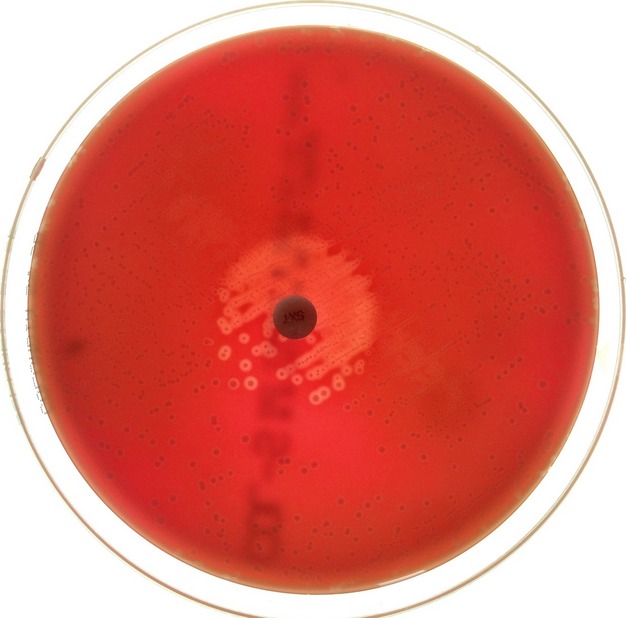
Enhancing effect of folate inhibitors on GBS haemolysin. Depicted is a picture of a blood agar plate inoculated with a haemolytic GBS strain and with a sulfamethoxazole/trimethoprim (23.75/1.25 μg) antibiotic paper disc.
Polyenes display a high affinity to sterols and phospholipids of the cell membrane, and they are haemolytic as well as cytolytic (Bolard, 1986; Knopik-Skrocka & Bielawski, 2002; Aparicio et al., 2004; Knopik-Skrocka et al., 2007). The toxicity of polyenes against mammalian cells is well documented in polyenic antibiotics (Bolard, 1986), and cytotoxicity is observed even in polyene molecules harbouring only four conjugated double bonds (Bae et al., 2013). The formation of polyene–lipid complexes can lead to changes in membrane permeability, resulting in haemolysis and cytolysis (Hsuchen & Feingold, 1973; Siegel, 1977; Brajtburg et al., 1980; Bolard, 1986; Aparicio et al., 2004). However, cholesterol, which inhibits the haemolytic activity of some polyenic antibiotics (Hsuchen & Feingold, 1973; Strom et al., 1979), had no inhibitory effect on the GBS haemolysin that is inhibited by phospholipids (Marchlewicz & Duncan, 1980; Ferrieri, 1982; Tapsall & Phillips, 1991).
It is also possible that the rhamnose tail of the GBS pigment may contribute to its membrane-damaging activity, as bacterial di-rhamnolipids are haemolytic. This haemolytic activity has been attributed to their biosurfactant characteristics as well as their cone-shaped configuration (Abdel-Mawgoud et al., 2010; Ortiz et al., 2010; Sanchez et al., 2010). While there appear to be structural differences between di-rhamnolipids and granadaene, it is possible that the rhamnose tail of GBS pigment may play a role in the membrane-damaging effect. The available data strongly support the hypothesis that GBS haemolysin and GBS polyenic pigment share a common metabolic pathway encoded in the cyl operon and that the haemolysin is a molecule closely related or identical to the granadaene polyenic pigment.
Summary
Research on the GBS β-h/c and pigment, two major phenotypic traits used for bacterial identification, has been being conducted for almost a century. GBS harbour a unique set of 12 genes, the cyl operon, responsible for both haemolysin and pigment production. Expression of the cyl operon is controlled by the CovS/R two-component system, which coordinates the expression of multiple virulence factors. Several publications have shown the important role of β-h/c in virulence. However, due to its small molecular size, the apparent lack of immunogenicity and a rapid loss of activity, the biochemical nature of the GBS β-h/c has remained elusive for many years. In 2000, the cyl operon was shown to be responsible for haemolysin and pigment production, and in 2006 the structure of the pigment was solved and demonstrated to be an ornithine-rhamnolipid designated ‘granadaene’. Recently, this rhamnolipid was shown to be haemolytic under certain experimental conditions. These data strongly support the idea that the GBS β-h/c and pigment are identical or very closely related molecules. Purification of β-h/c and determination of its structure will definitely prove this hypothesis.
Acknowledgments
Electron micrographs were produced by Prof. Paul Walther of the Central Electron Microscopy Facility of the University of Ulm and Dr Gerd Bröker. Dr Rodriguez-Granger from the Microbiology Service of Virgen de las Nieves Hospital and Prof. J. M. Cuerva and Dr A. Haidour-Benamin of the University of Granada identified the molecular structure of granadaene.
References
- Abdel-Mawgoud AM, Lépine F, Déziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol. 2010;86:1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler A, Block C, Engelstein D, Hochner-Celnikcier D, Drai-Hassid R, Moses AE. Culture-based methods for detection and identification of Streptococcus agalactiae in pregnant women–what are we missing? Eur J Clin Microbiol Infect Dis. 2008;27:241–243. doi: 10.1007/s10096-007-0421-2. [DOI] [PubMed] [Google Scholar]
- Alkuwaity K, Taylor A, Heckels JE, Doran KS, Christodoulides M. Group B streptococcus interactions with human meningeal cells and astrocytes in vitro. PLoS One. 2012;7:e42660. doi: 10.1371/journal.pone.0042660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparicio JF, Mendes MV, Antón N, Recio E, Martín JF. Polyene macrolide antibiotic biosynthesis. Curr Med Chem. 2004;11:1645–1656. doi: 10.2174/0929867043365044. [DOI] [PubMed] [Google Scholar]
- Ayers SH, Rupp P. Differentiation of hemolytic streptococci from human and bovine sources by the hydrolysis of sodium hippurate. J Infect Dis. 1922;30:388–399. [Google Scholar]
- Bae M, Kim H, Shin Y, Kim BY, Lee SK, Oh KB, Shin J, Oh DC. Separacenes A–D, novel polyene polyols from the marine actinomycete, Streptomyces sp. Mar Drugs. 2013;11:2882–2893. doi: 10.3390/md11082882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebien M, Hensler ME, Davanture S, Hsu LC, Karin M, Park JM, Alexopoulou L, Liu GY, Nizet V, Lawrence T. The pore-forming toxin β hemolysin/cytolysin triggers p38 MAPK-dependent IL-10 production in macrophages and inhibits innate immunity. PLoS Pathog. 2012;8:e1002812. doi: 10.1371/journal.ppat.1002812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BeMiller JN. Organic complexes and coordination compounds of carbohydrates. In: Paschall E, Whistler RL, editors. Starch: Chemistry and Technology. New York, NY: Academic Press; 1965. pp. 309–329. [Google Scholar]
- Billington SJ, Jost BH, Songer JG. Thiol-activated cytolysins: structure, function and role in pathogenesis. FEMS Microbiol Lett. 2000;182:197–205. doi: 10.1016/s0378-1097(99)00536-4. [DOI] [PubMed] [Google Scholar]
- Blazek J. 2008. Role of amylose in structure-function relationship in starches from Australian wheat varieties. PhD Thesis, Faculty of Agriculture, Food and Natural Resources, University of Sydney, Sydney, NSW, Australia.
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Brajtburg J, Medoff G, Kobayashi GS, Elberg S, Finegold C. Permeabilizing and hemolytic action of large and small polyene antibiotics on human erythrocytes. Antimicrob Agents Chemother. 1980;18:586–592. doi: 10.1128/aac.18.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt CM, Spellerberg B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin Infect Dis. 2009;49:766–772. doi: 10.1086/605085. [DOI] [PubMed] [Google Scholar]
- Brglez I. Pigment production in human and bovine Streptococcus agalactiae strains. Zentralbl Bakteriol Mikrobiol Hyg B. 1983;177:533–538. [PubMed] [Google Scholar]
- Brimil N, Barthell E, Heindrichsd U, Kuhn M, Lutticken R, Spellerberg B. Epidemiology of Streptococcus agalactiae colonization in Germany. Int J Med Microbiol. 2006;296:39–44. doi: 10.1016/j.ijmm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Britton G. UV/visible spectroscopy. In: Pfander H, Britton G, Jensen L, editors. Carotenoids Volume 1B. Spectroscopy. Basel: Birkhauser Verlag; 1995. pp. 13–62. [Google Scholar]
- Burger MM, Glaser L, Burton RM. The enzymatic synthesis of a rhamnose-containing glycolipid by extracts of Pseudomonas aeruginosa. J Biol Chem. 1963;238:2595–2602. [PubMed] [Google Scholar]
- Burnside K, Lembo A, Harrell MI, et al. Serine/Threonine phosphatase Stp1 mediates post-transcriptional regulation of hemolysin, autolysis, and virulence of group B streptococcus. J Biol Chem. 2011;286:44197–44210. doi: 10.1074/jbc.M111.313486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie R, Atkins NE, Munch-Petersen E. A note on the lytic phenomenon shown by group B streptococci. Aust J Exp Biol Med Sci. 1944;22:197–200. doi: 10.1038/icb.1945.30. [DOI] [PubMed] [Google Scholar]
- Costa A, Gupta R, Signorino G, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, Draper LA, Lawton EM, Daly KM, Groeger DS, Casey PG, Ross RP, Hill C. Listeriolysin S, a novel peptide haemolysin associated with a subset of lineage I Listeria monocytogenes. PLoS Pathog. 2008;4:e1000144. doi: 10.1371/journal.ppat.1000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumley NJ, Smith LM, Anthony M, May RC. The CovS/CovR acid response regulator is required for Intracellular survival of group B Streptococcus in macrophages. Infect Immun. 2012;80:1650–1661. doi: 10.1128/IAI.05443-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal MC, Monteil H. Hemolysin produced by group B Streptococcus agalactiae. FEMS Microbiol Lett. 1983;6:89–94. [Google Scholar]
- Datta V, Myskowski SM, Kwinn LA, Chiem DN, Varki N, Kansal RG, Kotb M, Nizet V. Mutational analysis of the group A streptococcal operon encoding streptolysin S and its virulence role in invasive infection. Mol Microbiol. 2005;56:681–695. doi: 10.1111/j.1365-2958.2005.04583.x. [DOI] [PubMed] [Google Scholar]
- Delannoy CMJ, Zadoks RN, Lainson FA, Ferguson HW, Crumlish M, Turnbull JF, Fontaine MC. Draft genome sequence of a nonhemolytic fish-pathogenic Streptococcus agalactiae strain. J Bacteriol. 2012;194:6341–6342. doi: 10.1128/JB.01552-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Turnbull JF, Zadoks RN. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiol. 2013;13:41. doi: 10.1186/1471-2180-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doran KS, Nizet V. Molecular pathogenesis of neonatal group B streptococcal infection: no longer in its infancy. Mol Microbiol. 2004;54:23–31. doi: 10.1111/j.1365-2958.2004.04266.x. [DOI] [PubMed] [Google Scholar]
- Doran KS, Chang JC, Benoit VM, Eckmann L, Nizet V. Group B streptococcal beta-hemolysin/cytolysin promotes invasion of human lung epithelial cells and the release of interleukin-8. J Infect Dis. 2002;185:196–203. doi: 10.1086/338475. [DOI] [PubMed] [Google Scholar]
- Doran KS, Liu GY, Nizet V. Group B streptococcal beta-hemolysin/cytolysin activates neutrophil signalling pathways in brain endothelium and contributes to development of meningitis. J Clin Invest. 2003;112:736–744. doi: 10.1172/JCI17335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dramsi S, Cossart P. Listeriolysin O: a genuine cytolysin optimized for an intracellular parasite. J Cell Biol. 2002;156:943–946. doi: 10.1083/jcb.200202121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand P, Giraud P. Les streptocoques chromogenes. CR Acad Sci Paris. 1923;177:1333–1335. [Google Scholar]
- Edwards MS. Group B streptococcal infections. In: Maldonado YA, Nizet V, Remington JS, Klein JO, Wilson CB, Nizet V, editors. Infectious Diseases of the Fetus and Newborn Infant. 7th edn. Amsterdam: Elsevier; 2011. pp. 419–469. [Google Scholar]
- Evans JJ, Bohnsack JF, Klesius PH, Whiting AA, Garcia JC, Shoemaker CA, Takahashi S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: a dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J Med Microbiol. 2008;57:1369–1376. doi: 10.1099/jmm.0.47815-0. [DOI] [PubMed] [Google Scholar]
- Ewards MS. Streptococcus agalactiae (Group B Streptococcus. In: Dolin R, Baker CJ, Mandell GL, Bennett JE, editors. Mandell, Douglas and Bennett's. Principles & Practice of Infectious Diseases. 7th edn. Vol. 2. Amsterdam: Elsevier; 2010. pp. 2655–2666. [Google Scholar]
- Facklam R. What happened to the streptococci: overview of taxonomic and nomenclature changes. Clin Microbiol Rev. 2002;15:613–630. doi: 10.1128/CMR.15.4.613-630.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facklam RR, Padula JF, Wortham EC, Cooksey RC, Rountree HA. Presumptive identification of group A, B, and D streptococci on agar plate media. J Clin Microbiol. 1979;9:665–672. doi: 10.1128/jcm.9.6.665-672.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon RJ. The rapid recognition of Lancefield group B haemolytic streptococci. J Clin Pathol. 1974;27:902–905. doi: 10.1136/jcp.27.11.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley MM. Group B streptococcal disease in nonpregnant adults. Clin Infect Dis. 2001;33:556–561. doi: 10.1086/322696. [DOI] [PubMed] [Google Scholar]
- Ferrieri P. Characterization of a hemolysin isolated from group B streptococci. In: Christebnsen P, Holm SE, editors. Basic Concepts of Streptococci and Streptococcal Diseases. Chertsey: Reedbooks; 1982. pp. 142–143. [Google Scholar]
- Fettucciari K, Rosati E, Scaringi L, Cornacchione P, Migliorati G, Sabatini R, Fetriconi I, Rossi R, Marconi P. Group B streptococcus induces apoptosis in macrophages. J Immunol. 2000;165:3923–3933. doi: 10.4049/jimmunol.165.7.3923. [DOI] [PubMed] [Google Scholar]
- Fettucciari K, Fetriconi I, Mannucci R, Nicoletti I, Bartoli A, Coaccioli S, Marconi P. Group B streptococcus induces macrophage apoptosis by calpain activation. J Immunol. 2006;176:7542–7556. doi: 10.4049/jimmunol.176.12.7542. [DOI] [PubMed] [Google Scholar]
- Fettucciari K, Quotadano F, Noce R, Palumbo C, Modesti A, Rosati E, Mannucci R, Bartoli A, Marconi P. Group B streptococcus (GBS) disrupts by calpain activation the actin and microtubule cytoskeleton of macrophages. Cell Microbiol. 2011;13:859–884. doi: 10.1111/j.1462-5822.2011.01584.x. [DOI] [PubMed] [Google Scholar]
- Firon A, Tazi A, Da Cunha V, Brinster S, Sauvage E, Dramsi S, Golenbock DT, Glaser P, Poyart C, Trieu-Cuot P. The Abi-domain protein Abx1 interacts with the CovS histidine kinase to control virulence gene expression in group B Streptococcus. PLoS Pathog. 2013;9:e1003179. doi: 10.1371/journal.ppat.1003179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluegge K, Wons J, Spellerberg B, Swoboda S, Siedler A, Hufnagel M, Berner R. Genetic differences between invasive and noninvasive neonatal group B streptococcal isolates. Pediatr Infect Dis J. 2011;30:1027–2031. doi: 10.1097/INF.0b013e31822a2a1f. [DOI] [PubMed] [Google Scholar]
- Forquin MP, Tazi A, Rosa-Fraile M, Poyart C, Trieu-Cuot P, Dramsi S. The putative glycosyltransferase-encoding gene cylJ and the group B streptococcus (GBS)-specific gene cylK modulate hemolysin production and virulence of GBS. Infect Immun. 2007;75:2063–2066. doi: 10.1128/IAI.01565-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry RM. Fatal infections by hemolytic streptococcus group B. Lancet. 1938;I:199–201. [Google Scholar]
- Fuller JD, Camus AC, Duncan CL, Nizet V, Bast DJ, Thune RL, Low DE, De Azavedo JC. Identification of a streptolysin S-associated gene cluster and its role in the pathogenesis of Streptococcus iniae disease. Infect Immun. 2002;70:5730–5739. doi: 10.1128/IAI.70.10.5730-5739.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JC, Klesius PH, Evans JJ, Shoemaker CA. Non-infectivity of cattle Streptococcus agalactiae in Nile tilapia, Oreochromis niloticus and channel catfish, Ictalurus punctatus. Aquaculture. 2008;281:151–154. [Google Scholar]
- Gibson RL, Nizet V, Rubens CE. Group B Streptococcal beta-hemolysin promotes injury of lung microvascular endothelial cells. Pediatr Res. 1999;45:626–634. doi: 10.1203/00006450-199905010-00003. [DOI] [PubMed] [Google Scholar]
- Glaser P, Rusniok C, Buchrieser C, et al. Genome sequence of Streptococcus agalactiae, a pathogen causing invasive neonatal disease. Mol Microbiol. 2002;45:1499–1513. doi: 10.1046/j.1365-2958.2002.03126.x. [DOI] [PubMed] [Google Scholar]
- Goel AK, Rajagopal L, Nagesh N, Sonti RV. Genetic locus encoding functions involved in biosynthesis and outer membrane localization of xanthomonadin in Xanthomonas oryzae pv. oryzae. J Bacteriol. 2002;184:3539–3548. doi: 10.1128/JB.184.13.3539-3548.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez DJ, Lee SW, Hensler ME, Markley AL, Dahesh S, Mitchell DA, Bandeira N, Nizet V, Dixon JE, Dorrestein PC. Clostridiolysin S, a post-translationally modified biotoxin from Clostridium botulinum. J Biol Chem. 2010;285:28220–28228. doi: 10.1074/jbc.M110.118554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottschalk B, Broker G, Kuhn M, Aymanns S, Gleich-Theurer U, Spellerberg B. Transport of multidrug resistance substrates by the Streptococcus agalactiae hemolysin transporter. J Bacteriol. 2006;188:5984–5992. doi: 10.1128/JB.00768-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug RH, Soderlund E. Pigment production in group B streptococcus. Acta Pathol Microbiol Scand B. 1977;85:286–288. doi: 10.1111/j.1699-0463.1977.tb01976.x. [DOI] [PubMed] [Google Scholar]
- Hensler ME, Liu GY, Sobczak S, Benirschke K, Nizet V, Heldt GP. Virulence role of group B streptococcus beta-hemolysin/cytolysin in a neonatal rabbit model of early-onset pulmonary infection. J Infect Dis. 2005;191:1287–1291. doi: 10.1086/428946. [DOI] [PubMed] [Google Scholar]
- Hensler ME, Miyamoto S, Nizet V. Group B streptococcal beta-hemolysin/cytolysin directly impairs cardiomyocyte viability and function. PLoS One. 2008a;3:e2446. doi: 10.1371/journal.pone.0002446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensler ME, Quach D, Hsieh CJ, Doran KS, Nizet V. CAMP factor is not essential for systemic virulence of group B streptococcus. Micro Pathog. 2008b;44:84–88. doi: 10.1016/j.micpath.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood M, Janney A, Dameron G. Beta hemolytic streptococcus group B associated with problems of the perinatal period. Am J Obstet Gynecol. 1961;82:809–818. doi: 10.1016/s0002-9378(16)36146-4. [DOI] [PubMed] [Google Scholar]
- Hsuchen CC, Feingold DS. Selective membrane toxicity of the polyene antibiotics: studies on natural membranes. Antimicrob Agents Chemother. 1973;4:316–319. doi: 10.1128/aac.4.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humar D, Datta V, Bast DJ, Beall B, De Azavedo JC, Nizet V. Streptolysin S and necrotising infections produced by group G Streptococcus. Lancet. 2002;359:124–129. doi: 10.1016/S0140-6736(02)07371-3. [DOI] [PubMed] [Google Scholar]
- Islam AKMS. Rapid recognition of group B streptococci. Lancet. 1977;I:256–257. doi: 10.1016/s0140-6736(77)91055-8. [DOI] [PubMed] [Google Scholar]
- Jansen TL, Janssen M, Traksel R, Jong AJL. A clinical and serological comparison of group A versus non-group A streptococcal reactive arthritis and throat culture negative cases of post-streptococcal reactive arthritis. Ann Rheum Dis. 1999;58:410–414. doi: 10.1136/ard.58.7.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SM, Cieslewicz MJ, Kasper DL, Wessels MR. Regulation of virulence by a two-component system in group B streptococcus. J Bacteriol. 2005;187:1105–1113. doi: 10.1128/JB.187.3.1105-1113.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang SM, Ishmael N, Dunning Hotopp J, et al. Variation in the group B Streptococcus CsrRS regulon and effects on pathogenicity. J Bacteriol. 2008;190:1956–1965. doi: 10.1128/JB.01677-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A, Chung K, Kocak H, Bertolotto C, Uh A, Hobel CJ, Simmons CF, Doran K, Liu GY, Equils O. Group B streptococcus induces trophoblast death. Microb Pathog. 2008;45:231–235. doi: 10.1016/j.micpath.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe GP. Streptococcus agalactiae mastitis: a review. Can Vet J. 1997;38:429–437. [PMC free article] [PubMed] [Google Scholar]
- Knopik-Skrocka A, Bielawski J. The mechanism of the hemolytic activity of polyene antibiotics. Cell Mol Biol Lett. 2002;7:31–48. [PubMed] [Google Scholar]
- Knopik-Skrocka A, Bielawski J, Wrzeszcz K, Bochanysz A, Nowak K. Modifications of hemolytic activity of polyene antibiotics by mono and disaccharides in mammalian erythrocytes. Biol Lett. 2007;44:17–30. [Google Scholar]
- Kornblatt AN, Adams RL, Barthold SW, Cameron GA. Canine neonatal deaths associated with group B streptococcal septicemia. J Am Vet Med Assoc. 1983;183:700–701. [PubMed] [Google Scholar]
- Krinsky NI. Carotenoid protection against oxidation. Pure Appl Chem. 1979;51:649–660. [Google Scholar]
- Krinsky NI, Yeum KJ. Carotenoid-radical interactions. Biochem Biophys Res Commun. 2003;305:754–760. doi: 10.1016/s0006-291x(03)00816-7. [DOI] [PubMed] [Google Scholar]
- Lämmler C, Schaufuss P, Blobel H. Pigment production by streptococci of serological group B. IRCS Med Sci. 1985;13:396. [Google Scholar]
- Lamy MC, Zouine M, Fert J, et al. CovS/CovR of group B Streptococcus: a two-component global regulatory system involved in virulence. Mol Microbiol. 2004;54:1250–1268. doi: 10.1111/j.1365-2958.2004.04365.x. [DOI] [PubMed] [Google Scholar]
- Lancefield RC. A serological differentiation of humans and others groups of hemolytic streptococci. J Exp Med. 1933;57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield RC. Loss of the properties of haemolysin and pigment formation without change in immunological specificity in a strain of Streptococcus haemolyticus. J Exp Med. 1934;59:459–469. doi: 10.1084/jem.59.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancefield R, Hare R. The serological differentiation of pathogenic and non-pathogenic strains of hemolitic streptococci from parturient women. J Exp Med. 1935;61:335–349. doi: 10.1084/jem.61.3.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo A, Gurney MA, Burnside K, et al. Regulation of CovR expression in group B streptococcus impacts blood-brain barrier penetration. Mol Microbiol. 2010;77:431–443. doi: 10.1111/j.1365-2958.2010.07215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin WJ, Walthers D, Connelly JE, Burnside K, Jewell KA, Kenney LJ, Rajagopal L. Threonine phosphorylation prevents promoter DNA binding of the Group B Streptococcus response regulator CovR. Mol Microbiol. 2009;71:1477–1495. doi: 10.1111/j.1365-2958.2009.06616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GY, Nizet V. Extracellular virulence factors of group B streptococci. Front Biosci. 2004;9:1794–1802. doi: 10.2741/1296. [DOI] [PubMed] [Google Scholar]
- Liu GY. The group B streptococcal β-haemolysin/cytolysin. In: Popoff NR, Nizet V, Alouf JE, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. 3rd edn. Oxford: Elsevier; 2006. pp. 9737–9745. [Google Scholar]
- Liu GY, Doran KS, Lawrence T, Turkson N, Puliti M, Tissi L, Nizet V. Sword and shield: linked group B streptococcal beta-hemolysin/cytolysin and carotenoid pigment function to subvert host phagocyte defense. P Natl Acad Sci USA. 2004;101:14491–14496. doi: 10.1073/pnas.0406143101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang W, Lu C. Complete genome sequence of Streptococcus agalactiae GD201008-001, isolated in China from tilapia with meningoencephalitis. J Bacteriol. 2012;194:6653. doi: 10.1128/JB.01788-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang W, Lu C. Comparative genomics analysis of Streptococcus agalactiae reveals that isolates from cultured tilapia in China are closely related to the human strain A909. BMC Genomics. 2013;14:775. doi: 10.1186/1471-2164-14-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence. Expert Rev Mol Med. 2009;10:e27. doi: 10.1017/S1462399408000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz DA, Duncan JL. Properties of a haemolysin produced by group B streptococci. Infect Immun. 1980;30:805–813. doi: 10.1128/iai.30.3.805-813.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchlewicz DA, Duncan JL. Lysis of erythrocites by a haemolysin produced by group B streptococcus sp. Infect Immun. 1981;34:787–794. doi: 10.1128/iai.34.3.787-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick JK, Peterson ML. Toxins and superantigens of group A streptococci. In: Rood JI, Schlievert PM, Fischetti VA, Novick RP, Ferretti JJ, Portnoy A, editors. Gram-Positive Pathogens. 2nd edn. Washington, DC: ASM Press; 2006. pp. 47–58. [Google Scholar]
- Merrit K, Jacobs NJ. Improved medium for detecting pigment production by group B streptococci. J Clin Microbiol. 1976;4:379–380. doi: 10.1128/jcm.4.4.379-380.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrit K, Treadwell Tl, Jacobs JN. Rapid recognition of group B streptococci by pigment production and counterimmunoelectrophoresis. J Clin Microbiol. 1976;3:287–290. doi: 10.1128/jcm.3.3.287-290.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K, Jacobs NJ. Characterization and incidence of pigment production by human clinical group B streptococci. J Clin Microbiol. 1978;8:105–107. doi: 10.1128/jcm.8.1.105-107.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messier S, Daminet S, Lemarchand T. Streptococcus agalactiae endocarditis with embolization in a dog. Can Vet J. 1995;36:73–74. [PMC free article] [PubMed] [Google Scholar]
- Mhalu FS. Infection with Streptococcus agalactiae in a London hospital. J Clin Pathol. 1976;29:309–312. doi: 10.1136/jcp.29.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikus FF, Hixon RM, Rundle RE. The complexes of fatty acids with amylose. J Am Chem Soc. 1946;68:1115–1123. doi: 10.1021/ja01210a062. [DOI] [PubMed] [Google Scholar]
- Molloy EM, Cotter PD, Hill C, Mitchell DA, Ross RP. Streptolysin S-like virulence factors: the continuing sagA. Nat Rev Microbiol. 2011;9:670–681. doi: 10.1038/nrmicro2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemergut RA, Merritt K. Protective effects of the group B streptococcal carotenoid pigment again superoxide. Abstr Annu Meet Am Soc Microbiol. 1982;147:42. [Google Scholar]
- Nemergut RA, Merritt K. Neutralization of oxidative killing by group B streptococcal carotenoid pigment. Abstr Annu Meet Am Soc Microbiol. 1983;32:38. [Google Scholar]
- Nizet V. Streptococcal β-haemolysins: genetics and role in disease pathogenesis. Trends Microbiol. 2002;10:575–580. doi: 10.1016/s0966-842x(02)02473-3. [DOI] [PubMed] [Google Scholar]
- Nizet V. Pathogenic mechanisms and virulence factors of group B streptococci. In: Rood JI, Rubens CE, Fischetti VA, Novick RP, Ferretti JJ, Portnoy A, editors. Gram Positive Pathogens. 2 edn. Washington, DC: ASM Press; 2006. pp. 152–168. [Google Scholar]
- Nizet W, Gibson RL, Chi EM, Framson PE, Hulse M, Ribens CE. Group B streptococcal beta-hemolysin expression is associated with injury of lung epithelial cells. Infect Inmun. 1996;64:3818–3826. doi: 10.1128/iai.64.9.3818-3826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Gibson RL, Rubens CE. The role of group B streptococci beta-hemolysin expression in newborn lung injury. Adv Exp Med Biol. 1997a;418:627–630. doi: 10.1007/978-1-4899-1825-3_146. [DOI] [PubMed] [Google Scholar]
- Nizet V, Kim KS, Stins M, Jonas M, Chi EY, Nguyen D, Rubens CE. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997b;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Beall B, Bast DJ, Datta V, Kilburn L, Low DE, De Azavedo JC. Genetic locus for streptolysin S production by group A streptococcus. Infect Immun. 2000;68:4245–4254. doi: 10.1128/iai.68.7.4245-4254.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble MA, Bent JM, West AB. Detection and identification of group B streptococci by use of pigment production. J Clin Pathol. 1983;36:350–352. doi: 10.1136/jcp.36.3.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner UA, Fiechter A, Reiser J. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. J Biol Chem. 1994;269:19787–19795. [PubMed] [Google Scholar]
- Ortiz A, Aranda FJ, Teruel JA. Interaction of dirhamnolipid biosurfactants with phospholipid membranes: a molecular level study. Adv Exp Med Biol. 2010;672:42–53. doi: 10.1007/978-1-4419-5979-9_3. [DOI] [PubMed] [Google Scholar]
- Paradas M, Jurado R, Haidour A, Rodríguez-Granger J, Sampedro Martínez A, Rosa-Fraile M, Robles R, Justicia J, Cuerva JM. Clarifying the structure of granadaene: total synthesis of related analogue 2-granadaene and confirmation of its absolute stereochemistry. Bioorg Med Chem. 2012;20:6655–6661. doi: 10.1016/j.bmc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Patras KA, Wang NY, Fletcher EM, Cavaco CK, Jimenez A, Garg M, Fierer J, Sheen TR, Rajagopal L, Doran KS. Group B Streptococcus CovR regulation modulates host immune signalling pathways to promote vaginal colonization. Cell Microbiol. 2013;15:1154–1167. doi: 10.1111/cmi.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazur JH, Shuey EW. Thymidine diphosphate rhamnose diphosphate dlucose and Its conversion to the enzymatic synthesis of thymidine. J Biol Chem. 1961;236:1780–1785. [PubMed] [Google Scholar]
- Pereira UP, Santos AR, Aburjaile FF, et al. Complete genome sequence of Streptococcus agalactiae strain SA20-06, a fish pathogen associated to meningoencephalitis outbreaks. Stand Genomic Sci. 2013;8:188–197. doi: 10.4056/sigs.3687314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt MW. In vivo hemolytic activity of group B streptococcus is dependent on erythrocyte-bacteria contact and independent of a carrier molecule. Curr Microbiol. 1995;31:5–9. doi: 10.1007/BF00294625. [DOI] [PubMed] [Google Scholar]
- Plummer H. A serological and biochemical study of haemolytic streptococci. J Inmunol. 1941;42:91–107. [Google Scholar]
- Poyart C, Réglier-Poupet H, Tazi A, Billoët A, Dmytruk N, Bidet P, Bingen E, Raymond J, Trieu-Cuot P. Invasive group B streptococcal infections in infants, France. Emerg Infect Dis. 2008;14:1647–1649. doi: 10.3201/eid1410.080185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritzlaff CA, Chang JC, Kuo SP, Tamura GS, Rubens CE, Nizet V. Genetic basis for the beta-haemolytic/cytolytic activity of group B streptococcus. Mol Microbiol. 2001;39:236–248. doi: 10.1046/j.1365-2958.2001.02211.x. [DOI] [PubMed] [Google Scholar]
- Puliti M, Nizet V, Hunolstein C, Bistoni F, Mosci P, Orefici G, Tiss S. Severity of group B streptococcal arthritis is correlated with b-hemolysin expression. J Infect Dis. 2000;182:824–832. doi: 10.1086/315773. [DOI] [PubMed] [Google Scholar]
- Rajagopal L. Understanding the regulation of group B streptococcal virulence factors. Future Microbiol. 2009;4:201–221. doi: 10.2217/17460913.4.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal L, Sundari CS, Balasubramanian D, Sonti RV. The bacterial pigment xanthomonadin offers protection against photodamage. FEBS Lett. 1997;415:125–128. doi: 10.1016/s0014-5793(97)01109-5. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Clancy C, Rubens CE. A eukaryotic type serine/threonine kinase and phosphatase in Streptococcus agalactiae reversibly phosphorylate an inorganic pyrophosphatase and affect growth, cell segregation, and virulence. J Biol Chem. 2003;278:14429–14441. doi: 10.1074/jbc.M212747200. [DOI] [PubMed] [Google Scholar]
- Rajagopal L, Vo A, Silvestroni A, Rubens CE. Regulation of cytotoxin expression by converging eukaryotic-type and two-component signalling mechanisms in Streptococcus agalactiae. Mol Microbiol. 2006;62:941–957. doi: 10.1111/j.1365-2958.2006.05431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rato MG, Nerlich A, Bergmann R, Bexiga R, Nunes SF, Vilela CL, Santos-Sanches I, Chhatwal GS. Virulence gene pool detected in bovine group C Streptococcus dysgalactiae subsp. dysgalactiae isolates by use of a group A S .pyogenes virulence microarray. J Clin Microbiol. 2011;49:2470–2479. doi: 10.1128/JCM.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon EP, Noble MA, Luther ER, Wort AJ, Bent J, Swift M. Evaluation of a rapid method for the detection of vaginal group B streptococci in women in labor. Am J Obstet Gynecol. 1984;148:575–578. doi: 10.1016/0002-9378(84)90751-8. [DOI] [PubMed] [Google Scholar]
- Ring A, Braun JS, Pohl J, Nizet V, Stremmel W, Shenep JL. Group B streptococcal beta-hemolysin induces mortality and liver injury in experimental sepsis. J Infect Dis. 2002;185:1745–1753. doi: 10.1086/340818. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Granger J, Baldassarri L, Hufnagel M, Lara-Oya A, Liebana C. Non-haemolytic/non-pigmented GBS strains as a cause of early onset neonatal disease. In: Baldassarri L, Rosa-Fraile M, editors. An Update on Diagnosis, Management and Treatment of Neonatal Group B Streptococcal Infections. Rome: Istituto Superiore di Sanita; 2011. pp. 24–25. [Google Scholar]
- Rodriguez-Granger J, Alvar-Gonzalez JC, Berardi A, et al. Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur J Clin Microbiol Infect Dis. 2012;31:2097–2104. doi: 10.1007/s10096-012-1559-0. [DOI] [PubMed] [Google Scholar]
- Rosa M, Villarreal R, Vega D, Miranda C, Martinez-Brocal A. Granada Medium for detection and identification of group B streptococci. J Clin Microbiol. 1983;18:779–785. doi: 10.1128/jcm.18.4.779-785.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa M, Perez M, Carazo C, Peis JI, Pareja L, Hernandez L. New Granada Medium for detection and identification of group B streptococci. J Clin Microbiol. 1992;30:1019–1021. doi: 10.1128/jcm.30.4.1019-1021.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Fraile M, Sampedro A, Varela J, Garcia-Peña M, Gimenez-Gallego G. Identification of a peptide from mammal albumins responsible for enhanced pigment production by group B streptococci. Clin Diagn Lab Immunol. 1999a;6:425–426. doi: 10.1128/cdli.6.3.425-426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Fraile M, Rodriguez-Granger J, Cueto-Lopez M, Sampedro A, Biel Gaye E, Haro M, Andreu A. Use of Granada medium to detect group B streptococcal colonization in pregnant women. J Clin Microbiol. 1999b;37:2674–2677. doi: 10.1128/jcm.37.8.2674-2677.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa-Fraile M, Rodríguez-Granger J, Haidour-Benamin A, Cuerva JM, Sampedro A. Granadaene: proposed structure of the group B streptococcus polyenic pigment. Appl Environ Microbiol. 2006;72:6367–6370. doi: 10.1128/AEM.00756-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosinski-Chupin I, Sauvage E, Mairey B, Mangenot S, Ma L, Da Cunha V, Rusniok C, Bouchier C, Barbe V, Glaser P. Reductive evolution in Streptococcus agalactiae and the emergence of a host adapted lineage. BMC Genomics. 2013;14:252. doi: 10.1186/1471-2164-14-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotta J. Pyogenic hemolytic streptococci. In: Holt JG, Sneath PHA, Nicholas NS, Sherpe ME, editors. Bergey's Manual of Systematic Bacteriology. Baltimore, MD: Willians and Wilkins; 1986. pp. 1047–1054. Vol 2. [Google Scholar]
- Rühlmann J, Wittmann-Liebold B, Jürgens D, Fehrenbach FJ. Complete amino acid sequence of protein B. FEBS Lett. 1988;235:262–266. doi: 10.1016/0014-5793(88)81275-4. [DOI] [PubMed] [Google Scholar]
- Sagar A, Klemm C, Hartjes L, Mauerer S, van Zandbergen G, Spellerberg B. The β-haemolysin and intracellular survival of Streptococcus agalactiae in human macrophages. PLoS One. 2013;8:e60160. doi: 10.1371/journal.pone.0060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samen UM, Eikmanns BJ, Reinscheid DJ. The transcriptional regulator RovS controls the attachment of Streptococcus agalactiae to human epithelial cells and the expression of virulence genes. Infect Immun. 2006;74:5625–5635. doi: 10.1128/IAI.00667-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Aranda FJ, Teruel JA, Espuny MJ, Marqués A, Manresa A, Ortiz A. Permeabilization of biological and artificial membranes by a bacterial dirhamnolipid produced by Pseudomonas aeruginosa. J Colloid Interface Sci. 2010;341:240–247. doi: 10.1016/j.jcis.2009.09.042. [DOI] [PubMed] [Google Scholar]
- Schaufuβ P, Lämmler CH, Blobel H. Effects of glucose and trimethoprim on pigment production of group B streptococci. IRCS Med Sci. 1985;13:842. [Google Scholar]
- Schiedt K. Isolation and analysis. In: Pfander H, Liaaen-Jensen S, Britton G, Jensen L, editors. Carotenoids Volume 1A. Isolation and Analysis. Basel: Birkhauser Verlag; 1995. pp. 81–108. [Google Scholar]
- Schweizer E. Biosynthesis of fatty acids and related compounds. In: Wilkinson SG, Ratledge C, editors. Microbial Lipids. Vol. 2. London: Academic Press; 1989. pp. 3–50. [Google Scholar]
- Sendi P, Johansson L, Dahesh S, Van-Sorge NM, Darenberg J, Norgren M, Sjölin J, Nizet V, Norrby-Teglund A. Bacterial phenotype variants in group B streptococcal toxic shock syndrome. Emerg Infect Dis. 2009;15:223–232. doi: 10.3201/eid1502.080990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman JM. The streptococci. Bacteriol Rev. 1937;1:3–97. doi: 10.1128/br.1.1.3-97.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuster KA, Hish GA, Selles LA, Chowdhury MA, Wiggins RC, Dysko RC, Bergin IL. Naturally occurring disseminated group B Streptococcus Infections in postnatal rats. Comp Med. 2013;63:55–61. [PMC free article] [PubMed] [Google Scholar]
- Siegel EB. Measurement of polyene antibiotic-mediated erythrocyte damage by release of hemoglobin and radioactive chromium. Antimicrob Agents Chemother. 1977;11:675–678. doi: 10.1128/aac.11.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieiro C, Poza M, de Miguel T, Villa TG. Genetic basis of microbial carotenogenesis. Int Microbiol. 2003;6:11–16. doi: 10.1007/s10123-003-0097-0. [DOI] [PubMed] [Google Scholar]
- Sigge A, Schmid M, Mauerer S, Spellerberg B. Heterogeneity of hemolysin expression during neonatal Streptococcus agalactiae sepsis. J Clin Microbiol. 2008;46:807–809. doi: 10.1128/JCM.01963-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA. Fatty acid binding to plasma albumin. J Lipid Res. 1975;16:165–179. [PubMed] [Google Scholar]
- Spector AA, John K, Fletcher JE. Binding of long-chain fatty acids to bovine serum albumin. J Lipid Res. 1969;10:56–67. [PubMed] [Google Scholar]
- Spellerberg B. Streptococcus. In: Warnock DW, Brandt C, Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, editors. Manual of Clinical Microbiology. 10th edn. Vol. 1. Washington, DC: ASM Press; 2011. pp. 331–334. [Google Scholar]
- Spellerberg B, Pohl B, Haase G, Martin S, Weber-Heynemann J, Lütticken R. Identification of genetic determinants for the hemolytic activity of Streptococcus agalactiae by ISS1 transposition. J Bacteriol. 1999;181:3212–3219. doi: 10.1128/jb.181.10.3212-3219.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellerberg B, Martin S, Brandt C, Lutticken R. The cyl genes of Streptococcus agalactiae are involved in the production of pigment. FEMS Microbiol Lett. 2000a;188:125–128. doi: 10.1111/j.1574-6968.2000.tb09182.x. [DOI] [PubMed] [Google Scholar]
- Spellerberg B, Martin S, Franken C, Berner R, Lütticken R. Identification of a novel insertion sequence element in Streptococcus agalactiae. Gene. 2000b;241:51–56. doi: 10.1016/s0378-1119(99)00469-2. [DOI] [PubMed] [Google Scholar]
- Stock AM, Robinson VL, Goudreaux PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- Strom R, Crifo C, Bozzi A. A comparative study of the hemolytic effect of polyenic antibiotics and of other cholesterol-binding agents. Biochem Pharmcol. 1979;28:2427–2432. doi: 10.1016/0006-2952(79)90003-0. [DOI] [PubMed] [Google Scholar]
- Tapsall JW. Pigment production by Lancefield-group B streptococci (Streptococcus agalactiae) J Med Microbiol. 1986;21:75–81. doi: 10.1099/00222615-21-1-75. [DOI] [PubMed] [Google Scholar]
- Tapsall JW. Relationship between pigment production and haemolysin formation by Lancefield group B streptococci. J Med Microbiol. 1987;24:83–87. doi: 10.1099/00222615-24-1-83. [DOI] [PubMed] [Google Scholar]
- Tapsall JW, Phillips EA. Presumptive identification of group B streptococci by rapid detection of CAMP factor and pigment production. Diagn Microbiol Infect Dis. 1987;7:225–228. doi: 10.1016/0732-8893(87)90010-1. [DOI] [PubMed] [Google Scholar]
- Tapsall JW, Phillips EA. The hemolytic and cytolytic activity of group B streptococcal hemolysin and its possible role in early onset group B streptococcal disease. Pathology. 1991;23:139–144. doi: 10.3109/00313029109060813. [DOI] [PubMed] [Google Scholar]
- Tazi A, Bellais S, Tardieux I, Dramsi S, Trieu-Cuot P, Poyart C. Group B Streptococcus surface proteins as major determinants for meningeal tropism. Curr Opin Microbiol. 2012;15:44–49. doi: 10.1016/j.mib.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Tettelin H, Masignani V, Cieslewicz MJ, et al. Complete genome sequence and comparative genomic analysis of an emerging human pathogen, serotype V Streptococcus agalactiae. P Natl Acad Sci USA. 2002;99:12391–12396. doi: 10.1073/pnas.182380799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettelin H, Masignani V, Cieslewicz MJ, et al. Genome analysis of multiple pathogenic isolates of Streptococcus agalactiae: implications for the microbial “pan-genome”. P Natl Acad Sci USA. 2005;102:13950–13955. doi: 10.1073/pnas.0506758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd EW. The differentiation of two distinct serologic varieties of streptolysin, streptolysin O and streptolysin S. J Pathol Bacteriol. 1938;47:423–445. [Google Scholar]
- Tsaihong JC, Wennerstrom DE. Effect of carrier molecules on production and properties of extracellular hemolysin produced by Streptococcus agalactiae. Curr Microbiol. 1983;9:333–338. [Google Scholar]
- Vanberg C, Lutnaes BF, Langsrud T, Nees IF, Holo H. Propionibacterium jensenii produces the polyene pigment granadaene and has hemolytic properties similar to those of Streptococcus agalactiae. Appl Environ Microbiol. 2007;75:5501–5506. doi: 10.1128/AEM.00545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedamuthu ER, Washam CJ, Reinbold GW. Isolation of inhibitory factor in raw milk whey active against propionibacteria. Appl Microbiol. 1971;22:552–556. doi: 10.1128/am.22.4.552-556.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verani JR, McGee L, Schrag SJ. Prevention of perinatal group B streptococcal disease revised guidelines from CDC. MMWR Recomm Rep. 2010;59:1–32. [PubMed] [Google Scholar]
- Wang B, Jian J, Lu Y, Cai S, Huang Y, Tang J, Wub Z. Complete genome sequence of Streptococcus agalactiae ZQ0910, a pathogen causing meningoencephalitis in the GIFT strain of Nile tilapia (Oreochromis niloticus. J Bacteriol. 2013;194:5132–5133. doi: 10.1128/JB.01080-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser JN, Rubens CE. Transposon mutagenesis of group B streptococcus beta-hemolysin biosynthesis. Infect Immun. 1987;55:2314–2316. doi: 10.1128/iai.55.9.2314-2316.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerstrom DE, Tsaihong JC. Evaluation of the role of hemolysin and pigment in pathogenesis of early-onset group B streptococcal infection. In: Shiokawa Y, Crawford JT, Kimura Y, Kotami S, editors. Recent Advances in Streptococci and Streptococcal Diseases. Bracknell: Redbooks; 1985. pp. 155–156. [Google Scholar]
- Wennerstrom DE, Lee LN, Basenan AG, Leblamc DJ, Cerniglia CE. Genetics and characterization of group B streptococcal pigment. In: McKey LL, Trotter KM, Dunny GM, Cleary PP, editors. Genetics and Molecular Biology of Streptococcus, Lactococcus and Enterococci. Washington, DC: ASM Press; 1991. pp. 224–227. [Google Scholar]
- Whidbey C, Harrell MI, Burnside K, Ngo L, Becraft AK, Iyer LM, Aravind L, Hitti J, Adams Waldorf KM, Rajagopal L. A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J Exp Med. 2013;210:1265–1281. doi: 10.1084/jem.20122753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair S, de Villiers EP, Fuxelius HH, Andersson G, Johansson KE, Bishop RP, Bongcam-Rudloffa E. Genome sequence of Streptococcus agalactiae strain 09mas018883, isolated from a Swedish cow. Genome Announc. 2013;1:e00456–13. doi: 10.1128/genomeA.00456-13. [DOI] [PMC free article] [PubMed] [Google Scholar]