Abstract
Gaucher's disease is caused by a deficiency of the lysosomal enzyme glucocerebrosidase (glucosylceramidase; D-glucosyl-N-acylsphingosine glucohydrolase, EC 3.2.1.45); this disorder has been a leading candidate for enzyme replacement trials. A rapid, high-yield method for purification of glucocerebrosidase has been developed. Detergent extraction of human placenta was followed by salt fractionation, concanavalin A-Sepharose chromatography, organic solvent precipitation, and affinity chromatography on phosphatidylserine-agarose; the total yield is 60% with 6000-fold purification. Purified glucocerebrosidase has been administered intravenously to a volunteer Gaucher's patient on two separate occasions. For the first injection, the enzyme was entrapped in resealed erythrocytes; for the second injection, the enzyme was given without any carrier. The enzyme infusions caused no untoward effects.
Full text
PDF
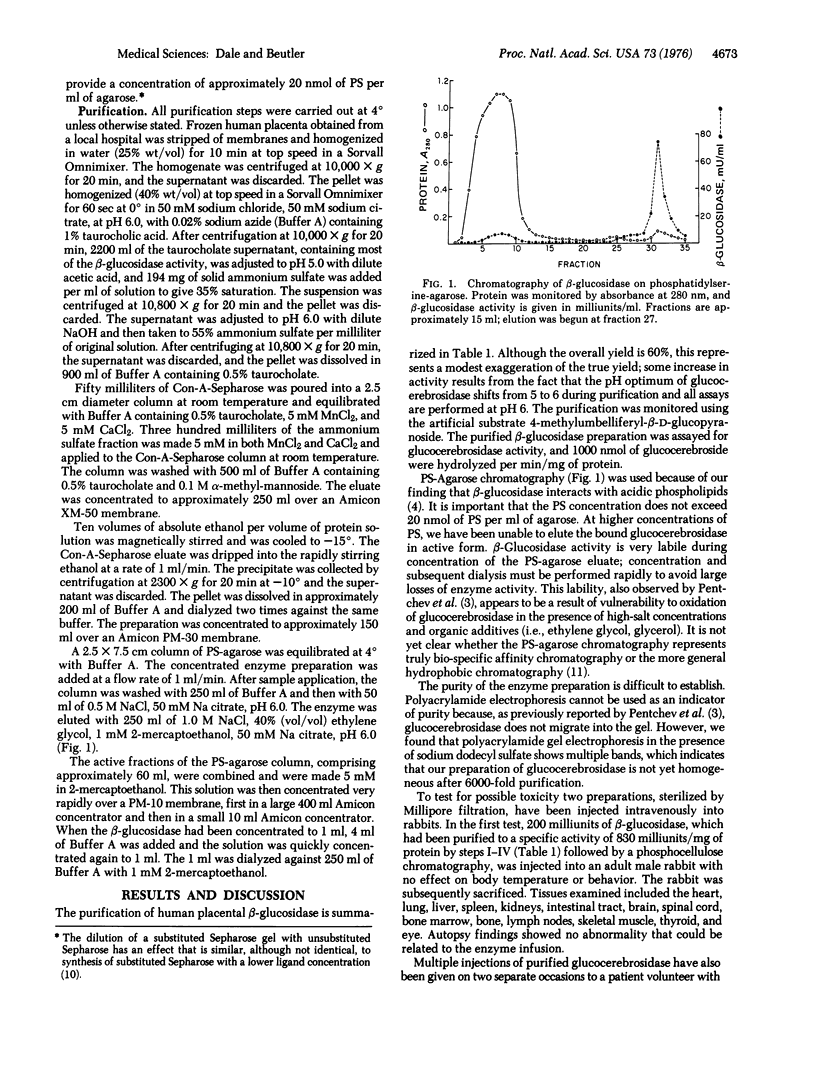
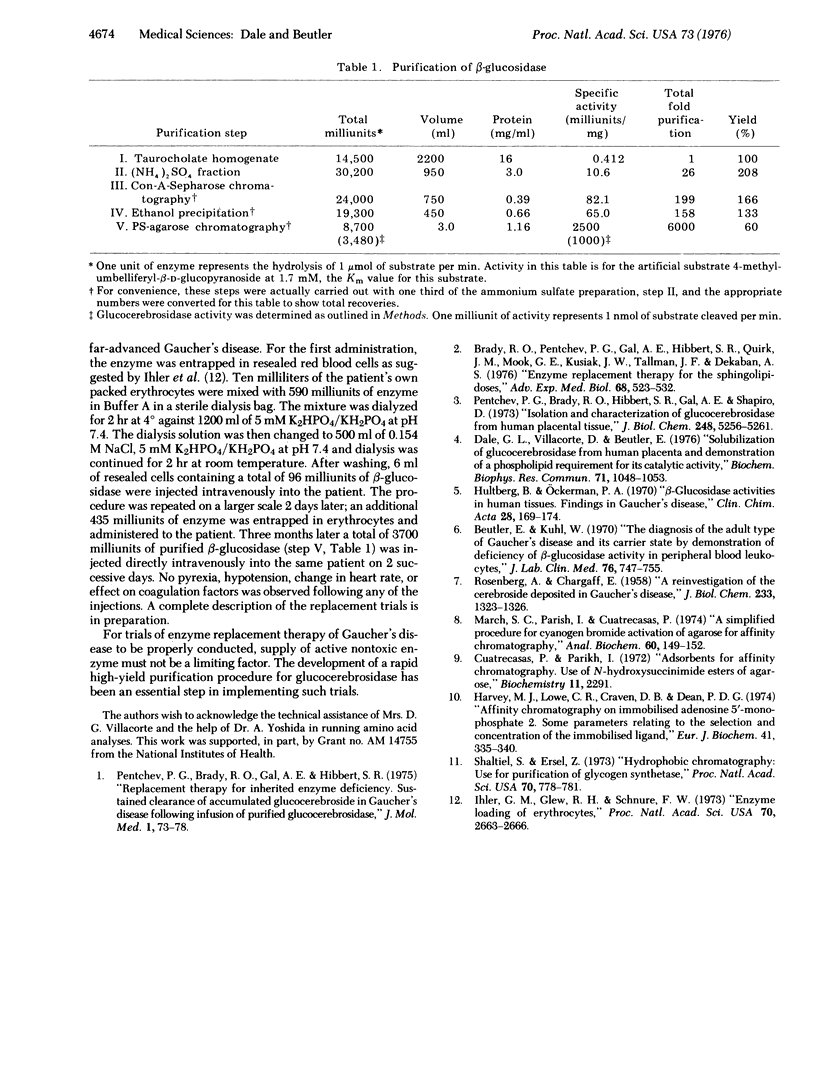
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E., Kuhl W. The diagnosis of the adult type of Gaucher's disease and its carrier state by demonstration of deficiency of beta-glucosidase activity in peripheral blood leukocytes. J Lab Clin Med. 1970 Nov;76(5):747–755. [PubMed] [Google Scholar]
- Brady R. O., Pentchev P. G., Gal A. E., Hibbert S. R., Quirk J. M., Mook G. E., Kusiak J. W., Tallman J. F., Dekaban A. S. Enzyme replacement therapy for the sphingolipidoses. Adv Exp Med Biol. 1976;68:523–532. doi: 10.1007/978-1-4684-7735-1_34. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Adsorbents for affinity chromatography. Use of N-hydroxysuccinimide esters of agarose. Biochemistry. 1972 Jun 6;11(12):2291–2299. doi: 10.1021/bi00762a013. [DOI] [PubMed] [Google Scholar]
- Dale G. L., Villacorte D. G., Beutler E. Solubilization of glucocerebrosidase from human placenta and demonstration of a phospholipid requirement for its catalytic activity. Biochem Biophys Res Commun. 1976 Aug 23;71(4):1048–1053. doi: 10.1016/0006-291x(76)90760-9. [DOI] [PubMed] [Google Scholar]
- Harvey M. J., Lowe C. R., Craven D. B., Dean P. D. Affinity chromatography on immobilised adenosine 5'-monophosphate. 2. Some parameters relating to the selection and concentration of the immobilised ligand. Eur J Biochem. 1974 Jan 16;41(2):335–340. doi: 10.1111/j.1432-1033.1974.tb03274.x. [DOI] [PubMed] [Google Scholar]
- Hultberg B., Ockerman P. A. Beta-glucosidase activities in human tissues. Findings in Gaucher's disease. Clin Chim Acta. 1970 Apr;28(1):169–174. doi: 10.1016/0009-8981(70)90176-2. [DOI] [PubMed] [Google Scholar]
- Ihler G. M., Glew R. H., Schnure F. W. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2663–2666. doi: 10.1073/pnas.70.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March S. C., Parikh I., Cuatrecasas P. A simplified method for cyanogen bromide activation of agarose for affinity chromatography. Anal Biochem. 1974 Jul;60(1):149–152. doi: 10.1016/0003-2697(74)90139-0. [DOI] [PubMed] [Google Scholar]
- Pentchev P. G., Brady R. O., Hibbert S. R., Gal A. E., Shapiro D. Isolation and characterization of glucocerebrosidase from human placental tissue. J Biol Chem. 1973 Aug 10;248(15):5256–5261. [PubMed] [Google Scholar]
- ROSENBERG A., CHARGAFF E. A reinvestigation of the cerebroside deposited in Gaucher's disease. J Biol Chem. 1958 Dec;233(6):1323–1326. [PubMed] [Google Scholar]
- Shaltiel S., Er-El Z. Hydrophobic chromatography: use for purification of glycogen synthetase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):778–781. doi: 10.1073/pnas.70.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]



