Abstract
Morphogenesis in fungi is often induced by extracellular factors and executed by fungal genetic factors. Cell surface changes and alterations of the microenvironment often accompany morphogenetic changes in fungi. In this review, we will first discuss the general traits of yeast and hyphal morphotypes and how morphogenesis affects development and adaptation by fungi to their native niches, including host niches. Then we will focus on the molecular machinery responsible for the two most fundamental growth forms, yeast and hyphae. Last, we will describe how fungi incorporate exogenous environmental and host signals together with genetic factors to determine their morphotype and how morphogenesis, in turn, shapes the fungal microenvironment.
Fungi integrate exogenous and host signals with genetic factors to determine their morphotype, allowing them to rapidly adapt to changing environmental conditions.
PROPERTIES OF MORPHOGENESIS IN FUNGAL CELLS
The defining morphogenetic characteristic of fungal cells is their polarity, whereby cell surface expansion and wall deposition are confined to discrete sites on the cell surface. Although hyphae and yeasts show obvious differences in their modes of growth (Fig. 1), they share three basic properties that enable polarized growth and the formation of a diverse array of cell shapes. The first is symmetry breaking, in which an initially isotropic cell generates an axis of polarized growth. The second is polarity maintenance, which refers to the stabilization of the polarity axis such that polar growth ensues. The third is depolarization, in which polarity is lost in a controlled manner. The balance between polarity maintenance and depolarization generates the diversity of fungal cell shapes.
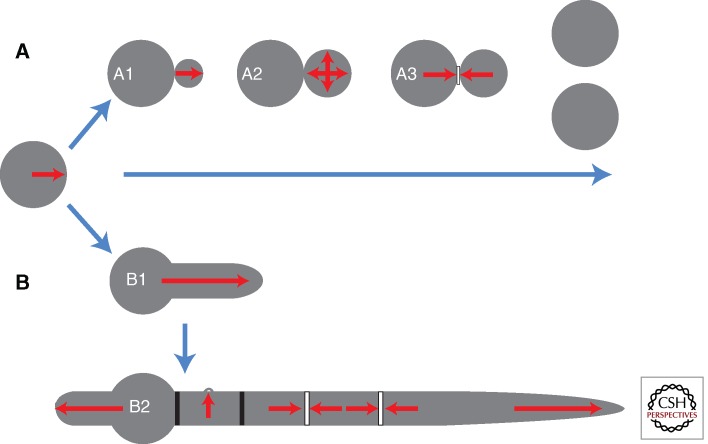
Figure 1.
Distinct patterns of morphogenesis in yeast and hyphal cells. Yeasts and filamentous fungi typically initiate growth as nonpolarized cells or spores. Budding yeasts such as Saccharomyces cerevisiae or Cryptococcus neoformans (A) establish an axis of polarity that directs the emergence of a new bud (A1). Following a period of polarized growth, depolarization enables the formation of an ellipsoidal bud (A2). Following nuclear division, the construction and controlled degradation of a septum (A3) results in cell separation. Filamentous fungi such Aspergillus nidulans and Candida albicans (B) establish a polarity axis that directs the emergence of a germ tube (B1). Unlike yeasts, sustained polar growth leads to the formation of a hypha that grows by apical extension. Moreover, hyphae are able to simultaneously support multiple polarity axes to allow the formation of septal cross-walls (bars) and lateral branches (B2). For many filamentous fungi, spores also generate secondary germ tubes once they are partitioned from the primary hypha by a septum.
Fungal cells, typically, are not polarized during the earliest phases of development. They usually undergo an initial period of nonpolar isotropic expansion (e.g., yeast mother cells, spores). Ultimately, however, cellular symmetry must be broken and a polarity axis generated, either for bud site selection or the development of polar structures, such as hyphae. One form of symmetry breaking relies on preexisting spatial landmarks that direct the recruitment of the morphogenetic machinery to a discrete site on the cell surface. The best-known example is the bud site selection process in Saccharomyces cerevisiae, which uses a set of landmark proteins to communicate positional information to the morphogenetic machinery via sequential GTPase modules (Chant 1999). In contrast, the fission yeast Schizosaccharomyces pombe uses cell end markers coupled to a microtubule-based delivery system to demarcate polarization sites (Martin 2009). Another form of symmetry breaking refers to the formation of a polar axis without any detectable, preexisting landmark (Wedlich-Soldner et al. 2003). Although a full consensus has yet to be reached regarding the specific details of this form of symmetry breaking (Johnson et al. 2011), it does involve the simultaneous action of multiple feedback loops that direct the accumulation of the activated form of the Cdc42 GTPase at a discrete site on the cell surface (Freisinger et al. 2013). Such feedback loops can be regulated to permit formation of multiple polarity axes based on studies in S. cerevisiae (Wu and Lew 2013). This might provide a basis for understanding how filamentous fungi can generate several hyphae from a single cell.
Once an axis of polarized growth has been generated, it must be stabilized to ensure that cell surface expansion and wall deposition are confined to a discrete site. The existence of a stable polarity axis is reflected by the resulting asymmetry of cellular organization (Riquelme 2013). Vesicle exocytosis is largely confined to that discrete site, which is flanked by endocytic zones in which membrane material and proteins are retrieved for recycling. Localized actin filaments support exocytosis, whereas actin patches mediate endocytosis. Arrays of cytoplasmic microtubules are organized in parallel to the polarity axis and, generally, mediate longer-range vesicle movements to and from the growth site. Localized changes in plasma membrane composition might be one broadly applied mechanism for maintaining polarity in both yeasts and hyphae. This is consistent with the recent observation that polarity factors are spatially segregated into distinct clusters on the cell surface in both S. pombe and S. cerevisiae (Dodgson et al. 2013). Although some of these features vary across different growth forms (i.e., yeast cells vs. hyphae), they appear to be universally coordinated by Cdc42/Rho1-related GTPase modules and their numerous effectors.
Whereas a great deal of attention has focused on how polarity is established in fungal cells, little has been devoted to understanding how fungal cells depolarize. Nevertheless, careful regulation of depolarization presumably underlies much of the variation in cell shape that is observed in fungi. In S. cerevisiae, depolarization manifests as the “apical-to-isotropic” switch that enables the formation of an ellipsoidal yeast cell as opposed to more elongated pseudohyphae (Bi and Park 2012). In contrast, hyphae, by definition, undergo sustained polarized growth. During subsequent developmental phases, such as conidiation, however, regulated depolarization undoubtedly plays a vital role in dictating the diverse patterns of cell division that result in taxonomically informative characters, such as conidial ontogeny and spore shape (Cole and Samson 1979).
Hyphal Morphogenesis
Hyphae are multicellular filaments in which symmetry breaking leads to the formation of a new hypha referred to as a germ tube or branch initial (Fig. 1). To date, there is no evidence supporting the existence of spatial landmarks that designate sites of germ tube or branch emergence. It seems reasonable that these sites are selected stochastically (e.g., spontaneous polarization). On the other hand, there is evidence suggesting that secondary polarization events in germinating spores are biased toward the pole that is opposite to the original polarization site (i.e., a bipolar polarization pattern) (Fig. 1) (Harris 1999). The basis of this bias remains unknown.
The continued growth of germ tubes and branch initials results in their maturation into hyphae. This generally correlates with an increase in the rate of apical extension (Horio and Oakley 2005), as well as the appearance of a Spitzenkörper (apical body). The latter structure is typically located proximal to the hyphal tip (Girbardt 1957; Sudbery 2011a), largely composed of vesicles of different sizes, microfilaments, and ribosomes (Harris et al. 2005; Verdin et al. 2009). An extensive body of data support the view that Spitzenkörper dynamics determine the shape of the hyphal tip, dictate the orientation of hyphal extension (Bartnicki-Garcia et al. 1995; Riquelme 2013), and help maximize rates of apical extension (Kohli et al. 2008). Nevertheless, the presence of a Spitzenkörper is not an obligate requirement for the formation of polarized hyphae. It should be noted that the Spitzenkörper exists within the context of a larger assembly that has been referred to as the hyphal tip complex (Taheri-Talesh et al. 2008). Components of this complex include the polarisome and exocyst, which localize to the extreme hyphal apex in which they regulate exocytosis, as well as the subapical collar that consists of actin patches and the endocytic machinery (Sudbery 2011a). As characterized in Aspergillus nidulans, precise spatial organization of the hyphal tip complex does seem to be a strict requirement for polarized hyphal growth. Thus, the Spitzenkörper might be a highly structured hyphal tip complex required for efficient apical growth.
A single fungal spore is capable of generating a mycelium composed of an extensive network of branched hyphae. At the margins of the colony, individual hyphae show an avoidance response, whereas in the colony interior, hyphal fusion (anastomosis) permits the exchange of nutrients and perhaps growth signals (Rayner 1996; Simonin et al. 2012). An important concept that underlies mycelium formation is apical dominance, which refers to the suppression of secondary polarity axes in the vicinity of an actively extending hyphal tip (Rayner 1991). Apical dominance enables the precise regulation of lateral branching by minimizing the competition between hyphal tips for existing resources. Although not well understood, the enforcement of apical dominance in some filamentous fungi requires the accumulation of reactive oxygen species at the hyphal tip (Tanaka et al. 2006; Semighini and Harris 2008). It seems likely that calcium gradients would also play a role in this process (Schmid and Harold 1988). Because of apical dominance, lateral branches often emerge from subapical hyphal compartments separated from the tip by a septum. In some filamentous fungi, septa conceivably could play a role as “branching landmarks” (Harris 2011a), although there appears to be no universal pattern of lateral branching. One distinct branching pattern observed in some hyphae is apical branching, whereby an existing hyphal tip “splits” into two distinct tips. This pattern of branching is shown by rapidly growing hyphae of Ashbya gossypii (Philippsen et al. 2005).
Yeast Morphogenesis in Ascomycetes
Yeasts generally divide either by budding or fission (Martin and Arkowitz 2014). Even in nearly spherical and symmetric yeast cells, cell polarity plays a major role in cell division and growth. The establishment of subcellular asymmetry is required in the context of the most polar events of yeast cell growth, for example, establishing the site of new bud emergence (Fig. 1). In S. cerevisiae, symmetry breaking leads to localized cell surface expansion and wall deposition at the incipient bud site. As a new daughter cell begins to form, the mother cell must direct the trafficking of new cellular material to this growing cell. Subsequently, the morphogenetic machinery then relocalizes to the mother–bud junction to enable septum formation and cytokinesis. With cytokinesis, there is a transition to the more symmetric phase of isotropic growth until the cells are ready for new budding events. Therefore, yeast cell division is dependent on a dynamic and ordered transition between cytoskeletal polarization and loss of polarization to complete its budding cell cycle (Fig. 1).
A fundamental feature that distinguishes yeasts from hyphae is the coupling of cell polarization to subsequent depolarization. The obstruction of polarized growth leads to a unicellular lifestyle. Moreover, nuclear division is tightly coordinated with cellular morphogenesis in yeast growth, such that the same cyclin-dependent protein kinase (CDK) complexes that drive cell-cycle progression also trigger the transitions between polar and nonpolar growth (Wang 2009; Howell and Lew 2012). Furthermore, morphogenetic checkpoints maintain the coordination of nuclear division with polar growth to prevent the formation of anucleate or binucleate cells (Howell and Lew 2012). In contrast, early morphogenetic events during germ tube emergence or branching initiation occur largely independent of nuclear division (Momany and Taylor 2000). It seems that coordination between septation and nuclear division and migration is dispensable in vegetative multinucleate hyphae. However, it is important to note that such coordination is required for the growth of heterokaryotic hyphae generated after mating events and critical for subsequent nuclear fusion and meiosis during sexual reproduction in filamentous fungi (both ascomycetes and basidiomycetes).
Yeast Morphogenesis in a Basidiomycete
Much of the work studying the cell division events for common pathogenic yeasts uses paradigms established in the ascomycete S. cerevisiae. However, basidiomycete yeasts show both conserved and distinct features of morphogenesis. By definition, a basidiomycete fungus (e.g., Cryptococcus neoformans) produces hyphal structures during sexual differentiation that distinguish it from ascomycetes (Kwon-Chung 1975, 1976). Spores produced by Cryptococcus hyphae are quite infectious and may be the primary particle inhaled during a natural infection (Giles et al. 2009; Velagapudi et al. 2009). Therefore, the transition to hyphal growth that supports sporulation is required for the wide dissemination of Cryptococcus in the environment, as well as for infection of its mammalian hosts.
Once inhaled in the lung, cryptococcal spores germinate to produce yeast cells. During the context of infection, this fungus grows within the human host almost exclusively in a budding, yeast-like form. Detailed histopathological studies have shown that hyphal forms are rarely encountered during human C. neoformans infections, with elongated fungal morphologies observed only as rare variants among clinical strains (Baker and Haugen 1955; Shadomy and Utz 1966). In addition to the typical yeast-cell budding dynamics, other morphological events are required for specific aspects of C. neoformans pathogenesis. In the lungs, small ovoid cryptococcal yeasts can undergo a morphological transition to form “giant” or “titan” cells (Zaragoza et al. 2010; Zaragoza and Nielsen 2013). In contrast to typical yeast cells, which measure 5 µm in diameter, C. neoformans titan cells can enlarge to sizes ranging from 15 to 100 µm. These giant cells are highly resistant to phagocytosis and reactive oxygen/nitrogen species, thus providing a more resilient form for early infection. Titan cells have polyploid nuclei, and undergo nuclear reduction to generate haploid progeny by unknown mechanisms (Zaragoza and Nielsen 2013). The process of titan cell formation is dependent on many central signaling proteins that control many aspects of cellular differentiation and morphogenesis, including pheromone receptors, PKA, the Rim101 transcription factor, GTPase-activating proteins Gap1 and Cnc1560, G1 cyclin Pcl103, and Rho104 GTPase (Okagaki et al. 2011).
The cellular events during C. neoformans yeast-cell morphogenesis have been well described. In general, cells proceed through a cycle of cell division with repeated asexual, clonal budding of a haploid yeast cell (Moore 2000). Several features distinguish the C. neoformans budding cycle from those of other pathogenic yeast cells. The single nucleus of the mother cell migrates into the daughter cell after initial budding begins. Mitosis then culminates in nuclear division with one of the new nuclei migrating back into the mother cell before final cell separation occurs. Also, in contrast to S. cerevisiae in which subsequent budding events occur adjacent to prior bud scars, C. neoformans seems to preferentially and repeatedly bud from the same site. This results in a feathering appearance of cell wall material accumulated at the site of a recent bud (Moore 2000; Adams 2004). For this reason, counting bud scars is unlikely to provide an accurate measure of cryptococcal cell age.
Evolution of Fungal Morphogenesis
An intriguing question in fungal morphogenesis is the evolutionary relationship between hyphae and yeasts. It seems likely that the cell morphology of ancestral fungi resembled protists (Jones et al. 2011), and hyphal growth emerged as the dominant growth form that facilitated the colonization of terrestrial habitats (Stajich et al. 2009). Yet, many filamentous fungi also show yeast-like patterns of cellular morphogenesis, particularly during development (Cole and Samson 1979). It seems reasonable that specific ecological niches favored the loss of hyphal growth in the subphylla Saccharomycotina and Taphrinamycotina (i.e., the clades containing S. cerevisiae and S. pombe, respectively), resulting in the evolution of clades in which yeast growth forms dominate (Harris 2011a,b). Nevertheless, many members of these clades retained the capacity to undergo hyphal (or pseudohyphal) growth in response to different environmental triggers. These so-called dimorphic fungi include many prominent human and plant pathogens whose morphotype transitions play critical roles in virulence.
THE MORPHOGENETIC MACHINERY
Vesicle Trafficking
Both yeasts and filamentous fungi possess exocytic vesicles of distinct classes based on their size and presumed content (Harsay and Bretscher 1995). In filamentous fungi, each class of exocytic vesicles appears to transit through the Spitzenkörper on its way to the apex of the hyphal tip. The localization of exocyst components to the apex suggests that this is the ultimate site in which vesicles fuse to the target plasma membrane (Taheri-Talesh et al. 2008). However, recent studies with Aspergillus oryzae point to the existence of additional secretion sites, including septa and subapical sites (Hayakawa et al. 2011). These observations imply that the trafficking of exocytic vesicles is far more complex than previously imagined.
The specificity of vesicle interactions with their target plasma membranes is likely governed by interactions between vesicle and target soluble NSF (N-ethylmaleimide-sensitive factor) attachment protein receptors (v- or t-SNAREs). Filamentous fungi possess a large complement of v-SNAREs and there appears to be some degree of redundancy in their function (Kuratsu et al. 2007). It should be noted that alternative routes for the delivery of contents to the cell surface might not rely on the classical secretion pathway (Rodrigues et al. 2011).
The Golgi complex serves a pivotal role in vesicle trafficking, as it is the source of exocytic vesicles and terminus of many endosomes. Thus, in the Golgi, different classes of vesicles must be sorted and matched to their correct cargoes. The importance of the Golgi to vesicle trafficking and morphogenesis has long been established in S. cerevisiae (Schekman and Novick 2004), but only recently has its importance for sustained polar growth been described for filamentous fungi (Pinar et al. 2013). Indeed, whereas the Spitzenkörper appears to be required primarily for rapid rates of hyphal extension, polarized growth fails completely in the absence of a functional Golgi complex.
The Cytoskeleton
The organization and function of both microfilaments and -tubules have been well characterized in yeasts and filamentous fungi. Formins assist nucleating microfilament formation at polarization sites (Evangelista et al. 2003; Pearson et al. 2004; Schmitz et al. 2006), but the formation of a stable axis of hyphal polarity can occur in their absence, as shown for A. nidulans (Sharpless and Harris 2002). On the other hand, regulators of actin patch formation and dynamics appear to be essential for the formation of a stable axis (Araujo-Bazan et al. 2008; Upadhyay and Shaw 2008; Hervas-Aguilar and Penalva 2010). These observations underscore the importance of endocytosis in polarized growth, but also imply that the localized delivery of cell wall material to polarization sites can occur in the absence of actin filaments. Limited evidence suggests that microtubules can potentially compensate for the absence of actin filaments (Virag et al. 2007). Normally, the key function of microtubules in hyphae appears to be positioning of the Spitzenkörper to enable proper orientation of hyphal extension (Fischer et al. 2008). However, in the absence of actin filaments at the tip, microtubules likely ensure sufficient vesicle flux to support apical extension, albeit at less than optimal rates.
Septins are highly conserved throughout fungi and animals, wherein they play multiple roles in cellular morphogenesis (Hall et al. 2008). In S. cerevisiae, septins form scaffolds that are essential for normal polarized growth, septation, and cytokinesis (Oh and Bi 2011). In filamentous fungi, septins are needed for normal septum formation and appear to delimit growth sites (DeMay et al. 2009; Lindsey et al. 2010; Ryder et al. 2013). For example, the absence of septins in A. nidulans leads to the simultaneous emergence of multiple germ tubes and hyperbranching. However, further maturation of branch initials into secondary hyphae is blocked. In general, septins function as scaffolds that coordinate localized cell wall biosynthesis with the cytoskeleton and vesicle trafficking machinery. Thus, septins likely consolidate and stabilize polarity axes, thereby preventing the formation of spurious ones.
Positional Markers
The only spatial landmarks that have been characterized in filamentous fungi are those that also function in S. cerevisiae and S. pombe. In budding yeast, the cell surface protein Axl2 acts in conjunction with the septin-associated proteins Bud3 and Bud4 to specify the axial budding pattern of mating-type a or α cells, whereas a distinct set of cell surface proteins (i.e., Bud8, Bud9, Rax1, Rax2) specify the bipolar pattern observed in a/α cells (Chant 1999). In either case, the landmarks trigger local activation of Cdc42 via the Ras-like GTPase Bud1 (Bi and Park 2012). In fission yeast, the plasma membrane-anchored protein Mod5 provides a target for the delivery of Tea1 via the plus ends of cytoplasmic microtubules (Snaith and Sawin 2003). This serves to orient microtubules and allows the accumulation of a formin-containing complex that enables localized microfilament formation (Martin 2009). The relationship between this pathway and Cdc42 in S. pombe is not clear, although some evidence suggests that they may act in parallel to promote cell polarity (Das et al. 2009).
Signal Transduction
Small GTPases, such as Ras, Rho, Cdc42, and Rac, play a fundamental role in the regulation of fungal morphogenesis. Typically, they act via multiple effectors to coordinate organization of the cytoskeleton and vesicle trafficking at polarization sites. Cdc42 effectors include p21-associated kinases such as Cla4, the Borg-related proteins Gic1 and Gic2, formins such as Bni1, Wiscott–Aldrich syndrome protein homologs such as Las17, and the exocyst (Park and Bi 2007). Rho GTPases have well-established roles in the regulation of β-glucan synthesis and cell wall integrity (Levin 2011). Less is known about potential effectors of Ras that might mediate polarized growth, particularly in filamentous fungi. It should be noted that there are examples of effectors with important roles in polarized hyphal growth that are not well conserved in S. cerevisiae. A particularly prominent example is NADPH oxidase, which appears to be an effector of Rac1 in filamentous fungi (Tanaka et al. 2006; Semighini and Harris 2008), in which it regulates seemingly diverse morphogenetic processes, such as lateral branching and infection-related morphogenesis (Tanaka et al. 2006; Egan et al. 2007). Small GTPases, such as Ras, Rho, and Cdc42, do not operate in isolation, but rather in a sequential manner as suggested by studies in S. cerevisiae and S. pombe. For example, considerable evidence shows that Ras GTPases function upstream of Cdc42 to regulate polarized growth (Chang et al. 1994), whereas Cdc42 and Rho1 may antagonize each other to spatially and temporally coordinate localized cell wall deposition (Gao et al. 2004). Collectively, these observations reinforce the central role of small GTPase modules as regulators of hyphal morphogenesis in fungi. Nevertheless, the extent to which individual GTPases contribute to specific aspects of morphogenesis appears to vary across fungal species.
The molecular events directing C. neoformans yeast cell polarity also possess conserved and unique features compared with ascomycetous yeasts. As in other fungi, the Ras1-Cdc24-signaling pathway appears to control many aspects of yeast-cell polarization and budding. Ras proteins control the activity of Cdc24, a guanine–nucleotide exchange factor, which, in turn, controls the activation of downstream effector proteins, such as Cdc42 (Zhao et al. 1995; Bassilana et al. 2003). In C. neoformans, mutation of either the RAS1 or CDC24 gene results in a mutant strain that cannot efficiently repolarize its actin cytoskeleton after exposure to stresses, such as elevated temperatures (37°C). This defect in cell polarity manifests as temperature sensitivity because of unchecked isotropic growth, with eventual arrest as a large, unbudded yeast (Alspaugh et al. 2000; Nichols et al. 2007). In addition to temperature elevations, the ras1 and cdc24 mutants are more susceptible to other physiologically relevant stresses, such as hypoxia (Nichols et al. 2007). Together, impaired resistance to conditions encountered in the host make these strains avirulent in animal models of cryptococcosis (Alspaugh et al. 2000; Nichols et al. 2007). Studies in these mutant strains, therefore, emphasize the role of proper cell morphogenesis and budding in survival within the host.
In S. cerevisiae, the Cdc42 protein is the major effector of Cdc24, as well as the primary determinant of cell polarity (Johnson 1999). In other fungi, especially those with more prominent hyphal morphological forms, the related Rac and Cdc42 proteins together direct cell polarity (Hurtado et al. 2000; Boyce et al. 2003; Chen and Dickman 2004; Vallim et al. 2005; Bassilana and Arkowitz 2006). C. neoformans is unique in that it possesses duplicated genes for Ras, Rac, and Cdc42 proteins (Ballou et al. 2013a). Recent studies have indicated that the Rac and Cdc42 proteins together serve as the downstream effectors of morphogenesis for C. neoformans Ras1 (Ballou et al. 2010, 2013a,b). For example, the CnRac proteins are required for the highly polar state of filamentous growth. Deletion of either the RAC1 or RAC2 gene results in the production of dysmorphic hyphae during mating (Ballou et al. 2013b). Moreover, overexpression of RAC genes complements the ras1 mutant defect in mating hyphal development (Ballou et al. 2013a). Subcellular localization of a Gfp–Rac2 fusion protein indicates enrichment at the site of early bud emergence and growing tip of the daughter cell, both sites of cell polarity (Fig. 2). Also, CnRac proteins are required for intracellular trafficking events, which are also important in the polarization process before cell division (Shen et al. 2012). The double rac1/rac2 mutation appears to be synthetically lethal, suggesting a central role for Rac proteins in cell division and basic growth (Ballou et al. 2013b).
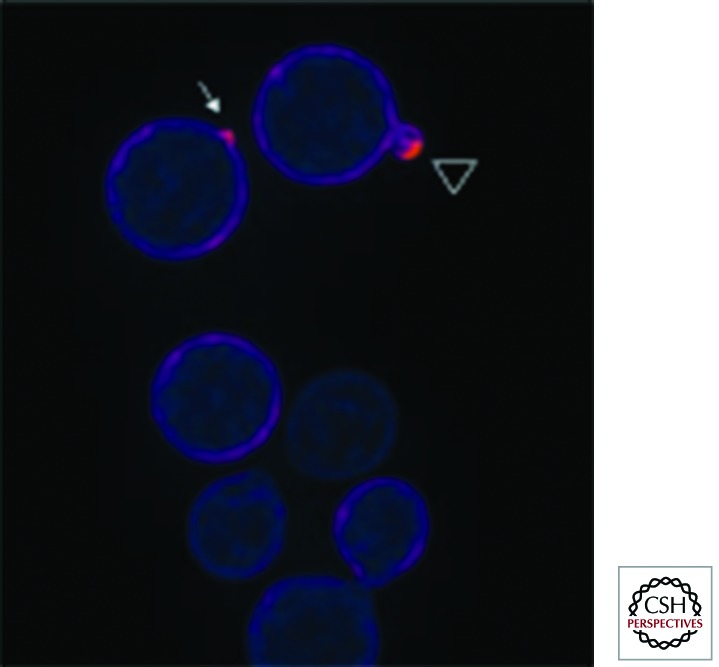
Figure 2.
C. neoformans Rac2 localization suggests a role in cell polarity. A Gfp–Rac2 fusion protein was expressed in C. neoformans and visualized using an Olympus (Center Valley, PA) IX70 microscope. After image acquisition and deconvolution of serial images in the Z-coordinate using Deltavision (GE Healthcare, Issaquah, WA) software, pseudocolored, merged images were produced using Fiji (Madison, WI) software. The most intense fluorescent signal, representing enrichment of Rac2 localization, is present at the site of incipient bud emergence (arrow) and distal edge of a small daughter cell (arrowhead). (Image provided by S. Esher and K. Selvig, Duke University.)
The duplicated Cdc42/Cdc420 proteins in C. neoformans are also important for cellular morphogenesis. These proteins direct the localization of septins to the site of cytokinesis (Ballou et al. 2013a). C. neoformans contains genes encoding homologs to the Cdc3, Cdc10, Cdc11, and Cdc12 septin proteins in S. cerevisiae (amino acid identity to S. cerevisiae proteins: 42%, 56%, 42%, and 49%, respectively). Unlike their S. cerevisiae orthologs, none of the C. neoformans septin genes is essential. However, the cdc3, cdc11, and cdc12 mutants fail to grow at 37°C, and the cdc10 mutant is modestly growth impaired at this higher temperature (Kozubowski and Heitman 2010). Similarly, mutation of either C. neoformans Ras or Cdc42 proteins results in mislocalized septins and failed cell separation under stress conditions. These strains are, therefore, poorly viable in animal models of cryptococcosis (Ballou et al. 2013a). Thus, Cdc42-mediated septin assembly is important for growth of Cryptococcus in vivo.
The complementary roles in morphogenesis for Cdc42 and Rac proteins have also been explored in C. albicans. Although the single Cdc42 ortholog is required for hyphal formation and pathogenesis, the C. albicans Rac protein is required only for polarized growth in a semisolid matrix. Moreover, despite high sequence conservation, overexpression of Rac or Cdc42-encoding genes does not cross-complement mutations of the other GTPase. Therefore, these two highly related proteins appear to serve distinct functions in the physiology of C. albicans (Bassilana and Arkowitz 2006).
MORPHOTYPE, NICHE ADAPTATION, AND FUNGAL VIRULENCE
Unlike in multicellular eukaryotes, in which most cells are typically embedded in a relatively constant environment, fungal cells face the challenge of unpredictable environmental fluctuations. Appropriate responses, including morphological changes, are often needed to survive, reproduce, and disperse. Cells in the hyphal form are inherently more effective in penetrating physical barriers and expanding colony growth three-dimensionally. Hyphae are also more likely to show gravitropism, thigmotropism, phototropism, aerotropism, and galvanotropism (Hoch et al. 1987; Crombie et al. 1990; Moore 1991; Moore et al. 1996; Aoki et al. 1998; Idnurm and Heitman 2005; Brand et al. 2007). In contrast, yeasts or yeast-like cells (including conidia, endospores, etc.) are superior in amplification, stress tolerance, and dispersal in liquid or air. The ability to switch morphotypes helps fungi to survive or escape otherwise suppressive environments, contributing to the success of this kingdom in the tree of life (Institute of Medicine (US) Forum on Microbial Threats 2011).
Morphotype switching is a prominent strategy adopted by phytopathogenic fungi for host invasion and dissemination. In Ophiostoma ulmi, the causal agent of Dutch elm disease, the mycelial form is required to penetrate adjacent xylem vessels. In contrast, the conidia/yeast form (conidia bud in a yeast-like fashion) is used to translocate within the individual xylem vessels via the host transpiration stream (Kulkarni and Nickerson 1981). Production of conidia or yeast-like blastoconidia in planta by Verticillium species is also implicated in the rapid dissemination of the pathogen within the host vascular tissue in diverse plant species (Schnathorst 1981; Pegg and Brady 2002). Mycosphaerella graminicola, an important wheat pathogen, switches from a yeast-like form to the infectious hyphal form to invade leaf tissue through stomata (Mehrabi and Kema 2006). In Taphrina deformans, the causal agent of leaf curl in peach and almond, yeast cells land on leaves and undergo a mitotic nuclear division to establish a binucleate condition. The resulting dikaryotic cells subsequently switch to parasitic filamentous growth in planta (Rodrigues and Fonseca 2003). Similarly, in the biotrophic maize pathogen Ustilago maydis, haploid yeast cells of compatible mating types fuse to form a dikaryon, which subsequently generates the infectious dikaryotic hyphae to continue growth and development in vivo (Banuett 1991). Although the hyphal form is used by many plant pathogens to invade the host, filamentous growth itself may not be adequate, or even required, for pathogenesis. For example, Holleya sinecauda, the dimorphic pathogen of mustard seeds, is isolated almost exclusively in the yeast form in plant lesions (Holley et al. 1984). Mutations that enable U. maydis to grow solely in a filamentous form do not support Ustilago pathogenicity (Barrett et al. 1993). Thus, either or both of the morphotypes could be associated with infection, depending on the infection strategy adopted by a particular species in a particular host microenvironment.
Likewise, the ability to undergo morphotype transitions is often required for human fungal pathogens to adapt to the host environment, elude host immune defense systems, and inhabit different niches in the host. The classic thermally dimorphic fungi, such as Histoplasma capsulatum, Blastomyces dermatitidis, Paracoccidioides brasiliensis, Coccidioides immitis, and Penicillium marneffei, grow as saprophytic molds in the environment. After host infection, conidia or hyphal fragments convert to yeasts to replicate intracellularly and disseminate through the host (Gauthier and Klein 2008). (See Sil and Andrianopoulos [2015] for details about the association between morphotype changes and virulence in these dimorphic human fungal pathogens.) However, the role of morphotype switching in pathogenesis goes beyond these classic dimorphic pathogens. For instance, Malassezia yeasts typically superficially colonize the skin and can also be taken up by keratinocytes to exist as facultative intracellular parasites. Excessive sebum from the host could induce hyphal formation in Malassezia globosa to reroot the infection in nutrient-rich, deep cornified layers (Brand 2012).
Similarly, the yeast form of C. albicans is associated with the commensal state, and the appearance of filaments is associated with invasive disease. The escape of C. albicans from phagocytic cells requires the switch from the yeast form to the hyphal form (Lorenz et al. 2004). Failure to sustain polarized hyphal growth leads to the entrapment of C. albicans intracellularly (Zakikhany et al. 2007). Although C. albicans hyphae predominate at the infiltration site, yeast cells are seen on the epithelial cell surface, as well as emerging from penetrating hyphae (Scherwitz 1982; Ray and Payne 1988). Yeast cells also produce proteins (e.g., Ywp1) that weaken adherence and thus promote dissemination (Granger et al. 2005). This bidirectional morphotype conversion likely boosts the ability of C. albicans to invade and disseminate.
Dermatophytes (e.g., Trichophyton rubrum) are known to grow as hyphae when infecting the stratum corneum or hair follicle. However, during invasive or deep dermal infections, dermatophytes also manifest morphological diversity as broad, pleomorphic hyphae with budding yeast–like arthrospores are observed (King et al. 1975; Bibel et al. 1977; Lillis et al. 2010; Marconi et al. 2010; Brand 2012). Such morphological switching in the dermatophyte Trichophyton mentagrophytes can also be observed under certain in vitro conditions (Bibel et al. 1977). In contrast, Cryptococcus species grow as encapsulated yeasts in animals/humans. Its filamentous form is only occasionally seen in host tissues and considered less virulent (Lin 2009; Magditch et al. 2012; Wang et al. 2012). For this intracellular pathogen, the ability to hijack host cells bypasses the requirement of the hyphal form for host invasion. Thus, Cryptococcus can translocate, invade, escape, and disseminate in the host, all in the yeast form.
In term of pathogenesis, fungal morphotype transitions can alter the host–pathogen interaction by the differential presentation of pathogen-associated molecular patterns in different morphotypes. For example, in B. dermatitidis, H. capsulatum, and P. brasiliensis, the transition from the hyphal to the pathogenic yeast form is accompanied by increased α-1,3-glucan deposition in the cell wall (Kanetsuna and Carbonell 1971; Seider et al. 2010), which masks the immunostimulatory β-glucan (Rappleye et al. 2007). Additionally, these different fungal morphotypes can display altered tolerance of certain host physiological conditions, as well as varying abilities to disseminate in different host tissues (Klein and Tebbets 2007). The pathogenic Histoplasma yeast cells, and not the saprophytic hyphal cells, secrete Cbp1 to acquire calcium to better survive within phagolysosomes (Batanghari et al. 1998). Cbp1 supports the growth of intracellular yeast cells and is required for Histoplasma virulence in animal models (Sebghati et al. 2000). Histoplasma yeast cells also produce Yps3 to promote extrapulmonary dissemination (Holbrook and Rappleye 2008). Similarly, the pathogenic Blastomyces yeast cells produce adhesin WI-1/Bad1 to suppress phagocyte proinflammatory responses, and Bad1 is indispensable for virulence of the fungus (Klein 2000; Rooney et al. 2001; Finkel-Jimenez et al. 2002).
The yeast and hyphal forms of C. albicans also differentially interact with the host and its immune cells. For example, the hyphal form of this fungus does not expose the immunostimulatory β-glucan (Gantner et al. 2005). Instead, it produces a suite of hypha-specific factors such as Als3, Hyr1, and Hwp1 to assist in host invasion (Staab et al. 1999; Luo et al. 2010). Als3 is an adhesin and invasin that induces host endocytosis (Hoyer et al. 1998; Argimon et al. 2007; Phan et al. 2007; Liu and Filler 2011). Als3 also helps Candida acquire iron by binding to host ferritin (Almeida et al. 2008).
In Aspergillus fumigatus, both hydrophobins and melanin coat the conidial surface to help the cell type evade host detection and remain protected within host phagolysosomes (Aimanianda et al. 2009; Volling et al. 2011; Carrion et al. 2013). On germination and hyphal growth, cell wall protein CspA is unmasked, helping to mitigate hyphal damage induced by neutrophils (Levdansky et al. 2010). Thus, factors produced during morphogenesis, in addition to the morphotype transition itself, help fungi adapt to host conditions.
ENVIRONMENTAL REGULATION OF HYPHAL MORPHOGENESIS
Sensing Nutritional and Environmental Signals
Pathogens must be proficient at sensing and adapting to their surroundings to survive changing host microenvironments. C. albicans cells undergo the yeast to hypha transition in response to many nutritional and environmental signals, including an increase in temperature to 37°C, neutral pH, serum, nutrients, N-acetylglucosamine (GlcNAc), hypoxia, and CO2. Sensors for many of these signals have been identified in C. albicans (Fig. 3) (Cottier and Muhlschlegel 2009). Many of the strong hypha-inducing signals are sensed and integrated by the adenylate cyclase Cyr1, which is indispensable for hyphal growth under all conditions (Bahn and Sundstrom 2001; Rocha et al. 2001; Hogan and Sundstrom 2009; Zou et al. 2009). CO2/HCO3− directly stimulates Cyr1p activity by binding to the catalytic domain of Cyr1 (Klengel et al. 2005). The cyclase activity is also regulated by the small GTPases (Ras1, Ras2), G-protein coupled receptor Gpr1, and Gα protein Gpa2 in response to nutrients (Feng et al. 1999; Sanchez-Martinez and Perez-Martin 2002; Miwa et al. 2004; Maidan et al. 2005a; Zhu et al. 2009). Gpr1 in C. albicans is responsive to methionine, but not to glucose (Maidan et al. 2005b), suggesting that this signal is likely encountered by this fungus in vivo. The rapid increase in temperature to 37°C is also essential for hyphal induction, likely by relieving Hsp90-mediated repression of the Ras1-Cyr1 pathway (Shapiro et al. 2009, 2012). The cAMP-dependent protein kinase A (PKA) is a major target of Cyr1, consisting of one regulatory subunit (Bcy1) and two catalytic subunits (Tpk1 and Tpk2). Each PKA subunit has distinct functions in C. albicans hyphal development, indicating even further adaptability of this otherwise highly conserved signaling system (Sonneborn et al. 2000; Bockmuhl et al. 2001; Cassola et al. 2004). A major function of the C. albicans cAMP-PKA pathway is to down-regulate the expression level of NRG1 (Lu et al. 2011), the major repressor of hyphal morphogenesis (Braun et al. 2001; Murad et al. 2001). This down-regulation of NRG1 expression requires the transcription factors Efg1 and Flo8 (Lu et al. 2011).
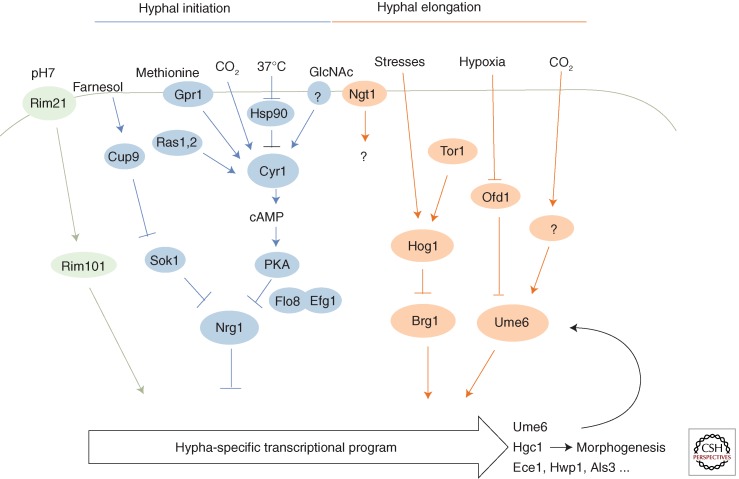
Figure 3.
Signal transduction pathways integrating various signals for morphogenesis in C. albicans. Selective signal transduction pathways and regulators of morphogenesis are shown. Arrows indicate activation. Bars indicate inhibition. Hyphal-specific regulator Ume6 sustains the hypha-specific transcriptional program and Hgc1 promotes hyphal morphogenesis.
The yeast-to-hypha transition in C. albicans must be initiated and then maintained (Lu et al. 2011). Hyphal initiation requires temporary clearing of Nrg1, which needs a transient activation of the cAMP-PKA pathway. In contrast, hyphal maintenance requires continuous and active sensing of the surrounding environment. During hyphal initiation, when the Nrg1 protein disappears, the expression of a GATA family transcription factor Brg1 is activated in response to serum, starvation, or treatment with rapamycin via reduced Tor1 signaling (Lu et al. 2011, 2012; Su et al. 2013). The accumulated Brg1 recruits the Hda1 histone deacetylase to promoters of hypha-specific genes, leading to nucleosome repositioning, obstruction of Nrg1 binding sites, and sustained hyphal development (Lu et al. 2011, 2012). The conserved Tor1-signaling pathway functions as a global regulator of cellular growth in response to nutrient availability, and it controls different cellular processes in fungi (Rohde and Cardenas 2004; Rohde et al. 2008). Therefore, hyphal development is controlled by two major nutrient-responsive and growth-regulating pathways.
These same signaling pathways have been co-opted by other pathogens to regulate different phenotypic outputs that are required for pathogenesis. In contrast to C. albicans, in which the yeast-hyphal morphological transition is essential for pathogenesis, Cryptococcus uses the cAMP-signaling axis to control expression of the polysaccharide capsule, a predominantly virulence-associated phenotype (Alspaugh et al. 1997). The hyphal transition during cryptococcal mating is also dependent on cAMP-Nrg1 signaling (Cramer et al. 2006), although the detailed signaling mechanisms of this observation have yet to be defined.
In addition to PKA signaling, other kinase-directed pathways also control fungal morphological transitions and host response. The high osmolarity glycerol (HOG) mitogen-associated protein kinase pathway plays a central role in stress responses in C. albicans (Alonso-Monge et al. 1999; Smith et al. 2004; Arana et al. 2005; Enjalbert et al. 2006). Hog1 is activated by osmotic, oxidative, and heavy metal stress. Hog1 is required for the survival of C. albicans cells when they encounter these host-relevant stresses. In contrast to stress-induced rapid Hog1 activation, rapamycin treatment leads to a down-regulation of Hog1 basal activity for a prolonged period of time through the functions of the two Hog1 tyrosine phosphatases, Ptp2 and Ptp3 (Su et al. 2013). Hog1 and its upstream kinases, Pbs2 and Ssk2, play a repressive role in hyphal elongation (Alonso-Monge et al. 1999; Eisman et al. 2006). Hog1 phosphorylation/activation in response to stresses, such as osmotic or oxidative stress, blocks hyphal maintenance (Su et al. 2013). This effect is dominant over rapamycin-induced Hog1 dephosphorylation and hyphal elongation. Active Hog1 represses the expression of BRG1 via the transcriptional repressor Sko1 (Su et al. 2013), which in turn represses the yeast-to-hypha transition (Alonso-Monge et al. 2010; Su et al. 2013). Therefore, reduced Tor1 signaling lowers Hog1 basal activity via Hog1 phosphatases to activate BRG1 expression for hyphal elongation. The HOG pathway is required for similar stress resistance phenotypes in other fungi such as C. neoformans. Also, mutations in HOG signaling result in repression of hyphal differentiation in C. neoformans mating reactions (Bahn et al. 2005), similar to its hyphal suppressive effects in C. albicans.
Other environmental factors also control morphological decisions in human fungal pathogens. The combination of hypoxia and high CO2, but neither condition alone, maintains C. albicans hyphal elongation, even in mutants lacking the nutrient responsive chromatin-remodeling pathway (Lu et al. 2013). One key downstream target of Hda1-mediated chromatin regulation is Ume6, a hypha-specific transcription factor that controls the level and duration of hypha-specific transcription (Banerjee et al. 2008; Carlisle et al. 2009; Zeidler et al. 2009; Lu et al. 2012). Ume6 is stabilized via regulation by Ofd1, a prolyl hydroxylase family member that is inhibited by hypoxia and an uncharacterized pathway that senses high CO2. The Ume6 stabilization and chromatin-remodeling pathways act in parallel to govern hyphal maintenance and elongation. Virulence and hyphal elongation in vivo are attenuated only when both pathways are blocked. C. albicans Ofd1 acts as an oxygen sensor that regulates hyphal development in a mechanism similar to that of S. pombe Ofd1. Both Ofd1 proteins have two functional domains: an amino-terminal dioxygenase domain with a conserved Fe2+ binding motif essential for O2 sensing and a carboxy-terminal domain that promotes protein degradation. The amino-terminal domain inhibits the degradative activity of the carboxy-terminal domain in hypoxia (Hughes and Espenshade 2008; Henri et al. 2010). The closest structural homolog to the amino-terminal dioxygenase domain is human PHD2cat, a prolyl 4-hydroxylase that acts as an oxygen-sensing component and hydroxylates the hypoxia-inducible transcription factor HIF1α in the presence of oxygen, thus leading to its degradation by the proteasome (Schofield and Ratcliffe 2005; Ozer and Bruick 2007; Henri et al. 2010). Ofd1 orthologs with sequence conservation in both amino- and carboxy-terminal domains are found only in fungi, including C. neoformans and A. fumigatus. The mechanism of CO2 sensing during hyphal elongation is not clear. CO2 is hydrolyzed into HCO3− inside the cell naturally and through the activity of carbonic anhydrase (Klengel et al. 2005). HCO3− then regulates hyphal morphogenesis through its activation of the adenylyl cyclase Cyr1 and, subsequently, the cAMP-PKA pathway (Klengel et al. 2005). HCO3− also signals independently of Cyr1 to regulate levels of carbonic anhydrase (Cottier et al. 2012) and promote hyphal development and cell-fate transition (Du et al. 2012a). Stabilization of Ume6 by CO2 is likely mediated through a Cyr1-independent pathway.
GlcNAc, unlike other sugars, stimulates the yeast-to-hypha transition in C. albicans by activating the Cyr1-PKA pathway (Castilla et al. 1998; Gunasekera et al. 2010) through unknown sensors/receptors. GlcNAc also activates a cAMP-independent pathway to induce the expression of genes needed to catabolize itself (Fig. 3) (Yamada-Okabe et al. 2001; Kumar et al. 2009; Gunasekera et al. 2010). One such gene, NGT1, encodes a GlcNAc transporter that mediates hyphal induction (Alvarez and Konopka 2007), suggesting that GlcNAc has to be internalized to induce signaling. GlcNAc is also a potent and specific inducer of the yeast-to-filament transition in the thermally dimorphic pathogens, H. capsulatum and B. dermatitidis (Gilmore et al. 2013). GlcNAc transporters, NGT1 and NGT2, are necessary for H. capsulatum cells to robustly filament in response to GlcNAc and in standard glucose medium, suggesting that Ngt1 and Ngt2 monitor endogenous levels of GlcNAc to control filamentous growth in response to temperature (Gilmore et al. 2013). Interestingly, GlcNAc metabolism is not required for GlcNAc signaling in these fungi (Naseem et al. 2011; Gilmore et al. 2013). Current data suggest that either internal GlcNAc acts as a signal or GlcNAc transporters can also function as a signal transducer. Future experiments are needed to differentiate these possibilities.
Extracellular pH is another important environmental factor that regulates fungal growth (Fig. 3). A fungal-specific signaling pathway, characterized by the activation of the Rim101/pacC transcription factor, controls the cellular response to changes in pH. First identified in S. cerevisiae and A. nidulans, this pathway is also conserved in fungal pathogens and controls important microbial interactions with the host environment. C. albicans grows in the yeast form in acidic conditions and forms hyphae in neutral/alkaline conditions. At neutral/alkaline pH, such as that encountered in the host, the Rim101 transcription factor is activated by proteolysis of its carboxy-terminal tail (Li and Mitchell 1997; Davis et al. 2000). Rim8, Rim13, Rim20, and Rim21 are upstream components required for Rim101 activation (Davis et al. 2000; Gomez-Raja and Davis 2012). The 7-transmembrane domain protein, Rim21, in the plasma membrane is the predicted sensor of pH. Mutation of any of these pH-responsive signaling elements in C. albicans results in failed morphogenesis and reduced virulence. In other ascomycetes, such as A. fumigatus, the Rim/Pal-signaling cascade is conserved and required for pathogenesis (Bignell 2012). Interestingly, the most upstream elements of this pathway are not clearly present in basidiomycetes, such as U. maydis and C. neoformans (Arechiga-Carvajal and Ruiz-Herrera 2005; O’Meara et al. 2013). Moreover, unlike in Candida species, in C. neoformans, the Rim/Pal-signaling cascade is not a significant regulator of the yeast–hyphal transition, although it does control major cell wall events, thereby influencing the host–pathogen interface.
Quorum Sensing
Quorum sensing is the regulation of gene expression and group behavior in response to changes in cell-population density. Quorum sensing in C. albicans was established based on the observation that dense cultures display a reduced propensity for the yeast-to-hypha switch (Hornby et al. 2001). The inhibitory activity is caused by the accumulation of a sesquiterpine alcohol, farnesol (Hornby et al. 2001), formed from an intermediate of the sterol biosynthesis pathway (Hornby et al. 2003). Therefore, farnesol is a quorum-sensing molecule secreted to the medium by C. albicans cells as a cell density signal (Hornby et al. 2001). At concentrations of 10–250 µm, farnesol inhibits hyphal initiation, but it does not suppress hyphal elongation (Mosel et al. 2005). Farnesol is reported to exert its inhibitory effects on germ-tube formation through Ras1-Cyr1 (Davis-Hanna et al. 2008). The release from farnesol inhibition when cells are inoculated from dense cultures into fresh media is essential for Nrg1 degradation, which requires the kinase Sok1 (Lu et al. 2014). The SOK1 transcriptional repressor, Cup9, is rapidly degraded on release from farnesol inhibition through the E3 ubiquitin ligase, Ubr1, leading to the derepression of SOK1 transcription and Nrg1 degradation (Lu et al. 2014). Therefore, the temporary clearing of Nrg1 protein during hyphal initiation is achieved by two independent regulations: the cAMP-PKA-dependent transcriptional down-regulation of NRG1 and degradation of Nrg1 protein triggered by the release from farnesol inhibition. Both pathways are required for the rapid clearing of Nrg1 protein and, thus, neither one is sufficient for hyphal initiation.
Contact Sensing
In C. albicans, the growth direction of hyphae can be dictated through contact with a surface (thigmotropism) (Gow et al. 1994). Thigmotropism is seen when growing hyphae come into contact with a ridge. Instead of continuing through the ridge, hyphae modify their growth direction (Brand et al. 2007). This response involves two plasma membrane proteins, Mid1 and Cch1, which are components of the high-affinity calcium uptake system, and Fig. 1, a member of the low-affinity calcium system (Brand et al. 2007). The Mid1/Cch1 complex has been suggested to act as a mechanosensitive channel that takes up calcium in response to contact. Future work needs to elucidate how a localized calcium signal can modulate the activity of the Cdc42-GTPase module at the cell apex for tip reorientation.
From Transcription to Hyphal Morphogenesis
Hyphal growth requires sustained activation of Cdc42 at the growing tips. This is achieved by hypha-specific expression of a G1-type cyclin protein Hgc1. The hypha-specific Cdk1Hgc1 phosphorylates and prevents Rgt2 GTPase-activating protein (GAP) from localizing to hyphal tips in which Cdc42 is concentrated, resulting in a local increase of Cdc42-GTP at hyphal tips (Zheng and Wang 2004; Zheng et al. 2007). Therefore, hypha-specific expression of Hgc1 is responsible for maintaining Cdc42-GTP at the hyphal tips and for sustained polarized growth in hyphae. In S. cerevisiae, Bem3 and Rga2 GAPs are hypophosphorylated at the time of bud emergence by Cdk1G1, and the inhibition of GAP activity by Cdk1G1 phosphorylation leads to increased amounts of Cdc42-GTP and actin polarization for bud emergence (Knaus et al. 2007; Sopko et al. 2007). Therefore, inhibition of GAPs via phosphorylation by G1/CDKs may be a conserved mechanism that promotes polarized growth in fungi (Wang 2009).
MORPHOGENESIS IN THE FUNGAL LIFE CYCLE
The morphotype switch in fungi is often a bidirectional reversible process. The plasticity in fungal morphological differentiation is very different from that in higher eukaryotes, in which cellular differentiation is often unidirectional and irreversible. The “totipotency” allows fungi to vary the mode of differentiation in response to external signals and timing of morphological transition in their life cycle. For instance, Aspergillus competent hyphae can maintain vegetative growth indefinitely, or switch to conidiophores for sporogenesis when environmental conditions become permissible (light and air, etc.). Conversely, Aspergillus conidiophores transferred to submerged culture will revert to vigorous hyphal growth. In the black ink mushroom Coprinopsis cinerea and S. cerevisiae, the addition of ammonium can revert a reproductive state to a vegetative one (Chiu and Moore 1988a). Because morphotype changes in fungi are often an environmentally controlled flexible process, continued development requires genetic reinforcement appropriate for the cellular microenvironment. For instance, sustained polarized hyphal growth requires hyphal initiation factors followed by hyphal maintenance factors, and some factors function at both stages of hyphal development (Momany et al. 1999; Harris and Momany 2004; Lu et al. 2011; Sudbery 2011b). In Cryptococcus, MATa and MATα isolates are both capable of initiating yeast to hypha transition (Tscharke et al. 2003; Lin et al. 2006), but the MATα allele is much more efficient in sustaining hyphal growth (Wickes et al. 1996; Lin et al. 2006), resulting in more efficient and robust fruiting in α isolates. In C. albicans, Ras1 and Efg1 are necessary to establish the hyphal growth; Bin1, Hda1, Cln1, Pes1, Eed1, and Ume6 are required to maintain hyphal growth; and Yap1 and Spa2 are involved in both the establishment and the maintenance of hyphal growth (Martin et al. 2005; Goyard et al. 2008; Lu et al. 2011; Jacobsen et al. 2012). Similarly, initiation and maintenance factors are required for sustained hyphal growth in Aspergillus (Momany 2002, 2005; Fortwendel et al. 2011).
It is important to note that not all fungal development is reversible. Irreversible commitment does happen, in some cases, during fungal differentiation. Fungal cells become competent when they have internalized necessary molecules in preparation for the next developmental stage. Morphogenesis is initiated when these competent cells are exposed to inducing conditions. Continued differentiation depends on the interaction of environmental factors, like light and nutrients, with genetic factors controlling differentiation. Commitment occurs when cells undergo certain differentiation steps irrespective of culture conditions. An irreversible state can be established when morphogenesis is coupled with the completion of the fungal life cycle (Madhani and Fink 1998). For example, U. maydis forms a dikaryon after two mating-compatible yeast cells fuse. The dikaryon switches to hyphal growth only after signals from the host plant release the fungus from cell-cycle arrest (Garcia-Muse et al. 2004; Heimel et al. 2010). Coprinopsis basidia are committed to meiosis and sporulation, and they will continue that development pathway even when excised from their parental fruit body (Chiu and Moore 1988b). (Please see Heitman et al. [2014] for more discussion on the link between morphogenesis and sexual reproduction.)
MORPHOGENESIS IN COMMUNITY DEVELOPMENT
Microbial communities are comprised of a heterogeneous population with variations in morphological or physiological states (Fig. 4). A population with cells poised for different contingencies permits the exploration of new niches or an increase in population fitness during perturbations (i.e., a bet-hedging strategy). For instance, a mature Aspergillus colony growing on a solid substrate is comprised of several hyphal types (invasive, interface, and aerial hyphae). The formation of septa allows contrasting patterns of differentiation to exist in neighboring cells in an interconnected mycelium that was derived from a single cell. Furthermore, mycelia give rise to aerial conidiophores with multiple cell types (stalks, vesicles, metulae, phialides, and conidia). Highly polarized growth occurs exclusively at hyphal tips, whereas budding/isotropic growth occurs at conidiophores. Similarly, C. albicans biofilm colonies often include coexisting cells of varying morphologies such as yeast, pseudohyphae, and hyphae. The proportion of each morphotype and architecture of the biofilm greatly depend on the environmental conditions and genetic makeup of the population (Daniels et al. 2013; Lin et al. 2013). A Cryptococcus mating colony also contains yeasts, hyphae, and basidiospores, with yeasts predominantly localized in the colony center and hyphae predominating at the periphery (Fig. 4).
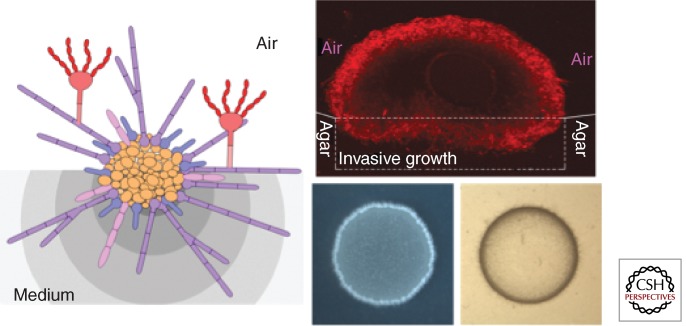
Figure 4.
A fungal colony is a heterogeneous population. The left panel shows a diagram of a fungal colony composed of multiple morphotypes based on what is known about Cryptococcus. Yeast cells (orange) populate the colony center, mixed with some pseudohyphae (pink), germ tubes (blue), and hyphae (purple), which predominate at the periphery of the colony. The filamentous cells also invade the medium (invasive hyphae) or extend into the air (aerial hyphae). Some aerial hyphae further develop into fruiting bodies and produce sexual spores (red). Different microenvironments in the medium are indicated with different shades of gray. Different cell types have different cell surface composition and structure. Even for the same morphotype, cells are phenotypically and physiologically different, based on their microenvironment and spatial position. The right upper panel shows a confocal image of a Cryptococcus colony derived from a single yeast cell. The bifunctional adhesion protein Cfl1 fused with m-Cherry fluorescent protein is highly expressed in the hyphal subpopulation located at the colony periphery. Yeast cells appear dark because of low levels of Cfl1 expression. The right lower panel shows the colony that developed after a yeast-cell suspension was dropped on the filamentation agar. The white fluffy appearance at the colony edge is caused by the presence of aerial hyphae. Invasive growth, detected as cells that remain when the surface is washed, is shown on the bottom right. The invasive cells, mostly in the germ tube or hyphal form at the periphery of the colony, penetrate into agar medium forming a ring-like footprint. (Images provided by L. Wang and X. Tian, Texas A&M University.)
Heterogeneity in gene expression serves as a foundation for the phenotypic heterogeneity in a community derived from otherwise genetically identical cells (Levy et al. 2012; Stovicek et al. 2012). The difference in gene expression among cells within a community could be caused by stochastic factors, determinant factors (e.g., age, cell-cycle stage), or a response to the microenvironment created by gradients of signaling molecules and the extracellular matrix (Kerr et al. 2002; Kim et al. 2008; Shank et al. 2011). For instance, stochastic expression of Wor1 determines white-opaque switch in Candida (Zordan et al. 2006; Tuch et al. 2010; Porman et al. 2013), and adhesin Cfl1 in the extracellular matrix stimulates morphogenesis from yeast to hypha in Cryptococcus (Wang et al. 2013). The ability to have mixed yeast and hyphal morphotypes was recently shown to enhance Cryptococcus competitiveness in vitro (Phadke et al. 2013).
There is an intimate connection between fungal morphogenesis and fungal community development. For instance, genes and compounds that control morphogenesis often also control biofilm development (Giacometti et al. 2011; Du et al. 2012b; Tsang et al. 2012; Connolly et al. 2013; Fazly et al. 2013). Not surprisingly, 68 out of the 122 genes that are involved in biofilm formation are also involved in filamentous growth in C. albicans (Inglis et al. 2012). Some of these factors, such as Ras1, Cyr1/Cdc35, and Efg1, regulate Candida biofilm formation, filamentous growth, phenotypic switching, and pathogenesis (Inglis et al. 2012). In Cryptococcus, Znf2, the decision maker of the yeast to hypha transition and the mediator of the cryptococcal ability to cause disease, regulates more than half of the potential adhesins encoded in the genome (Wang et al. 2012, 2013; L Wang and X Lin, unpubl.). The Znf2 downstream adhesin Cfl1 also regulates the yeast–hypha transition (Fig. 4) (Wang et al. 2013). Although filamentous growth is not essential for the formation of biofilms (Hawser and Douglas 1994; Reynolds and Fink 2001; Martinez and Casadevall 2007; Cushion et al. 2009), hyphae strengthen the biofilm structure and provide strong and expanded scaffolds for the deposition of extracellular matrix materials and other cells (Ramage et al. 2002; Richard et al. 2005). Hyphae of C. albicans are known to penetrate host tissues and even denture materials, and thus it is not surprising that there are many hyphal-specific adhesins that help anchor hyphae firmly to the substrate. Similarly, in T. mentagrophytes, hyphae produce adhesion molecules to help the fungus attach to the skin surface (Kaufman et al. 2007). Even in biofilms composed of Aspergillus hyphae, the hyphal organization in hyphae formed during aspergilloma infection differs from that formed during invasive aspergillosis. A spheroid mass of highly agglutinated hyphae with increased α-1,3-glucans is present in the former, whereas individual separated hyphae predominate in the latter (Loussert et al. 2010). An in-depth discussion of the genetic regulation of biofilms can be found in Desai et al. (2014).
CONCLUSION
Morphological transitions are commonly observed among diverse fungal species. These cellular responses are accompanied by equally profound changes in cell physiology and structure. As noted above, the highly regulated process of fungal morphogenesis represents an adaptive response to specific stresses encountered in various microenvironments, including that of the infected host. Therefore, morphological and physiological plasticity allows fungi to rapidly adapt to changing extracellular conditions. Species-specific signaling and morphological features appear to be a direct result of fungal attempts to survive as new microenvironments, and their particular cell stresses, were encountered (Hogan and Klein 1994; Newman et al. 1995; Batanghari et al. 1998; Sebghati et al. 2000; Gow et al. 2002; Brandhorst et al. 2004; Rappleye et al. 2004, 2007; Gantner et al. 2005; Nemecek et al. 2006; Gauthier and Klein 2008; Nather and Munro 2008; Mora-Montes et al. 2011; Wang and Lin 2012; Wang et al. 2012). The concerted action of morphotype and physiological changes in the context of a particular environment are therefore critical for successful fungal adaptation (Butler et al. 2009; O’Connor et al. 2010). Defining the cellular machinery controlling fungal morphogenesis offers unique insight into our basic understanding of fungal life cycles and pathogenesis.
Footnotes
Editors: Arturo Casadevall, Aaron P. Mitchell, Judith Berman, Kyung J. Kwon-Chung, John R. Perfect, and Joseph Heitman
Additional Perspectives on Human Fungal Pathogens available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Adams DJ 2004. Fungal cell wall chitinases and glucanases. Microbiol 150: 2029–2035. [DOI] [PubMed] [Google Scholar]
- Aimanianda V, Bayry J, Bozza S, Kniemeyer O, Perruccio K, Elluru SR, Clavaud C, Paris S, Brakhage AA, Kaveri SV, et al. 2009. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nature 460: 1117–1121. [DOI] [PubMed] [Google Scholar]
- Almeida RS, Brunke S, Albrecht A, Thewes S, Laue M, Edwards JE, Filler SG, Hube B 2008. The hyphal-associated adhesin and invasin Als3 of Candida albicans mediates iron acquisition from host ferritin. PLoS Pathog 4: e1000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Monge R, Navarro-Garcia F, Molero G, Diez-Orejas R, Gustin M, Pla J, Sanchez M, Nombela C 1999. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol 181: 3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Monge R, Roman E, Arana DM, Prieto D, Urrialde V, Nombela C, Pla J 2010. The Sko1 protein represses the yeast-to-hypha transition and regulates the oxidative stress response in Candida albicans. Fungal Genet Biol 47: 587–601. [DOI] [PubMed] [Google Scholar]
- Alspaugh JA, Perfect JR, Heitman J 1997. Cryptococcus neoformans mating and virulence are regulated by the G-protein α subunit GPA1 and cAMP. Genes Dev 11: 3206–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alspaugh JA, Cavallo LM, Perfect JR, Heitman J 2000. RAS1 regulates filamentation, mating and growth at high temperature of Cryptococcus neoformans. Mol Microbiol 36: 352–365. [DOI] [PubMed] [Google Scholar]
- Alvarez FJ, Konopka JB 2007. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol Biol Cell 18: 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki S, Ito-Kuwa S, Nakamura K, Vidotto V, Takeo K 1998. Oxygen as a possible tropic factor in hyphal growth of Candida albicans. Mycoscience 39: 231–238. [Google Scholar]
- Arana DM, Nombela C, Alonso-Monge R, Pla J 2005. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiol 151: 1033–1049. [DOI] [PubMed] [Google Scholar]
- Araujo-Bazan L, Penalva MA, Espeso EA 2008. Preferential localization of the endocytic internalization machinery to hyphal tips underlies polarization of the actin cytoskeleton in Aspergillus nidulans. Mol Microbiol 67: 891–905. [DOI] [PubMed] [Google Scholar]
- Arechiga-Carvajal ET, Ruiz-Herrera J 2005. The RIM101/pacC homologue from the basidiomycete Ustilago maydis is functional in multiple pH-sensitive phenomena. Eukaryot Cell 4: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argimon S, Wishart JA, Leng R, Macaskill S, Mavor A, Alexandris T, Nicholls S, Knight AW, Enjalbert B, Walmsley R, et al. 2007. Developmental regulation of an adhesin gene during cellular morphogenesis in the fungal pathogen Candida albicans. Eukaryot Cell 6: 682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn YS, Sundstrom P 2001. CAP1, an adenylate cyclase-associated protein gene, regulates bud-hypha transitions, filamentous growth, and cyclic AMP levels and is required for virulence of Candida albicans. J Bacteriol 183: 3211–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn YS, Kojima K, Cox GM, Heitman J 2005. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Mol Biol Cell 16: 2285–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RD, Haugen RK 1955. Tissue changes and tissue diagnosis in cryptococcosis: A study of twenty-six cases. Am J Clin Pathol 25: 14–24. [DOI] [PubMed] [Google Scholar]
- Ballou ER, Nichols CB, Miglia KJ, Kozubowski L, Alspaugh JA 2010. Two CDC42 paralogues modulate Cryptococcus neoformans thermotolerance and morphogenesis under host physiological conditions. Mol Microbiol 75: 763–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou ER, Kozubowski L, Nichols CB, Alspaugh JA 2013a. Ras1 acts through duplicated Cdc42 and Rac proteins to regulate morphogenesis and pathogenesis in the human fungal pathogen Cryptococcus neoformans. PLoS Genet 9: e1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou ER, Selvig K, Narloch JL, Nichols CB, Alspaugh JA 2013b. Two Rac paralogs regulate polarized growth in the human fungal pathogen Cryptococcus neoformans. Fungal Genet Biol 57: 58–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M, Thompson DS, Lazzell A, Carlisle PL, Pierce C, Monteagudo C, Lopez-Ribot JL, Kadosh D 2008. UME6, a novel filament-specific regulator of Candida albicans hyphal extension and virulence. Mol Biol Cell 19: 1354–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F 1991. Identification of genes governing filamentous growth and tumor induction by the plant pathogen Ustilago maydis. Proc Natl Acad Sci 88: 3922–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett KJ, Gold SE, Kronstad JW 1993. Identification and complementation of a mutation to constitutive filamentous growth in Ustilago maydis. Mol Plant Microbe Interact 6: 274–283. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S, Bartnicki DD, Gierz G, Lopez-Franco R, Bracker CE 1995. Evidence that Spitzenkorper behavior determines the shape of a fungal hypha: A test of the hyphoid model. Exp Mycol 19: 153–159. [DOI] [PubMed] [Google Scholar]
- Bassilana M, Arkowitz RA 2006. Rac1 and Cdc42 have different roles in Candida albicans development. Eukaryot Cell 5: 321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassilana M, Blyth J, Arkowitz RA 2003. Cdc24, the GDP-GTP exchange factor for Cdc42, is required for invasive hyphal growth of Candida albicans. Eukaryot Cell 2: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batanghari JW, Deepe GS Jr, Di Cera E, Goldman WE 1998. Histoplasma acquisition of calcium and expression of CBP1 during intracellular parasitism. Mol Microbiol 27: 531–539. [DOI] [PubMed] [Google Scholar]
- Bi E, Park HO 2012. Cell polarization and cytokinesis in budding yeast. Genetics 191: 347–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel DJ, Crumrine DA, Yee K, King RD 1977. Development of arthrospores of Trichophyton mentagrophytes. Infect Immun 15: 958–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell EM 2012. Conservation in Aspergillus fumigatus of pH-signaling seven transmembrane domain and arrestin proteins, and implications for drug discovery. Ann NY Acad Sci 1273: 35–43. [DOI] [PubMed] [Google Scholar]
- Bockmuhl DP, Krishnamurthy S, Gerads M, Sonneborn A, Ernst JF 2001. Distinct and redundant roles of the two protein kinase A isoforms Tpk1p and Tpk2p in morphogenesis and growth of Candida albicans. Mol Microbiol 42: 1243–1257. [DOI] [PubMed] [Google Scholar]
- Boyce KJ, Hynes MJ, Andrianopoulos A 2003. Control of morphogenesis and actin localization by the Penicillium marneffei RAC homolog. J Cell Sci 116: 1249–1260. [DOI] [PubMed] [Google Scholar]
- Brand A 2012. Hyphal growth in human fungal pathogens and its role in virulence. Int J Microbiol 2012: 517529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A, Shanks S, Duncan VM, Yang M, Mackenzie K, Gow NA 2007. Hyphal orientation of Candida albicans is regulated by a calcium-dependent mechanism. Current Biol 17: 347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhorst TT, Wuthrich M, Finkel-Jimenez B, Warner T, Klein BS 2004. Exploiting type 3 complement receptor for TNF-α suppression, immune evasion, and progressive pulmonary fungal infection. J Immunol 173: 7444–7453. [DOI] [PubMed] [Google Scholar]
- Braun BR, Kadosh D, Johnson AD 2001. NRG1, a repressor of filamentous growth in C. albicans, is down-regulated during filament induction. EMBO J 20: 4753–4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, et al. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459: 657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle PL, Banerjee M, Lazzell A, Monteagudo C, Lopez-Ribot JL, Kadosh D 2009. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc Natl Acad Sci 106: 599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion Sd J, Leal SM Jr, Ghannoum MA, Aimanianda V, Latgé JP, Pearlman E 2013. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol 191: 2581–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassola A, Parrot M, Silberstein S, Magee BB, Passeron S, Giasson L, Cantore ML 2004. Candida albicans lacking the gene encoding the regulatory subunit of protein kinase A displays a defect in hyphal formation and an altered localization of the catalytic subunit. Eukaryot Cell 3: 190–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castilla R, Passeron S, Cantore ML 1998. N-acetyl-d-glucosamine induces germination in Candida albicans through a mechanism sensitive to inhibitors of cAMP-dependent protein kinase. Cell Signal 10: 713–719. [DOI] [PubMed] [Google Scholar]
- Chang EC, Barr M, Wang Y, Jung V, Xu HP, Wigler MH 1994. Cooperative interaction of S. pombe proteins required for mating and morphogenesis. Cell 79: 131–141. [DOI] [PubMed] [Google Scholar]
- Chant J 1999. Cell polarity in yeast. Annu Rev Cell Dev Biol 15: 365–391. [DOI] [PubMed] [Google Scholar]
- Chen C, Dickman MB 2004. Dominant active Rac and dominant negative Rac revert the dominant active Ras phenotype in Colletotrichum trifolii by distinct signalling pathways. Mol Microbiol 51: 1493–1507. [DOI] [PubMed] [Google Scholar]
- Chiu SW, Moore D 1988a. Ammonium ions and glutamine inhibit sporulation of Coprinus cinereus basidia assayed in vitro. Cell Biol Int Rep 12: 519–526. [DOI] [PubMed] [Google Scholar]
- Chiu SW, Moore D 1988b. Evidence for developmental commitment in the differentiating fruit body of Coprinus cinereus. Trans Br Mycol Soc 90: 247–253. [Google Scholar]
- Cole GT, Samson RA 1979. Patterns of development in conidial fungi. Pitman, London. [Google Scholar]
- Connolly LA, Riccombeni A, Grozer Z, Holland LM, Lynch DB, Andes DR, Gacser A, Butler G 2013. The APSES transcription factor Efg1 is a global regulator that controls morphogenesis and biofilm formation in Candida parapsilosis. Mol Microbiol 90: 36–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottier F, Muhlschlegel FA 2009. Sensing the environment: Response of Candida albicans to the X factor. FEMS Microbiol Lett 295: 1–9. [DOI] [PubMed] [Google Scholar]
- Cottier F, Raymond M, Kurzai O, Bolstad M, Leewattanapasuk W, Jimenez-Lopez C, Lorenz MC, Sanglard D, Vachova L, Pavelka N, et al. 2012. The bZIP transcription factor Rca1p is a central regulator of a novel CO2 sensing pathway in yeast. PLoS Pathog 8: e1002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer KL, Gerrald QD, Nichols CB, Price MS, Alspaugh JA 2006. Transcription factor Nrg1 mediates capsule formation, stress response, and pathogenesis in Cryptococcus neoformans. Eukaryot Cell 5: 1147–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombie T, Gow NA, Gooday GW 1990. Influence of applied electrical fields on yeast and hyphal growth of Candida albicans. J Gen Microbiol 136: 311–317. [DOI] [PubMed] [Google Scholar]
- Cushion MT, Collins MS, Linke MJ 2009. Biofilm formation by Pneumocystis spp. Eukaryot Cell 8: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels KJ, Park YN, Srikantha T, Pujol C, Soll DR 2013. Impact of environmental conditions on the form and function of Candida albicans biofilms. Eukaryot Cell 12: 1389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Wiley DJ, Chen X, Shah K, Verde F 2009. The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42. Curr Biol 19: 1314–1319. [DOI] [PubMed] [Google Scholar]
- Davis D, Wilson RB, Mitchell AP 2000. RIM101-dependent and -independent pathways govern pH responses in Candida albicans. Mol Cell Biol 20: 971–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Hanna A, Piispanen AE, Stateva LI, Hogan DA 2008. Farnesol and dodecanol effects on the Candida albicans Ras1-cAMP signalling pathway and the regulation of morphogenesis. Mol Microbiol 67: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMay BS, Meseroll RA, Occhipinti P, Gladfelter AS 2009. Regulation of distinct septin rings in a single cell by Elm1p and Gin4p kinases. Mol Cell Biol 20: 2311–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Desai JV, Mitchell AP, Andes DR 2014. Fungal biofilms, drug resistance, and recurrent infection. Cold Spring Harbor Perspect Med 4: a019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson J, Chessel A, Yamamoto M, Vaggi F, Cox S, Rosten E, Albrecht D, Geymonat M, Csikasz-Nagy A, Sato M, et al. 2013. Spatial segregation of polarity factors into distinct cortical clusters is required for cell polarity control. Nat Commun 4: 1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guan G, Xie J, Cottier F, Sun Y, Jia W, Muhlschlegel FA, Huang G 2012a. The transcription factor Flo8 mediates CO2 sensing in the human fungal pathogen Candida albicans. Mol Biol Cell 23: 2692–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Guan G, Xie J, Sun Y, Tong Y, Zhang L, Huang G 2012b. Roles of Candida albicans Gat2, a GATA-type zinc finger transcription factor, in biofilm formation, filamentous growth and virulence. PloS ONE 7: e29707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ 2007. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci 104: 11772–11777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisman B, Alonso-Monge R, Roman E, Arana D, Nombela C, Pla J 2006. The Cek1 and Hog1 mitogen-activated protein kinases play complementary roles in cell wall biogenesis and chlamydospore formation in the fungal pathogen Candida albicans. Eukaryot Cell 5: 347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJ, Quinn J 2006. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 17: 1018–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evangelista M, Zigmond S, Boone C 2003. Formins: Signaling effectors for assembly and polarization of actin filaments. J Cell Sci 116: 2603–2611. [DOI] [PubMed] [Google Scholar]
- Fazly A, Jain C, Dehner AC, Issi L, Lilly EA, Ali A, Cao H, Fidel PL Jr, Kaufman RPRPD 2013. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci 110: 13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q, Summers E, Guo B, Fink G 1999. Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol 181: 6339–6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel-Jimenez B, Wuthrich M, Klein BS 2002. BAD1, an essential virulence factor of Blastomyces dermatitidis, suppresses host TNF-α production through TGF-β-dependent and -independent mechanisms. J Immunol 168: 5746–5755. [DOI] [PubMed] [Google Scholar]
- Fischer R, Zekert N, Takeshita N 2008. Polarized growth in fungi—Interplay between the cytoskeleton, positional markers and membrane domains. Mol Microbiol 68: 813–826. [DOI] [PubMed] [Google Scholar]
- Fortwendel JR, Juvvadi PR, Rogg LE, Steinbach WJ 2011. Regulatable Ras activity is critical for proper establishment and maintenance of polarity in Aspergillus fumigatus. Eukaryot Cell 10: 611–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freisinger T, Klunder B, Johnson J, Muller N, Pichler G, Beck G, Costanzo M, Boone C, Cerione RA, Frey E, et al. 2013. Establishment of a robust single axis of cell polarity by coupling multiple positive feedback loops. Nat Commun 4: 1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantner BN, Simmons RM, Underhill DM 2005. Dectin-1 mediates macrophage recognition of Candida albicans yeast but not filaments. EMBO J 24: 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XD, Caviston JP, Tcheperegine SE, Bi E 2004. Pxl1p, a paxillin-like protein in Saccharomyces cerevisiae, may coordinate Cdc42p and Rho1p functions during polarized growth. Mol Biol Cell 15: 3977–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Muse T, Steinberg G, Perez-Martin J 2004. Characterization of B-type cyclins in the smut fungus Ustilago maydis: Roles in morphogenesis and pathogenicity. J Cell Sci 117: 487–506. [DOI] [PubMed] [Google Scholar]
- Gauthier G, Klein BS 2008. Insights into fungal morphogenesis and immune evasion. Microbe 3: 416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti R, Kronberg F, Biondi RM, Passeron S 2011. Candida albicans Tpk1p and Tpk2p isoforms differentially regulate pseudohyphal development, biofilm structure, cell aggregation and adhesins expression. Yeast 28: 293–308. [DOI] [PubMed] [Google Scholar]
- Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun 77: 3491–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore SA, Naseem S, Konopka JB, Sil A 2013. N-acetylglucosamine (GlcNAc) triggers a rapid, temperature-responsive morphogenetic program in thermally dimorphic fungi. PLoS Genet 9: e1003799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girbardt M 1957. Der Spitzenkörper von Polystictus versicolor [The Spitzenkorper of Polystictus versicolor]. Planta 50: 47–59. [Google Scholar]
- Gomez-Raja J, Davis DA 2012. The β-arrestin-like protein Rim8 is hyperphosphorylated and complexes with Rim21 and Rim101 to promote adaptation to neutral-alkaline pH. Eukaryot Cell 11: 683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow NA, Perera TH, Sherwood-Higham J, Gooday GW, Gregory DW, Marshall D 1994. Investigation of touch-sensitive responses by hyphae of the human pathogenic fungus Candida albicans. Scanning Microsc 8: 705–710. [PubMed] [Google Scholar]
- Gow NA, Brown AJ, Odds FC 2002. Fungal morphogenesis and host invasion. Curr Opin Microbiol 5: 366–371. [DOI] [PubMed] [Google Scholar]
- Goyard S, Knechtle P, Chauvel M, Mallet A, Prevost MC, Proux C, Coppee JY, Schwarz P, Dromer F, Park H, et al. 2008. The Yak1 kinase is involved in the initiation and maintenance of hyphal growth in Candida albicans. Mol Biol Cell 19: 2251–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger BL, Flenniken ML, Davis DA, Mitchell AP, Cutler JE 2005. Yeast wall protein 1 of Candida albicans. Microbiol 151: 1631–1644. [DOI] [PubMed] [Google Scholar]
- Gunasekera A, Alvarez FJ, Douglas LM, Wang HX, Rosebrock AP, Konopka JB 2010. Identification of GIG1, a GlcNAc-induced gene in Candida albicans needed for normal sensitivity to the chitin synthase inhibitor nikkomycin Z. Eukaryot Cell 9: 1476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall PA, Russell SEH, Pringle JR 2008. The septins. Wiley, Hoboken, NJ. [Google Scholar]
- Harris SD 1999. Morphogenesis is coordinated with nuclear division in germinating Aspergillus nidulans conidiospores. Microbiology 145: 2747–2756. [DOI] [PubMed] [Google Scholar]
- Harris SD 2011a. Cdc42/Rho GTPases in fungi: Variations on a common theme. Mol Microbiol 79: 1123–1127. [DOI] [PubMed] [Google Scholar]
- Harris SD 2011b. Hyphal morphogenesis: An evolutionary perspective. Fungal Biol 115: 475–484. [DOI] [PubMed] [Google Scholar]
- Harris SD, Momany M 2004. Polarity in filamentous fungi: Moving beyond the yeast paradigm. Fungal Genet Biol 41: 391–400. [DOI] [PubMed] [Google Scholar]
- Harris SD, Read ND, Roberson RW, Shaw B, Seiler S, Plamann M, Momany M 2005. Polarisome meets spitzenkorper: Microscopy, genetics, and genomics converge. Eukaryot Cell 4: 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay E, Bretscher A 1995. Parallel secretory pathways to the cell surface in yeast. J Cell Biol 131: 297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawser SP, Douglas LJ 1994. Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun 62: 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y, Ishikawa E, Shoji JY, Nakano H, Kitamoto K 2011. Septum-directed secretion in the filamentous fungus Aspergillus oryzae. Mol Microbiol 81: 40–55. [DOI] [PubMed] [Google Scholar]
- Heimel K, Scherer M, Schuler D, Kamper J 2010. The Ustilago maydis Clp1 protein orchestrates pheromone and b-dependent signaling pathways to coordinate the cell cycle and pathogenic development. Plant Cell 22: 2908–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Heitman J, Carter DA, Dyer PS, Soll DR 2014. Sexual reproduction of human fungal pathogens. Cold Spring Harbor Perspect Med 4: a019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henri J, Rispal D, Bayart E, van Tilbeurgh H, Seraphin B, Graille M 2010. Structural and functional insights into Saccharomyces cerevisiae Tpa1, a putative prolylhydroxylase influencing translation termination and transcription. J Biol Chem 285: 30767–30778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hervas-Aguilar A, Penalva MA 2010. Endocytic machinery protein SlaB is dispensable for polarity establishment but necessary for polarity maintenance in hyphal tip cells of Aspergillus nidulans. Eukaryot Cell 9: 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch HC, Staples RC, Whitehead B, Comeau J, Wolf ED 1987. Signaling for growth orientation and cell differentiation by surface topography in Uromyces. Science 235: 1659–1662. [DOI] [PubMed] [Google Scholar]
- Hogan LH, Klein BS 1994. Altered expression of surface α-1,3-glucan in genetically related strains of Blastomyces dermatitidis that differ in virulence. Infect Immun 62: 3543–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DA, Sundstrom P 2009. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol 4: 1263–1270. [DOI] [PubMed] [Google Scholar]
- Holbrook ED, Rappleye CA 2008. Histoplasma capsulatum pathogenesis: Making a lifestyle switch. Curr Opin Microbiol 11: 318–324. [DOI] [PubMed] [Google Scholar]
- Holley RA, Allanwojtas P, Phippstodd BE 1984. Nematospora-sinecauda sp. nov., a yeast pathogen of mustard seeds. Antonie Van Leeuwenhoek 50: 305–320. [DOI] [PubMed] [Google Scholar]
- Horio T, Oakley BR 2005. The role of microtubules in rapid hyphal tip growth of Aspergillus nidulans. Mol Biol Cell 16: 918–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW 2001. Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67: 2982–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby JM, Kebaara BW, Nickerson KW 2003. Farnesol biosynthesis in Candida albicans: Cellular response to sterol inhibition by zaragozic acid B. Antimicrob Agents Chemother 47: 2366–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AS, Lew DJ 2012. Morphogenesis and the cell cycle. Genetics 190: 51–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer LL, Payne TL, Bell M, Myers AM, Scherer S 1998. Candida albicans ALS3 and insights into the nature of the ALS gene family. Curr Genet 33: 451–459. [DOI] [PubMed] [Google Scholar]
- Hughes BT, Espenshade PJ 2008. Oxygen-regulated degradation of fission yeast SREBP by Ofd1, a prolyl hydroxylase family member. EMBO J 27: 1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtado CA, Beckerich JM, Gaillardin C, Rachubinski RA 2000. A rac homolog is required for induction of hyphal growth in the dimorphic yeast Yarrowia lipolytica. J Bacteriol 182: 2376–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm A, Heitman J 2005. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol 3: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis DO, Skrzypek MS, Arnaud MB, Binkley J, Shah P, Wymore F, Sherlock G 2012. Improved gene ontology annotation for biofilm formation, filamentous growth, and phenotypic switching in Candida albicans. Eukaryot Cell 12: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (US) Forum on Microbial Threats. 2011. Fungal diseases: An emerging threat to human, animal, and plant health: Workshop summary. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10: 85–93. [DOI] [PubMed] [Google Scholar]
- Johnson DI 1999. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev 63: 54–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Jin M, Lew DJ 2011. Symmetry breaking and the establishment of cell polarity in budding yeast. Curr Opin Genet Dev 21: 740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MD, Forn I, Gadelha C, Egan MJ, Bass D, Massana R, Richards TA 2011. Discovery of novel intermediate forms redefines the fungal tree of life. Nature 474: 200–203. [DOI] [PubMed] [Google Scholar]
- Kanetsuna F, Carbonell LM 1971. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J Bacteriol 106: 946–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman G, Horwitz BA, Duek L, Ullman Y, Berdicevsky I 2007. Infection stages of the dermatophyte pathogen Trichophyton: Microscopic characterization and proteolytic enzymes. Med Mycol 45: 149–155. [DOI] [PubMed] [Google Scholar]
- Kerr B, Riley MA, Feldman MW, Bohannan BJ 2002. Local dispersal promotes biodiversity in a real-life game of rock-paper-scissors. Nature 418: 171–174. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Boedicker JQ, Choi JW, Ismagilov RF 2008. Defined spatial structure stabilizes a synthetic multispecies bacterial community. Proc Natl Acad Sci 105: 18188–18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King RD, Khan HA, Foye JC, Greenberg JH, Jones HE 1975. Transferrin, iron, and dermatophytes. I. Serum dematophyte inhibitory component definitively identified as unsaturated transferrin. J Lab Clin Med 86: 204–212. [PubMed] [Google Scholar]
- Klein BS 2000. Molecular basis of pathogenicity in Blastomyces dermatitidis: The importance of adhesion. Curr Opin Microbiol 3: 339–343. [DOI] [PubMed] [Google Scholar]
- Klein BS, Tebbets B 2007. Dimorphism and virulence in fungi. Curr Opin Microbiol 10: 314–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klengel T, Liang WJ, Chaloupka J, Ruoff C, Schroppel K, Naglik JR, Eckert SE, Mogensen EG, Haynes K, Tuite MF, et al. 2005. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol 15: 2021–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus M, Pelli-Gulli MP, van Drogen F, Springer S, Jaquenoud M, Peter M 2007. Phosphorylation of Bem2p and Bem3p may contribute to local activation of Cdc42p at bud emergence. EMBO J 26: 4501–4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli M, Galati V, Boudier K, Roberson RW, Philippsen P 2008. Growth-speed-correlated localization of exocyst and polarisome components in growth zones of Ashbya gossypii hyphal tips. J Cell Sci 121: 3878–3889. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Heitman J 2010. Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans. Mol Microbiol 75: 658–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni RK, Nickerson KW 1981. Nutritional control of dimorphism in Ceratocystis ulmi. Exp Mycol 5: 148–154. [Google Scholar]
- Kumar H, Kumagai Y, Tsuchida T, Koenig PA, Satoh T, Guo Z, Jang MH, Saitoh T, Akira S, Kawai T 2009. Involvement of the NLRP3 inflammasome in innate and humoral adaptive immune responses to fungal β-glucan. J Immunol 183: 8061–8067. [DOI] [PubMed] [Google Scholar]
- Kuratsu M, Taura A, Shoji JY, Kikuchi S, Arioka M, Kitamoto K 2007. Systematic analysis of SNARE localization in the filamentous fungus Aspergillus oryzae. Fungal Genet Biol 44: 1310–1323. [DOI] [PubMed] [Google Scholar]
- Kwon-Chung KJ 1975. A new genus, Filobasidiella, the perfect state of Cryptococcus neoformans. Mycologia 67: 1197–1200. [PubMed] [Google Scholar]
- Kwon-Chung KJ 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68: 821–833. [PubMed] [Google Scholar]
- Levdansky E, Kashi O, Sharon H, Shadkchan Y, Osherov N 2010. The Aspergillus fumigatus cspA gene encoding a repeat-rich cell wall protein is important for normal conidial cell wall architecture and interaction with host cells. Eukaryot Cell 9: 1403–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin DE 2011. Regulation of cell wall biogenesis in Saccharomyces cerevisiae: The cell wall integrity signaling pathway. Genetics 189: 1145–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy SF, Ziv N, Siegal ML 2012. Bet hedging in yeast by heterogeneous, age-correlated expression of a stress protectant. PLoS Biol 10: e1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Mitchell AP 1997. Proteolytic activation of Rim1p, a positive regulator of yeast sporulation and invasive growth. Genetics 145: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillis JV, Dawson ES, Chang R, White CR Jr 2010. Disseminated dermal Trichophyton rubrum infection—An expression of dermatophyte dimorphism? J Cutan Pathol 37: 1168–1169. [DOI] [PubMed] [Google Scholar]
- Lin X 2009. Cryptococcus neoformans: Morphogenesis, infection, and evolution. Infect Genet Evol 9: 401–416. [DOI] [PubMed] [Google Scholar]
- Lin X, Huang JC, Mitchell TG, Heitman J 2006. Virulence attributes and hyphal growth of C. neoformans are quantitative traits and the MATα allele enhances filamentation. PLoS Genet 2: e187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Kabrawala S, Fox EP, Nobile CJ, Johnson AD, Bennett RJ 2013. Genetic control of conventional and pheromone-stimulated biofilm formation in Candida albicans. PLoS Pathog 9: e1003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey R, Cowden S, Hernandez-Rodriguez Y, Momany M 2010. Septins AspA and AspC are important for normal development and limit the emergence of new growth foci in the multicellular fungus Aspergillus nidulans. Eukaryot Cell 9: 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Filler SG 2011. Candida albicans Als3, a multifunctional adhesin and invasin Eukaryot Cell 10: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz MC, Bender JA, Fink GR 2004. Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loussert C, Schmitt C, Prevost MC, Balloy V, Fadel E, Philippe B, Kauffmann-Lacroix C, Latgé JP, Beauvais A 2010. In vivo biofilm composition of Aspergillus fumigatus. Cell Microbiol 12: 405–410. [DOI] [PubMed] [Google Scholar]
- Lu Y, Su C, Wang A, Liu H 2011. Hyphal development in Candida albicans requires two temporally linked changes in promoter chromatin for initiation and maintenance. PLoS Biol 9: e1001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Liu H 2012. A GATA transcription factor recruits Hda1 in response to reduced Tor1 signaling to establish a hyphal chromatin state in Candida albicans. PLoS Pathog 8: e1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Solis NV, Filler SG, Liu H 2013. Synergistic regulation of hyphal elongation by hypoxia, CO2, and nutrient conditions controls the virulence of Candida albicans. Cell Host Microbe 14: 499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Su C, Unoje O, Liu H 2014. Quorum sensing controls hyphal initiation in Candida albicans through Ubr1-mediated protein degradation. Proc Natl Acad Sci 111: 1975–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Ibrahim AS, Spellberg B, Nobile CJ, Mitchell AP, Fu Y 2010. Candida albicans Hyr1p confers resistance to neutrophil killing and is a potential vaccine target J Infect Dis 201: 1718–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani HD, Fink GR 1998. The control of filamentous differentiation and virulence in fungi. Trends Cell Biol 8: 348–353. [DOI] [PubMed] [Google Scholar]
- Magditch DA, Liu TB, Xue C, Idnurm A 2012. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog 8: e1002936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan MM, De Rop L, Serneels J, Exler S, Rupp S, Tournu H, Thevelein JM, Van Dijck P 2005a. The G protein-coupled receptor Gpr1 and the Gα protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans. Mol Biol Cell 16: 1971–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maidan MM, Thevelein JM, Van Dijck P 2005b. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem Soc Trans 33: 291–293. [DOI] [PubMed] [Google Scholar]
- Marconi VC, Kradin R, Marty FM, Hospenthal DR, Kotton CN 2010. Disseminated dermatophytosis in a patient with hereditary hemochromatosis and hepatic cirrhosis: Case report and review of the literature. Med Mycol 48: 518–527. [DOI] [PubMed] [Google Scholar]
- Martin SG 2009. Microtubule-dependent cell morphogenesis in the fission yeast. Trends Cell Biol 19: 447–454. [DOI] [PubMed] [Google Scholar]
- Martin SG, Arkowitz RA 2014. Cell polarization in budding and fission yeasts. FEMS Microbiol Rev 38: 228–253. [DOI] [PubMed] [Google Scholar]
- Martin R, Walther A, Wendland J 2005. Ras1-induced hyphal development in Candida albicans requires the formin Bni1. Eukaryot Cell 4: 1712–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez LR, Casadevall A 2007. Cryptococcus neoformans biofilm formation depends on surface support and carbon source and reduces fungal cell susceptibility to heat, cold, and UV light. Appl Environ Microbiol 73: 4592–4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrabi R, Kema GH 2006. Protein kinase A subunits of the ascomycete pathogen Mycosphaerella graminicola regulate asexual fructification, filamentation, melanization and osmosensing. Mol Plant Pathol 7: 565–577. [DOI] [PubMed] [Google Scholar]
- Miwa T, Takagi Y, Shinozaki M, Yun CW, Schell WA, Perfect JR, Kumagai H, Tamaki H 2004. Gpr1, a putative G-protein-coupled receptor, regulates morphogenesis and hypha formation in the pathogenic fungus Candida albicans. Eukaryot Cell 3: 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momany M 2002. Polarity in filamentous fungi: Establishment, maintenance and new axes. Curr Opin Microbiol 5: 580–585. [DOI] [PubMed] [Google Scholar]
- Momany M 2005. Growth control and polarization. Med Mycol 43: S23–S25. [DOI] [PubMed] [Google Scholar]
- Momany M, Taylor I 2000. Landmarks in the early duplication cycles of Aspergillus fumigatus and Aspergillus nidulans: Polarity, germ tube emergence and septation. Microbiol 146: 3279–3284. [DOI] [PubMed] [Google Scholar]
- Momany M, Westfall PJ, Abramowsky G 1999. Aspergillus nidulans swo mutants show defects in polarity establishment, polarity maintenance and hyphal morphogenesis Genetics 151: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D 1991. Perception and response to gravity in higher fungi—A critical appraisal. New Phytol 117: 3–23. [DOI] [PubMed] [Google Scholar]
- Moore RT 2000. Cytology and ultrastructure of yeasts and yeastlike fungi. In The yeasts: A taxonomic study (ed. Kurtzman CP, Fell JW), pp. 33–44 Elsevier, Amsterdam. [Google Scholar]
- Moore D, Hock B, Greening JP, Kern VD, Novak Frazer L, Monzer J 1996. Centenary review. Gravimorphogenesis in agarics. Mycol Res 100: 257–273. [DOI] [PubMed] [Google Scholar]
- Mora-Montes HM, Netea MG, Ferwerda G, Lenardon MD, Brown GD, Mistry AR, Kullberg BJ, O’Callaghan CA, Sheth CC, Odds FC, et al. 2011. Recognition and blocking of innate immunity cells by Candida albicans chitin. Infect Immun 79: 1961–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosel DD, Dumitru R, Hornby JM, Atkin AL, Nickerson KW 2005. Farnesol concentrations required to block germ tube formation in Candida albicans in the presence and absence of serum. Appl Environ Microbiol 71: 4938–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murad AM, Leng P, Straffon M, Wishart J, Macaskill S, MacCallum D, Schnell N, Talibi D, Marechal D, Tekaia F, et al. 2001. NRG1 represses yeast-hypha morphogenesis and hypha-specific gene expression in Candida albicans. EMBO J 20: 4742–4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naseem S, Gunasekera A, Araya E, Konopka JB 2011. N-acetylglucosamine (GlcNAc) induction of hyphal morphogenesis and transcriptional responses in Candida albicans are not dependent on its metabolism. J Biol Chem 286: 28671–28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nather K, Munro CA 2008. Generating cell surface diversity in Candida albicans and other fungal pathogens. FEMS Microbiol Lett 285: 137–145. [DOI] [PubMed] [Google Scholar]
- Nemecek JC, Wuthrich M, Klein BS 2006. Global control of dimorphism and virulence in fungi. Science 312: 583–588. [DOI] [PubMed] [Google Scholar]
- Newman SL, Chaturvedi S, Klein BS 1995. The WI-1 antigen of Blastomyces dermatitidis yeasts mediates binding to human macrophage CD11b/CD18 (CR3) and CD14. J Immunol 154: 753–761. [PubMed] [Google Scholar]
- Nichols CB, Perfect Z, Alspaugh JA 2007. A Ras1-Cdc24 signal transduction pathway mediates thermotolerance in the fungal pathogen Cryptococcus neoformans. Mol Microbiol 63: 1118–1130. [DOI] [PubMed] [Google Scholar]
- O’Connor L, Caplice N, Coleman DC, Sullivan DJ, Moran GP 2010. Differential filamentation of Candida albicans and Candida dubliniensis is governed by nutrient regulation of UME6 expression. Eukaryot Cell 9: 1383–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh Y, Bi E 2011. Septin structure and function in yeast and beyond. Trends Cell Biol 21: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okagaki LH, Wang Y, Ballou ER, O’Meara TR, Bahn YS, Alspaugh JA, Xue C, Nielsen K 2011. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell 10: 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara TR, Holmer SM, Selvig K, Dietrich F, Alspaugh JA 2013. Cryptococcus neoformans Rim101 is associated with cell wall remodeling and evasion of the host immune responses. MBio 4: e00522-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer A, Bruick RK 2007. Non-heme dioxygenases: Cellular sensors and regulators jelly rolled into one? Nat Chem Biol 3: 144–153. [DOI] [PubMed] [Google Scholar]
- Park HO, Bi E 2007. Central roles of small GTPases in the development of cell polarity in yeast and beyond. Microbiol Mol Biol Rev 71: 48–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CL, Xu K, Sharpless KE, Harris SD 2004. MesA, a novel fungal protein required for the stabilization of polarity axes in Aspergillus nidulans. Mol Biol Cell 15: 3658–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg GF, Brady BL 2002. Veticillium wilts. CABI, Wallingford, UK. [Google Scholar]
- Phadke SS, Feretzaki M, Heitman J 2013. Unisexual reproduction enhances fungal competitiveness by promoting habitat exploration via hyphal growth and sporulation. Eukaryot Cell 12: 1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan QT, Myers CL, Fu Y, Sheppard DC, Yeaman MR, Welch WH, Ibrahim AS, Edwards JE Jr, Filler SG 2007. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5: e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P, Kaufmann A, Schmitz HP 2005. Homologues of yeast polarity genes control the development of multinucleated hyphae in Ashbya gossypii. Curr Opin Microbiol 8: 370–377. [DOI] [PubMed] [Google Scholar]
- Pinar M, Pantazopoulou A, Arst HN Jr, Penalva MA 2013. Acute inactivation of the Aspergillus nidulans Golgi membrane fusion machinery: Correlation of apical extension arrest and tip swelling with cisternal disorganization. Mol Microbiol 89: 228–248. [DOI] [PubMed] [Google Scholar]
- Porman AM, Hirakawa MP, Jones SK, Wang N, Bennett RJ 2013. MTL-independent phenotypic switching in Candida tropicalis and a dual role for Wor1 in regulating switching and filamentation PLoS Genet 9: e1003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage G, VandeWalle K, Lopez-Ribot JL, Wickes BL 2002. The filamentation pathway controlled by the Efg1 regulator protein is required for normal biofilm formation and development in Candida albicans. FEMS Microbiol Lett 214: 95–100. [DOI] [PubMed] [Google Scholar]
- Rappleye CA, Engle JT, Goldman WE 2004. RNA interference in Histoplasma capsulatum demonstrates a role for α-(1,3)-glucan in virulence. Mol Microbiol 53: 153–165. [DOI] [PubMed] [Google Scholar]
- Rappleye CA, Eissenberg LG, Goldman WE 2007. Histoplasma capsulatum α-(1,3)-glucan blocks innate immune recognition by the β-glucan receptor. Proc Natl Acad Sci 104: 1366–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray TL, Payne CD 1988. Scanning electron microscopy of epidermal adherence and cavitation in murine candidiasis: A role for Candida acid proteinase. Infect Immun 56: 1942–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner ADM 1991. The challenge of the individualistic mycelium. Mycologia 83: 48–71. [Google Scholar]
- Rayner ADM 1996. Interconnectedness and individualism in fungal mycelia. In A century of mycology (ed. Sutton BC), pp. 193–232 Cambridge University Press, Cambridge. [Google Scholar]
- Reynolds TB, Fink GR 2001. Bakers’ yeast, a model for fungal biofilm formation. Science 291: 878–881. [DOI] [PubMed] [Google Scholar]
- Richard ML, Nobile CJ, Bruno VM, Mitchell AP 2005. Candida albicans biofilm-defective mutants Eukaryot Cell 4: 1493–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme M 2013. Tip growth in filamentous fungi: A road trip to the apex. Annu Rev Microbiol 67: 587–609. [DOI] [PubMed] [Google Scholar]
- Rocha CR, Schroppel K, Harcus D, Marcil A, Dignard D, Taylor BN, Thomas DY, Whiteway M, Leberer E 2001. Signaling through adenylyl cyclase is essential for hyphal growth and virulence in the pathogenic fungus Candida albicans. Mol Biol Cell 12: 3631–3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues MG, Fonseca A 2003. Molecular systematics of the dimorphic ascomycete genus Taphrina. Int J Syst Evol Micr 53: 607–616. [DOI] [PubMed] [Google Scholar]
- Rodrigues ML, Nosanchuk JD, Schrank A, Vainstein MH, Casadevall A, Nimrichter L 2011. Vesicular transport systems in fungi. Future Microbiol 6: 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde JR, Cardenas ME 2004. Nutrient signaling through TOR kinases controls gene expression and cellular differentiation in fungi. Curr Top Microbiol 279: 53–72. [DOI] [PubMed] [Google Scholar]
- Rohde JR, Bastidas R, Puria R, Cardenas ME 2008. Nutritional control via Tor signaling in Saccharomyces cerevisiae. Curr Opin Microbiol 11: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney PJ, Sullivan TD, Klein BS 2001. Selective expression of the virulence factor BAD1 upon morphogenesis to the pathogenic yeast form of Blastomyces dermatitidis: Evidence for transcriptional regulation by a conserved mechanism. Mol Microbiol 39: 875–889. [DOI] [PubMed] [Google Scholar]
- Ryder LS, Dagdas YF, Mentlak TA, Kershaw MJ, Thornton CR, Schuster M, Chen J, Wang Z, Talbot NJ 2013. NADPH oxidases regulate septin-mediated cytoskeletal remodeling during plant infection by the rice blast fungus. Proc Natl Acad Sci 110: 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Martinez C, Perez-Martin J 2002. Gpa2, a G-protein α subunit required for hyphal development in Candida albicans. Eukaryot Cell 1: 865–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R, Novick P 2004. 23 genes, 23 years later. Cell 116: S13–S15, 1 p. following S19. [DOI] [PubMed] [Google Scholar]
- Scherwitz C 1982. Ultrastructure of human cutaneous candidosis. J Invest Dermatol 78: 200–205. [DOI] [PubMed] [Google Scholar]
- Schmid J, Harold FM 1988. Dual roles for calcium ions in apical growth of Neurospora crassa. J Gen Microbiol 134: 2623–2631. [DOI] [PubMed] [Google Scholar]
- Schmitz HP, Kaufmann A, Kohli M, Laissue PP, Philippsen P 2006. From function to shape: A novel role of a formin in morphogenesis of the fungus Ashbya gossypii. Mol Biol Cell 17: 130–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnathorst WC 1981. Life cycle and epidemiology of Verticillium dahliae. In Fungal wilt diseases of plants (ed. Mace ME, Bell AA, Beckman CH), pp. 81–111 Academic, New York. [Google Scholar]
- Schofield CJ, Ratcliffe PJ 2005. Signalling hypoxia by HIF hydroxylases. Biochem Biophys Res Commun 338: 617–626. [DOI] [PubMed] [Google Scholar]
- Sebghati TS, Engle JT, Goldman WE 2000. Intracellular parasitism by Histoplasma capsulatum: Fungal virulence and calcium dependence. Science 290: 1368–1372. [DOI] [PubMed] [Google Scholar]
- Seider K, Heyken A, Luttich A, Miramon P, Hube B 2010. Interaction of pathogenic yeasts with phagocytes: Survival, persistence and escape. Curr Opin Microbiol 13: 392–400. [DOI] [PubMed] [Google Scholar]
- Semighini CP, Harris SD 2008. Regulation of apical dominance in Aspergillus nidulans hyphae by reactive oxygen species. Genetics 179: 1919–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadomy HJ, Utz JP 1966. Preliminary studies on a hypha-forming mutant of Cryptococcus neoformans. Mycologica 58: 383–390. [PubMed] [Google Scholar]
- Shank EA, Klepac-Ceraj V, Collado-Torres L, Powers GE, Losick R, Kolter R 2011. Interspecies interactions that result in Bacillus subtilis forming biofilms are mediated mainly by members of its own genus. Proc Natl Acad Sci 108: E1236–E1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, Heitman J, Cowen LE 2009. Hsp90 orchestrates temperature-dependent Candida albicans morphogenesis via Ras1-PKA signaling. Curr Biol 19: 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro RS, Zaas AK, Betancourt-Quiroz M, Perfect JR, Cowen LE 2012. The Hsp90 co-chaperone Sgt1 governs Candida albicans morphogenesis and drug resistance. PloS ONE 7: e44734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless KE, Harris SD 2002. Functional characterization and localization of the Aspergillus nidulans formin SEPA. Mol Biol Cell 13: 469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G, Zhou E, Alspaugh JA, Wang P 2012. Wsp1 is downstream of Cin1 and regulates vesicle transport and actin cytoskeleton as an effector of Cdc42 and Rac1 in Cryptococcus neoformans. Eukaryot Cell 11: 471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sil A, Andrianopoulos A 2015. Thermally dimorphic human fungal pathogens—Polyphyletic pathogens with a convergent pathogenicity trait. Cold Spring Harbor Perspect Med 10.1101/cshperspect.a019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonin A, Palma-Guerrero J, Fricker M, Glass NL 2012. Physiological significance of network organization in fungi. Eukaryot Cell 11: 1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DA, Nicholls S, Morgan BA, Brown AJ, Quinn J 2004. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell 15: 4179–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith HA, Sawin KE 2003. Fission yeast mod5p regulates polarized growth through anchoring of tea1p at cell tips. Nature 423: 647–651. [DOI] [PubMed] [Google Scholar]
- Sonneborn A, Bockmuhl DP, Gerads M, Kurpanek K, Sanglard D, Ernst JF 2000. Protein kinase A encoded by TPK2 regulates dimorphism of Candida albicans. Mol Microbiol 35: 386–396. [DOI] [PubMed] [Google Scholar]
- Sopko R, Huang D, Smith JC, Figeys D, Andrews BJ 2007. Activation of the Cdc42p GTPase by cyclin-dependent protein kinases in budding yeast. EMBO J 26: 4487–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staab JF, Bradway SD, Fidel PL, Sundstrom P 1999. Adhesive and mammalian transglutaminase substrate properties of Candida albicans Hwp1. Science 283: 1535–1538. [DOI] [PubMed] [Google Scholar]
- Stajich JE, Berbee ML, Blackwell M, Hibbett DS, James TY, Spatafora JW, Taylor JW 2009. The fungi. Curr Biol 19: R840–R845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stovicek V, Vachova L, Palkova Z 2012. Yeast biofilm colony as an orchestrated multicellular organism. Commun Integr Biol 5: 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Lu Y, Liu H 2013. Reduced TOR signaling sustains hyphal development in Candida albicans by lowering Hog1 basal activity. Mol Biol Cell 24: 385–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudbery P 2011a. Fluorescent proteins illuminate the structure and function of the hyphal tip apparatus. Fungal Genet Biol 48: 849–857. [DOI] [PubMed] [Google Scholar]
- Sudbery PE 2011b. Growth of Candida albicans hyphae. Nat Rev 9: 737–748. [DOI] [PubMed] [Google Scholar]
- Taheri-Talesh N, Horio T, Araujo-Bazan L, Dou X, Espeso EA, Penalva MA, Osmani SA, Oakley BR 2008. The tip growth apparatus of Aspergillus nidulans. Mol Biol Cell 19: 1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B 2006. Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 18: 1052–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang PW, Bandara HM, Fong WP 2012. Purpurin suppresses Candida albicans biofilm formation and hyphal development. PloS ONE 7: e50866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscharke RL, Lazera M, Chang YC, Wickes BL, Kwon-Chung KJ 2003. Haploid fruiting in Cryptococcus neoformans is not mating type α-specific. Fungal Genet Biol 39: 230–237. [DOI] [PubMed] [Google Scholar]
- Tuch BB, Mitrovich QM, Homann OR, Hernday AD, Monighetti CK, De La Vega FM, Johnson AD 2010. The transcriptomes of two heritable cell types illuminate the circuit governing their differentiation. PLoS Genet 6: e1001070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay S, Shaw BD 2008. The role of actin, fimbrin and endocytosis in growth of hyphae in Aspergillus nidulans. Mol Microbiol 68: 690–705. [DOI] [PubMed] [Google Scholar]
- Vallim MA, Nichols CB, Fernandes L, Cramer KL, Alspaugh JA 2005. A Rac homolog functions downstream of Ras1 to control hyphal differentiation and high-temperature growth in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell 4: 1066–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J 2009. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 77: 4345–4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdin J, Bartnicki-Garcia S, Riquelme M 2009. Functional stratification of the Spitzenkorper of Neurospora crassa. Mol Microbiol 74: 1044–1053. [DOI] [PubMed] [Google Scholar]
- Virag A, Lee MP, Si H, Harris SD 2007. Regulation of hyphal morphogenesis by cdc42 and rac1 homologues in Aspergillus nidulans. Mol Microbiol 66: 1579–1596. [DOI] [PubMed] [Google Scholar]
- Volling K, Thywissen A, Brakhage AA, Saluz HP 2011. Phagocytosis of melanized Aspergillus conidia by macrophages exerts cytoprotective effects by sustained PI3K/Akt signalling. Cell Microbiol 13: 1130–1148. [DOI] [PubMed] [Google Scholar]
- Wang Y 2009. CDKs and the yeast–hyphal decision. Curr Opin Microbiol 12: 644–649. [DOI] [PubMed] [Google Scholar]
- Wang L, Lin X 2012. Morphogenesis in fungal pathogenicity: Shape, size, and surface. PLoS Pathog 8: e1003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhai B, Lin X 2012. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog 8: e1002765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Tian X, Gyawali R, Lin X 2013. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc Natl Acad Sci 111: 11571–11576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner R, Altschuler S, Wu L, Li R 2003. Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299: 1231–1235. [DOI] [PubMed] [Google Scholar]
- Wickes BL, Mayorga ME, Edman U, Edman JC 1996. Dimorphism and haploid fruiting in Cryptococcus neoformans: Association with the α-mating type. Proc Natl Acad Sci 93: 7327–7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CF, Lew DJ 2013. Beyond symmetry-breaking: Competition and negative feedback in GTPase regulation. Trends Cell Biol 23: 476–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada-Okabe T, Sakamori Y, Mio T, Yamada-Okabe H 2001. Identification and characterization of the genes for N-acetylglucosamine kinase and N-acetylglucosamine-phosphate deacetylase in the pathogenic fungus Candida albicans. Eur J Biochem 268: 2498–2505. [DOI] [PubMed] [Google Scholar]
- Zakikhany K, Naglik JR, Schmidt-Westhausen A, Holland G, Schaller M, Hube B 2007. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol 9: 2938–2954. [DOI] [PubMed] [Google Scholar]
- Zaragoza O, Nielsen K 2013. Titan cells in Cryptococcus neoformans: Cells with a giant impact. Curr Opin Microbiol 16: 409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A 2010. Fungal cell gigantism during mammalian infection. PLoS Pathog 6: e1000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidler U, Lettner T, Lassnig C, Muller M, Lajko R, Hintner H, Breitenbach M, Bito A 2009. UME6 is a crucial downstream target of other transcriptional regulators of true hyphal development in Candida albicans. FEMS Yeast Res 9: 126–142. [DOI] [PubMed] [Google Scholar]
- Zhao Z-S, Leung T, Manser E, Lim L 1995. Pheromone signalling in Saccharomyces cerevisiae requires the small GTP-binding protein Cdc42p and its activator CDC24. Mol Cell Biol 15: 5246–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X, Wang Y 2004. Hgc1, a novel hypha-specific G1 cyclin-related protein regulates Candida albicans hyphal morphogenesis. EMBO J 23: 1845–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng XD, Lee RT, Wang YM, Lin QS, Wang Y 2007. Phosphorylation of Rga2, a Cdc42 GAP, by CDK/Hgc1 is crucial for Candida albicans hyphal growth. EMBO J 26: 3760–3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Fang HM, Wang YM, Zeng GS, Zheng XD, Wang Y 2009. Ras1 and Ras2 play antagonistic roles in regulating cellular cAMP level, stationary-phase entry and stress response in Candida albicans. Mol Microbiol 74: 862–875. [DOI] [PubMed] [Google Scholar]
- Zordan RE, Galgoczy DJ, Johnson AD 2006. Epigenetic properties of white-opaque switching in Candida albicans are based on a self-sustaining transcriptional feedback loop. Proc Natl Acad Sci 103: 12807–12812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou H, Fang HM, Zhu Y, Wang Y 2009. Candida albicans Cyr1, Cap1 and G-actin form a sensor/effector apparatus for activating cAMP synthesis in hyphal growth. Mol Microbiol 75: 579–591. [DOI] [PubMed] [Google Scholar]