Abstract
Despite the availability of effective chemotherapy, tuberculosis (TB) killed 1.3 million people in 2012. Alongside HIV, it remains a top cause of death from an infectious disease. Global targets for reductions in the epidemiological burden of TB have been set for 2015 and 2050 within the context of the Millennium Development Goals (MDGs) and by the Stop TB Partnership. Achieving these targets is the focus of national and international efforts in TB control, and showing whether or not they are achieved is of major importance to guide future and sustainable investments. This article provides a short overview of sources of data to estimate TB disease burden; presents estimates of TB incidence, prevalence, and mortality in 2012 and an assessment of progress toward the 2015 targets for reductions in these indicators based on trends since 1990 and projections up to 2015; analyzes trends in TB notifications and in the implementation of the Stop TB Strategy; and considers prospects for elimination of TB after 2015.
Alongside HIV, tuberculosis (TB) remains a major cause of death worldwide, despite the discovery of chemotherapy more than 50 years ago. The Stop TB Strategy aims to dramatically reduce the global burden of TB by 2015.
Tuberculosis (TB) is likely to have affected humans for most of their history (Holloway et al. 2011; Comas et al. 2013) and remains a major cause of death worldwide despite the discovery of effective and affordable chemotherapy more than 50 years ago. With 1.3 million TB deaths (including TB deaths in HIV-positive individuals) in 2012 (WHO 2013a), TB and the human immunodeficiency virus (HIV) are the top causes of death from a single infectious agent worldwide (Lozano et al. 2012; Ortblad et al. 2013). TB is a leading killer among adults in the most economically productive age groups and people living with HIV (Lopez et al. 2006), and even those cured from TB can be left with lifetime sequelae that substantially reduce their quality of life (Miller et al. 2009). Recognition of these facts has kept TB control high on the international public health agenda since the early 1990s (Zumla et al. 2009; Lienhardt et al. 2012a), following years of neglect during the 1980s (Raviglione and Pio 2002).
Evidence that chemotherapy is among the most cost-effective of all health-care interventions (Murray et al. 1991; Dye and Floyd 2006), the catastrophic impact of the HIV epidemic on TB in Africa, and the global concern about the growth of multidrug-resistant TB (MDR-TB) have emphasized the need to improve TB prevention and control. Global targets for reductions in the epidemiological burden of TB have been set for 2015 and 2050 within the context of the Millennium Development Goals (MDGs) and separately by the Stop TB Partnership, a global coalition of stakeholders established to coordinate international efforts (Box 1). The WHO’s recommended approach for achieving these targets is the Stop TB Strategy (Raviglione and Uplekar 2006), which comprises best practices in the diagnosis and treatment of patients with active TB, approaches to address major epidemiological and system challenges, and the promotion of research for innovations (Box 2). It was launched in 2006 and underpins the Global Plan 2011–2015, a comprehensive and budgeted plan to reach the global targets (Raviglione 2006b; 2007; Korenromp et al. 2012).
BOX 1. GOALS, TARGET, AND INDICATORS FOR TB CONTROL.
Health in the Millennium Development Goals
Goal 6: Combat HIV/AIDS, Malaria, and Other Diseases
Target 6c: Halt and begin to reverse the incidence of malaria and other major diseases
Indicator 6.9: Incidence, prevalence, and death rates associated with TB
Indicator 6.10: Proportion of TB cases detected and cured under DOTS
Stop TB Partnership Targets
By 2005: At least 70% of people with sputum smear-positive TB will be diagnosed (i.e., under the DOTS strategy), and at least 85% will be successfully treated. The targets of a case detection rate of at least 70% and a treatment success rate of at least 85% were first set by the World Health Assembly of WHO in 1991.
By 2015: The global burden of TB (per capita prevalence and death rates) will be reduced by 50% relative to 1990 levels.
By 2050: The global incidence of active TB will be less than one case per million population per year.
BOX 2. STOP TB STRATEGY (Raviglione and Uplekar 2006; World Health Organization 2007).
Vision, Goal, Objectives, and Targets
Vision: A world free of TB
Goal: To dramatically reduce the global burden of TB by 2015 in line with the Millennium Development Goals and the Stop TB Partnership targets
Objectives:
Achieve universal access to high-quality diagnosis and patient-centered treatment
Reduce the human suffering and socioeconomic burden associated with TB
Protect poor and vulnerable populations from TB, TB/HIV, and multidrug-resistant TB
Support development of new tools and enable their timely and effective use
Targets:
MDG 6, Target 8: …halted by 2015 and begun to reverse the incidence….
- Targets linked to the MDGs and endorsed by the Stop TB Partnership:
- by 2005: detect at least 70% of new sputum smear-positive TB cases and cure at least 85% of these cases
- by 2015: reduce prevalence of and death due to TB by 50% relative to 1990
- by 2050: eliminate TB as a public health problem (less than one case per million population)
Components of the Stop TB Strategy
- Pursue high-quality DOTS expansion and enhancement
- Secure political commitment, with adequate and sustained financing
- Ensure early case detection, and diagnosis through quality-assured bacteriology
- Provide standardized treatment with supervision, and patient support
- Ensure effective drug supply and management
- Monitor and evaluate performance and impact
- Address TB-HIV, MDR-TB, and the needs of poor and vulnerable populations
- Scale-up collaborative TB/HIV activities
- Scale-up prevention and management of multidrug-resistant TB (MDR-TB)
- Address the needs of TB contacts, and of poor and vulnerable populations
- Contribute to health system strengthening based on primary health care
- Help improve health policies, human resource development, financing, supplies, service delivery, and information
- Strengthen infection control in health services, other congregate settings, and households
- Upgrade laboratory networks, and implement the Practical Approach to Lung Health (PAL)
- Adapt successful approaches from other fields and sectors, and foster action on the social determinants of health
- Engage all care providers
- Involve all public, voluntary, corporate, and private providers through Public-Private Mix (PPM) approaches
- Promote use of the International Standards for Tuberculosis Care (ISTC)
- Empower people with TB, and communities through partnership
- Pursue advocacy, communication, and social mobilization
- Foster community participation in TB care
- Promote use of the Patients’ Charter for Tuberculosis Care
- Enable and promote research
- Conduct program-based operational research, and introduce new tools into practice
Reaching the 2015 targets has been the focus of national and international efforts in TB control for more than a decade, and the World Health Organization (WHO) has established a Global Task Force on TB Impact Measurement (Dye et al. 2008) to ensure the best possible evaluation of whether or not they are achieved. Every year, WHO publishes estimates of TB incidence, prevalence, and mortality at global, regional, and country levels, along with an analysis of progress toward achievement of global targets (WHO 2013a).
This article provides an overview of sources of data to estimate the burden of disease; presents estimates of the global burden of TB in 2012 and trends in incidence, prevalence, and mortality from 1990 to 2012, with projections up to 2015; analyzes trends in TB notifications and in the implementation of the Stop TB Strategy; and considers prospects for elimination of TB after 2015. Special attention is given to drug-resistant TB, the association of TB with HIV, and TB in children.
GLOBAL MONITORING OF THE BURDEN OF TB
TB incidence has never been measured at the national level because this would require long-term studies among large cohorts of people (hundreds of thousands) at high cost and with challenging logistics. The best approach to estimating TB incidence is from routine surveillance systems in which case reports are more or less complete, such that notifications can be considered a close proxy of incidence. This is possible in settings with universal health-care coverage (Moreno-Serra and Smith 2012; O’Neill et al. 2013), and in which operational research has been used to quantify the small fraction of cases that are treated but not notified to surveillance systems (Van Hest et al. 2008). However, surveillance systems in many countries are not yet capable of providing a direct measure of TB incidence because the numbers of cases who are treated but not notified, or undiagnosed at all, are unknown. The major reasons why cases are missed from official notification data include laboratory errors (Botha et al. 2008), lack of notification of cases by public (Dye et al. 1999) and private providers (Uplekar et al. 2001), failure of health-care staff to recognize TB signs and symptoms among people accessing health services (Meintjes et al. 2008), and lack of access to health services (Veron et al. 2004). Operational research (such as capture-recapture studies) as well as supporting evidence (such as whether prescriptions for TB drugs are available in the private sector, and practices of staff managing people suspected of having TB in primary health-care facilities) can be used to assess the plausible range of the fraction of cases that are unaccounted for in case notification data (Borgdorff et al. 2004; Cailhol et al. 2005; Baussano et al. 2006; Crofts et al. 2008; Van Hest et al. 2008; WHO 2013a). Duplicated or misclassified records (Bierrenbach et al. 2007), inconsistent case notification data at the subnational level, inconsistent time trends, or inconsistencies with existing knowledge about TB epidemiology (Dye et al. 2007) all contribute to uncertainty about TB incidence estimates obtained from case notification data.
In contrast to incidence, TB prevalence can be directly measured in nationwide population-based surveys in countries with a high burden of TB (Glaziou et al. 2008; WHO 2011c). Since 2002, 19 countries have successfully measured the prevalence of TB disease through such surveys (Hong et al. 1998; Tupasi et al. 1999; Dye et al. 2000; Soemantri et al. 2007; WHO 2013a) including seven in Africa, and 14 more (eight in Africa) have planned to implement a survey by 2015. Repeat surveys conducted about every 10 years allow trends in disease burden to be assessed. Countries that have completed repeat surveys in the last 10 years include Cambodia, China, the Philippines, and Thailand, and repeat surveys are planned in Myanmar and Vietnam (WHO 2013a). Prevalence surveys have proved extremely useful for designing and adapting national TB control program strategies. A large proportion of the global burden of TB as expressed in terms of TB prevalence will be directly measured from population-based surveys by 2015, as opposed to indirectly estimated from statistical modeling.
TB mortality among HIV-negative people can be directly measured using data from national vital registration (VR) systems, provided that these systems have high coverage and causes of death are accurately coded according to the latest revision of the International classification of diseases (ICD-10) (Korenromp et al. 2009). Sample VR systems covering representative areas of the country (e.g., China) provide an interim solution. Direct measurements of TB mortality from 123 countries were used in 2012 (WHO 2013a). The parts of the world where there are major gaps in the availability of VR data are the African region and parts of the Southeast Asia region; in the latter, Indonesia is currently building a sample VR system.
TB mortality among HIV-positive people is hard to measure even when national VR systems with standard coding of causes of death are in place, because deaths among HIV-positive people are coded as HIV deaths and contributory causes (such as TB) are generally not reliably recorded. This will need to be corrected to permit a comprehensive assessment of TB mortality, especially in countries with a high prevalence of HIV. In the interim, well-monitored cohorts of HIV-positive people who are enrolled in care and/or autopsy studies provide very useful data on contributory causes of AIDS deaths. Starting in 2013, estimates of TB mortality among HIV-positive people are obtained using models implemented in the Spectrum software (see http://www.futuresinstitute.org/spectrum.aspx) (WHO 2013a).
The incidence of and mortality from MDR-TB can be estimated from periodic surveys or from routine drug-susceptibility testing (DST) if the coverage of patient testing is sufficiently comprehensive (WHO 2013a). The global surveillance of resistance to at least the two most important first-line anti-TB drugs—isoniazid and rifampicin—has been coordinated by the WHO since 1994 (Pablos-Mendez et al. 1998; Zignol et al. 2012). Extensively drug-resistant TB (XDR-TB) is defined as MDR-TB plus resistance to the two most effective classes of second-line drugs— the fluoroquinolones and second-line injectable drugs (the aminoglycosides amikacin and kanamycin, and the polypeptide capreomycin) (WHO 2006).
TB EPIDEMIOLOGY
TB is contagious and airborne (Riley 1983). The risk of acquiring Mycobacterium tuberculosis infection is essentially determined by exogenous factors. TB is most commonly transmitted from a person with infectious pulmonary TB to others by droplet nuclei, which are aerosolized by coughing, sneezing, or speaking. Other routes of transmission are uncommon and of no epidemiologic significance. The probability of contact with a person who has an infectious form of TB, the intimacy and duration of that contact, the degree of infectiousness of the case, and the shared environment in which the contact takes place are important determinants of the likelihood of transmission. About one-third of the world’s population is estimated to have been exposed to TB bacteria and potentially infected (WHO 2013a). Of those infected, only a small proportion will become sick with TB (Vynnycky and Fine 2000; Borgdorff et al. 2011) but people living with HIV, people with weakened immune systems caused by the prolonged use of medicines such as steroids or TNF-α inhibitors, and patients with diabetes, renal insufficiency, and silicosis (among other morbidities) have a much greater risk of falling ill from TB. Among HIV-uninfected the risk of death after 10 years has been variously reported between 53% and 86%, with a weighted mean of 70% (Tiemersma et al. 2011), compared with ∼3% of HIV-uninfected treated tuberculosis patients expected to die because of TB (Straetemans et al. 2011). TB is a disease of poverty that thrives where social and economic determinants of ill health prevail, and that affects mostly young adults in their most productive years living in the developing world (WHO 2013a).
Incidence
In 2012, there were an estimated 8.6 million incident cases of TB (range, 8.3 million–9.0 million) in the world, equivalent to 122 cases per 100,000 population. The absolute number of incident cases has been decreasing, albeit slowly, since the early 2000s (WHO 2013a). Most of the estimated cases in 2012 occurred in the WHO regions of Southeast Asia, the western Pacific (58%), and the African region (27%); smaller proportions of cases occurred in the eastern Mediterranean region (8%), the European region (4%), and the region of the Americas (3%). The largest number of incident cases in 2012 was in India (uncertainty range 2.0 million–2.4 million) corresponding to 26% of global cases, China (0.9 million–1.1 million), and South Africa (0.4 million–0.6 million). Of the 8.6 million incident cases, an estimated 0.5 million were children and 2.9 million (2.7–3.1 million) occurred among women.
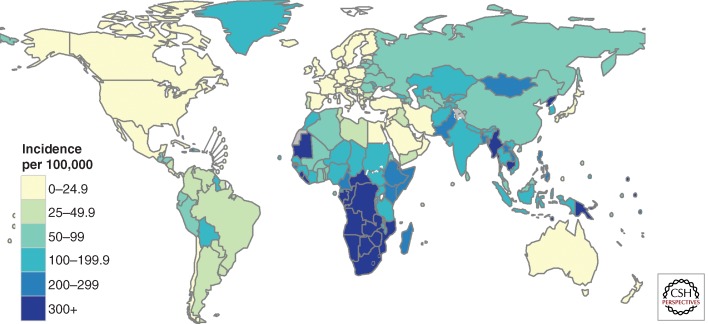
The incidence rate varies widely among countries (Fig. 1). The lowest rates are found predominantly in high-income countries including most countries in Western Europe, Canada, the United States of America, Australia, and New Zealand. In these countries, the incidence rate is less than 10 cases per 100,000 population. Most countries in the region of the Americas have rates below 50 per 100,000 population and this is the region with the lowest burden of TB on average. Countries in the top 10 worldwide in terms of incidence rates are mostly in Africa. In South Africa and Swaziland, the best estimate is that at least one in every 100 people develops TB each year.
Figure 1.
Estimated tuberculosis incidence rates per 100,000 population, 2012.
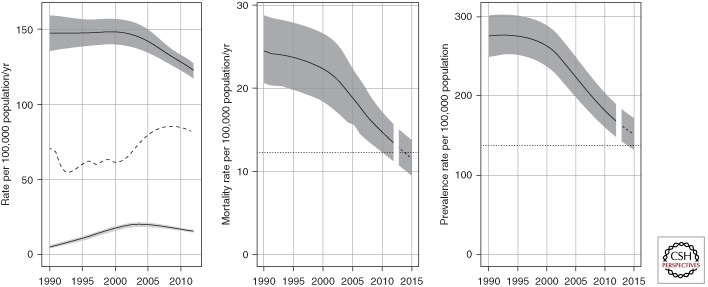
Globally, the incidence rate was relatively stable from 1990 up to around 2001, and then started to decrease, achieving the MDG target ahead of the 2015 deadline. Between 2011 and 2012, the rate of decline was 2% (Fig. 2, left panel). This downward trend needs to be sustained to ensure that the MDG target is met in 2015. Incidence rates are also declining in all of six WHO regions, fastest in the European region (6.5% per year) and slowest in the eastern Mediterranean and Southeast Asia regions (<1% per year and 2% per year, respectively). Incidence rates have been decreasing since the mid-1990s in the eastern Mediterranean region and since around 2000 in the Southeast Asia region; they peaked around 1997 in the European region and around 2002 in the African region, and have been decreasing since 1990 in the region of the Americas and the western Pacific region.
Figure 2.
Global trends in estimated TB incidence and estimated TB mortality. (Left) Global trends in estimated incidence including HIV-negative and HIV-positive TB (dark gray, top) and estimated incidence of HIV-positive TB (light gray, bottom). The dashed line shows global trends in case notification rates (all forms of TB). (Middle) Global trends in estimated TB mortality excluding TB-associated AIDS deaths. The dotted line represents the Stop TB Partnership targets of halving mortality by 2015 compared with the level of 1990. (Right) Global trends in estimated TB prevalence. The dotted line represents the Stop TB Partnership targets of halving prevalence by 2015 compared with the level of 1990. Shaded areas represent uncertainty bands.
Mortality
There were an estimated 1.3 million TB deaths in 2012: 940,000 among HIV-negative people and 320,000 among HIV-positive people. These deaths included 410,000 among women and 74,000 among HIV-negative children. Approximately 75% of total TB deaths occurred in the African and South-East Asia Regions in 2012 (both including and excluding TB deaths among HIV-positive people). India and South Africa accounted for about one-third of global TB deaths.
The number of TB deaths per 100,000 population averaged 13 globally in 2012 and 17.6 when TB deaths among HIV-positive people are included. There is considerable variation among countries, ranging from less than one TB death per 100,000 population (examples include most countries in western Europe, Canada, the United States of America, Australia and New Zealand) to more than 40 deaths per 100,000 population in much of the African region as well as three high burden countries (HBCs) in Asia (Bangladesh, Cambodia, and Myanmar).
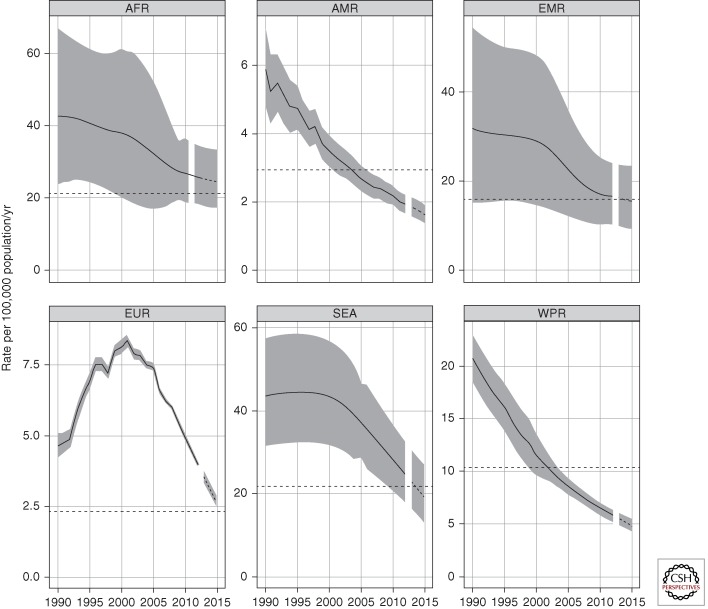
Globally, mortality rates (excluding deaths among HIV-positive people) have decreased by 45% since 1990 (Fig. 2, central panel); the current forecast suggests that the international target of a 50% reduction in TB mortality by 2015 compared with a baseline of 1990 will be achieved. Mortality rates are declining in all six WHO regions (Fig. 3). The 2015 target has already been surpassed in the region of the Americas (since 2004) and the western Pacific region (since 2002), and may have been reached in the eastern Mediterranean region. Among the other three regions, the Southeast Asia region appears best placed to achieve the target. The resurgence of TB mortality in Eastern European countries during the 1990s can be explained by the major political, economic, and social disruption associated with the breakup of the Soviet Union in 1991 and related failures in TB control and provision of health care (Shilova and Dye 2001).
Figure 3.
Trends in estimated TB mortality rates excluding HIV during the period 1990–2012 and forecast TB mortality rates 2013–2015 by WHO region. Estimated TB mortality excludes TB deaths among HIV-positive people (which are classified as AIDS deaths). Shaded areas represent uncertainty bands. The horizontal dashed lines represent the Stop TB Partnership target of a 50% reduction in the mortality rate by 2015 compared with 1990. The other dashed lines show projections up to 2015.
Prevalence
There were an estimated 12 million prevalent cases (11 million–13 million) of TB in 2012, equivalent to 169 cases per 100,000 population. By 2012, the prevalence rate had decreased 37% globally since 1990 (Fig. 2, right panel). Current forecasts suggest that the Stop TB Partnership target of halving TB prevalence by 2015 compared with a baseline of 1990 will not be met worldwide.
Regionally, prevalence rates are declining in all six WHO regions. The region of the Americas halved the 1990 level of TB prevalence by around 2004, well in advance of the target year of 2015, and the best estimate suggests that the Western Pacific Region achieved the 50% reduction target in 2012. Reaching the 50% reduction target by 2015 appears feasible in the Southeast Asia region and also in the European region with a relatively small acceleration in the current rate of progress. The target appears out of reach in the African and eastern Mediterranean regions. In Africa, prevalence rates increased substantially during the 1990s, and by 2007 were still far above the 1990 level. With limited access to antiretroviral therapy (ART), HIV has probably had a smaller effect on TB prevalence than on incidence because the duration of TB among HIV-infected patients is relatively short: for people with advanced HIV infection, the progression to severe tuberculosis is rapid, with a marked reduction in life expectancy (Corbett et al. 2004).
Case Notifications and Treatment Success
Routine recording and reporting of the numbers of TB cases diagnosed and treated by NTPs and monitoring of treatment outcomes was one of the five components of the global TB strategy (DOTS) launched by WHO in the mid-1990s and it remains a core element of its successor, the Stop TB Strategy (Box 2). With the standard definitions of cases and treatment outcomes recommended by WHO and associated recording and reporting framework as a foundation, global monitoring of trends in case notifications and treatment outcomes has been possible since 1995.
In 2012, 6.1 million cases of TB were notified by NTPs and reported to WHO. 5.7 million were individuals newly diagnosed in 2012 and 0.4 million were previously diagnosed TB patients whose treatment regimen was changed. India and China accounted for 39% of notified cases of TB worldwide in 2012, African countries for 23% and the 22 HBCs for 82%. Notifications of TB cases have stabilized in recent years, and in 2012 represented 66% (64%–69%) of estimated incident cases (Fig. 2).
Most cases (88%) were aged 15–64 yr. Children (aged <15 yr) accounted for <10% of notified cases. The sex ratio (M/F) was 1.7, ranging from 1.0 to 2.1 among the six WHO regions. The reasons for higher TB notification rates in men are poorly understood (Ottmani and Uplekar 2008). Possible explanations include biological differences between men and women in certain age groups that affect the risk of being infected as well as the risk of infection progressing to active disease (Thorson et al. 2007), and differences in the societal roles of men and women that influence their risk of exposure to TB and access to care (gender differences) (Connolly and Nunn 1996; Borgdorff et al. 2000; Somma et al. 2008). The observation that TB notification rates tend to be more equal between men and women in countries with a high prevalence of HIV supports the hypothesis of biological differences that can be lessened by immunological suppression caused by HIV, but other nonbiological factors may play a role. Variation in the sex ratio among countries may reflect real differences in epidemiology as well as differential access to health care and use of services linked to the NTP. In some settings (e.g., Afghanistan and the neighboring provinces of Iran and Pakistan), the sex ratio among notified cases is less than one. However, the recent national population-based survey in Pakistan (E Qadeer, R Fatima, S Tahseen, et al., unpubl.) confirmed findings from all other recent prevalence surveys to date that there are more men than women among prevalent TB cases (in Asian surveys conducted since 1990, the sex ratio has been between around 3 and 4), suggesting that women access TB care services linked to the NTP to a greater extent than men in some settings or that disease progression in those settings could be slower on average in women.
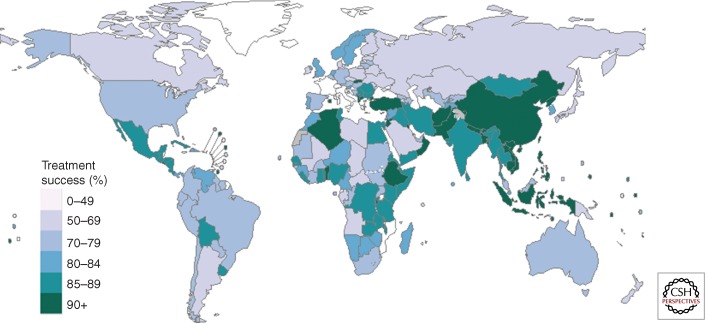
Globally, 87% of the new cases in 2011 were successfully treated (Fig. 4). The lowest treatment success rates were in Africa (79%, despite steady improvement since 2006), in the region of the Americas (75%), and in the European region (72%); in the latter case probably because of a high prevalence of multidrug-resistant TB. Treatment success for MDR-TB patients is in general much lower (see below).
Figure 4.
Treatment success rate, 2011 cohort of newly diagnosed TB cases (all forms). Countries shown in white did not report data to WHO for the 2011 cohort.
The provision of diagnosis and treatment according to the DOTS/Stop TB Strategy has resulted in major achievements in TB care and control. Between 1995 and 2012, 56 million people were successfully treated for TB in countries that had adopted the DOTS/Stop TB Strategy, saving 22 million lives.
HIV-Associated TB
The 8.6 million incident TB cases in 2012 included 1.0 million–1.2 million (12%–14%) among people living with HIV, with a best estimate of 1.1 million (13%). The probability of developing TB among people living with HIV divided by the probability of developing TB among HIV-negative people is the incidence rate ratio (IRR), a measure of the relative risk of TB in HIV-positive individuals compared with HIV-negative individuals. The estimated global IRR in 2012 was 29.6 (27.1–32.1).
The proportion of TB cases coinfected with HIV was highest in countries in the African region. Overall, 37% of TB cases were estimated to be coinfected with HIV in this region, which accounted for 75% of TB cases among people living with HIV worldwide. In parts of southern Africa, >50% of TB cases were coinfected with HIV. The number of people dying from HIV-associated TB has been decreasing since 2003. However, there were still 320,000 deaths from HIV-associated TB in 2012 and further efforts are needed to reduce this burden.
Globally, the percentage of notified TB patients with a documented HIV test result was 46% in 2012. In the African region, 74% of notified TB patients had an HIV test result in 2012. Among the 41 countries with the highest TB/HIV burden, 15 achieved HIV testing levels of ≥85%. There was an encouraging increase in ART coverage among HIV-positive TB patients between 2011 and 2012, from 49% worldwide in 2011 to 57% in 2012. Nonetheless, given the WHO recommendation that all HIV-positive TB patients are eligible for ART, the coverage of ART for HIV-positive TB patients still needs to be greatly improved. Antiretroviral therapy (ART) is expected to play an increasing role in preventing TB deaths as coverage expands (∼57% of notified TB cases estimated to be infected with HIV globally were on ART in 2012, compared with 16% in 2010) (Straetemans et al. 2010; Williams et al. 2010; Glaziou et al. 2011; WHO 2013a). ART and INH prophylaxis will also increasingly contribute to prevention of TB in individuals with both HIV infection and latent TB infection.
Multidrug-Resistant TB
Drug resistance, which develops as a result of inadequate treatment creating a selection pressure on spontaneously occurring mutants, complicates the management of TB; MDR- and XDR-TB represent one of the most important challenges in the control of TB worldwide (Raviglione and Uplekar 2006; Migliori et al. 2007a; Velayati et al. 2009; WHO 2011b; Udwadia et al. 2012).
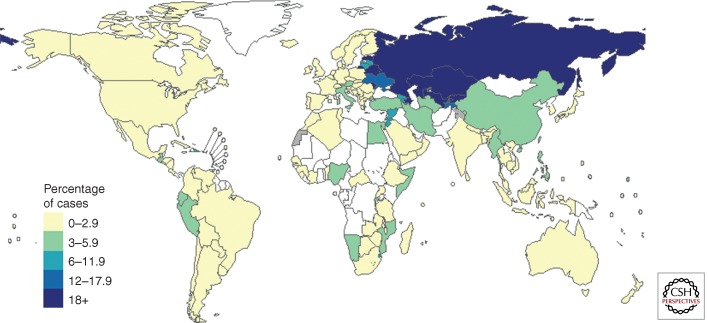
Globally among notified TB cases, an estimated 3.6% of new cases and 20.2% of those previously treated have MDR-TB. Eastern Europe and central Asia have the highest MDR-TB percentage globally (up to 35% among new cases in some oblasts of Russia and Belarus), whereas important data gaps exist in the African region (Fig. 5). There were an estimated 450,000 (300,000–600,000) new cases of MDR-TB in 2012. These emerged either among incident TB cases (primary MDR-TB) or prevalent TB cases currently on treatment (acquired MDR-TB) in 2012. Among new and retreated TB patients known to National TB Programs that could have tested for drug resistance, there were an estimated 300,000 (220,000–380,000) MDR-TB cases in 2012; more than half of these cases were in India, China, and the Russian Federation. Of these 300,000 estimated cases, 94,000 were detected as having MDR-TB and 82% of those started on second-line treatment. However, treatment success globally remains well behind the conventional target of 75% set in the Global Plan to Stop TB (WHO 2011a), at only 48% in the 2010 patient cohort (the latest cohort for which data were available at in 2013, given treatment duration of around 2 years). Finally, deaths from MDR-TB in 2012 were estimated at 170,000 (100,000–240,000). Extensively drug-resistant TB (XDR-TB) has been so far reported from 92 countries world-wide. It is estimated that ∼10% of the MDR-TB cases are XDR-TB.
Figure 5.
Percentage of new TB cases with MDR-TB, based on the most recent year for which data have been reported, which varies between countries. Countries and territories shown in white have reported no data. In Brazil, Spain, Central African Republic, Russia, Turkmenistan, India, Malaysia, and Indonesia, drug resistance surveillance data was only subnational.
By the end of June 2013, new diagnostics based on molecular techniques had started to be deployed in 88 low- and middle-income countries (WHO 2013a) and 49% of low and middle-income countries had incorporated WHO policy guidance on new diagnostics (Weyer et al. 2013) in their national guidelines. New diagnostics will make it possible for more people with drug-resistant disease to be detected earlier on in the course of their disease, thus improving outcomes.
Childhood TB
In 2012, an estimated 530,000 (510,000–550,000) children (aged 15 or less) fell ill with TB representing ∼6% of the global burden and there were 74,000 (59,000–90,000) deaths among HIV-negative children (representing ∼8% of total TB mortality among HIV-negative people). Out of the ∼350,000 TB notifications among children, 70% had pulmonary TB; most of these cases (∼80%) were diagnosed without bacteriological confirmation.
The traditionally low prioritization given to mostly noninfectious TB cases among children in overburdened health systems is aggravated by and has contributed to inadequate availability of diagnostic and treatment tools tailored to the needs of children, as well as to limitations in the availability of robust and nationally representative surveillance data. Nonetheless, growing attention to childhood TB in recent years has resulted in the development of clearly articulated actions for all stakeholders to address historical shortcomings. These include (1) strengthening surveillance through better recording and reporting and engagement with the private sector, especially pediatricians; (2) integrating TB screening in existing maternal and child health services, especially in TB endemic settings; and (3) addressing knowledge and research gaps in epidemiology, basic and operational research; and the development of new tools, such as diagnostics, drugs, vaccines (WHO 2013b).
TB PREVENTION AND CONTROL AFTER 2015
Incidence rates comparable with those currently found in low- and middle-income countries with a high burden of TB were recorded in North America and Western Europe as late as the early 20th century. A few of these countries have reliably documented their substantial reductions in case rates for more than 100 years (Styblo et al. 1969). The fastest nationwide declines of ∼10% per year occurred in the post-1945 period immediately after the second World War, when continued improvements in living conditions were combined with rapid economic growth, the introduction of effective chemotherapy, and universal (and largely free or not impoverishing) access to health care. Steeper reductions of 16% per year were recorded in two subpopulations with incidence rates 10 times higher than the 2012 global average (Canadian Inuits [Enarson and Grzybowski 1986] and Alaskan Eskimos [Grzybowski et al. 1976]) in the 1950s and 1960s, thanks to effective treatment programs combined with mass screening and preventive therapy. The current best performers among high-burden countries, such as Cambodia, China, and Rwanda, have recently achieved reductions of ∼3%–5% per year and the global decline in incidence is only 2% per year. With current tools, effective TB control should be able to achieve faster reductions in incidence of 5%–10% per year (Dye et al. 2013). The 2050 target of TB elimination is most likely to be achieved if, besides early detection and treatment of active TB cases, latent infection is treated and active disease prevented with either such an easily administered preventive treatment or a new vaccine (Dye and Williams 2008). This will require new and better tools.
To date, only BCG vaccination, provided to >80% of the annual birth cohort, and treatment of active TB using first-line drugs, have been implemented on a large scale (Lonnroth et al. 2010; Raviglione et al. 2012a). With these interventions that result in a global decline of some 2% per year, elimination (less than one case per million population) will not be achieved by 2050 (Lonnroth and Raviglione 2008). A rapid reduction in TB incidence and mortality in excess of what has been achieved historically requires more effective implementation of existing tools and the development and widespread use of a highly effective and safe new vaccine or prophylactic treatment (PT) to prevent reactivation of TB disease among the estimated 2 billion infected people (Dye et al. 1999; WHO 2012b) already. Currently, the only vaccine for TB prevention (BCG) dates from the 1920s and is mainly effective in preventing severe forms of TB in children, whereas its efficacy in the infectious adult forms of TB varies according to settings and populations not resulting in any large-scale protection. The earliest date at which a new vaccine may become available is now estimated as early to mid 2020. Randomized controlled trials of PT have shown that up to 9 mo of daily INH for treatment of latent infection confers 25%–92% protection against developing active TB, with results toward the upper end of this range when patients adhere fully to the treatment regimen (Sterling 2008). Isoniazid, alone or in combination (Sterling et al. 2011), is also a relatively safe drug. Its main side effect, hepatitis, occurs in 1% of patients; however, four to seven treated individuals per 100,000 may develop fatal hepatitis (Millard et al. 1996). The high risk of TB among people coinfected with M. tuberculosis and HIV is a reason for promoting much greater use in settings with a high prevalence of HIV (Woldehanna and Volmink 2004; Churchyard et al. 2007). To date, however, PTs with INH or combinations of drugs are not widely used.
First-line treatments that cure ∼90% of drug-susceptible cases remain long (6 mo) and are based on drug combinations dating from the 1970s. In mid-2013, there were 12 vaccine candidates for TB prevention in clinical trials and 10 new or repurposed drugs in late phases of clinical development. Two new drugs belonging to two novel classes of antibiotics were approved by strict regulatory authorities in the past 15 mo—the dyarilquinoline, bedaquiline (Diacon et al. 2012) and the nitroimidazole, delamanid (Gler et al. 2012). These two drugs offer hope of better outcomes in severe cases of MDR-TB, but have not yet been tested to shorten treatment of drug-susceptible TB or as preventive treatment. Various new diagnostic tests and methods have been endorsed since 2007, although a point-of-care test remains elusive. Research and development related to new drugs, new diagnostics, and new vaccines has accelerated in recent years (Lonnroth and Raviglione 2008; Ly and McMurray 2008; Pai and O’Brien 2008; Lienhardt et al. 2012a,b; Nicolau et al. 2012; Raviglione et al. 2012a; WHO 2013a).
Besides PT, there is considerable scope to expand interventions to reduce TB-related mortality in HIV-positive people. ART decreases the incidence of TB among people living with HIV (although more research is needed to understand the impact of large scale ART programs on TB epidemics) and reduces mortality. Cotrimoxazole preventive therapy (CPT) for HIV-positive TB patients reduces mortality (Grimwade et al. 2005; Harries et al. 2009). Recent expansion of TB control in areas in which the prevalence of HIV is high has also exposed a neglect of the basic principles of infection control, in which immunosuppressed patients have been exposed to patients with active TB in clinics and hospitals (Bock et al. 2007; Wells et al. 2007).
Provision of diagnosis and treatment for cases of MDR-TB is mostly confined to the European region, North America, and South Africa (WHO 2013a). Urgent improvements in the provision of services for laboratory culture and DST, new rapid drug susceptibility tests as well as treatment are needed in many countries. The emergence of XDR-TB (Migliori et al. 2007b; Shah et al. 2007) has reinforced the need for updated plans to improve the performance of TB control, including prevention of additional acquisition of resistance, transmission of resistant strains, and the capacity to diagnose and provide adequate treatment for drug-resistant TB (Manissero and Fernandez de la Hoz 2006; Raviglione 2006a; Raviglione and Smith 2007).
In addition to scaling up interventions related to treatment of latent infection, TB/HIV, and MDR-TB, the Stop TB Strategy emphasizes the importance of engaging the full range of health-care providers. These include those in prisons and the armed forces, private clinicians, services provided by nongovernmental organizations, faith-based organizations, and mission services and hospitals, and clinics in the corporate sector (Lonnroth et al. 2004; Ambe et al. 2005; Lonnroth and Uplekar 2005; Floyd et al. 2006; Pantoja et al. 2009). Such engagement can shorten delays to diagnosis and ensure that treatment for all patients (in both the public and the nonstate sector) meets international standards (Hopewell et al. 2006).
As in the immediate post–World War II period, a paradigm shift in the tools available for TB control is now needed, in the context of Universal Health Coverage (WHO 2012a). Incidence would need to decline as fast as 20% per year on average for the elimination target to be achieved by 2050. Wider application of existing interventions combined with development and implementation of new tools is required, as set out in the Stop TB Strategy and the Global Plan to Stop TB (Raviglione 2006b, 2007). In addition, TB control and prevention need to be placed in the wider context of health and development (Dye and Raviglione 2013). The challenge will be to incorporate new tools into TB control practices while also strengthening overall health systems and ensuring wider access to prevention, diagnostic, and treatment. In 2012, WHO initiated work on a new post-2015 global TB strategy that incorporates all of these elements; it is anticipated that the strategy and associated operational guidance will be finalized by 2015.
Recognition that TB depends largely on social and economic determinants of ill health is crucial to achieve full control and elimination. Factors such as smoking, alcohol abuse, diabetes, indoor air pollution, and malnutrition that, like HIV, are impacting on TB epidemiology impeding faster progress toward its control (Lonnroth et al. 2009; Lienhardt et al. 2012a). Preventive strategies may result in remarkable outcomes among certain vulnerable groups, such as people with diabetes and those infected with HIV. Reductions in levels of smoking, alcohol abuse, malnutrition, and indoor air pollution will likely be associated with reduction in TB infection and disease. Ultimately, education, health promotion, universal health coverage (Moreno-Serra and Smith 2012), and poverty-reduction strategies will have an effect on the TB epidemic by removing progressively individuals from the vulnerable pool.
CONCLUSIONS
Major gains were achieved in TB control over the past two decades. Between 1995 and 2012, 22 million lives were saved in countries that had adopted the DOTS/Stop TB Strategy (WHO 2013a) and international TB targets for 2015 will likely be met at the global level (WHO 2013a). Nonetheless, the burden of TB remains high and affects disproportionately poor and disadvantaged populations worldwide (Squire et al. 2006), and whereas the burden of TB is declining, the rate of progress is too slow. The accelerated development of new tools allows for a more optimistic view of the future course of the TB pandemic. A post-2015 global strategy to control TB will necessarily include the successful development and application of new and safe prophylactic treatments and effective vaccines (Raviglione et al. 2012b) administered on a very large scale. While pursuing new tool research and development, global adoption and rapid implementation of the universal health-care agenda and effectively addressing determinants of TB will be the key to accelerating the decline in incidence.
ACKNOWLEDGMENTS
We sincerely thank Matteo Zignol and Dennis Falzon for their contribution to the section on drug-resistant tuberculosis. The World Health Organization (WHO) has graciously granted the authors and publishers permission to publish this material, which remains exclusively vested in WHO.
Footnotes
Editors: Stefan H.E. Kaufmann, Eric J. Rubin, and Alimuddin Zumla
Additional Perspectives on Tuberculosis available at www.perspectivesinmedicine.org
REFERENCES
- Ambe G, Lonnroth K, Dholakia Y, Copreaux J, Zignol M, Borremans N, Uplekar M 2005. Every provider counts: Effect of a comprehensive public-private mix approach for TB control in a large metropolitan area in India. Int J Tuberc Lung Dis 9: 562–568. [PubMed] [Google Scholar]
- Baussano I, Bugiani M, Gregori D, van Hest R, Borraccino A, Raso R, Merletti F 2006. Undetected burden of tuberculosis in a low-prevalence area. Int J Tuberc Lung Dis 10: 415–421. [PubMed] [Google Scholar]
- Bierrenbach AL, Stevens AP, Gomes AB, Noronha EF, Glatt R, Carvalho CN, Oliveira Junior JG, Souza Mde F 2007. [Impact on tuberculosis incidence rates of removal of repeat notification records]. Revista de saude publica 41: 67–76. [DOI] [PubMed] [Google Scholar]
- Bock NN, Jensen PA, Miller B, Nardell E 2007. Tuberculosis infection control in resource-limited settings in the era of expanding HIV care and treatment. J Infect Dis 196: S108–S113. [DOI] [PubMed] [Google Scholar]
- Borgdorff MW, Nagelkerke NJ, Dye C, Nunn P 2000. Gender and tuberculosis: A comparison of prevalence surveys with notification data to explore sex differences in case detection. Int J Tuberc Lung Dis 4: 123–132. [PubMed] [Google Scholar]
- Borgdorff MW, Glynn JR, Vynnycky E 2004. Using capture-recapture methods to study recent transmission of tuberculosis. Int J Epidemiol 33: 905–906. [DOI] [PubMed] [Google Scholar]
- Borgdorff MW, Sebek M, Geskus RB, Kremer K, Kalisvaart N, van Soolingen D 2011. The incubation period distribution of tuberculosis estimated with a molecular epidemiological approach. Int J Epidemiol 40: 964–970. [DOI] [PubMed] [Google Scholar]
- Botha E, den Boon S, Lawrence KA, Reuter H, Verver S, Lombard CJ, Dye C, Enarson DA, Beyers N 2008. From suspect to patient: Tuberculosis diagnosis and treatment initiation in health facilities in South Africa. Int J Tuberc Lung Dis 12: 936–941. [PubMed] [Google Scholar]
- Cailhol J, Che D, Jarlier V, Decludt B, Robert J 2005. Incidence of tuberculous meningitis in France, 2000: A capture-recapture analysis. Int J Tuberc Lung Dis 9: 803–808. [PubMed] [Google Scholar]
- Churchyard GJ, Scano F, Grant AD, Chaisson RE 2007. Tuberculosis preventive therapy in the era of HIV infection: Overview and research priorities. J Infect Dis 196: S52–S62. [DOI] [PubMed] [Google Scholar]
- Comas I, Coscolla M, Luo T, Borrell S, Holt KE, Kato-Maeda M, Parkhill J, Malla B, Berg S, Thwaites G, et al. 2013. Out-of-Africa migration and Neolithic coexpansion of Mycobacterium tuberculosis with modern humans. Nat Genet 45: 1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M, Nunn P 1996. Women and tuberculosis. World Health Stat Q 49: 115–119. [PubMed] [Google Scholar]
- Corbett EL, Charalambous S, Moloi VM, Fielding K, Grant AD, Dye C, De Cock KM, Hayes RJ, Williams BG, Churchyard GJ 2004. Human immunodeficiency virus and the prevalence of undiagnosed tuberculosis in African gold miners. Am J Respir Crit Care Med 170: 673–679. [DOI] [PubMed] [Google Scholar]
- Crofts JP, Pebody R, Grant A, Watson JM, Abubakar I 2008. Estimating tuberculosis case mortality in England and Wales, 2001–2002. Int J Tuberc Lung Dis 12: 308–313. [PubMed] [Google Scholar]
- Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, van Niekerk C, Everitt D, Winter H, Becker P, et al. 2012. 14-day bactericidal activity of PA-824, bedaquiline, pyrazinamide, and moxifloxacin combinations: A randomised trial. Lancet 380: 986–993. [DOI] [PubMed] [Google Scholar]
- Dye C, Floyd K 2006. Tuberculosis. In Disease Control Priorities Priorities in Developing Countries (ed. Jamison DT et al. ), pp. 289–309 Oxford University Press, Washington, DC. [Google Scholar]
- Dye C, Raviglione M 2013. Weigh all TB risks. Nature 502: S13. [DOI] [PubMed] [Google Scholar]
- Dye C, Williams BG 2008. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface 5: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC 1999. Consensus statement. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282: 677–686. [DOI] [PubMed] [Google Scholar]
- Dye C, Fengzeng Z, Scheele S, Williams B 2000. Evaluating the impact of tuberculosis control: Number of deaths prevented by short-course chemotherapy in China. Int J Epidemiol 29: 558–564. [PubMed] [Google Scholar]
- Dye C, Ottmani S, Laasri L, Bencheikh N 2007. The decline of tuberculosis epidemics under chemotherapy: A case study in Morocco. Int J Tuberc Lung Dis 11: 1225–1231. [PubMed] [Google Scholar]
- Dye C, Bassili A, Bierrenbach AL, Broekmans JF, Chadha VK, Glaziou P, Gopi PG, Hosseini M, Kim SJ, Manissero D, et al. 2008. Measuring tuberculosis burden, trends, and the impact of control programmes. Lancet Infect Dis 8: 233–243. [DOI] [PubMed] [Google Scholar]
- Dye C, Glaziou P, Floyd K, Raviglione M 2013. Prospects for tuberculosis elimination. Annu Rev Public Health 34: 271–286. [DOI] [PubMed] [Google Scholar]
- Enarson DA, Grzybowski S 1986. Incidence of active tuberculosis in the native population of Canada. CMAJ 134: 1149–1152. [PMC free article] [PubMed] [Google Scholar]
- Floyd K, Arora VK, Murthy KJ, Lonnroth K, Singla N, Akbar Y, Zignol M, Uplekar M 2006. Cost and cost-effectiveness of PPM-DOTS for tuberculosis control: Evidence from India. Bull World Health Organ 84: 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaziou P, van der Werf MJ, Onozaki I, Dye C, Borgdorff MW, Chiang CY, Cobelens F, Enarson DA, Gopi PG, Holtz TH, et al. 2008. Tuberculosis prevalence surveys: Rationale and cost. Int J Tuberc Lung Dis 12: 1003–1008. [PubMed] [Google Scholar]
- Glaziou P, Floyd K, Korenromp EL, Sismanidis C, Bierrenbach AL, Williams BG, Atun R, Raviglione M 2011. Lives saved by tuberculosis control and prospects for achieving the 2015 global target for reducing tuberculosis mortality. Bull World Health Organ 89: 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park SK, Shim TS, et al. 2012. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med 366: 2151–2160. [DOI] [PubMed] [Google Scholar]
- Grimwade K, Sturm AW, Nunn AJ, Mbatha D, Zungu D, Gilks CF 2005. Effectiveness of cotrimoxazole prophylaxis on mortality in adults with tuberculosis in rural South Africa. AIDS (London, England) 19: 163–168. [DOI] [PubMed] [Google Scholar]
- Grzybowski S, Styblo K, Dorken E 1976. Tuberculosis in Eskimos. Tubercle 57: S1–S58. [DOI] [PubMed] [Google Scholar]
- Harries AD, Zachariah R, Lawn SD 2009. Providing HIV care for co-infected tuberculosis patients: A perspective from sub-Saharan Africa. Int J Tuberc Lung Dis 13: 6–16. [PubMed] [Google Scholar]
- Holloway KL, Henneberg RJ, de Barros Lopes M, Henneberg M 2011. Evolution of human tuberculosis: A systematic review and meta-analysis of paleopathological evidence. Homo 62: 402–458. [DOI] [PubMed] [Google Scholar]
- Hong YP, KSJ, Lew WJ, Lee EK, Han YC 1998. The seventh nationwide tuberculosis prevalence survey in Korea, 1995. Int J Tuberc Lung Dis 2: 27–36. [PubMed] [Google Scholar]
- Hopewell PC, Pai M, Maher D, Uplekar M, Raviglione MC 2006. International standards for tuberculosis care. Lancet Infect Dis 6: 710–725. [DOI] [PubMed] [Google Scholar]
- Korenromp EL, Williams BG, Dye C 2009. The measurement and estimation of tuberculosis mortality. Int J Tuberc Lung Dis 13: 283–303. [PubMed] [Google Scholar]
- Korenromp EL, Glaziou P, Fitzpatrick C, Floyd K, Hosseini M, Raviglione M, Atun R, Williams B 2012. Implementing the global plan to stop TB, 2011–2015—Optimizing allocations and the Global Fund’s contribution: A scenario projections study. PLoS ONE 7: e38816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lienhardt C, Glaziou P, Uplekar M, Lonnroth K, Getahun H, Raviglione M 2012a. Global tuberculosis control: Lessons learnt and future prospects. Nat Rev Microbiol 10: 407–416. [DOI] [PubMed] [Google Scholar]
- Lienhardt C, Raviglione M, Spigelman M, Hafner R, Jaramillo E, Hoelscher M, Zumla A, Gheuens J 2012b. New drugs for the treatment of tuberculosis: Needs, challenges, promise, and prospects for the future. J Infect Dis 205: S241–S249. [DOI] [PubMed] [Google Scholar]
- Lonnroth K, Raviglione M 2008. Global epidemiology of tuberculosis: Prospects for control. Semin Respir Crit Care Med 29: 481–491. [DOI] [PubMed] [Google Scholar]
- Lonnroth K, Uplekar M 2005. Invest in breaking the barriers of public-private collaboration for improved tuberculosis care. Bull World Health Organ 83: 558–559. [PMC free article] [PubMed] [Google Scholar]
- Lonnroth K, Uplekar M, Arora VK, Juvekar S, Lan NT, Mwaniki D, Pathania V 2004. Public-private mix for DOTS implementation: What makes it work? Bull World Health Organ 82: 580–586. [PMC free article] [PubMed] [Google Scholar]
- Lonnroth K, Jaramillo E, Williams BG, Dye C, Raviglione M 2009. Drivers of tuberculosis epidemics: The role of risk factors and social determinants. Soc Sci Med 68: 2240–2246. [DOI] [PubMed] [Google Scholar]
- Lonnroth K, Castro KG, Chakaya JM, Chauhan LS, Floyd K, Glaziou P, Raviglione MC 2010. Tuberculosis control and elimination 2010–50: Cure, care, and social development. Lancet 375: 1814–1829. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ 2006. Global and regional burden of disease and risk factors, 2001: Systematic analysis of population health data. Lancet 367: 1747–1757. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly LH, McMurray DN 2008. Tuberculosis: Vaccines in the pipeline. Expert Rev Vaccines 7: 635–650. [DOI] [PubMed] [Google Scholar]
- Manissero D, Fernandez de la Hoz K 2006. Surveillance methods and case definition for extensively drug resistant TB (XDR-TB) and relevance to Europe: Summary update. Euro Surveill 11: E061103.1. [DOI] [PubMed] [Google Scholar]
- Meintjes G, Schoeman H, Morroni C, Wilson D, Maartens G 2008. Patient and provider delay in tuberculosis suspects from communities with a high HIV prevalence in South Africa: A cross-sectional study. BMC Infect Dis 8: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliori GB, De Iaco G, Besozzi G, Centis R, Cirillo DM 2007a. First tuberculosis cases in Italy resistant to all tested drugs. Euro Surveill 12: E070517.1. [DOI] [PubMed] [Google Scholar]
- Migliori GB, Loddenkemper R, Blasi F, Raviglione MC 2007b. 125 years after Robert Koch’s discovery of the tubercle bacillus: The new XDR-TB threat. Is “science” enough to tackle the epidemic? Eur Respir J 29: 423–427. [DOI] [PubMed] [Google Scholar]
- Millard PS, Wilcosky TC, Reade-Christopher SJ, Weber DJ 1996. Isoniazid-related fatal hepatitis. West J Med 164: 486–491. [PMC free article] [PubMed] [Google Scholar]
- Miller TL, McNabb SJ, Hilsenrath P, Pasipanodya J, Weis SE 2009. Personal and societal health quality lost to tuberculosis. PLoS ONE 4: e5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Serra R, Smith PC 2012. Does progress towards universal health coverage improve population health? Lancet 380: 917–923. [DOI] [PubMed] [Google Scholar]
- Murray CJ, DeJonghe E, Chum HJ, Nyangulu DS, Salomao A, Styblo K 1991. Cost effectiveness of chemotherapy for pulmonary tuberculosis in three sub-Saharan African countries. Lancet 338: 1305–1308. [DOI] [PubMed] [Google Scholar]
- Nicolau I, Ling D, Tian L, Lienhardt C, Pai M 2012. Research questions and priorities for tuberculosis: A survey of published systematic reviews and meta-analyses. PLoS ONE 7: e42479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill K, Takane M, Sheffel A, Abou-Zahr C, Boerma T 2013. Monitoring service delivery for universal health coverage: The Service Availability and Readiness Assessment. Bull World Health Organ 91: 923–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortblad KF, Lozano R, Murray CJ 2013. The burden of HIV: Insights from the Global Burden of Disease Study 2010. AIDS 27: 2003–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottmani SE, Uplekar MW 2008. Gender and TB: Pointers from routine records and reports. Int J Tuberc Lung Dis 12: 827–828. [PubMed] [Google Scholar]
- Pablos-Mendez A, Raviglione MC, Laszlo A, Binkin N, Rieder HL, Bustreo F, Cohn DL, Lambregts-van Weezenbeek CS, Kim SJ, Chaulet P, et al. 1998. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med 338: 1641–1649. [DOI] [PubMed] [Google Scholar]
- Pai M, O’Brien R 2008. New diagnostics for latent and active tuberculosis: State of the art and future prospects. Semin Respir Crit Care Med 29: 560–568. [DOI] [PubMed] [Google Scholar]
- Pantoja A, Floyd K, Unnikrishnan KP, Jitendra R, Padma MR, Lal SS, Uplekar M, Chauhan LS, Kumar P, Sahu S, et al. 2009. Economic evaluation of public-private mix for tuberculosis care and control, India. Part I. Socio-economic profile and costs among tuberculosis patients. Int J Tuberc Lung Dis 13: 698–704. [PubMed] [Google Scholar]
- Raviglione M 2006a. XDR-TB: Entering the post-antibiotic era? Int J Tuberc Lung Dis 10: 1185–1187. [PubMed] [Google Scholar]
- Raviglione MC 2006b. The Global Plan to Stop TB, 2006–2015. Int J Tuberc Lung Dis 10: 238–239. [PubMed] [Google Scholar]
- Raviglione MC 2007. The new Stop TB Strategy and the Global Plan to Stop TB, 2006–2015. Bull World Health Organ 85: 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviglione MC, Pio A 2002. Evolution of WHO policies for tuberculosis control, 1948–2001. Lancet 359: 775–780. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, Smith IM 2007. XDR tuberculosis—Implications for global public health. N Engl J Med 356: 656–659. [DOI] [PubMed] [Google Scholar]
- Raviglione MC, Uplekar MW 2006. WHO’s new Stop TB Strategy. Lancet 367: 952–955. [DOI] [PubMed] [Google Scholar]
- Raviglione M, Marais B, Floyd K, Lonnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, et al. 2012a. Scaling up interventions to achieve global tuberculosis control: Progress and new developments. Lancet 379: 1902–1913. [DOI] [PubMed] [Google Scholar]
- Raviglione M, Zumla A, Marais B, Horton R, Motsoaledi A 2012b. A sustainable agenda for tuberculosis control and research. Lancet 379: 1077–1078. [DOI] [PubMed] [Google Scholar]
- Riley RL 1983. The contagiosity of tuberculosis. Schweiz Med Wochenschr 113: 75–79. [PubMed] [Google Scholar]
- Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martin-Casabona N, Drobniewski F, Gilpin C, Havelkova M, Lepe R, et al. 2007. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis 13: 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilova MV, Dye C 2001. The resurgence of tuberculosis in Russia. Philos Trans R Soc Lond B Biol Sci 356: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soemantri S, Senewe FP, Tjandrarini DH, Day R, Basri C, Manissero D, Mehta F, Dye C 2007. Three-fold reduction in the prevalence of tuberculosis over 25 years in Indonesia. Int J Tuberc Lung Dis 11: 398–404. [PubMed] [Google Scholar]
- Somma D, Thomas BE, Karim F, Kemp J, Arias N, Auer C, Gosoniu GD, Abouihia A, Weiss MG 2008. Gender and socio-cultural determinants of TB-related stigma in Bangladesh, India, Malawi and Colombia. Int J Tuberc Lung Dis 12: 856–866. [PubMed] [Google Scholar]
- Squire SB, Obasi A, Nhlema-Simwaka B 2006. The Global Plan to Stop TB: A unique opportunity to address poverty and the Millennium Development Goals. Lancet 367: 955–957. [DOI] [PubMed] [Google Scholar]
- Sterling TR 2008. New approaches to the treatment of latent tuberculosis. Semin Respir Crit Care Med 29: 532–541. [DOI] [PubMed] [Google Scholar]
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, et al. 2011. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med 365: 2155–2166. [DOI] [PubMed] [Google Scholar]
- Straetemans M, Bierrenbach AL, Nagelkerke N, Glaziou P, van der Werf MJ 2010. The effect of tuberculosis on mortality in HIV positive people: A meta-analysis. PLoS ONE 5: e15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straetemans M, Glaziou P, Bierrenbach AL, Sismanidis C, van der Werf MJ 2011. Assessing tuberculosis case fatality ratio: A meta-analysis. PLoS ONE 6: e20755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo K, Meijer J, Sutherland I 1969. [The transmission of tubercle bacilli: Its trend in a human population]. Bull World Health Organ 41: 137–178. [PMC free article] [PubMed] [Google Scholar]
- Thorson A, Long NH, Larsson LO 2007. Chest X-ray findings in relation to gender and symptoms: A study of patients with smear positive tuberculosis in Vietnam. Scand J Infect Dis 39: 33–37. [DOI] [PubMed] [Google Scholar]
- Tiemersma EW, van der Werf MJ, Borgdorff MW, Williams BG, Nagelkerke NJ 2011. Natural history of tuberculosis: Duration and fatality of untreated pulmonary tuberculosis in HIV negative patients: A systematic review. PLoS ONE 6: e17601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tupasi TE, Radhakrishna S, Rivera AB, Pascual MLG, Quelapio MI, Co VM, Villa MLA, Beltran G, Legaspi JD, Mangubat NV, et al. 1999. The 1997 nationwide tuberculosis prevalence survey in the Philippines. Int J Tuberc Lung Dis 3: 471–477. [PubMed] [Google Scholar]
- Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C 2012. Totally drug-resistant tuberculosis in India. Clin Infect Dis 54: 579–581. [DOI] [PubMed] [Google Scholar]
- Uplekar M, Pathania V, Raviglione M 2001. Private practitioners and public health: Weak links in tuberculosis control. Lancet 358: 912–916. [DOI] [PubMed] [Google Scholar]
- Van Hest N, Story A, Grant AD, Antoine D, Crofts JP, Watson JM 2008. Record-linkage and capture-recapture analysis to estimate the incidence and completeness of reporting of tuberculosis in England 1999–2002. Epidemiol Infect 136: 1606–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velayati AA, Masjedi MR, Farnia P, Tabarsi P, Ghanavi J, Ziazarifi AH, Hoffner SE 2009. Emergence of new forms of totally drug-resistant tuberculosis bacilli: Super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136: 420–425. [DOI] [PubMed] [Google Scholar]
- Veron LJ, Blanc LJ, Suchi M, Raviglione MC 2004. DOTS expansion: Will we reach the 2005 targets? Int J Tuberc Lung Dis 8: 139–146. [PubMed] [Google Scholar]
- Vynnycky E, Fine PE 2000. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 152: 247–263. [DOI] [PubMed] [Google Scholar]
- Wells CD, Cegielski JP, Nelson LJ, Laserson KF, Holtz TH, Finlay A, Castro KG, Weyer K 2007. HIV infection and multidrug-resistant tuberculosis: The perfect storm. J Infect Dis 196: S86–S107. [DOI] [PubMed] [Google Scholar]
- Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D’Ambrosio L, Zignol M, Floyd K, Centis R, Cirillo DM, Tortoli E, et al. 2013. Rapid molecular TB diagnosis: Evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J 42: 252–271. [DOI] [PubMed] [Google Scholar]
- WHO. 2006. WHO Global Task Force outlines measures to combat XDR-TB worldwide [Internet]. WHO, Geneva. [Google Scholar]
- WHO. 2011a. The Global Plan to Stop TB 2011–2015. [Google Scholar]
- WHO. 2011b. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015. WHO progress report 2011. (ed. WHO). WHO, Geneva. [Google Scholar]
- WHO ed. 2011c. Tuberculosis prevalence surveys: A handbook. World Health Organization, Geneva. [Google Scholar]
- WHO. 2012a. Health financing for universal coverage. WHO, Geneva. [Google Scholar]
- WHO. 2012b. Tuberculosis fact sheet. WHO, Geneva. [Google Scholar]
- WHO. 2013a. Global TB report 2013 WHO, Geneva. [Google Scholar]
- WHO. 2013b. Roadmap for childhood tuberculosis. (ed. WHO). WHO, Geneva. [Google Scholar]
- Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C 2010. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Natl Acad Sci 107: 19485–19489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldehanna S, Volmink J 2004. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane database of systematic reviews (Online): CD000171. [DOI] [PubMed] [Google Scholar]
- World Health Organization. 2007. Global tuberculosis control: Surveillance, planning, financing. World Health Organization, Geneva. [Google Scholar]
- Zignol M, van Gemert W, Falzon D, Sismanidis C, Glaziou P, Floyd K, Raviglione M 2012. Surveillance of anti-tuberculosis drug resistance in the world: An updated analysis, 2007–2010. Bull World Health Organ 90: 111–119D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A, Mwaba P, Huggett J, Kapata N, Chanda D, Grange J 2009. Reflections on the white plague. Lancet Infect Dis 9: 197–202. [DOI] [PubMed] [Google Scholar]