Abstract
Signaling pathways regulate contraction of striated (skeletal and cardiac) and smooth muscle. Although these are similar, there are striking differences in the pathways that can be attributed to the distinct functional roles of the different muscle types. Muscles contract in response to depolarization, activation of G-protein-coupled receptors and other stimuli. The actomyosin fibers responsible for contraction require an increase in the cytosolic levels of calcium, which signaling pathways induce by promoting influx from extracellular sources or release from intracellular stores. Rises in cytosolic calcium stimulate numerous downstream calcium-dependent signaling pathways, which can also regulate contraction. Alterations to the signaling pathways that initiate and sustain contraction and relaxation occur as a consequence of exercise and pathophysiological conditions.
In all muscle cells, contraction depends on a rise in cytosolic calcium. Signaling pathways control the release of calcium from intracellular stores, as well as the contraction of muscle fibers after the calcium is released.
1. Introduction
Muscle can be subdivided into two general categories: striated muscle, which includes skeletal and cardiac muscles; and nonstriated muscle, which includes smooth muscle such as vascular, respiratory, uterine, and gastrointestinal muscles. In all muscle types, the contractile apparatus consists of two main proteins: actin and myosin. Striated muscle is so called because the regular arrangement of alternating actomyosin fibers gives it a striped appearance. This arrangement allows coordinated contraction of the whole muscle in response to neuronal stimulation through a voltage- and calcium-dependent process known as excitation–contraction coupling. The coupling enables the rapid and coordinated contraction required of skeletal muscles and the heart. Smooth muscle does not contain regular striations or undergo the same type of excitation–contraction coupling. Instead, it typically uses second messenger signaling to open intracellular channels that release the calcium ions that control the contractile apparatus. These processes, in contrast to excitation–contraction coupling, are slow and thus suitable for the slower and more sustained contractions required of smooth muscle. The actomyosin contractile apparatus is both calcium- and phosphorylation-dependent, and restoration of basal calcium levels or its phosphorylation status returns an actively contracting muscle to a noncontractile state. Muscle-specific signals modulate these processes, depending on the type of muscle, its function, and the amount of force required.
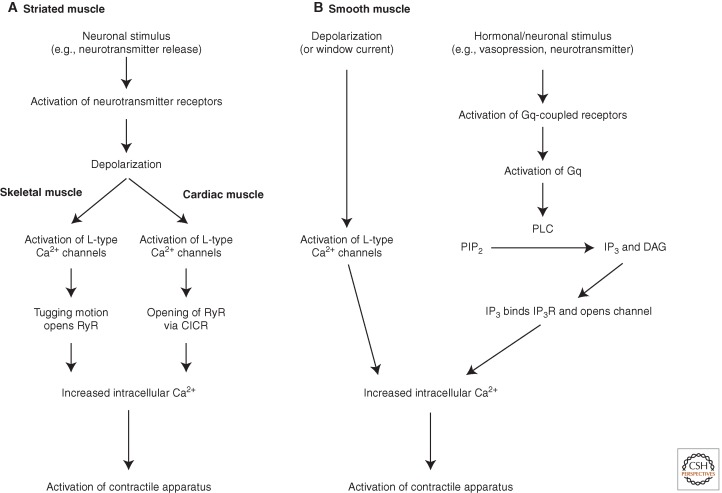
In all muscle cells, contraction thus depends on an increase in cytosolic calcium concentration (Fig. 1). Calcium has an extracellular concentration of 2–4 mm and a resting cytosolic concentration of ∼100 nm. It is also stored inside cells within the sarcoplasmic (SR, referring to skeletal and cardiac muscle) and endoplasmic reticulum (ER, referring to smooth muscle) at a concentration of ∼0.4 mm (Bootman 2012). In striated muscle, the increase in calcium levels is due to its release from the SR stores via ryanodine receptor (RyRs). Neurotransmitters such as acetylcholine bind to receptors on the muscle surface and elicit a depolarization by causing sodium/calcium ions to enter through associated channels. This shifts the resting membrane potential to a more positive value, which in turn activates voltage-gated channels, resulting in an action potential (the “excitation” part). The action potential stimulates L-type calcium channels (also known as dihydropyridine receptors). In skeletal muscle, these are mechanically coupled to the SR RyRs and open them directly. In cardiac muscle, calcium influx through the L-type channels opens RyRs via calcium-induced calcium release (CICR) (Bootman 2012). The RyR is a large tetrameric six-transmembrane-span calcium-release channel. Of the three RyR subtypes, RyR1 is predominantly found in skeletal muscle (see review by Klein et al. 1996), and RyR2 is predominantly found in cardiac muscle (Cheng et al. 1993).
Figure 1.
Overview of muscle contraction signals in striated (A), and smooth (B) muscle.
Smooth muscle also contains voltage-gated calcium channels and RyRs responsible for increases in intracellular calcium concentration (see below). Depolarization causes L-type calcium channels to open, enabling calcium to enter down its concentration gradient into the cell (Fig. 1B). Opening of RyRs is usually associated with CICR. As the intracellular calcium concentration rises, calcium binds to RyRs, whose consequent opening further enhances the increase in cytoplasmic calcium concentration. Another major mechanism controlling contraction in these cells, however, involves a different tetrameric six-transmembrane-span calcium channel: the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R). Circulating hormones (e.g., vasopressin and bradykinin) and neurotransmitters released by sympathetic nerves (e.g., endothelin and norepinephrine) act through G-protein-coupled receptors (GPCRs) to generate the second messenger IP3 via activation of phospholipase C (PLC). IP3 binds to and opens IP3Rs on the ER/SR, causing the calcium release that drives contraction. IP3Rs are present in both skeletal and cardiac muscle; however, they do not contribute significantly to the excitation–contraction coupling in striated muscle. Note that both RyRs and IP3Rs are stimulated by low concentrations of cytoplasmic calcium but close when the concentration gets higher, showing bell-shaped response curves (Bezprozvanny et al. 1991; Finch et al. 1991).
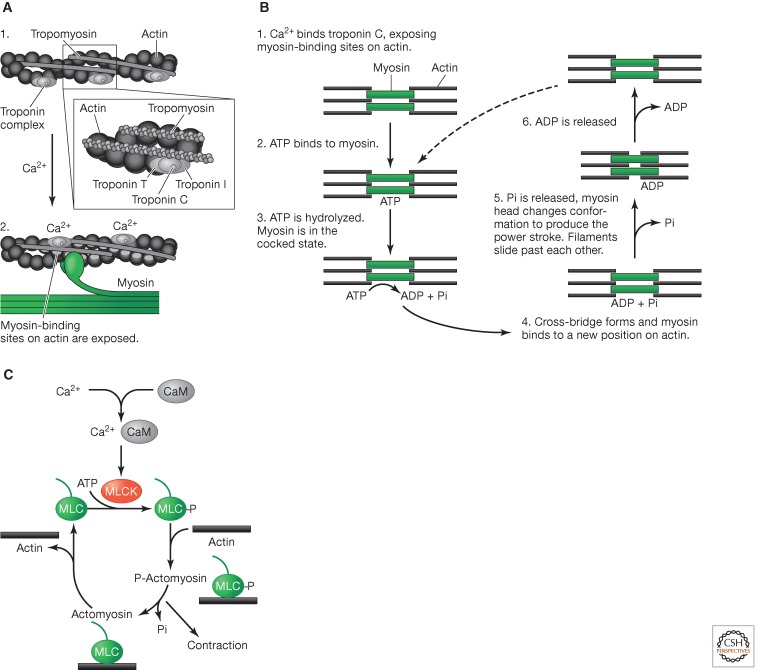
Once intracellular calcium levels are raised, calcium binds to either troponin C on actin filaments (in striated muscle) or calmodulin (CaM), which regulates myosin filaments (in smooth muscle). In striated muscle, calcium causes a shift in the position of the troponin complex on actin filaments, which exposes myosin-binding sites (Fig. 2A). Myosin bound by ADP and inorganic phosphate (Pi) can then form cross-bridges with actin, and the release of ADP and Pi produces the power stroke that drives contraction. This force causes the thin actin filament to slide past the thick myosin filament and shortens the muscle. Binding of ATP to myosin then releases myosin from actin, and myosin hydrolyzes ATP to repeat the process (Fig. 2B).
Figure 2.
Calcium triggers contraction in striated muscle. (A) Actomyosin in striated muscle. (1) Striated muscle in the relaxed state has tropomyosin covering myosin-binding sites on actin. (2) Calcium binds to troponin C, which induces a conformational change in the troponin complex. This causes tropomyosin to move deeper into the actin groove, revealing the myosin-binding sites. (B) Cross-bridge cycle in striated muscle. (1) Calcium binds to troponin C, causing the conformational shift in tropomyosin that reveals myosin-binding sites on actin. (2) ATP then binds to myosin. (3) ATP is then hydrolyzed. (4) A cross-bridge forms and myosin binds to a new position on actin. (5) Pi is released and myosin changes conformation, resulting in the power stroke that causes the filaments to slide past each other. (6) ADP is then released. (C) Contraction in smooth muscle. In smooth muscle, calcium binds to calmodulin and causes the activation of myosin light chain (MLC) kinase (MLCK). This phosphorylates MLC, which then binds to actin to form phosphorylated actomyosin, enabling the cross-bridge cycle to start.
In smooth muscle, by contrast, calcium binds to CaM, which then interacts with myosin light-chain kinase (MLCK), causing it to phosphorylate the myosin light-chain (MLC) at S19 or Y18. The phosphorylated MLC then forms cross-bridges with actin, producing phosphorylated actomyosin, which leads to contraction (Fig. 2C). Note that striated muscle contraction can also be regulated by calcium-bound CaM and MLCK; however, this is not the dominant mechanism. Finally, calcium and calcium-CaM also bind to various other proteins in muscle cells, including the phosphatase calcineurin and protein kinases such as CaMKIV, respectively. These regulate other cellular targets, including transcription factors such as NFAT and CREB, which control gene expression programs that can have longer-term effects on muscle physiology.
These different calcium-release mechanisms all also stimulate the pumping of calcium from the cytoplasm back into intracellular stores via the SR/ER calcium ATPase (SERCA) pump. The plasma membrane calcium ATPase (PMCA) pump and the sodium/calcium exchanger (NCX), both of which reside on the plasma membrane, can also remove calcium from the cytosol. Calcium dissociates from troponin C or calmodulin as the cytosolic calcium concentration decreases as a consequence, which terminates the contraction process.
The main pathways promoting muscle relaxation involve the second messengers cAMP and cyclic guanosine monophosphate (cGMP). cAMP is generated by adenylyl cyclases, downstream from the β-adrenergic GS-coupled receptor, which is activated by noradrenaline. Note that the cAMP pathway generally promotes contraction in cardiac muscle; however, in smooth muscle, activation of cAMP causes relaxation. The cGMP pathway can be activated either by nitric oxide (NO) or natriuretic peptides (NPs). In the case of blood vessels and other smooth muscles, NO produced by endothelial NO synthase (eNOS) diffuses across the muscle cell membrane to activate soluble guanylyl cyclase (sGC), which in turn increases levels of cGMP. NPs, such as atrial (ANP, released by the heart atria under high blood pressure), brain (BNP, primarily released by the heart ventricle), and c-type (CNP, mainly involved in pathological conditions, and released by the vascular and central nervous system), instead bind to transmembrane guanylyl cyclase, whose intracellular domain possesses the enzymatic activity (Nishikimi et al. 2011). The cAMP and cGMP generated act via the protein kinase PKA and PKG on the contractile process in multiple ways: (1) their phosphorylation of calcium pumps leads to increased activity; (2) activation of MLC phosphatase (MLCP) by PKG antagonizes MLCK; and (3) both PKA and PKG cause a reduction in the sensitivity of the contractile machinery by inhibiting the GTPase RhoA (this increases MLCP activity and causes MLC dephosphorylation and muscle relaxation). The levels of cAMP and cGMP are in turn regulated through their degradation by phosphodiesterases to yield the inactive metabolites 5′-AMP and 5′-GMP.
Below, we examine the key differences between the signaling mechanisms controlling contraction of skeletal, cardiac, and smooth muscle, and how these relate to their differing functions. In addition, we discuss the changes to the signaling pathways that occur as a consequence of exercise and pathological situations.
2. Skeletal muscle contraction
Skeletal muscles comprise multiple individual muscle fibers that are stimulated by motor neurons stemming from the spinal cord. They are grouped together to form “motor units” and more than one type of muscle fiber can be present within each motor unit. Muscle fibers can be divided into fast- and slow-twitch muscles. Fast-twitch muscles use glycolytic metabolism and are recruited for phasic activity (an active contraction). Slow-twitch muscles (also known as red muscles) are rich in myoglobin, mitochondria, and oxidative enzymes and specialized for sustained or tonic activity. See Schiaffino and Reggiani (2011) for a more complete discussion of skeletal muscle types and the types of myosin isoforms that make up fast- and slow-twitch muscles.
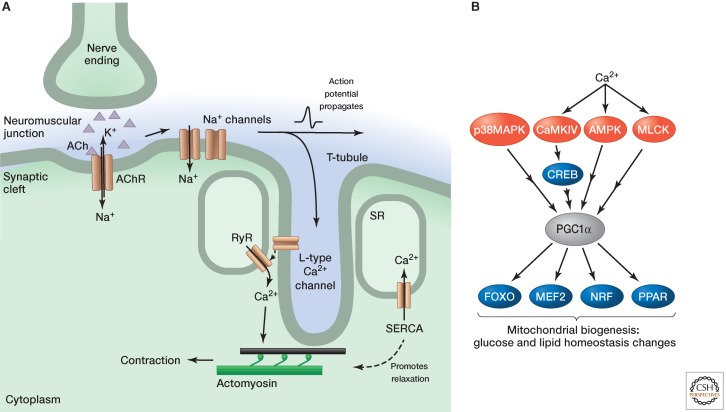
The neuromuscular junction (NMJ) that connects skeletal muscle with the nerves that innervate them consists of three distinct parts: the distal motor nerve ending, the synaptic cleft, and the postsynaptic region, located on the muscle membrane. Motor neurons branch into multiple termini, which are juxtaposed to motor endplates, specialized regions of muscle where neurotransmitter receptors are concentrated (Fig. 3A). The transfer of information between the nerve and muscle is mediated by the release of acetylcholine from the motor neuron, which diffuses across the synaptic cleft, and binds to and activates the ligand-gated, nicotinic acetylcholine receptors (nAChRs) on the endplate. Activation of the nAChR leads to an influx of cations (sodium and calcium) that causes depolarization of the muscle cell membrane. This depolarization in turn activates a high density of voltage-gated sodium channels on the muscle membrane, eliciting an action potential.
Figure 3.
Skeletal muscle contraction and changes with exercise. (A) Neurotransmitter (acetylcholine, ACh) released from nerve endings binds to receptors (AChRs) on the muscle surface. The ensuing depolarization causes sodium channels to open, which elicits an action potential that propagates along the cell. The action potential invades T-tubules and causes the L-type calcium channels to open, which in turn causes ryanodine receptors (RyRs) in the SR to open and release calcium, which stimulates contraction. Calcium is pumped back into the SR by (SR/ER calcium ATPase SERCA) pumps. The decreasing cytosolic calcium levels cause calcium to disassociate from troponin C and, consequently, tropomyosin reverts to a conformation that covers the myosin-binding sites. (B) Signaling in exercised skeletal muscle. Both calcium and calcium-independent signals stimulate the transcriptional coactivator PGC1α. This activates a number of transcription factors that regulate genes associated with mitochondrial biogenesis, glucose, and lipid homeostasis.
The action potential runs along the top of the muscle and invades the T-tubules (specialized invaginations of the membrane containing numerous ion channels). The opening of voltage-gated sodium channels activates L-type voltage-gated calcium channels lining the T-tubule. A conformational change in these enables release of calcium on the closely apposed SR via activation of RyR1. Calcium then binds to troponin as described above, initiating the contraction process. Calcium-bound CaM also activates MLCK, whose phosphorylation of the MLC changes cross-bridge properties. This modulates the troponin-dependent contraction, although there is no effect on the ATPase activity of MLC. MLC phosphorylation instead enhances force development at submaximal saturating calcium concentrations (see below). The phosphate group is subsequently removed by protein phosphatase 1 (PP1).
3. Skeletal muscle fiber types and exercise
Skeletal muscle is plastic. Exercise can lead to pronounced changes in its metabolic properties and, sometimes, a change in the fiber type. Physical differences between fast- and slow-twitch muscles underlie the functional roles of these fibers, including the type of myosin used and differing resting calcium levels. The free calcium level is twofold higher in slow-twitch muscle, even though the SR volume is greater. The level of MLC phosphorylation is higher in fast-twitch muscle, however, because of higher levels of expression of MLCK (Bozzo et al. 2005). The force enhancement produced by MLC phosphorylation, under submaximal saturating calcium concentrations, counteracts the reduction in force caused by fatigue in fast-twitch muscle fibers (Schiaffino and Reggiani 2011).
Fast- and slow-twitch fibers also have different calcium-sequestering and -buffering systems. Different SERCA isoforms are present: SERCA2A is the main isoform in slow-twitch muscle fibers, whereas SERCA1A is expressed in fast-twitch muscle fibers. Similarly, different cytosolic calcium buffers are expressed. Calsequestrin (CSQ) is the main SR-luminal calcium-buffering protein. It is a high-capacity, low-affinity calcium-binding protein that binds calcium cooperatively (Campbell et al. 1983). When the muscle is at rest, the SR is primed to release large amounts of calcium, because CSQ is polymerized, which reduces its ability to bind calcium. In cardiac muscle, only CSQ2 is expressed. In skeletal muscle, CSQ1 and CSQ2 are found in slow-twitch muscle fibers, but only CSQ1 is found in fast-twitch muscle fibers. The two isoforms differ in their carboxy-terminal tail; functionally, CSQ1 reduces the activity of RyR1, whereas CSQ2 increases the open probability of RyR1 and RyR2 (Wei et al. 2009).
Other differences between the muscle fiber types include posttranslational modifications such as phosphorylation of RyR by PKA, and interactions between RyR and other proteins, such as CaM and FK506-binding protein (FKBP) 12 and FKBP12.6. Phosphorylation of RyR by either PKA or CaMKII fully activates the channel. PKA and CaMKII can also phosphorylate phospholamban, a protein that inhibits SERCA; phosphorylation causes phospholamban to dissociate from SERCA. The FKBPs are immunophilins that bind to immunosuppressants such as rapamycin and FK506. FKBP12 and FKBP12.6 have differing expression levels in muscle tissue, but both bind all three forms of the RyR and stabilize its closed state. Collectively, these calcium-dependent differences between fast- and slow-twitch muscle fibers, in addition to differences in the myosin isoform used and the number of mitochondria, account for the different functional outputs of the two muscle fiber types.
Long-term exercise causes a general shift in muscle fiber type from slow twitch to fast twitch. It induces a number of changes, including altered expression and activity of membrane transporters and mitochondrial metabolic enzymes, together with increased blood supply to skeletal muscle (Hardie 2012). These, in turn, enhance the oxidative capacity and increase expression of enzymes preventing damage by reactive oxygen species (ROS). One major signaling pathway is through the peroxisome-proliferator-activated receptor (PPAR) γ coactivator (PGC) 1α (Fig. 3B). PGC1α coactivates a number of transcription factors that regulate genes important for muscle function. These include PPARs (which regulate glucose and lipid homeostasis, proliferation, and differentiation), nuclear respiratory factors (NRFs, which regulate metabolism and mitochondrial biosynthesis), myocyte enhancer factor 2 (MEF2, which is involved in development and hematopoesis), and Forkhead box O (FoxO) family transcription factors (which counter oxidative stress and promote cell-cycle arrest and apoptosis) (Handschin and Spiegelman 2006; Ronnebaum and Patterson 2010). In addition to PGC1α, calcium-dependent processes are also involved. Rises in cytosolic calcium result in the activation of calcineurin, which then dephosphorylates NFAT. Translocation of NFAT to the nucleus results in activation of slow-fiber gene expression. Rises in nuclear calcium levels also cause calcium-dependent signaling molecules to become active. These include the phosphorylation of histone deacetylases (HDACs) by nuclear calmodulin-dependent protein kinase. HDACs repress transcription by causing DNA to be tightly wrapped around histones. Removal of HDACs enables transcription factors such as MEF2 to bind and enable induction of genes encoding proteins found in slow fibers (Liu et al. 2005).
As mentioned above, exercise induces an increase in the levels of mitochondrial metabolic enzymes to compensate for the increased metabolic demand on skeletal muscle. Unsurprisingly, PGC1α is a potent stimulator of mitochondrial biogenesis (see review by Olesen et al. 2010). This was shown elegantly by experiments in which overexpression of PGC1α in white, glycolytic skeletal muscle could turn it into red, oxidative muscle by increasing the levels and activity of a number of mitochondrial proteins (Lin et al. 2002; Wenz et al. 2009). These proteins include most components of the mitochondrial respiratory chain and ATP synthase, as well as several enzymes in the Krebs cycle and enzymes involved in fatty acid oxidation.
4. Malignant hyperthermia in skeletal muscle
Mutations in RyR and CSQ isoforms cause malignant hyperthermia, demonstrating the importance of proteins involved in calcium signaling in skeletal muscle. The mutations in RyR1 appear to increase its open probability when levels of luminal calcium are low and account for the majority of malignant hyperthermia cases (80%); the remainder are caused by mutations to CSQ1.
In the case of RyR1 mutations, volatile anesthetics (inhaled anesthetics such as isoflurane or halothane) lead to a rapid opening of RyR1 and an uncontrolled release of calcium from the SR, which in turn leads to sustained skeletal muscle contraction (Robinson et al. 2006). In response to the elevated calcium levels, there is activation of SERCA to pump calcium, using ATP, back into the SR. However, the continual activation of SERCA consumes excessive ATP, leading to hypermetabolism. This then leads to a drop in ATP levels, acidosis, tachycardia, and an abnormal increase in body temperature. These symptoms can be treated with dantrolene, an inhibitor of the RyR signaling pathway. The mutations in RyR1 associated with malignant hyperthermia are clustered in three hot spots on the 500 kDa protein (Lanner et al. 2010). The first cluster is near the amino terminus and the second cluster is in the middle of the protein. The third cluster lies in the carboxy-terminal region surrounding the channel-forming domains. How mutations in all three regions exert similar effects is yet to be determined.
Mutations in CSQ can also result in malignant hyperthermia. A lack of buffering causes uncontrolled calcium transients that lead to lethal malignant hyperthermia in response to heat stress and volatile anesthetics (Dainese et al. 2009).
5. Cardiac muscle contraction
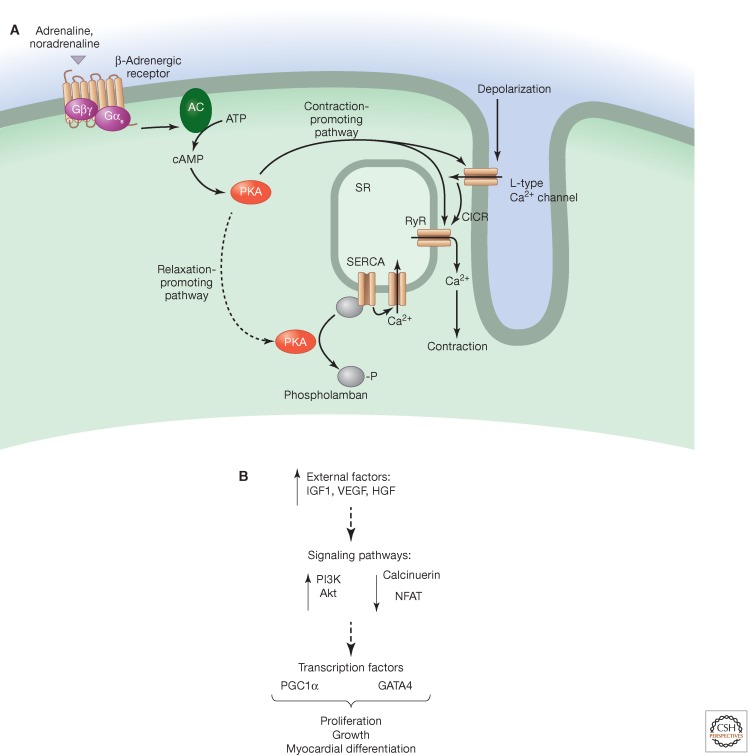
In cardiac muscle, depolarization starts in the pacemaker cells (modified cardiac myocytes that set the heart rate and are rich in signaling molecules) in the sinoatrial node, which is innervated by both parasympathetic and sympathetic nerves. The external stimuli modulate the activity of the pacemaker cells—they undergo spontaneous self-depolarization to produce action potentials. This is achieved by a slow leak of potassium ions and a concurrent influx of sodium and calcium ions. The action potential then traverses to the cardiac myocytes, where it invades the T-tubule. However, unlike skeletal muscle, where L-type calcium channels are directly coupled to RyRs, in cardiomyocytes the influx of calcium across the plasma membrane elicits calcium release from the SR via RyRs by CICR (Fig. 4B). The predominant isoform in the heart is RyR2. As in skeletal muscle, contraction is controlled by phosphorylation of troponin but can also be modulated by calcium-CaM and MLCK. Mice with a nonphosphorylatable MLC in ventricular myocytes display depressed contractile function and develop atrial hypertrophy and dilatation (Sanbe et al. 1999).
Figure 4.
Cardiac muscle contraction and changes with exercise. (A) Cardiac muscle contraction can occur as a consequence of calcium entry through L-type calcium channels, which activate ryanodine receptor (RyR) channels in the SR. Alternatively, β-adrenergic receptors on the cell membrane lead to activation of adenylyl cyclase (AC), which stimulates PKA. This can promote contraction by phosphorylating RyR and L-type calcium channels or relaxation by phosphorylating the SERCA pump inhibitor phospholamban. (B) Changes with exercise lead to an activation of the PI3K/Akt pathway, and a down-regulation of NFAT and calcinurin.
Catecholamines, such as adrenaline and noradrenaline, act on β-adrenergic receptors (metabotropic GPCRs) to release cAMP that in turn activates PKA. PKA can be viewed as a primary regulator of the contractile pathway, as it phosphorylates a number of targets, including L-type calcium channels and RyRs. In most cases, phosphorylation of these proteins increases calcium release (for example, phosphorylation of RyR increases its open probability), and thus the outcome is to stimulate contraction (Ibrahim et al. 2011). Another target of PKA is phospholamban (an inhibitor of SERCA), which, when phosphorylated, loses its inhibitory effect on SERCA.
6. Exercise hypertrophy in cardiac muscle
Cardiac hypertrophy is an abnormal enlargement of the heart that occurs because of increases in cell size and proliferation of nonmuscle cells. These changes can either be beneficial (e.g., exercised hearts), in which changes are correlated with increased contractility, or detrimental, in which changes lead to decreased contractility and subsequent heart failure.
Exercised hearts develop a form of mild cardiac hypertrophy that does not lead to cardiac failure. The main structural changes include a thickening of the ventricle wall, which leads to increased contractility and thus a greater ability to pump blood. Within myocytes, expression of the α myosin heavy chain increases, which leads to high ATPase activity and increased contractility (Fig. 4B). Various signals are involved, including growth factors such as insulin-like growth factor (IGF1), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF) (Fig. 4B) (Hemmings and Restuccia 2012). There is increased signaling through the phosphoinositide 3 kinase (PI3K)/Akt pathway, which leads to proliferation and growth of cardiomyocytes (Matsui et al. 2003). Transcription factors up-regulated in exercised hearts include GATA4, which regulates genes involved in myocardial differentiation. Other pathways, such as the calcineurin/NFAT pathway are down-regulated (Oliveira et al. 2009). Cardiac muscle, like skeletal muscle, consumes tremendous amounts of ATP. Thus, PGC1α is also up-regulated in exercised hearts, facilitating transcription of metabolic and oxidative genes (Ventura-Clapier et al. 2007; Watson et al. 2007).
7. Pathophysiological cardiac hypertrophy
The main pathways driving pathological cardiac hypertrophy are overstimulation of the sympathetic nervous system, increased oxidative stress, and inflammatory signaling (Balakumar and Jagadeesh 2010). These collectively lead to induction of fetal isoforms of heart proteins and a corresponding decrease in adult forms (Chien 1999), including the myosin heavy chain (see below). The signals responsible include the GPCR agonist endothelin 1, peptide growth factors such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and cytokines such as cardiotrophin and leukemia inhibitory factor (LIF). Mechanical stress can also induce hypertrophy. In each case, activation of the ERK mitogen-activated protein kinase (MAPK) pathway (Morrison 2012) is often observed in hypertrophy and leads to regulation of transcription factors that alter expression of the myosin heavy chain, IP3R2, and other proteins (see below).
In hypertrophy, paracrine and autocrine neurohormonal factors that activate the heterotrimeric G protein Gq, and consequently PLCβ, are released. This results in an increase in cytosolic calcium levels and activation of PKC by diacylglycerol (DAG) as well as activation of CaMKII (Mishra et al. 2010). The importance of the Gq pathway in hypertrophy has been shown in studies of transgenic mice: mice overexpressing Gq have heart failure (D’Angelo et al. 1997), whereas mice with reduced Gq levels are protected against hypertrophy (Wettschureck et al. 2001). There is also a switch from the α form of the myosin heavy chain to the fetal β isoform (Miyata et al. 2000). This has a lower ATPase activity and a lower rate of contraction. Other changes include increased SERCA2A activity (Hasenfuss et al. 1994; Meyer et al. 1995), up-regulation of IP3R2 (Harzheim et al. 2010), and changes to a neuronal calcium sensor (NCS1). NCS1 is a calcium-binding protein that also interacts with IP3R (Schlecker et al. 2006).
8. Heart failure
The structural organization of the T-tubules breaks down in heart failure. This breakdown, caused by myocardial insults (such as myocardial infarction causing ischemia) among other factors, leads to impaired contractility owing to reduced, asynchronous, and chaotic calcium release. Several signaling pathways are compromised in heart failure. Initially, there can be reorganization of the β-adrenergic system. Activation of the β2-adrenergic receptor is normally limited to the T-tubule, whereas in heart failure, there is a redistribution of the receptor across the entire plasma membrane (Nikolaev et al. 2010). With chronic adrenergic activation, the hyperphosphorylation of RyRs results in leaky RyR channels, leading to a reduction of SR calcium and, thus, weaker contractions.
Other alterations to RyR2 that are observed include increased nitrosylation and loss of the regulatory protein FKBP12.6 (Andersson and Marks 2010). Both of these changes result in increased RyR activity. Moreover, mutations in RyR2 that result in leaky channels have been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT) and arrhythmogenic right ventricular dysplasia type 2.
Mutations in CSQ2 cause CPVT (Postma et al. 2002) by lowering buffering capacity within the SR, which results in premature calcium release and thus arrhythmias. Another important modulator of RyRs is junctin. Its levels are reduced in heart failure, which may be a compensatory mechanism to increase contractility (Pritchard and Kranias 2009).
Heart failure also leads to up-regulation of molecules that may have a protective function. One pathway is through cGMP, which promotes relaxation. The cGMP pathway is regulated by cGMP-targeted phosphodiesterases, of which one, PDE5A, looks to be a promising target for protective therapy against hypertrophy.
9. Smooth muscle types
Smooth muscle is found lining the walls of various organs and tubular structures in the body, including the intestine, bladder, airway, uterus, blood vessels, and stomach. It receives neural innervation from the autonomic nervous system, and its contractile state is also controlled by hormonal and autocrine/paracrine stimuli. Smooth muscle can be divided into two types: unitary and multiunit smooth muscles. In unitary smooth muscle, individual smooth muscle cells are coupled to neighboring cells by gap junctions. These gap junctions permit cell-to-cell passage of small molecules such as ATP and ions. These include those mobilized in response to electrical signals causing depolarization, which enable the whole area (known as a syncytium) to coordinate activity. In contrast, in multiunit smooth muscle, cells are not coupled to each other and are intermingled with connective tissue. Smooth muscle can undergo tonic (sustained) or phasic contractions. In the case of vascular smooth muscle, a sustained contraction is required to provide vessel tone. This enables the regulation of blood flow. Blood vessels are divided into the larger diameter conduit vessels (e.g., the thoracic aorta) and the smaller diameter resistance vessels. The resistance blood vessels display a myogenic response, in which increasing pressures over the physiological range (∼70–100 mmHg) result in a sustained contractile state. However, overconstriction of the vessels leads to hypertension (see below). Other smooth muscle, such as that found in the gut, including the stomach, small intestine, or gall bladder, shows variable tone and rhythmic contractions known as slow waves.
10. The contractile process in smooth muscle
The important distinction between striated muscle and smooth muscle is that calcium mediates contraction by regulating the availability of actin filaments in striated muscle, whereas in smooth muscle MLC is the target (Fig. 5). The source of the increase in cytosolic calcium levels can be extracellular or intracellular, or a combination of the two. In the case of tonic constriction of blood vessels, a constant supply of calcium comes from influx via the L-type calcium channels. The resting membrane potential of smooth muscle (between −50 and −40 mV) is such that it lies in an overlap (the window current) between the activation and inactivation curves of the L-type channel. Thus a small population of the L-type channels is always open. An alternatively spliced high-voltage-gated form of T-type channels may also contribute to calcium influx (Kuo et al. 2011), along with stretch-activated channels residing on the plasma membrane, such as TRPC6.
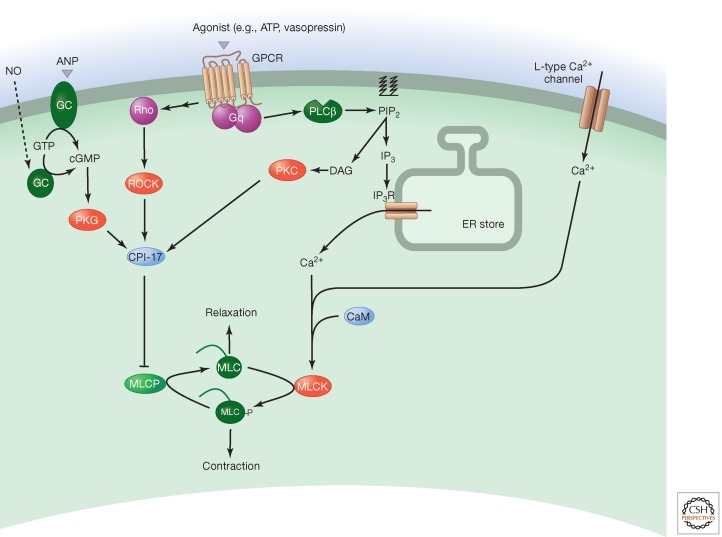
Figure 5.
Smooth muscle contraction. Calcium released by L-type calcium channels or IP3Rs downstream from Gq-coupled cell-surface receptors causes smooth muscle contraction. It binds to calmodulin (CaM) and the resulting complex stimulates myosin light-chain (MLC) kinase (MLCK). This phosphorylates MLC to promote contraction. A RhoA/ROCK pathway and a diacylglycerol (DAG) pathway contribute to calcium sensitization by altering the phosphorylation status of myosin light-chain phosphatase (MLCP). Relaxation is mediated through the cGMP/PKG pathway downstream from nitric oxide (NO) and agonists such as atrial natriuretic peptide (ANP).
In stomach muscle, the rhythmic contractions are due to the activity of pacemaker cells, but activation of voltage-gated calcium channels can trigger calcium entry and contraction. Sympathetic nerves run along the vascular smooth muscle and can release stimuli such as acetylcholine, norepinephrine, angiotensin, and endothelin. Moreover, circulating blood factors such as cytokines and diffusible factors such as nitric oxide can also act on receptors in the plasma membrane or cross the plasma membrane, respectively, to regulate pathways controlling intracellular calcium levels. The activation of receptor-operated channels (ROCs) also causes calcium influx, which enables additional calcium release from intracellular stores. GPCRs activate PLCβ to generate IP3, which releases calcium via IP3Rs. In vascular smooth muscle and the circular smooth muscle of the gut, the main isoform is IP3R1. Note, however, that there is some heterogeneity. In longitudinal smooth muscle of the gut, RyRs, rather than IP3Rs, are expressed. Agonists such as cholecystokinin bind to the GPCR cholecystokinin A receptor (CCK-AR), which activates phospholipase A2, which in turn produces arachidonic acid. Arachidonic acid (AA) can also be generated through the cleavage of DAG. AA activates chloride channels, which depolarize the cell membrane, enabling the opening of voltage-gated calcium channels and an initial influx of calcium. This calcium can either act directly on the RyR causing CICR or enable the release of cyclic ADP ribose, which interacts with RyRs to enhance CICR.
In all smooth muscle, calcium-bound CaM then binds to MLCK, stimulating phosphorylation of MLC, which leads to muscle contraction. The necessity for MLCK has been shown in MLCK-knockout mice, in which smooth muscle MLC cannot be phosphorylated by other kinases (He et al. 2008; Zhang et al. 2010). The dephosphorylation of MLC is catalyzed by MLCP and a complex of the myosin-targeting protein MYPT1 and the phosphatase PP1 and results in relaxation.
11. Calcium sensitization
Calcium sensitization is an essentially calcium-independent process that enables the amount of constriction in smooth muscle to be tuned by an alteration in the sensitivity of MLC to calcium (Fig. 5). This process enables the muscle to sustain a contraction once the initial calcium transient has dissipated. There are two mechanisms for calcium sensitization: a DAG-PLC-PKC pathway and a RhoA pathway (Lincoln 2007).
Diacylglycerol (DAG) is produced by PLCβ downstream from certain GPCRs and activates the conventional and novel protein kinase C (cPKC and nPKC), but not atypical PKC (aPKC) (Steinberg 2008). PKC has a variety of downstream targets, such as MLCK and C-kinase potentiated protein phosphatase 1 inhibitor, molecular mass 17 kDa (CPI-17), both of which enhance constriction. CPI-17 is a smooth-muscle-specific inhibitor of MLCP that binds to its catalytic subunit and inhibits phosphatase activity, allowing contraction to persist.
Several agonists, including angiotensin II, norepinephrine, and endothelin, activate the small G protein RhoA. RhoA in turn activates Rho kinase (ROCK), which can mediate calcium sensitization through two main pathways. First, ROCK stimulates phosphorylation of MYPT1 (Feng et al. 1999). This can be direct, at T695 or T853, with a preference for T853. Alternatively, it can phosphorylate another kinase, zipper-interacting protein kinase (ZIPK, also known as DAPK3), which phosphorylates MYPT1 primarily at T695 (Kiss et al. 2002). ZIPK also phosphorylates MLC at T18/S19. Phosphorylation of MYPT1 interferes with binding of MLCP to MLC, and thus is believed to decrease phosphatase activity. ROCK can also phosphorylate CPI-17 (MacDonald et al. 2001).
The preference for the MYPT1 or CPI-17 pathway depends on the type of smooth muscle. Whereas MYPT1 is ubiquitously expressed in smooth muscle, CPI-17 is differentially expressed. Moreover, RhoA and associated proteins are expressed at lower levels in phasic smooth muscle compared with tonic smooth muscle (Patel and Rattan 2006). Note that PKC can also phosphorylate CPI-17 to prevent MLCP activity. Within resistance arteries, an increase in vascular pressure also activates the RhoA pathway; however, the signaling intermediates linking the change in vascular pressure and the activation of RhoA remain unknown (Cole and Welsh 2011).
12. Vascular smooth muscle in disease
Smooth muscle cells are remarkably plastic, altering their phenotype in response to conditions such as vascular injury, altered blood flow conditions, or disease states. The changes in phenotype that can occur include cell proliferation, apoptosis, and cell migration and are induced by many factors, including cytokines and growth factors, mechanical forces, neuronal stimuli, and genetic factors. Here we limit our discussion to hypertension.
In hypertension, there is often a change in the sympathetic nervous system and the renin–angiotensin system that leads to increased blood pressure. Angiotensinogen is converted to angiotensin I by renin, which in turn is converted to angiotensin II by angiotensin-converting enzyme (ACE). Increased circulating angiotensin II acts on the angiotensin receptors (AT1 and AT2), which, when activated, cause increased peripheral resistance. The consequence for smooth muscle cells is they become hypercontractile. Treatments include ACE inhibitors (which inhibit the conversion of angiotensin I to angiotensin II), α1-adrenergic antagonists (which block the AT1 and AT2 GPCRs), and calcium channel blockers (such as dihydropyridines, which inhibit the voltage-gated calcium channels). All of these treatments aim to reduce the contractility of smooth muscle. Interfering with downstream targets such as RhoA signaling in hypertensive animals has also been shown to be effective (Uehata et al. 1997; Seko et al. 2003; Moriki et al. 2004).
The sustained contractile state of vascular smooth muscle is associated with the activation of calcium-dependent transcription factors. These include SRF, FOS, NFAT, and CREB. SRF, which is activated by the RhoA pathway, promotes the expression of genes encoding components of the contractile apparatus. Calcium-stimulated CaMKII activates and causes the translocation of CaMKIV to the nucleus, where it can activate CREB, which promotes transcription of components of the contractile apparatus and other targets. However, CaMKII can also activate a phosphatase that dephosphorylates and thus inactivates CREB (Matchkov et al. 2012). NFAT is activated on dephosphorylation by calcium-activated calcineurin, which induces genes associated with proliferation and migration.
NO produced by eNOS in endothelial cells protects against the changes observed in hypertension: the cGMP pathway inhibits DNA synthesis, mitogenesis, and cell proliferation (Forstermann and Sessa 2012). However, endothelial dysfunction is a hallmark of vascular disease, including hypertension. In many types of vascular diseases, eNOS is up-regulated but owing to reduced oxygen availability it is converted to a dysfunctional enzyme that produces superoxides, which contribute to vascular oxidative stress (Forstermann and Sessa 2012).
In some disease states, smooth muscle cells adopt a noncontractile phenotype. Although these cells still have signaling machinery that increases intracellular calcium levels, they have significantly reduced calcium influx through voltage-gated calcium channels. Thus, there is a shift to intracellular-store-operated calcium release, similar to the changes observed in cardiac hypertrophy. Concomitant with decreases in the levels of SERCA, RyR2, PMCA1, and the sodium/calcium exchanger, the levels of STIM, ORAI (proteins associated with refilling of intracellular calcium stores; see Bootman 2012), SERCA2B, and IP3R increase and there is a change in RyR receptor subtypes from RyR2 to RyR3 (Lipskaia and Lompre 2004; Berra-Romani et al. 2008; Baryshnikov et al. 2009; Matchkov et al. 2012). These changes collectively reflect a less contractile phenotype.
13. Concluding remarks
Signal transduction is essential for the function of contractile cells. The stimulatory signal results in an increase in cytosolic calcium levels, which activates muscle contraction. We now know the main contributors to the various types of muscle contraction, and have a better appreciation of the changes that occur to the contractile apparatus under exercise and pathophysiological conditions. For example, the identification of PGC1α as a master regulator of transcription factors up-regulated in both exercised and pathological striated muscle provides new avenues to modulate muscles in a therapeutic setting. It is also apparent that many signaling proteins in both smooth and striated muscles are activated by changes in cytosolic calcium levels, and these signaling pathways often lead to alterations in gene expression. Because we now have a better appreciation of the changes that occur to the contractile apparatus under pathophysiological conditions, this knowledge can be harnessed to allow us to treat disease strategically.
ACKNOWLEDGMENTS
Research in the Ehrlich Laboratory is supported by National Institutes of Health funds. I.Y.K. is an American Heart Association postdoctoral fellow.
Footnotes
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Andersson DC, Marks AR 2010. Fixing ryanodine receptor Ca leak—A novel therapeutic strategy for contractile failure in heart and skeletal muscle. Drug Discov Today 7: e151–e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakumar P, Jagadeesh G 2010. Multifarious molecular signaling cascades of cardiac hypertrophy: Can the muddy waters be cleared? Pharmacol Res 62: 365–383. [DOI] [PubMed] [Google Scholar]
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA 2009. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berra-Romani R, Mazzocco-Spezzia A, Pulina MV, Golovina VA 2008. Ca2+ handling is altered when arterial myocytes progress from a contractile to a proliferative phenotype in culture. Am J Physiol Cell Physiol 295: C779–C790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I, Watras J, Ehrlich BE 1991. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754. [DOI] [PubMed] [Google Scholar]
- *.Bootman MD 2012. Calcium signaling. Cold Spring Harb Perpsect Biol 4: a011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzo C, Spolaore B, Toniolo L, Stevens L, Bastide B, Cieniewski-Bernard C, Fontana A, Mounier Y, Reggiani C 2005. Nerve influence on myosin light chain phosphorylation in slow and fast skeletal muscles. FEBS J 272: 5771–5785. [DOI] [PubMed] [Google Scholar]
- Campbell KP, MacLennan DH, Jorgensen AO, Mintzer MC 1983. Purification and characterization of calsequestrin from canine cardiac sarcoplasmic reticulum and identification of the 53,000 dalton glycoprotein. J Biol Chem 258: 1197–1204. [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB 1993. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744. [DOI] [PubMed] [Google Scholar]
- Chien KR 1999. Stress pathways and heart failure. Cell 98: 555–558. [DOI] [PubMed] [Google Scholar]
- Cole WC, Welsh DG 2011. Role of myosin light chain kinase and myosin light chain phosphatase in the resistance arterial myogenic response to intravascular pressure. Arch Biochem Biophys 510: 160–173. [DOI] [PubMed] [Google Scholar]
- Dainese M, Quarta M, Lyfenko AD, Paolini C, Canato M, Reggiani C, Dirksen RT, Protasi F 2009. Anesthetic- and heat-induced sudden death in calsequestrin-1-knockout mice. FASEB J 23: 1710–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW 2nd 1997. Transgenic Gαq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci 94: 8121–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Ito M, Ichikawa K, Isaka N, Nishikawa M, Hartshorne DJ, Nakano T 1999. Inhibitory phosphorylation site for Rho-associated kinase on smooth muscle myosin phosphatase. J Biol Chem 274: 37385–37390. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM 1991. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252: 443–446. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Sessa WC 2012. Nitric oxide synthases: Regulation and function. Eur Heart J 33: 829–837, 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Spiegelman BM 2006. Peroxisome proliferator-activated receptor γ coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev 27: 728–735. [DOI] [PubMed] [Google Scholar]
- *.Hardie DG 2012. Organismal carbohydrate and lipid homeostasis. Cold Spring Harb Perspect Biol 4: a006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harzheim D, Talasila A, Movassagh M, Foo RS, Figg N, Bootman MD, Roderick HL 2010. Elevated InsP3R expression underlies enhanced calcium fluxes and spontaneous extra-systolic calcium release events in hypertrophic cardiac myocytes. Channels (Austin) 4: 67–71. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Reinecke H, Studer R, Meyer M, Pieske B, Holtz J, Holubarsch C, Posival H, Just H, Drexler H 1994. Relation between myocardial function and expression of sarcoplasmic reticulum Ca2+-ATPase in failing and nonfailing human myocardium. Circ Res 75: 434–442. [DOI] [PubMed] [Google Scholar]
- He WQ, Peng YJ, Zhang WC, Lv N, Tang J, Chen C, Zhang CH, Gao S, Chen HQ, Zhi G, et al. 2008. Myosin light chain kinase is central to smooth muscle contraction and required for gastrointestinal motility in mice. Gastroenterology 135: 610–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hemmings BA, Restuccia DF 2012. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4: a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM 2011. The structure and function of cardiac t-tubules in health and disease. Proc Biol Sci 278: 2714–2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss E, Muranyi A, Csortos C, Gergely P, Ito M, Hartshorne DJ, Erdodi F 2002. Integrin-linked kinase phosphorylates the myosin phosphatase target subunit at the inhibitory site in platelet cytoskeleton. Biochem J 365: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein MG, Cheng H, Santana LF, Jiang YH, Lederer WJ, Schneider MF 1996. Two mechanisms of quantized calcium release in skeletal muscle. Nature 379: 455–458. [DOI] [PubMed] [Google Scholar]
- Kuo IY, Wolfle SE, Hill CE 2011. T-type calcium channels and vascular function: The new kid on the block? J Physiol 589: 783–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner JT, Georgiou DK, Joshi AD, Hamilton SL 2010. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. 2002. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature 418: 797–801. [DOI] [PubMed] [Google Scholar]
- Lincoln TM 2007. Myosin phosphatase regulatory pathways: Different functions or redundant functions? Circ Res 100: 10–12. [DOI] [PubMed] [Google Scholar]
- Lipskaia L, Lompre AM 2004. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell 96: 55–68. [DOI] [PubMed] [Google Scholar]
- Liu Y, Shen T, Randall WR, Schneider MF 2005. Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J Muscle Res Cell Motil 26: 13–21. [DOI] [PubMed] [Google Scholar]
- MacDonald JA, Eto M, Borman MA, Brautigan DL, Haystead TA 2001. Dual Ser and Thr phosphorylation of CPI-17, an inhibitor of myosin phosphatase, by MYPT-associated kinase. FEBS Lett 493: 91–94. [DOI] [PubMed] [Google Scholar]
- Matchkov VV, Kudryavtseva O, Aalkjaer C 2012. Intracellular Ca2+ signalling and phenotype of vascular smooth muscle cells. Basic Clin Pharmacol Toxicol 110: 42–48. [DOI] [PubMed] [Google Scholar]
- Matsui T, Nagoshi T, Rosenzweig A 2003. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle 2: 220–223. [PubMed] [Google Scholar]
- Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, Kuwajima G, Mikoshiba K, Just H, Hasenfuss G, et al. 1995. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation 92: 778–784. [DOI] [PubMed] [Google Scholar]
- Mishra S, Ling H, Grimm M, Zhang T, Bers DM, Brown JH 2010. Cardiac hypertrophy and heart failure development through Gq and CaM kinase II signaling. J Cardiovasc Pharmacol 56: 598–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Minobe W, Bristow MR, Leinwand LA 2000. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res 86: 386–390. [DOI] [PubMed] [Google Scholar]
- Moriki N, Ito M, Seko T, Kureishi Y, Okamoto R, Nakakuki T, Kongo M, Isaka N, Kaibuchi K, Nakano T 2004. RhoA activation in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Hypertens Res 27: 263–270. [DOI] [PubMed] [Google Scholar]
- *.Morrison D 2012. MAP Kinase pathways. Cold Spring Harb Perspect Biol 4: a0011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev VO, Moshkov A, Lyon AR, Miragoli M, Novak P, Paur H, Lohse MJ, Korchev YE, Harding SE, Gorelik J 2010. β2-Adrenergic receptor redistribution in heart failure changes cAMP compartmentation. Science 327: 1653–1657. [DOI] [PubMed] [Google Scholar]
- Nishikimi T, Kuwahara K, Nakao K 2011. Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol 57: 131–140. [DOI] [PubMed] [Google Scholar]
- Olesen J, Kiilerich K, Pilegaard H 2010. PGC-1α-mediated adaptations in skeletal muscle. Pflugers Archiv Eur J Phys 460: 153–162. [DOI] [PubMed] [Google Scholar]
- Oliveira RS, Ferreira JC, Gomes ER, Paixao NA, Rolim NP, Medeiros A, Guatimosim S, Brum PC 2009. Cardiac anti-remodelling effect of aerobic training is associated with a reduction in the calcineurin/NFAT signalling pathway in heart failure mice. J Physiol 587: 3899–3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel CA, Rattan S 2006. Spontaneously tonic smooth muscle has characteristically higher levels of RhoA/ROK compared with the phasic smooth muscle. Am J Physiol Gastrointest Liver Physiol 291: G830–G837. [DOI] [PubMed] [Google Scholar]
- Postma AV, Denjoy I, Hoorntje TM, Lupoglazoff JM, Da Costa A, Sebillon P, Mannens MM, Wilde AA, Guicheney P 2002. Absence of calsequestrin 2 causes severe forms of catecholaminergic polymorphic ventricular tachycardia. Circ Res 91: e21–e26. [DOI] [PubMed] [Google Scholar]
- Pritchard TJ, Kranias EG 2009. Junctin and the histidine-rich Ca2+ binding protein: Potential roles in heart failure and arrhythmogenesis. J Physiol 587: 3125–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson R, Carpenter D, Shaw MA, Halsall J, Hopkins P 2006. Mutations in RYR1 in malignant hyperthermia and central core disease. Hum Mutat 27: 977–989. [DOI] [PubMed] [Google Scholar]
- Ronnebaum SM, Patterson C 2010. The FoxO family in cardiac function and dysfunction. Ann Rev Physiol 72: 81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanbe A, Fewell JG, Gulick J, Osinska H, Lorenz J, Hall DG, Murray LA, Kimball TR, Witt SA, Robbins J 1999. Abnormal cardiac structure and function in mice expressing nonphosphorylatable cardiac regulatory myosin light chain 2. J Biol Chem 274: 21085–21094. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C 2011. Fiber types in mammalian skeletal muscles. Physiol Rev 91: 1447–1531. [DOI] [PubMed] [Google Scholar]
- Schlecker C, Boehmerle W, Jeromin A, DeGray B, Varshney A, Sharma Y, Szigeti-Buck K, Ehrlich BE 2006. Neuronal calcium sensor-1 enhancement of InsP3 receptor activity is inhibited by therapeutic levels of lithium. J Clin Invest 116: 1668–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T 2003. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 92: 411–418. [DOI] [PubMed] [Google Scholar]
- Steinberg SF 2008. Structural basis of protein kinase C isoform function. Physiol Rev 88: 1341–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, et al. 1997. Calcium sensitization of smooth muscle mediated by a ρ-associated protein kinase in hypertension. Nature 389: 990–994. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Mettauer B, Bigard X 2007. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc Res 73: 10–18. [DOI] [PubMed] [Google Scholar]
- Watson PA, Reusch JE, McCune SA, Leinwand LA, Luckey SW, Konhilas JP, Brown DA, Chicco AJ, Sparagna GC, Long CS, et al. 2007. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol 293: H246–H259. [DOI] [PubMed] [Google Scholar]
- Wei L, Hanna AD, Beard NA, Dulhunty AF 2009. Unique isoform-specific properties of calsequestrin in the heart and skeletal muscle. Cell Calcium 45: 474–484. [DOI] [PubMed] [Google Scholar]
- Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT 2009. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci 106: 20405–20410. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wettschureck N, Rutten H, Zywietz A, Gehring D, Wilkie TM, Chen J, Chien KR, Offermanns S 2001. Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Gαq/Gα11 in cardiomyocytes. Nat Med 7: 1236–1240. [DOI] [PubMed] [Google Scholar]
- Zhang WC, Peng YJ, Zhang GS, He WQ, Qiao YN, Dong YY, Gao YQ, Chen C, Zhang CH, Li W, et al. 2010. Myosin light chain kinase is necessary for tonic airway smooth muscle contraction. J Biol Chem 285: 5522–5531. [DOI] [PMC free article] [PubMed] [Google Scholar]