Abstract
AIM: In the USA, Hawaii has the highest incidence of hepatocellular carcinoma (HCC) and a diverse population. It is an ideal place to characterize HCC in the context of ethnicity/risk factors.
METHODS: A total of 262 cases of HCC (1992-2003) were retrospectively reviewed for demographics, ethnicity, birthplace, viral hepatitis, alcohol use, diabetes, smoking and risk factors for viral hepatitis such as intravenous drug abuse (IVDA), transfusions, tattoos and vertical transmission. Tumor stage, Child’s class, Cancer of the Liver Italian Program (CLIP) score, α-fetoprotein level, treatment and survival were recorded.
RESULTS: Gender, age, viral hepatitis, alcohol, IVDA, and diabetes differed significantly in Asians, non-Asians and Pacific Islanders. There were also specific differences within Asian subgroups. Alpha-fetoprotein, smoking, transfusions, stage and resectability did not differ between groups. Asians were more likely to have hepatitis B, while non-Asians were more likely to have hepatitis C. Factors that decreased survival included hepatitis B, alcohol, elevated alpha-fetoprotein, CLIP >2 and increased Child’s class. When Asians were combined with Pacific Islanders, median survival (1.52 years vs 3.54 years), 1- and 3-year survival was significantly worse than those for non-Asians. After Cox regression analysis for hepatitis B and alcohol, there was no difference in survival by ethnicity.
CONCLUSION: Various ethnicities have different risk factors for HCC. Hepatitis B, alcohol, and α-fetoprotein are more important factors for survival than ethnicity.
Keywords: Hepatocellular cancer, Ethnicity, Hepatitis B
INTRODUCTION
The general consensus is that hepatocellular carcinoma (HCC) is a distinctly different risk-factor associated cancer in different geographic regions of the world. It differs in incidence, risk factors and natural history and these differences have been attributed to ethnicity, endemic viral hepatitis, and other risk factors[1-3]. HCC is the fifth most common cancer worldwide and is estimated to cause half a million deaths every year. The incidence in USA, though much less than in Asian and African countries, has been increasing over the last decade and is currently estimated at 2.4 per 100000[4]. The US Surveillance, Epidemiology and End Results Program (SEER) data indicate that HCC is the fourth most common cancer among Chinese and Korean males, second most common in Vietnamese males and fifth most common among Filipino males. HCC is also the second leading cause of cancer mortality among Chinese males and fourth leading cause of cancer mortality among Filipino males[5]. Literature from USA frequently mention the differences in HCC among Asians compared to Caucasians but often groups all Asians together, while literature from Asian countries distinctly describe each ethnic group separately[6-18]. Risk factors for viral hepatitis and HCC may be distinctly different in these subgroups of Asian patients, yet the data are frequently pooled into one seemingly heterogeneous group. Furthermore, little is written on HCC in Pacific Islanders and the Filipino population. Few study populations have enough patients in these ethnic groups to make any definite statement about them separately. Pacific Islanders are sometimes grouped with ‘Asians’ or ‘other’ and little has been done to describe HCC in this population. Hawaii has the highest incidence of HCC in USA, with a population that is approximately two-third of Asian or Pacific Islander ethnicity[19]. Furthermore, there is a large immigrant and visitor population, making it the ideal location to study HCC related difference in ethnicity, risk factors, and birthplace.
MATERIALS AND METHODS
This is a retrospective study on HCC. A total of 262 cases were referred either to the Liver Center (NT) or a group of surgeons (LW, WL) at St. Francis Medical Center, a tertiary medical center from August 1992 to August 2003. This medical center has the only clinic dedicated to liver diseases, the only transplant center in the state and the only referral center for liver diseases for American territories of the Pacific Basin (including American Samoa, Guam, Saipan, the Marshall Islands). A number of patients were nationals from Asian countries such as Japan, Korea, and the Philippines who desired medical care in USA.
HCC was diagnosed pathologically by percutaneous biopsy, liver biopsy at the time of surgery or explanted liver at transplant. We also included patients without a histologic confirmation, who had a history of chronic liver disease and one of the following; (1) discrete liver mass(es) and an alpha-fetoprotein (AFP) greater than 200 ng/dL; (2) discrete liver mass(es), which is(are) increasing in size with AFP less than 200 ng/dL; (3) discrete liver mass with rising AFP for three consecutive values, or (4) discrete liver mass with AFP less than 200 ng/dL that is also seen distinctly on Gallium scan or hepatic arteriogram.
We collected demographic data including age, sex, birthplace, and primary ethnicity as identified by the patient. We recorded a history of diabetes, other cancer, smoking, and family history of cancer. Risk factors for HCC including viral hepatitis, alcohol abuse (which we defined as greater than two drinks/daily for at least 10 years), and other chronic liver diseases were identified. We also looked specifically at risk factors for viral hepatitis such as vertical transmission, previous blood transfusions, intravenous drug use (IVDA), tattoo placement and high-risk occupations.
With respect to HCC, we recorded the size, number, and location of the tumor in order to determine tumor node metastases (TNM) stage. We also collected laboratory studies of bilirubin, albumin, and prothrombin time and ascites and encephalopathy in order to calculate Child-Turcotte-Pugh scores and CLIP scores. Serum alpha-fetoprotein (AFP) was also recorded. CTP scores were also expressed as Child’s class A (CTP 5-6), B (CTP 7-9) or C (CTP >9). Finally, we recorded the type of treatment, dates of diagnosis and death in order to calculate the survival since diagnosis. Liver resection was considered in Child’s A patients or early Child’s B (CTP score of 7) patients without any evidence of ascites or encephalopathy. Liver transplant was done on patients who were unresectable but had TNM T1 or T2 lesions (Milan criteria). Patients on the transplant waiting list underwent either radiofrequency ablation or transarterial chemoembolization while waiting for a donor.
The data were compiled and statistically analyzed. Ethnicity was also divided into three groups: Asians, Pacific Islanders, and non-Asians. Each factor was examined for differences by race using the χ2 test or Fisher’s exact test. Kaplan-Meier survival curves were then drawn for each factor and ethnic group. The log-rank test was used to compare survival differences. Cox regression analysis was used to assess the effects of ethnicity on survival while the other individual factors were controlled.
RESULTS
Study Population
During the period between August 1992 and 2003, 262 patients were referred for HCC. Racial distribution was as follows: Asians-193, Pacific Islanders-27 and non-Asians-42. Among the Asians: 84 were Japanese, 40 were Chinese, 38 were Filipino and 24 were Korean, 7 were Southeast Asian (Vietnamese, Laotian, Thai). All patients were assigned a primary ethnicity, though there were a number of patients who had several ethnic backgrounds. Among the 262 patients, 187 were males and 75 were females. Mean age at diagnosis was 60.7 years.
Risk factors by ethnicity
Age, gender, hepatitis B, hepatitis C, alcohol abuse, intravenous drug use and diabetes differed significantly by ethnicity when Asians, non-Asians and Pacific Islanders were compared (Table 1). In terms of age, Pacific Islanders presented with risk factors at a significantly earlier age (53.4 years) compared with Asians (61.3 years) and non-Asians (61.4 years) (P = 0.0035). Although all ethnic groups showed a male predominance over females, the M:F ratio was significantly different with non-Asians having the highest M:F ratio (P = 0.025).
Table 1.
Demographics, risk factors for HCC by ethnicity.
| Characteristic | Asians | Non-Asians | Pacific Isl | P |
| Patients | 193 | 42 | 27 | |
| Mean age (yr) | 61.4 | 61.3 | 53.4 | 0.0035 |
| Males:females | 2.06:1 | 7.4:1 | 2.86:1 | 0.025 |
| Hepatitis B, % | 46 | 4.8 | 50 | <0.0001 |
| Hepatitis C, % | 26.6 | 53.7 | 37 | 0.0024 |
| Alcohol abuse, % | 31.6 | 52.4 | 42.3 | 0.031 |
| Diabetes, % | 29.1 | 39 | 11.1 | 0.044 |
| Smoking, % | 47.8 | 48.6 | 59.1 | NS |
| IV drug use, % | 4.8 | 35 | 20 | <0.0001 |
| Tattoos, % | 1.1 | 12.5 | 4.2 | 0.001 |
| Vertical transmit, % | 24.8 | 2.6 | 11.1 | 0.0049 |
| Transfusions, % | 6.9 | 0 | 0 | NS |
There were differences in viral risk factors and alcohol usage among ethnicities and these differences also extended into subgroups of Asian ethnicity. In non-Asians, 53.7% of patients with HCC were hepatitis C positive, but only 4.8% were hepatitis B positive. If all Asians were grouped together, 46% had hepatitis B and 26.6% had hepatitis C. Incidence of hepatitis B and C differed significantly between Asians, non-Asians and Pacific Islanders (P<0.0001 and P = 0.0024, respectively). In Chinese, Koreans, and Filipinos, HCC was predominantly a hepatitis B-related disease, whereas in Japanese there was a slight predominance of hepatitis C (29.3%) compared to hepatitis B (26.8%). The incidence of alcohol use also differed significantly among the three ethnic groups (P = 0.031). Diabetes differed significantly between the three ethnic groups, occurring in 39.0% of non-Asians, 29.1% of Asians and 11.1% of Pacific Islanders (P = 0.044). There was no difference in smoking history among the three ethnic groups (Table 1).
Risk factors for viral hepatitis
Thirty-nine patients had definite vertical transmission of viral hepatitis, with at least one first degree relative with documented viral hepatitis. An additional 52 patients might have vertical transmission, as they were born in a country where hepatitis B is known to be endemic, however they were not certain of the hepatitis status of their immediate relatives. Twenty-eight patients admitted to intravenous drug use at some time, 12 patients had blood transfusions in the distant past (more than 10 years ago) and 8 patients had tattoos placed more than 10 years ago. Eight patients felt that their occupation was the source of viral hepatitis (military-4, police-2, emergency department worker-1, fire department-1). Five patients recalled having frequent intramuscular or subcutaneous injections as a child over 30 years ago in countries where needles may have been reused. Three of these patients were born in Japan, one in Vietnam and the other in the Philippines. One patient felt that frequent travel was the etiology of viral hepatitis and one patient admitted to promiscuity. In terms of ethnicity, there was a difference in IVDA with 4.8% Asians, 35% non-Asians and 20% Pacific Islanders (P<0.0001). There was also a significant difference in those with a history of tattoos or definite vertical transmission of viral hepatitis among the three ethnic groups. Transfusions did not vary by ethnicity and other possible etiologies, for viral hepatitis were too small in number to make adequate comparisons (Table 1).
Birthplace
There was a significant difference in distribution of birthplace among the three ethnic groups. A larger number of Asian patients (84 of 170) were born outside of the USA compared with non-Asians who were primarily born in the USA. Twelve of twenty-two Pacific Islanders were born in Hawaii while 10 on their native Pacific Islands, which may have included USA territories (foreigners in this group referred to outside of the 50 states of USA). Data on birthplace were not available for all patients and the exact time, they emigrated from their native countries was also not consistently available (Table 2).
Table 2.
Risk factors by subgroups of Asian ethnicities.
| Asian ethnicity | n | Hepatitis B, % | Hepatitis C, % | Alcohol abuse, % |
| Japanese | 84 | 26.8 | 29.3 | 36.9 |
| Chinese | 40 | 77 | 12.8 | 10 |
| Filipino | 38 | 67.6 | 22.2 | 44.7 |
| Korean | 24 | 75 | 30.4 | 29.2 |
| Southeast Asian | 7 | 57.1 | 71.4 | 28.6 |
Diagnosis/treatment
There was no difference in stage of disease or mean AFP among the three ethnic groups and no significant difference in the incidence of normal AFP (<20 ng/dL). The mean CTP score was 6.5 and the mean CLIP score was 1.6. CLIP score appeared to be distributed differently among the three ethnicities, when looking at the incidence of those patients with CLIP score below 2. Fewer Pacific Islanders (22.2%) had low CLIP scores <2 compared to Asians (53.1%) and non-Asians (56.1%) (P = 0.008). There was also a significantly different distribution of Child’s A-C classes among the three ethnicities (P = 0.036). In terms of surgical treatment, there was no difference in the patients who underwent surgical resection for HCC. On the other hand, there was a difference in those who underwent liver transplant, with no Pacific Islander patient undergoing liver transplant for HCC (P = 0.037, Table 3).
Table 3.
Stage and treatment by ethnicity.
| Asians | Non-Asians | Pac Islander | P | |
| Patients | 193 | 42 | 27 | |
| Stage I | 11 | 1 | 0 | NS |
| Stage II | 94 | 26 | 11 | NS |
| Stage III | 26 | 5 | 5 | NS |
| Stage IV | 62 | 9 | 11 | NS |
| AFP <20 | 61/189 (32) | 14/37 (37.8) | 5/26 (19.2) | NS |
| CLIP <2 | 102/192 (53.1) | 23/41 (56.1) | 6/27 (22.2) | 0.008 |
| Child’s A | 120 | 21 | 14 | |
| Child’s B | 61 | 11 | 9 | |
| Child’s C | 10 | 8 | 2 | 0.036 |
| Liver resection | 46 | 5 | 5 | NS |
| Liver transplant | 14 | 7 | 0 | 0.037 |
Survival statistics
The overall 1 and 3 year survival rates were 63% and 39%, respectively and the overall median survival time was 1.86 years. The following factors were significantly associated with survival: ethnicity, hepatitis B, alcohol, AFP, CLIP score and Child’s class. The presence of hepatitis B and the use of alcohol adversely affected the median and overall survival as seen on Kaplan-Meier curve (Figures 1 and 2). When considering the three ethnic groups, there was an early survival advantage for non-Asians, but long-term survival was not different between the groups (Figure 3). When Asians and Pacific Islanders were compared to non-Asians, the median survival was significantly different (1.52 years vs 3.54 years, P<0.05). The 1 and 3 year survival were also significantly different between Asians/Pacific Islanders vs non-Asians (P<0.05, Table 4, Figure 4).
Figure 1.
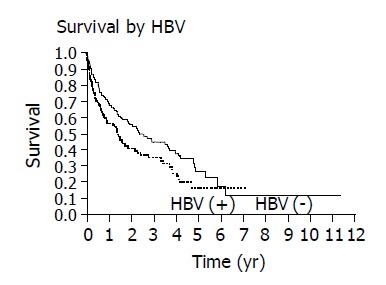
Kaplan-Meier curves demonstrate that the presence of hepatitis B adversely affects survival (P = 0.023).
Figure 2.
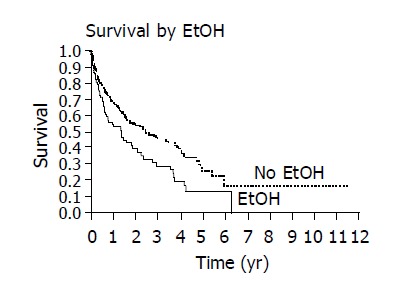
Kaplan-Meier curves demonstrate decreased survival with significant alcohol use (EtOH) (P = 0.005).
Figure 3.
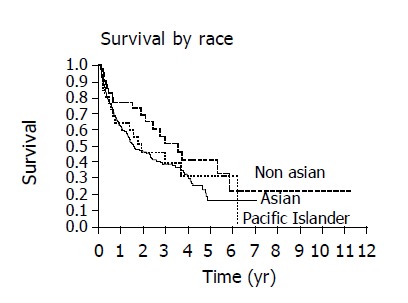
No difference in survival are noted when Kaplan-Meier curves are compared between Asians, Pacific Islanders and non-Asians.
Table 4.
Median/mean survival.
| Characteristic | Mean (yr) | Median (yr) | P |
| Race | |||
| Asians (n = 193) | 2.26±0.15 | 1.52 | NS |
| Non-Asians (n = 42) | 3.38±0.43 | 3.53 | |
| Pacific Islanders (n = 27) | 2.83±0.56 | 1.86 | |
| Asians+Pacific Islanders (n = 220) | 2.52±0.18 | 1.56 | = 0.045 |
| Non-Asians (n = 42) | 3.38±0.43 | 3.54 | |
| Birthplace | |||
| US born (n = 126) | 2.66±0.24 | 2.07 | NS |
| Foreign born (n = 99) | 2.64±0.22 | 2.39 | |
| Asian-US born (n = 85) | 2.25±0.22 | 1.60 | NS |
| Asian-foreign born (n = 84) | 2.52±0.23 | 2.29 | |
| ETOH | |||
| No (n = 166) | 2.90±0.20 | 2.40 | = 0.005 |
| Yes (n = 94) | 2.10±0.25 | 1.34 | |
| Hepatitis B | |||
| No (n = 141) | 2.98±0.23 | 2.40 | = 0.023 |
| Yes (n = 115) | 2.04±0.19 | 1.41 | |
| AFP | |||
| < 20 ng/dL (n = 79) | 3.31±0.29 | 3.69 | = 0.0013 |
| ≥20 ng/dL (n = 172) | 2.23±0.19 | 1.34 | |
| CLIP score | |||
| <2 (n = 130) | 3.26±0.21 | 3.69 | <0.0001 |
| ≥2 (n = 129) | 1.89±0.23 | 0.81 | |
| Child’s class | |||
| A (n = 154) | 2.97±0.21 | 2.73 | = 0.0055 |
| B (n = 81) | 2.07±0.26 | 1.29 | |
| C (n = 20) | 0.76±0.15 | 0.76 |
Figure 4.
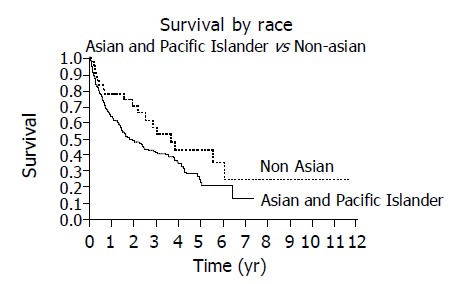
Survival is decreased when Asians and Pacific Islanders are compared to non-Asians as demonstrated by Kaplan-Meier curves (P = 0.045).
Patients with an elevated AFP ≥ 20 ng/dL had decreased survival compared to those with normal AFP<20 ng/dL (Figure 5). Those patients with CLIP score ≥ 2 had diminished survival compared to patients with CLIP score <2 (Figure 6). Finally, survival varied with Child’s class, Child’s C patients had the poorest survival, Child’s A patients had superior survival and Child’s B patients had an intermediate survival (Figure 7).
Figure 5.
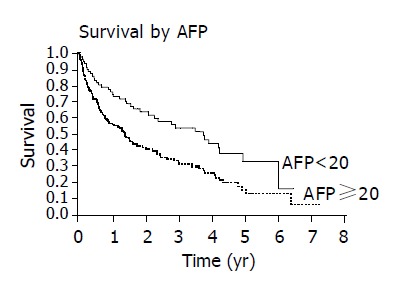
Patients with normal AFP levels (<20 ng/dL) have improved survival compared with those with elevated AFP levels (P = 0.0013).
Figure 6.
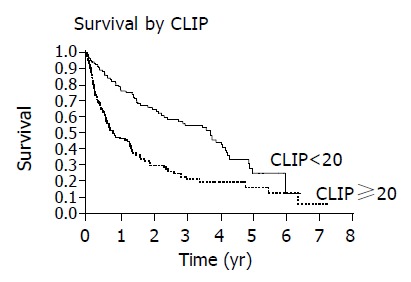
Patients with lower CLIP scores <2 have improved survival compared with those with CLIP score ≥ 2 as demonstrated by Kaplan-Meier curves (P<0.0001).
Figure 7.
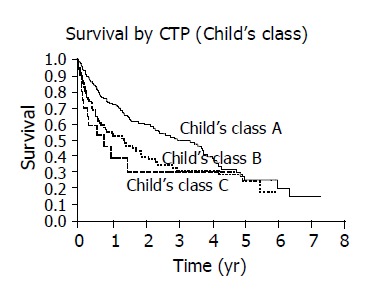
Increased Child’s class has progressively worse survival with Kaplan-Meier curves (P = 0.0055).
Cox regression analysis was then used to determine if ethnicity was significantly associated with survival. Once alcohol and hepatitis B were taken into account, ethnicity was no longer significantly associated with survival.
All other factors including age, gender, hepatitis C, IVDA, vertical transmission, transfusions, tattoo placement, birthplace, diabetes, and smoking did not affect survival. The mean and median survival were not significantly different in patients born in USA in comparison with those born outside USA. Also, when Asian patients were separated by birth place, there was no difference in median or mean survival.
DISCUSSION
HCC is a distinctly different risk-factor associated disease in various ethnic groups. The incidence of HCC increases with age and males are at a greater risk. Epidemiologic studies have also shown that the age at incidence is 10-20 years younger in those high incidence areas such as China and sub-Saharan Africa. The male to female predominance is also higher in those areas with a higher incidence of HCC[3]. Although USA is considered a low incidence area, our study showed that Pacific Islanders presented with HCC at a younger age than the other groups. Studies have demonstrated increased cancer incidence and mortality rates (up to 40%) in Hawaiians, however no studies indicate that cancer occurs at a younger age[5,20-22]. In our study, non-Asians had a much greater male: female predominance than the other ethnic groups which was much higher than generally reported in large epidemiologic studies [3].
It is well accepted that viral hepatitis plays a significant role in the incidence of HCC, but the type of viral hepatitis may vary in different ethnic groups and countries. Hepatitis C has been reported to account for much of the rise in HCC in USA[23]. Studies from Korea, China, and New Zealand indicate a predominance of hepatitis B as the risk factor for HCC. Hepatitis C does play a role in the development of HCC in these countries, especially in the absence of hepatitis B[6-13]. Japanese groups however, indicate that hepatitis C is the principal risk factor[14-18]. This rising importance of hepatitis C in Japan is thought to be due to increased blood transfusions post-World War II and contaminated needles used for medicinal purposes[2]. Hepatitis B and C are sometimes synergistic in the etiology of HCC in Asian countries, but there are regional differences.
When Asians are studied in USA, various Asian ethnicities are classed together. USA epidemiologic studies have indicated that Asian men have the highest age-adjusted incidence rate (23 per 100000) and Asians have the highest male to female ratio. Caucasians are 2-3 times less likely to have HCC compared to African Americans, who are in turn 2-3 times less affected than Asians, Pacific Islanders and native Americans[2]. In 110 patients with HCC, Hwang et al[24], reported that the incidence of Hepatitis B in Asian Americans is 80% compared to 19% in Caucasians. Hepatitis C is found in 20% of Asians and 32% of Caucasians. There is no difference in the rate of cirrhosis, AFP, or survival in patients with hepatitis B or C. They concluded that hepatitis B is the major etiologic factor in Asian Americans while hepatitis C and alcohol are the major factors in Caucasians[24].
Our study indicates that subgroups of Asian Americans with HCC may have similar risk factors to those in their native countries. Chinese and Korean-American patients have a predominance of hepatitis B, while Japanese-American patients have a slight predominance of hepatitis C. Filipino-Americans and Pacific Islanders in Hawaii also have a higher incidence of hepatitis B. This seems to indicate that viral hepatitis may be related to an exposure in their native countries many years prior to developing HCC. Recent immigrants may be more likely to have risk factors based on their native country, while a person who moved to USA as a child may have risk factors and exposures similar to a native American. Pyong et al[25], showed that 74% have hepatitis C while only 16.7% have hepatitis B in a study of 90 Koreans living in Japan who developed HCC. This group of Koreans has developed a risk factor profile similar to the Japanese living in Japan.
Whether Asian Americans with HCC have similar outcome is not completely clear. Chin et al[26], demonstrated a higher incidence of hepatitis B and poorer survival in the Asian American group in a study of 24 Asian Americans and 52 Non-Asian patients with HCC. El-Serag et al[27], demonstrated no differences in survival related to ethnicity in a review of the National Cancer Institute’s SEER data of 7398 patients with HCC between 1977 and 1996. So et al[28], found a high frequency of HCC (38%) in Asians who underwent liver transplantation due to hepatitis B but no difference was found in survival when compared to Caucasians with HCC.
Alcohol is known to contribute to the severity of chronic liver diseases and the etiology of HCC. Studies demonstrated that alcohol plays a role in the development of HCC both in the face of viral hepatitis and as an independent risk factor. It also results in a higher grade of malignancy and worse survival in those patients with HCC[29-32].
Our current study indicates that non-Asians have an early survival advantage but long-term survival is not different between the ethnic groups. When Asians and Pacific Islanders were combined, there was a significant disadvantage in survival. Hepatitis B, alcohol use, AFP, CLIP score and Child’s class are also factors affecting survival. When hepatitis B and alcohol were taken into account in regression analysis, survival was no longer different amongst the various ethnicities. Thus, being an Asian or Asian-American alone probably does not infer a worse prognosis with HCC.
Although there were differences in survival, there was no difference in the number of patients who underwent liver resection. There was a difference in liver transplant for HCC among the ethnic groups. This may reflect access to medical care, as many Pacific Islanders in our study had medical insurance that did not cover liver transplant. Access to medical care, in general, may be different in various ethnic groups. One study on cancer patterns among ethnic groups, noted that Asians and Pacific Islanders though less likely to have current tobacco use, obesity and chronic alcohol use, are less likely to have a health care plan and undergo screening Pap tests and mammograms[5]. Perhaps access to medical services and liver transplant may affect the overall outcome in patients with HCC.
When studying HCC in the USA, we often consider ethnicity and viral hepatitis in discussing prognosis. Ethnicity is a difficult factor to be studied. Patients sometimes have more than one ethnic background and as in this study, only one ethnicity was assigned, based on what the patient considered his/her primary ethnicity. There is much diversity within broad ethnic groups such as ‘Asian’ or ‘Pacific Islander’ and sometimes uncertainties in ethnicity are erroneously reported as ‘others’. Asians and Pacific Islanders had a similar survival pattern in this study, however there were distinct differences in risk factors among subgroups of Asian patients. Although it would be virtually impossible to get a complete and clear understanding of the role of ethnicity in HCC, this study indicates that viral hepatitis B and history of alcohol use are more important prognostic factors than ethnicity. This may also ultimately help physicians decide the value of screening for HCC for patients with specific risk factors. Universal hepatitis B vaccination may also change the risk factor profile in the future, thus we need to be aware that our current risk factor profile may change with time. We also need to continually educate patients with viral hepatitis or chronic liver diseases to abstain from alcohol as this appears to affect prognosis.
References
- 1.Simonetti RG, Cammà C, Fiorello F, Politi F, D'Amico G, Pagliaro L. Hepatocellular carcinoma. A worldwide problem and the major risk factors. Dig Dis Sci. 1991;36:962–972. doi: 10.1007/BF01297149. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35:S72–S78. doi: 10.1097/00004836-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 3.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107, vi. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 5.Parker SL, Davis KJ, Wingo PA, Ries LA, Heath CW. Cancer statistics by race and ethnicity. CA Cancer J Clin. 1998;48:31–48. doi: 10.3322/canjclin.48.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Huh K, Choi SY, Whang YS, Lee DS. Prevalence of viral hepatitis markers in Korean patients with hepatocellular carcinoma. J Korean Med Sci. 1998;13:306–310. doi: 10.3346/jkms.1998.13.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HS, Han CJ, Kim CY. Predominant etiologic association of hepatitis C virus with hepatocellular carcinoma compared with hepatitis B virus in elderly patients in a hepatitis B-endemic area. Cancer. 1993;72:2564–2567. doi: 10.1002/1097-0142(19931101)72:9<2564::aid-cncr2820720909>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Tsai JF, Jeng JE, Ho MS, Chang WY, Lin ZY, Tsai JH. Hepatitis B and C virus infection as risk factors for hepatocellular carcinoma in Chinese: a case-control study. Int J Cancer. 1994;56:619–621. doi: 10.1002/ijc.2910560502. [DOI] [PubMed] [Google Scholar]
- 9.Leung NW, Tam JS, Lai JY, Leung TW, Lau WY, Shiu W, Li AK. Does hepatitis C virus infection contribute to hepatocellular carcinoma in Hong Kong? Cancer. 1992;70:40–44. doi: 10.1002/1097-0142(19920701)70:1<40::aid-cncr2820700107>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 10.Lee SD, Lee FY, Wu JC, Hwang SJ, Wang SS, Lo KJ. The prevalence of anti-hepatitis C virus among Chinese patients with hepatocellular carcinoma. Cancer. 1992;69:342–345. doi: 10.1002/1097-0142(19920115)69:2<342::aid-cncr2820690211>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 11.Simmons GC, Yeong ML, Lee SP. The association of hepatitis B viral infection and hepatocellular carcinoma in New Zealand. N Z Med J. 1983;96:669–671. [PubMed] [Google Scholar]
- 12.Blakely TA, Bates MN, Baker MG, Tobias M. Hepatitis B carriage explains the excess rate of hepatocellular carcinoma for Maori, Pacific Island and Asian people compared to Europeans in New Zealand. Int J Epidemiol. 1999;28:204–210. doi: 10.1093/ije/28.2.204. [DOI] [PubMed] [Google Scholar]
- 13.Chung WK, Sun HS, Park DH, Minuk GY, Hoofnagle JH. Primary hepatocellular carcinoma and hepatitis B virus infection in Korea. J Med Virol. 1983;11:99–104. doi: 10.1002/jmv.1890110203. [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Ikematsu H, Hirohata T, Kashiwagi S. Hepatitis C virus infection and risk of hepatocellular carcinoma among Japanese: possible role of type 1b (II) infection. J Natl Cancer Inst. 1996;88:742–746. doi: 10.1093/jnci/88.11.742. [DOI] [PubMed] [Google Scholar]
- 15.Matsuda Y, Amuro Y, Higashino K, Hada T, Yamamoto T, Fujikura M, Yamaguchi K, Shimomura S, Iijima H, Nakano T. Relation between markers for viral hepatitis and clinical features of Japanese patients with hepatocellular carcinoma: possible role of alcohol in promoting carcinogenesis. Hepatogastroenterology. 1995;42:151–154. [PubMed] [Google Scholar]
- 16.Suga M, Senota A, Arima K, Kodama T, Ikuta S, Sakamoto H, Ohe Y, Sugai S, Yachi A. Prevalence of HBV and HCV infection in Japanese patients with hepatocellular carcinoma. Hepatogastroenterology. 1994;41:438–441. [PubMed] [Google Scholar]
- 17.Tanaka K, Hirohata T, Koga S, Sugimachi K, Kanematsu T, Ohryohji F, Nawata H, Ishibashi H, Maeda Y, Kiyokawa H. Hepatitis C and hepatitis B in the etiology of hepatocellular carcinoma in the Japanese population. Cancer Res. 1991;51:2842–2847. [PubMed] [Google Scholar]
- 18.Edamoto Y, Tani M, Kurata T, Abe K. Hepatitis C and B virus infections in hepatocellular carcinoma. Analysis of direct detection of viral genome in paraffin embedded tissues. Cancer. 1996;77:1787–1791. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1787::AID-CNCR5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Davila JA, Petersena NJ, Nelson HA, El-Serag HB. Geographic variation within the United States in the incidence of hepatocellular carcinoma. J Clin Epidemiol. 2003;56:487–493. doi: 10.1016/s0895-4356(02)00605-4. [DOI] [PubMed] [Google Scholar]
- 20.Hashibe M, Gao T, Li G, Dalbagni G, Zhang ZF. Comparison of bladder cancer survival among Japanese, Chinese, Filipino, Hawaiian and Caucasian populations in the United States. Asian Pac J Cancer Prev. 2003;4:267–273. [PubMed] [Google Scholar]
- 21.Pagano IS, Morita SY, Dhakal S, Hundahl SA, Maskarinec G. Time dependent ethnic convergence in colorectal cancer survival in Hawaii. BMC Cancer. 2003;3:5. doi: 10.1186/1471-2407-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163:49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Hassan MM, Frome A, Patt YZ, El-Serag HB. Rising prevalence of hepatitis C virus infection among patients recently diagnosed with hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2002;35:266–269. doi: 10.1097/00004836-200209000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Hwang SJ, Tong MJ, Lai PP, Ko ES, Co RL, Chien D, Kuo G. Evaluation of hepatitis B and C viral markers: clinical significance in Asian and Caucasian patients with hepatocellular carcinoma in the United States of America. J Gastroenterol Hepatol. 1996;11:949–954. [PubMed] [Google Scholar]
- 25.Pyong SJ, Tsukuma H, Hiyama T. Case-control study of hepatocellular carcinoma among Koreans living in Osaka, Japan. Jpn J Cancer Res. 1994;85:674–679. doi: 10.1111/j.1349-7006.1994.tb02413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chin PL, Chu DZ, Clarke KG, Odom-Maryon T, Yen Y, Wagman LD. Ethnic differences in the behavior of hepatocellular carcinoma. Cancer. 1999;85:1931–1936. [PubMed] [Google Scholar]
- 27.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Hepatology. 2001;33:62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 28.So SK, Esquivel CO, Imperial JC, Garcia G, Monge H, Keeffe EB. Does Asian race affect hepatitis B virus recurrence or survival following liver transplantation for hepatitis B cirrhosis? J Gastroenterol Hepatol. 1999;14 Suppl:S48–S52. doi: 10.1046/j.1440-1746.1999.01900.x. [DOI] [PubMed] [Google Scholar]
- 29.Okada S, Ishii H, Nose H, Okusaka T, Kyogoku A, Yoshimori M, Shimada K, Yamamoto J, Kosuge T, Yamasaki S, et al. Effect of heavy alcohol intake on long-term results after curative resection of hepatitis C virus-related hepatocellular carcinoma. Jpn J Cancer Res. 1996;87:867–873. doi: 10.1111/j.1349-7006.1996.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tagger A, Donato F, Ribero ML, Chiesa R, Portera G, Gelatti U, Albertini A, Fasola M, Boffetta P, Nardi G. Case-control study on hepatitis C virus (HCV) as a risk factor for hepatocellular carcinoma: the role of HCV genotypes and the synergism with hepatitis B virus and alcohol. Brescia HCC Study. Int J Cancer. 1999;81:695–699. doi: 10.1002/(sici)1097-0215(19990531)81:5<695::aid-ijc4>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 31.Kubo S, Kinoshita H, Hirohashi K, Tanaka H, Tsukamoto T, Shuto T, Kuroki T. High malignancy of hepatocellular carcinoma in alcoholic patients with hepatitis C virus. Surgery. 1997;121:425–429. doi: 10.1016/s0039-6060(97)90313-5. [DOI] [PubMed] [Google Scholar]
- 32.Yu H, Harris RE, Kabat GC, Wynder EL. Cigarette smoking, alcohol consumption and primary liver cancer: a case-control study in the USA. Int J Cancer. 1988;42:325–328. doi: 10.1002/ijc.2910420304. [DOI] [PubMed] [Google Scholar]


